Abstract
Background
There are important knowledge gaps in type 2 myocardial infarction (T2MI). Our primary objective was to compare the outcomes of patients with T2MI with those of patients with type 1 myocardial infarction (T1MI). Our secondary objective was to determine whether randomized controlled trials (RCTs) evaluating dual antiplatelets (DAPTs) have explicitly included patients with T2MI.
Methods
We performed a meta-analysis comparing outcomes of patients with T2MI with patients with T1MI and a separate systematic review to evaluate the inclusion of T2MI in RCTs evaluating DAPT. There were 19 cohorts enrolling 48,829 patients (40,604 with T1MI and 5361 with T2MI) and 51 RCTs enrolling 188,132 patients with acute coronary syndrome.
Results
Patients with T2MI had approximately 2-fold increases in unadjusted odds of long-term mortality compared with patients with T1MI (odds ratio, 2.47; 95% confidence interval, 2.06-2.96; P < 0.0001) and a 45% increase in adjusted odds of long-term mortality (odds ratio, 1.45; 95% confidence interval, 1.25-1.69; P < 0.0001, respectively). There was no published evaluation of efficacy, effectiveness, and safety of DAPT in patients with T2MI.
Conclusion
Patients with T2MI are at increased risk of adjusted all-cause long-term mortality compared with patients with T1MI. The role of DAPT remains unclear in T2MI.
Résumé
Contexte
Il existe d’importantes lacunes dans notre connaissance de l’infarctus du myocarde de type 2 (IMT2). Notre objectif principal était de comparer le devenir de patients ayant subi un IMT2 et celui de patients ayant subi un infarctus du myocarde de type 1 (IMT1). Notre objectif secondaire était de déterminer si des essais contrôlés randomisés (ECR) visant à évaluer des bithérapies antiplaquettaires (BA) avaient inclus explicitement des patients ayant subi un IMT2.
Méthodologie
Nous avons réalisé une méta-analyse afin de comparer le devenir de patients ayant subi un IMT2 et celui de patients ayant subi un IMT1. Nous avons aussi effectué une revue systématique distincte des données pour évaluer l’inclusion de cas d’IMT2 dans les ECR visant à évaluer des BA. Il y avait 19 cohortes regroupant 48 829 patients (40 604 ayant subi un IMT1 et 5 361 ayant subi un IMT2) et 51 ECR regroupant 188 132 patients atteints d’un syndrome coronarien aigu.
Résultats
Chez les patients ayant subi un IMT2, la probabilité non corrigée de mortalité à long terme était environ 2 fois plus élevée que chez les patients ayant subi un IMT1 (rapport de cotes : 2,47; intervalle de confiance à 95 % : 2,06-2,96; p < 0,0001), et la probabilité corrigée de mortalité à long terme était accrue de 45 % (rapport de cotes : 1,45; intervalle de confiance à 95 % : 1,25-1,69; p < 0,0001). Aucune évaluation de l’efficacité (potentielle ou réelle) et de l’innocuité des BA chez les patients ayant subi un IMT2 n’a été publiée.
Conclusion
Le risque corrigé de mortalité à long terme toutes causes confondues est plus élevé chez les patients ayant subi un IMT2 que chez les patients ayant subi un IMT1. Le rôle des BA reste à élucider dans les cas d’IMT2.
The term “type 2 myocardial infarction” (T2MI) was first defined by the Second Universal Definition of Myocardial Infarction 20071 and was recently updated in 2018 by the Task Force for the Fourth Universal Definition of Myocardial Infarction.2 T2MI was defined as myocardial infarction (MI) whereby a condition other than atherosclerotic coronary artery disease creates an imbalance between myocardial oxygen supply and demand.1 Currently, there are no formal management guidelines for patients with T2MI.
Dual antiplatelet therapy (DAPT) (aspirin plus a direct or an indirect P2Y12 inhibitor) is the cornerstone in the management of patients with myocardial infarctions secondary to atherosclerotic coronary plaque rupture (T1MI).3,4 However, it remains unclear to what extent DAPT has been evaluated in T2MI. Because platelet activation may be less prominent in T2MI, DAPT may not confer the same potential benefit in patients with T2MI as with T1MI. Notwithstanding, various causes of T2MI may predispose a prothrombotic state, suggesting a potential role for DAPT in patients with T2MI.5 On the other hand, patients with T2MI may have underlying conditions that can increase bleeding risk with DAPT. Considering the current knowledge gaps, we aim to compare the outcomes of patients with T2MI with patients with T1MI and to appraise the uses of DAPT in patients with T2MI enrolled in randomized controlled trials (RCTs) and observational cohorts.
