Abstract
Background
Smoking cessation improves morbidity and mortality among smokers who achieve long-term abstinence. Many smokers are using electronic cigarettes (e-cigarettes) to attempt to quit, despite a lack of data concerning their efficacy and safety for smoking cessation.
Methods
The Evaluating the Efficacy of E-Cigarette use for Smoking Cessation (E3) trial is a multicentre randomized controlled trial (NCT02417467) with a treatment period of 12 weeks and follow-up of 52 weeks. A total of 376 participants motivated to quit smoking were enrolled at 17 Canadian centres (November 2016 to September 2019). Participants were randomized (1:1:1) to 1 of 3 treatment arms: nicotine e-cigarettes, non-nicotine e-cigarettes, or no e-cigarettes. All groups received individual counselling. Treatment allocation was double-blind for the e-cigarette groups. The trial includes follow-ups by telephone at weeks 1, 2, 8, and 18, and clinic visits at weeks 4, 12, 24, and 52. The primary endpoint is to compare nicotine e-cigarettes to counselling alone in terms of biochemically validated point-prevalence smoking abstinence at 12 weeks; the primary endpoint was changed from 52 weeks after early termination (77% of targeted enrollment) due to a prolonged delay in e-cigarette manufacturing. The secondary objectives are to examine the efficacy of nicotine and non-nicotine e-cigarettes in terms of point-prevalence and continuous smoking abstinence, and reduction in daily cigarette consumption at all follow-ups through week 52, and to describe the occurrence of adverse events.
Conclusion
The E3 trial will provide regulators, health care professionals, and smokers with important information about the efficacy and safety of e-cigarettes for smoking cessation.
Résumé
Contexte
Le sevrage tabagique améliore la morbidité et la mortalité chez les fumeurs qui parviennent à une abstinence à long term. De nombreux fumeurs utilisent des cigarettes électroniques (e-cigarettes) pour tenter d'arrêter de fumer, malgré le manque de données concernant leur efficacité et leur sécurité pour le sevrage tabagique.
Méthodes
L'essai "Evaluation l’utilisation de la cigarette Électronique (E3)” pour cesser de fumer (E3)” est un essai contrôlé randomisé multicentrique (NCT02417467) avec une période de traitement de 12 semaines et un suivi de 52 semaines. Au total, 376 participants motivés à cesser de fumer ont été inscrits par 17 centres canadiens (de novembre 2016 à septembre 2019). Les participants ont été randomisés (1:1:1) dans l'un des trois groupes de traitement : e-cigarettes à la nicotine, e-cigarettes sans nicotine ou sans e-cigarette. Tous les groupes ont bénéficié de conseils individuels. Pour les groups avec une e-cigarettes, l'attribution des traitements a été faite en double aveugle. L'essai comprend de suivis par téléphone aux semaines 1, 2, 8 et 18, et des visites à la clinique aux semaines 4, 12, 24 et 52. L'objectif principal est de comparer les e-cigarettes nicotinique au conseils seul, en termes d’abstinence tabagique ponctuelle de 7 jours confirmée par un indicateur biochimique validée à 12 semaines; changé de la 52 semaines en raison d’une fin anticipée (77 % des inscriptions ciblées) dû à un retard prolongé dans la fabrication des e-cigarettes. Les objectifs secondaires sont d'examiner l'efficacité des e-cigarettes à la nicotine et sans nicotine en termes de prévalence ponctuelle et de sevrage tabagique continu, et la réduction quotidienne de cigarettes consommer pour l’ensemble des suivis jusqu'à la semaine 52, et de décrire l'occurrence des effets indésirables.
Conclusion
L'essai E3 fournira aux régulateurs, aux professionnels de la santé et aux fumeurs des informations importantes sur l'efficacité et la sécurité des e-cigarettes pour le sevrage tabagique.
More than half of adults who smoke conventional cigarettes attempted to quit in the past year, many using the increasingly popular electronic cigarette (e-cigarette).1 Between 2014 and 2016, North Americans who made a quit attempt were more likely to use an e-cigarette (35.3%) as a cessation tool than the nicotine patch or gum (24.5%), or an approved medication such as bupropion (Zyban) or varenicline (Champix/Chantix) (12.2%).2 The available data from randomized controlled trials (RCTs), which varied substantially in designs and populations (Supplemental Appendix S1), suggest that nicotine e-cigarettes may be modestly more efficacious for smoking cessation than conventional smoking cessation therapies.3, 4, 5, 6 However, many of these RCTs were limited by small sample sizes, conducted in smokers not motivated to quit, or were otherwise not designed to evaluate the efficacy of e-cigarettes compared with conventional therapies.3,5,7, 8, 9, 10, 11
The recent outbreak of e-cigarette, or vaping, product use-associated lung injury (EVALI) in the United States has also brought into question the safety of e-cigarettes. As of mid-January 2020, a total of 2668 people were hospitalized, and 60 had died after using e-cigarettes.12 Mounting evidence suggests that the outbreak is likely due to the use of e-cigarette liquids containing cannabis derivatives.13 Although most commercially available e-cigarettes are therefore unlikely to cause EVALI, additional data are required concerning their safety. The Evaluating the Efficacy of E-Cigarette use for Smoking Cessation (E3) trial will improve our understanding of the efficacy and safety of e-cigarettes for smoking cessation in North America.
Methods
Study design
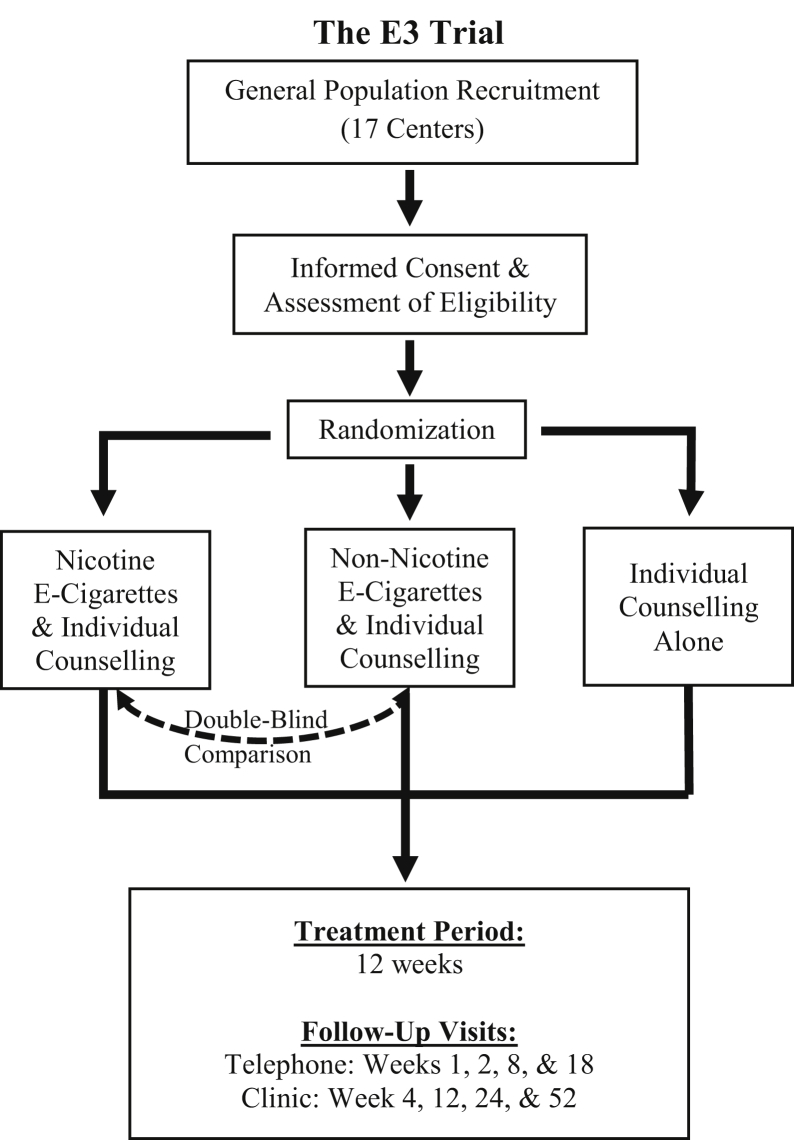
The E3 trial (clinicaltrials.gov registration NCT02417467) is a multicentre, three-arm RCT with a treatment period of 12 weeks and follow-up of 52 weeks (Fig. 1). Participants were randomized to 1 of 3 treatment arms: (1) nicotine e-cigarettes with individual counselling, (2) non-nicotine e-cigarettes with individual counselling, or (3) individual counselling alone. The primary objective is to compare the efficacy of nicotine and non-nicotine e-cigarettes, combined with individual counselling, with that of individual counselling alone, in terms of biochemically validated smoking abstinence at 12 weeks.