Methods
We performed a systematic review and meta-analysis following the standards set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement6 and the guidelines for reporting meta-analysis of observational studies as proposed by the MOOSE group.7 We conducted 2 independent literature searches in PubMed, EMBASE, and Science Direct. The first search aimed to identify any studies pertaining directly to T2MI. We used the following search terms: type 2 myocardial infarction, secondary MI, supply-demand mismatch, demand ischemia, secondary ischemia, myocardial ischemia, type 2 ischemia, myocardial injury, myocardial necrosis, and silent ischemia. The second search targeted all studies evaluating DAPT in acute coronary syndrome (ACS) using the keywords myocardial infarction, acute coronary syndrome, clopidogrel, prasugrel, ticagrelor, and heart attack. We specifically excluded RCTs evaluating ticlodipine because this drug is rarely if ever used in this contemporary era. Both searches had no language restriction and covered all studies published since 1999 (release of the first DAPT trial Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events [CURE]) to February 12, 2020.
We used the definitions of T1MI and T2MI as defined by the Fourth Universal Definition of Myocardial Infarction.2 We defined reinfarction as reported in each publication. We additionally included any available RCTs or observational studies of T2MI. We excluded editorials, reviews, letters, animal studies, case reports, and conference abstracts. We also excluded studies that evaluated exclusively postoperative myocardial infarction because the term “T2MI” vs postoperative troponin elevation and myocardial injury was often interchangeably used in these instances. Furthermore, the management and outcomes of these patients were inconsistently described. We excluded observational studies that did not report the rates or number of events for T2MI and T1MI separately. For the second search, we included all RCTs that evaluated DAPT in ACS to determine whether any of these trials specifically included patients with T2MI.
Three reviewers (CR, AAT, and TH) extracted data independently. Disagreements were resolved by consensus and the third reviewer (TH). We extracted data about baseline characteristics of study subjects (age, sex, and comorbidities), management, study inclusion and exclusion criteria, and in-hospital and long-term mortality and reinfarction.
We summarized the outcomes (short/intermediate and long-term all-cause mortality and reinfarction). We defined short/intermediate-term mortality as all deaths occurring at less than 1 year and long-term mortality as all deaths occurring during a follow-up of at least 1 year. We computed weighted means of baseline characteristics and rates of outcomes. We pooled the unadjusted and adjusted comparisons of long-term mortality of patients with T1MI and T2MI of the observational studies. We examined the funnel plot to identify potential publication bias. All meta-analyses were completed with random-effects models with Comprehensive Meta-Analysis, Version 3, 2014. We chose random-effect models because of the marked heterogeneity seen in the fixed-effect models.
Results
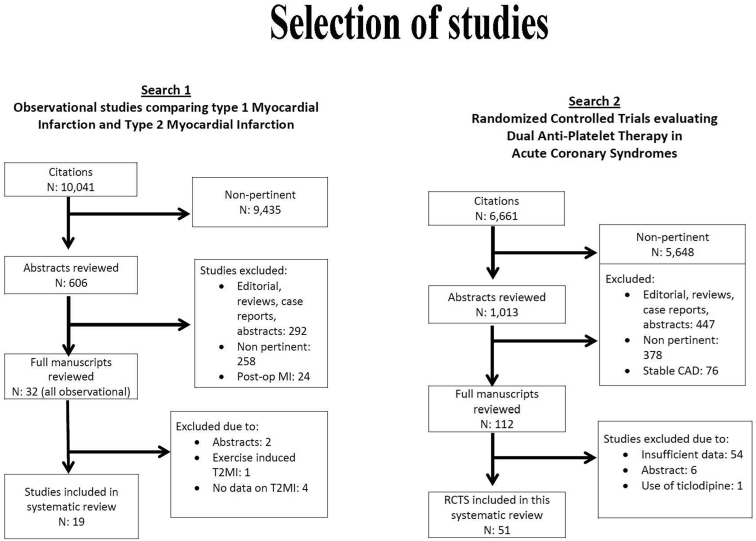
We retrieved 2048 citations of studies of T2MI and 1669 citations of studies evaluating DAPT in ACS (Fig. 1). For the final evaluation, we retained 19 cohorts enrolling 48,829 patients (43,468 with T1MI and 5361 with T2MI)8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 (Table 1) and 51 RCTs enrolling 188,132 patients25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77 (Fig. 1). We described the characteristics of the patients enrolled in the observational studies in Supplemental Table S1. No RCTs evaluating DAPT in ACS have explicitly included patients with T2MI. The effectiveness and safety of DAPT were also not appraised in any observational study of T2MI.
Figure 1.
Selection of studies. RCT, randomized controlled trial; T2MI, type 2 myocardial infarction.