Figure 1.
The Evaluating the Efficacy of E-Cigarette (E3) use for Smoking Cessation trial flow chart.
A total of 376 participants were enrolled at 17 centres across Canada (Supplemental Appendix S2) from November 2016 to September 2019. Because of a prolonged delay in e-cigarette manufacturing, the trial was terminated early with 77% of the target sample enrolled. The last participant enrolled will complete follow-up in September 2020. This article reporting the E3 trial protocol (E3-001, Version 5, January 8, 2020) conforms to the SPIRIT reporting guidelines.14
Recruitment
Participants were recruited through community-based advertisements (eg, printed fliers, newspaper ads) and online platforms (eg, Craigslist, Kijiji, Facebook), as well as in outpatient, smoking cessation, and walk-in clinics. Advertising material was approved by research ethics boards (REBs) at each participating centre. Preliminary screening was conducted in-person or over the phone. Potential participants were informed that nonidentifying screening data would be kept for research purposes and that consent could be withdrawn at any time (Supplemental Appendix S3). Interested potential participants were scheduled for an in-person baseline visit to provide informed consent and confirm eligibility.
Informed consent, eligibility, and randomization
Noninvestigator research personnel obtained informed consent, assessed eligibility, and randomized participants. Before the collection of data, written informed consent was obtained from all participants (Supplemental Appendix S4). Participants were given time and opportunity to ask questions and decide whether to participate before providing informed consent. Participants were informed that participation was voluntary and that they could withdraw consent for any reason at any time without penalty.
The inclusion criteria for the E3 trial are described in Figure 2. Briefly, to be included, participants must have smoked ≥10 cigarettes/day on average for the past year and be motivated to quit, as assessed by the Motivation To Stop Scale (level 5 or higher, Supplemental Appendix S5).15 Individuals were excluded if they had used an e-cigarette in the past 60 days or had ever used an e-cigarette for more than 7 consecutive days. Individuals were also excluded if they had used a smoking cessation therapy in the past 30 days.
Figure 2.
Inclusion and exclusion criteria for the Evaluating the Efficacy of E-Cigarette (E3) use for Smoking Cessation trial.
Participants who provided informed consent and met eligibility criteria were randomized (1:1:1) via an online data management system (Information Management Services of the Lady Davis Institute, Montréal, Québec, Canada) to (1) nicotine e-cigarettes with counselling, (2) non-nicotine e-cigarettes with counselling, or (3) counselling alone for 12 weeks. Randomization was stratified by centre and used permutated blocks (random block sizes of 6 and 9) to conceal the allocation sequence. For participants randomized into an e-cigarette group, participants and study personnel were blind to nicotine content. At baseline, demographic information and health and smoking history were collected. Participants also completed the Fagerström Test for Nicotine Dependence,16 the Smoking Cessation Quality of Life (SCQoL) questionnaire,17 the Beck Depression Inventory (BDI-II),18 and the Glover Nilsson Smoking Behavioral Questionnaire (GN-SBQ).19
Interventions
The treatment period was 12 weeks. Participants randomized to an e-cigarette arm received a closed-system NJOY (Scottsdale, AZ) rechargeable e-cigarette at baseline, with tobacco-flavoured cartridge refills (Fig. 3). Cartridges for nicotine (15 mg/mL) and non-nicotine (0 mg/mL) e-cigarettes were identical in appearance. Participants were not directed to use their e-cigarette for a particular number of sessions or puffs, as the optimal use was expected to naturally vary based on individuals’ smoking habits and level of addiction. E-cigarette use was monitored via self-report during follow-ups and the return of all used and unused cartridges at clinic visits. A total of 21 cartridges were supplied at baseline, with an additional 21 cartridges provided at week 4, if needed. Participants were advised of the signs and symptoms of nicotine toxicity and allergic reactions, and were instructed to discontinue use and seek medical attention if they suspected a nicotine overdose or allergic reaction.
Figure 3.
E-cigarette used in the Evaluating the Efficacy of E-Cigarette (E3) use for Smoking Cessation trial. Device and e-liquid characteristics can be found here: https://www.drugabuse.gov/research/research-data-measures-resources/nida-drug-supply-program/supplemental-information-nida-e-cig.
Individual smoking cessation/relapse prevention counselling was provided to all groups for a minimum of 30 minutes at baseline, 10 minutes during telephone follow-ups, and 15 minutes at clinic visits (20 minutes at week 4). The counselling script used (Supplemental Appendix S6) was based on the Program Training and Consultation Centre (Ontario) guidelines for tobacco use cessation counselling20 and provided by trained study personnel.
Nonstudy smoking cessation therapy use was recorded. Participants were encouraged to use only their randomized smoking cessation treatment during the treatment period, as there are limited safety data available concerning the concomitant use of e-cigarettes with other pharmacological smoking cessation therapies. In addition, the use of non-study therapies would render it more difficult to determine the efficacy of e-cigarettes, reducing the scientific validity (and therefore the ethical conduct) of the trial; abstaining from other smoking cessation therapies for a short period of time (12 weeks) was felt not to disadvantage trial participants in a meaningful way. Those who were still smoking at the end of the treatment period were permitted to use other therapies during follow-up. Participants were retained for the duration of follow-up regardless of the use of non-study smoking cessation therapy.
Individual counselling was selected as the comparator for several reasons. First, a behavioural intervention as the comparator provides greater assay sensitivity (ie, the ability to clearly determine if a treatment is efficacious), while ensuring that all participants receive an evidence-based therapy. Second, although there is evidence that combination therapy (a behavioural intervention with pharmacotherapy) is more efficacious than a behavioural intervention alone, many studies in this area included minimal clinical interventions as a behavioural intervention; there is strong evidence that minimal clinical intervention has modest efficacy relative to other behavioural interventions.21 There remains greater equipoise regarding the comparison of combination therapy vs individual counselling.21 Third, with a relatively short treatment period and participants encouraged to use non-study interventions after this period, the use of counselling was felt to be ethical. Finally, a comparison of a combination of e-cigarettes (with or without nicotine) with individual counselling vs another combination therapy would not have been feasible for a publicly funded trial, as it would have required a substantially larger sample size. Ultimately, the choice of individual counselling as the comparator balanced the scientific, ethical, and practical needs of the trial.
Follow-up
Study follow-ups were conducted by telephone at weeks 1, 2, 8, and 18, and clinic visits at weeks 4, 12, 24, and 52 after randomization. At all follow-ups, information was collected concerning self-reported smoking and e-cigarette use, withdrawal symptoms and potential side effects, use of non-study smoking cessation therapies, and the occurrence of adverse events. At clinic visits, weight, blood pressure, and heart rate were measured, and self-reported smoking abstinence was biochemically validated using exhaled carbon monoxide. At most clinic visits, participants completed the GN-SBQ, SCQoL, and BDI-II questionnaires.16,18,19 Participants randomized to e-cigarette arms were asked to guess their treatment allocation at week 12.
To limit losses to follow-up, participants provided multiple methods of contact (mail, phone, e-mail, and alternate person) at baseline. Study personnel were instructed to make up to 12 attempts to reach participants for a given follow-up, and to try again for each subsequent follow-up. Participants were not withdrawn for missing follow-ups. For follow-ups that would otherwise be missed, the collection of vital and smoking status, as well as serious adverse events (SAEs), was prioritized.
Endpoints
The primary endpoint is 7-day point-prevalence abstinence at week 12 (the end of the treatment period). The protocol was amended to change the timing of the primary endpoint from 52 weeks after early termination of enrolment that reduced power (see the “Sample Size Calculations” section). Point-prevalence abstinence is defined as self-reported abstinence in the past week, with biochemical validation using exhaled carbon monoxide ≤10 parts per million. Secondary endpoints include (1) 7-day point-prevalence abstinence (at all other follow-ups) (biochemically validated at weeks 4, 24, and 52); (2) continuous abstinence, defined as self-reported abstinence at the current and all preceding follow-ups (biochemically validated at weeks 4, 12, 24, and 52); (3) reduction in self-reported daily cigarette consumption; and (4) the incidence of adverse events throughout the treatment and follow-up periods.
Data collection, validation, and monitoring
Data are recorded on case report forms and questionnaires (considered source documents) provided to study centres. All collected data (except identifying information) are entered into a secure online data management system. These data are reviewed by the coordinating centre for inconsistencies, gross typological errors, and missing datapoints, and study centres are queried for identified errors. Study centres are selected for a monitoring visit based on their enrollment rate (≥10 participants). Sites that enroll ≥30 participants are monitored again. Monitoring is conducted to verify that (1) the rights and well-being of participants are protected; (2) reported data are accurate, complete, and verifiable from source documents; and (3) the study is conducted in compliance with the currently approved protocol, Good Clinical Practice (GCP), and applicable regulatory requirements.