Table 1.
Characteristics of observational studies comparing T2MI with T1MI
| Study first author (year of publication) | Design | Countries | Enrollment periods | No. of patients with MI | No. of centres/countries | Key inclusion criteria | Key exclusion criteria |
|---|---|---|---|---|---|---|---|
| Arora (2018) | Retrospective | United States | 2013-2014 | 1039 | Single centre | All patients with NSTEMI | STEMI, transferred in, no available troponins, cardiac arrest |
| Baron (2014) | Prospective (SWEDEHEART Study) | Sweden | 2011 | 18,891 | 73 Swedish hospitals | MI hospitalized in Sweden | None |
| Cediel (2016) | Retrospective | Spain | 2012-2013 | 570 | Single university centre | All adults with at least 1 value of troponin tested | Cardiac arrest, alternate diagnoses other than MI, lived far |
| Chapman (2018) | Prospective | Scotland | 2009-2009 | 1600 | 1 tertiary centre | All patients with elevated troponin values | Admitted for elective procedures, incomplete electronic hospital records, and nonresidents |
| Gonzalez (2011) | Retrospective | United States | 2004-2007 | 348 | 1 tertiary centre | All MI with ≥ 50% coronary stenosis on angiogram and ≥ 24-mo follow-up | Terminal diseases, refused standard MI treatment, no obstructive coronary artery disease |
| Greenslade (2017) | Pooled study of 1 prospective observational and 1 interventional study | Australia | 2008-2014 | 152 | Single tertiary centre | Adults with MI who could provide consent, enrollment during regular working hours | Pregnant, lived far |
| Javed (2009) | Prospective | United States | 2009 | 207 | Single centre | All adults with ≥ 1 abnormal troponin value who provided consent | Refusal to participate |
| Lambrecht (2018) | Prospective study | Denmark | 2010 | 479 | Single centre | All patients with at least 1 troponin ≥ 99th percentile normal value | Pregnant, lived outside catchment area |
| Lopez-Cuenca (2016) | Retrospective | Spain | 2012-2013 | 824 | Single veterans tertiary centre | All patients with MI | None |
| Nestelberger (2017) | Retrospective | Switzerland, Italy, Germany, Spain, Poland | 2006-2015 | 924 | 12 centres/5 countries | Adults within 12 h of ischemic symptoms | Unclear diagnosis |
| Neumann (2017) | Prospective | Germany | 2013-2016 | 287 | Single university centre | Adults with suspected MI who could provide consent | Missing troponins, STEMI |
| Radovanovic (2016) | Prospective (AMIS-PLUS) | Switzerland | 2009-2015 | 14,920 | 53 Swiss hospitals | All patients hospitalized with MI in Switzerland | None |
| Raphael (2020) | Prospective | United States | 2003-2012 | 2, 436 | Mayo Clinic and Olmstead Medical Center | Adults with ≥ 1 available troponin value | Prior MI, refused to consent, unclear cause for elevation of troponin |
| Saaby (2014) | Prospective | Denmark | 2010 | 488 | Single centre | Adults with ≥ 1 available troponin value | Outside catchment area, troponins administered outside the hospital |
| Sandoval (2015) | Retrospective | United States | 2013 | 310 | Single centre | Adults with ≥ 1 available troponin value | None |
| Sandoval (2017) | Prospective (UTROPIA Study) | United States | 2011 | 217 | Single centre | All patients who provided consent and with ≥ 2 troponins and 1 ECG within 24 h | Pregnant,transferred in patients, did not present to the emergency department |
| Shah (2015) | Prospective | Scotland | 2014 | 1600 | Single centre | All patients with troponin I ≥ 50 ng/L | None |
| Smilowitz (2018) | Prospective | United States | 2012-2013 | 283 | Single veterans tertiary centre | All patients with elevated troponin values | None |
| Stein (2014) | Prospective national Israel registry (ACSIS Registry) | Israel | 2008-2010 | 2818 | Nationwide Israel multicentres (26 intensive and 37 medical wards) | All patients with MI | None |
ACSIS, Acute Coronary Syndrome Israeli Survey; AMIS-PLUS, National Registry of Acute Myocardial Infarction in Switzerland; ECG, electrocardiogram; MI, myocardial infarction; NSTEMI, non–ST-segment myocardial infarction; STEMI, ST-segment elevation myocardial infarction; SWEDEHEART, Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction; UTROPIA, Use of Troponin in Acute Coronary Syndromes.