Participant safety
E-cigarette safety and tolerability data being collected include adverse events following the International Conference on Harmonization and GCP guidelines.22,23 An Endpoints Evaluation Committee (EEC, Supplemental Appendix S1) independently reviews all documentation pertaining to each reported SAE, classifies the SAE (eg, cardiovascular, respiratory, etc), and determines its potential causal relationship with the study intervention (e-cigarettes or counselling). The EEC is blinded to e-cigarette arms (nicotine vs non-nicotine). SAEs are also reported to the REBs of the enrolling and coordinating centres. Although unlikely, any site investigator, the EEC and/or REBs can request unblinding due to an adverse event. The Steering Committee has the final responsibility for deciding whether to unblind a participant.
The E3 trial has a Data and Safety Monitoring Board (DSMB, Supplemental Appendix S1) that acts as an external review committee to ensure the safety of participants and protect the scientific integrity of the trial. The DSMB reviews quality assurance data, including efficacy and safety outcomes and recruitment trends, and makes recommendations regarding the continuation of the trial every 6 months; quality assurance data are blind to the content of the e-cigarettes (ie, the nicotine and non-nicotine treatment arms were combined). An unblinded interim analysis was planned; however, given the nature of the trial and the sample size, the DSMB felt that formal stopping rules would not be appropriate.
Sample size calculations
We calculated a sample size of 486 participants (162 per arm) to have >80% power detect a ≥12% absolute difference in smoking abstinence at 52 weeks between the nicotine e-cigarette and counselling only arms. This calculation assumed a 52-week point-prevalence abstinence rate of 10% among participants randomized to counselling and a 2-tailed α of 0.05.24 However, because of an unexpected and prolonged delay in e-cigarette manufacturing, enrolment of new participants was terminated after 376 participants were enrolled. With 125 participants per arm, the estimated power to detect a ≥12% difference at 52 weeks was calculated to be <68%. Given this reduction in power, the time point of the primary endpoint was changed to 12 weeks; differences between treatment groups are expected to narrow over the course of follow-up as participants exit the treatment period and some relapse to smoking. Therefore, the greatest difference in abstinence between treatment groups is likely to be at the end of the 12-week treatment period. The decisions to stop enrollment and to change the timing of the primary endpoint were made by the Steering Committee, in consultation with the DSMB. At the time of these decisions, partially blinded quality assurance data were available regarding abstinence rates, which were broken down into the counselling only arm, and the nicotine and non-nicotine treatment arms combined. Importantly, no formal statistical analysis had been conducted and no inferential statistics were available.
Statistical analyses
The primary and secondary analyses will use an intention-to-treat approach in which participant data will be analysed according to the group to which they were randomized, regardless of treatment received. The initial descriptive analysis will examine the balance of demographic, clinical, and smoking variables between the 3 treatment groups; a variable will be considered imbalanced if the absolute value of the standardized difference is >0.1. Discrete data will be described using counts and proportions. Continuous data will be described using means and standard deviations or, in the presence of skewed distributions, medians and interquartile ranges.
Following the descriptive analyses, the primary analysis will compare point-prevalence abstinence at 12 weeks among participants randomized to nicotine e-cigarettes with that of participants randomized to counselling only using 95% confidence intervals based on the binomial distribution. The primary analysis will be unadjusted; if differences in baseline characteristics are identified between groups, we will use multivariable logistic regression to adjust for these differences in sensitivity analyses. Similar analyses will then be conducted as secondary analyses to compare the nicotine and non-nicotine e-cigarette groups and the non-nicotine e-cigarette and counselling only groups.
In secondary analyses, we will examine point-prevalence and continuous abstinence at weeks 1, 2, 4, 8, 18, 24, and 52 (biochemically validated at weeks 4, 12, 24, and 52; others self-report only) and between all treatment groups. In addition, we will examine the effect of the treatment group on daily cigarette consumption using linear regression, with cigarette consumption transformed using a log transformation to account for its skewed distribution. The occurrence of adverse events and SAEs will be compared between the 3 treatment groups, with cumulative events at 12 weeks representing the main safety measure. SAEs will be reported by the treatment group as well as type of event (eg, respiratory, cardiovascular, neurological, and other events). Although power is expected to be modest, we will perform hypothesis-generating subgroup analyses to investigate if treatment effects differ by age, sex, nicotine dependence (baseline Fagerström Test for Nicotine Dependence score), baseline cigarettes smoked per day, the influence of having another smoker at home, and quality of life (baseline SCQoL, BDI, and GN-SBQ scores). A priori, we anticipate potentially greater benefits with e-cigarettes among younger participants than older ones given their greater familiarity with e-cigarettes, but no differences in other subgroups. In addition, we will examine the effect of withdrawal symptoms, weight change, and therapy adherence (including frequency of e-cigarette use) on abstinence measures. In sensitivity analyses, we will use regression analyses to explore the effect of the use of non-study smoking cessation therapies on our results.
Several methods will be used to account for missing data. Outcome data may be missing from participants who withdrew or were lost to follow-up. These participants will be included in the intention-to-treat analyses by being classified as smokers. This is a standard assumption used in smoking cessation trials to account for these participants, as smokers attempting to quit unaided have a very low likelihood of abstinence. For smoking reduction analyses, these participants will be presumed to have returned to their baseline smoking amount. To examine the effect of this assumption on the robustness of our results, 2 sensitivity analyses will be performed: (1) restricted to those who returned for follow-up (ie, a complete case analysis); and (2) using multiple imputation. In the event of participant death, data will be censored at the time of death. Covariate data may also be missing. However, our primary analysis will be unadjusted; therefore, missing covariate data will not affect our primary analysis. Multiple imputation will be used to account for missing covariate data in sensitivity analyses that adjust for characteristic imbalances between groups.
Ethical considerations
The E3 trial is being conducted according to all applicable institutional, provincial, and federal regulations concerning clinical trials. The protocol was reviewed and approved by the REBs of each participating institution, and No Objection Letters were issued by Health Canada for the protocol and amendments. The study conforms to the International Conference on Harmonization, GCP guidelines, and the ethical principles embodied in the Declaration of Helsinki.22,23 The trial results will be published in peer-reviewed scientific journals and made available on clinicaltrials.gov. Requests for deidentified participant data will be handled on a case-by-case basis.
Discussion
The E3 trial will evaluate the efficacy of nicotine and non-nicotine e-cigarettes for smoking cessation in a general Canadian population of smokers motivated to quit. The trial will also contribute to what is known about the short-term safety and tolerability of e-cigarettes. Only a small number of RCTs examining e-cigarette efficacy have been conducted to date; these trials varied greatly in their specific objectives, study designs, and endpoints (Supplemental Appendix S1). Three RCTs were conducted in North America (all in the United States),8,9,11 and predominantly included smokers not motivated to quit; 2 were of very short duration with small sample sizes (<100).8,9,11 The larger RCT was a workplace-based study that randomized 6006 employees, who were automatically opted into a variety of smoking cessation interventions (eg, financial incentives and free therapies). Only 20% of the randomized employees logged into the trial’s website during the course of the study.9 The provision of free e-cigarettes was not found to be more efficacious than other interventions in terms of continuous abstinence.9 Trials conducted internationally have suggested that e-cigarettes may be modestly more efficacious for smoking cessation than conventional nicotine replacement therapies (NRTs).3, 4, 5, 6 However, the generalizability of these findings to a North American general population remains unclear.
The best available evidence for e-cigarette efficacy for smoking cessation to date comes from a parallel-group RCT by Hajek et al.4 This trial randomized 886 participants in the United Kingdom to nicotine e-cigarettes or NRT.4 The authors found that participants randomized to nicotine e-cigarettes were more likely to be continuously abstinent at 52 weeks compared with those randomized to NRT (18.0% vs 9.9%; relative risk, 1.83; 95% confidence interval, 1.30-2.58). However, this trial had several important limitations. Participants randomized to e-cigarettes were asked to purchase their own e-liquid once they ran out of the initial 30 mL bottle provided by the trial, and were encouraged to experiment with different flavours and nicotine strengths.4 This lack of standardized e-cigarette treatment makes it hard to determine whether the effectiveness of the e-cigarettes is related to behavioural smoking cues or is instead due to a particular nicotine strength or flavouring. In addition, the study-provided e-cigarette was not returned, and it is unclear if participants were instructed to stop e-cigarette use at a particular time. Among abstinent participants, 80% of participants in the e-cigarette group were still using an e-cigarette at 52 weeks, whereas only 9% of the NRT group were still using an NRT.4 This differential duration of use renders the results difficult to interpret, as it is unclear if the observed difference is due to the efficacy of e-cigarettes or the longer duration of treatment.
Three RCTs conducted internationally assessed the efficacy of both nicotine and non-nicotine e-cigarettes for smoking cessation, and included a comparator arm without e-cigarettes (Supplemental Appendix S1).5, 6, 7 Masiero et al.5 (n = 210) found that among smokers enrolled in a lung cancer screening program for high-risk individuals, both nicotine (25%) and non-nicotine e-cigarettes (23%) increased abstinence at 12 weeks compared with a low-intensity counselling control group (10%). Bullen et al.7 (n = 657) found that 7% of participants using nicotine e-cigarettes were continuously abstinent at 6 months, along with 6% of those using the nicotine patch, and 4% of those using non-nicotine e-cigarettes; however, the differences between groups were not statistically significant. Walker et al.6 (n = 1124) found that participants randomized to nicotine e-cigarettes with the nicotine patch were more likely to be abstinent at 6 months (7%) compared with those randomized to non-nicotine e-cigarettes with the patch (4%), or the patch alone (2%). However, this design does not address the efficacy of e-cigarettes alone (rather than in combination) compared with other smoking cessation therapies. Therefore, although some evidence suggests that e-cigarettes may be modestly efficacious for smoking cessation internationally, the available data address varied specific study objectives and are of limited generalizability. The E3 trial will contribute to what is known about the efficacy of both nicotine and non-nicotine e-cigarettes for smoking cessation in a general North American population.
The E3 trial has several limitations. First, the trial was terminated early because of an unexpected and prolonged delay in e-cigarette manufacturing, with 77% of the targeted study population enrolled. Given the reduced power to detect differences in smoking abstinence at 52 weeks, the protocol was amended to change the primary endpoint to 12 weeks (with subsequent reporting of 24- and 52-week data). Second, smoking cessation trials are known to have relatively high losses to follow-up of at least 20%-30%. However, participants who withdraw or are lost to follow-up will be assumed to have returned to smoking at their baseline amount. This is a standard assumption among smoking cessation trials, and more valid than the exclusion of these participants, given a low chance of successful abstinence when quitting unaided. Several sensitivity analyses will be conducted to assess the impact of this assumption on our results. Third, the counselling only arm could not be blinded. This could lead to differential losses to follow-up or increased use of non-study smoking cessation aids, the implications of which will be examined in sensitivity analyses. Lastly, the E3 trial is not powered to detect differences in the occurrence of SAEs between groups. However, given the ongoing cases of EVALI in North America and other respiratory concerns related to e-cigarette use,13 the E3 trial safety data collected will constitute some of the best available pieces of evidence concerning the safety of short-term e-cigarette use for smoking cessation.
Conclusions
The E3 trial will provide important information concerning the efficacy and safety of short-term nicotine and non-nicotine e-cigarette use for smoking cessation. The evidence generated by this trial is essential to aid in policy creation and clinical decision-making concerning the use of e-cigarettes for smoking cessation, and to improve smoking abstinence among individuals who smoke.
Acknowledgement
We would like to thank Natalie Zacchia for her assistance with trial initiation.
Funding Sources
This trial was funded by grants from the Canadian Institutes for Health Research (CIHR), Canada (Funding Reference Numbers 133727 and 155969). E-cigarettes were purchased from NJOY Inc., United States. NJOY had no role in the design, conduct, analysis, interpretation of data, or reporting of the E3 trial. K. B. Filion is supported by a Junior II salary support award from the Fonds de recherche du Québec – santé, Canada and a William Dawson Scholar award from McGill University, Canada.
Disclosures
M.J. Eisenberg received educational grants from Pfizer Inc., Canada to provide continuing medical education in cardiology. The rest of the authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported in this paper was conducted according to all applicable institutional, provincial, and federal regulations concerning clinical trials. The study conforms to the International Conference on Harmonization, GCP guidelines, and the ethical principles embodied in the Declaration of Helsinki.
See page 174 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.03.006.
Supplementary Material
References
- 1.Creamer M.R., Wang T.W., Babb S. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013. doi: 10.15585/mmwr.mm6845a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caraballo R.S., Shafer P.R., Patel D., Davis K.C., McAfee T.A. Quit methods used by US adult cigarette smokers, 2014–2016. Prevent Chronic Dis. 2017;14:E32. doi: 10.5888/pcd14.160600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caponnetto P., Campagna D., Cibella F. Efficiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PloS One. 2013;8 doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajek P., Phillips-Waller A., Przulj D. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629–637. doi: 10.1056/NEJMoa1808779. [DOI] [PubMed] [Google Scholar]
- 5.Masiero M., Lucchiari C., Mazzocco K. E-cigarettes may support smokers with high smoking-related risk awareness to stop smoking in the short run: preliminary results by randomized controlled trial. Nicotine Tobacco Res. 2018;21:119–126. doi: 10.1093/ntr/nty047. [DOI] [PubMed] [Google Scholar]
- 6.Walker N., Parag V., Verbiest M. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet Resp Med. 2020;8:54–64. doi: 10.1016/S2213-2600(19)30269-3. [DOI] [PubMed] [Google Scholar]
- 7.Bullen C., Howe C., Laugesen M. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet. 2013;382:1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter M.J., Heckman B.W., Wahlquist A.E. A naturalistic, randomized pilot trial of e-cigarettes: uptake, exposure, and behavioral effects. Cancer Epidemiol Prevent Biomarkers. 2017;26:1795–1803. doi: 10.1158/1055-9965.EPI-17-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpern S.D., Harhay M.O., Saulsgiver K. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. N Engl J Med. 2018;378:2302–2310. doi: 10.1056/NEJMsa1715757. [DOI] [PubMed] [Google Scholar]
- 10.Lee S.-H., Ahn S.-H., Cheong Y.-S. Effect of electronic cigarettes on smoking reduction and cessation in Korean male smokers: a randomized controlled study. J Am Board Family Med. 2019;32:567–574. doi: 10.3122/jabfm.2019.04.180384. [DOI] [PubMed] [Google Scholar]
- 11.Tseng T.-Y., Ostroff J.S., Campo A. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine Tobacco Res. 2016;18:1937–1943. doi: 10.1093/ntr/ntw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention Outbreak of Lung Injury Associated with the Use of E-Cigarette, or Vaping, Products. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html Available at: Accessed January 27, 2020.
- 13.Krishnasamy V.P. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90–94. doi: 10.15585/mmwr.mm6903e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan A.-W., Tetzlaff J.M., Altman D.G. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Internal Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hummel K., Brown J., Willemsen M.C., West R., Kotz D. External validation of the Motivation To Stop Scale (MTSS): findings from the International Tobacco Control (ITC) Netherlands Survey. Eur J Public Health. 2016;27:129–134. doi: 10.1093/eurpub/ckw105. [DOI] [PubMed] [Google Scholar]
- 16.Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerstrom K.O. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 17.Olufade A.O., Shaw J.W., Foster S.A. Development of the smoking cessation quality of life questionnaire. Clinical Therapeut. 1999;21:2113–2130. doi: 10.1016/s0149-2918(00)87242-2. [DOI] [PubMed] [Google Scholar]
- 18.Beck A.T., Steer R.A., Brown G.K. Beck depression inventory-II. San Antonio. 1996;78:490–498. [Google Scholar]
- 19.Glover E.D., Nilsson F., Å Westin. Developmental history of the Glover-Nilsson smoking behavioral questionnaire. Am J Health Behav. 2005;29:443–455. doi: 10.5555/ajhb.2005.29.5.443. [DOI] [PubMed] [Google Scholar]
- 20.Program Training and Consultation Centre. Brief Counselling for Tobacco Use Cessation: A Guide for Health Professionals; 2012.
- 21.Lancaster T., Stead L.F. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2017;3:CD001292. doi: 10.1002/14651858.CD001292. [DOI] [PubMed] [Google Scholar]
- 22.Dixon J.R. The international conference on harmonization good clinical practice guideline. Qual Assur. 1998;6:65–74. doi: 10.1080/105294199277860. [DOI] [PubMed] [Google Scholar]
- 23.Salako S.E. The declaration of Helsinki 2000: ethical principles and the dignity of difference. Med Law. 2006;25:341–354. [PubMed] [Google Scholar]
- 24.Eisenberg M.J., Filion K.B., Yavin D. Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. CMAJ. 2008;179:135–144. doi: 10.1503/cmaj.070256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.