Compared with patients with T1MI, patients with T2MI were older (69 vs 65 years, P = 0.02), more often female (44% vs 30%, P < 0.0001), and more often had diabetes mellitus (30% vs 27%) and hypertension (70% vs 67%, P = 0.03) (Table 2). DAPT use in patients with T2MI was reported in only 7 observational studies.7,8,13,15,17,19,21,23 The aggregate mean use was 20.8% in patients with T2MI and 74.2% in patients with T1MI (Table 3). Patients with T2MI were 91% less likely to use DAPT and 80% less likely to undergo percutaneous coronary intervention (Table 3).
Table 2.
Baseline characteristics of patients enrolled in RCTs and observational studies
| RCT |
Observational studies |
|||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | No. of studies (No. of patients) |
P values RCT vs observational studies |
T1MI (95% CI) | No. of studies (No. of patients) | T2MI (95% CI) | No. of studies (No. of patients) |
P values T1MI vs T2MI |
|
| Age, y | 62.1 (61.3-62.9) | 51 (188,132) | < 0.0001 | 64.9 (65.0-68.9) | 14 (36,592) | 69.2 (66.1-72.4) | 15 (3930) | 0.02 |
| Female, % | 25.5 (24.0-27.1) | 51 (188,132) | < 0.0001 | 29.8 (26.6-33.3) | 17 (38,352) | 44.2 (40.5-49.0) | 21 (4842) | < 0.0001 |
| Diabetes mellitus, % | 24.0 (22.0-26.1) | 49 (142,096) | 0.04 | 26.8 (23.3-30.7) | 17 (37,840) | 29.5 (25.5-33.9) | 18 (4771) | 0.05 |
| Hypertension, % | 63.3 (58.3-67.9) | 45 (170,988) | 0.32 | 67.1 (62.5-71.5) | 16 (35,276) | 69.9 (57.7-80.0) | 16 (8533) | 0.03 |
| Prior MI, % | 18.5 (15.0-22.5) | 34 (147,006) | 0.003 | 28.4 (25.2-31.8) | 11 (23,296) | 32.8 (25.9-40.6) | 11 (2877) | 0.21 |
| Heart failure, % | 8.3 (4.7-14.2) | 6 (27,556) | 0.25 | 14.7 (7.2-27.9) | 9 (32,619) | 21.1 (13.7-31.0) | 9 (3331) | 0.08 |
CI, confidence interval; DAPT, dual antiplatelet; MI, myocardial infarction; RCT, randomized controlled trial; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction.
Table 3.
Management and outcomes of patients
| RCT |
Observational studies |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. of studies (No. of patients) | Weighted mean, % (95% CI) |
P values RCT vs observational studies |
No. of studies comparing T1MI and T2MI (No. of patients) | T1MI weighted mean, % (95% CI) | T2MI observational studies % weighted mean, (95% CI) |
P values T1MI vs T2MI | ORs of T2MI compared with T1MI (95% CI) | |
| In-hospital initiation of DAPT | NA | NA | NA | 6 (19,480) | 74.2 (66.0-81.0) | 20.8 (4.1-34.2) | < 0.0001 | 0.09 (0.04-0.21) |
| Coronary angiography | 35 (83,466) | 99.8 (99.7-99.9) | < 0.0001 | 8 (35,795) | 82.9 (77.8-87.0) | 28.2 (18.5-40.4) | < 0.0001 | 0.28 (0.20-0.39) |
| PCI | 34 (78,358) | 99.8 (99.6-99.9) | < 0.0001 | 9 (36,825) | 64.4 (52.8-74.6) | 10.3 (4.3-22.6) | < 0.0001 | 0.17 (9.1-32.7) |
| Reinfarction | 4 (5,321) | 3.3 (2.6-4.2) | < 0.0001 | 5 (5396) | 9.8 (6.3-14.9) | 6.4 (4.0-10.1) | 0.002 | 0.62 (0.47-0.84)∗ |
| Short-term mortality | 12 (97,269) | 2.9 (1.7-4.9) | < 0.0001 | 8 (7249) | 7.1 (5.5-8.8) | 15.6 (10.3-20.8) | 0.0006 | 1.86 (1.20-2.88)∗ |
| Long-term mortality | 4 (33,593) | 3.6 (2.3-5.4) | < 0.0001 | 16 (46,947) | 11.3 (6.4-19.2) | 27.7 (20.6-36.1) | < 0.0001 | 2.47 (2.06-2.96)∗ |
| 11 (42,912) |
1.45 (1.25-1.69)† | |||||||
CI, confidence interval; DAPT, dual antiplatelet therapy; NA, nonapplicable (due to randomized comparison of dual antiplatelet therapy vs placebo); PCI, percutaneous coronary intervention; RCT, randomized controlled trials; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction.
Unadjusted comparison.
Adjusted comparison.
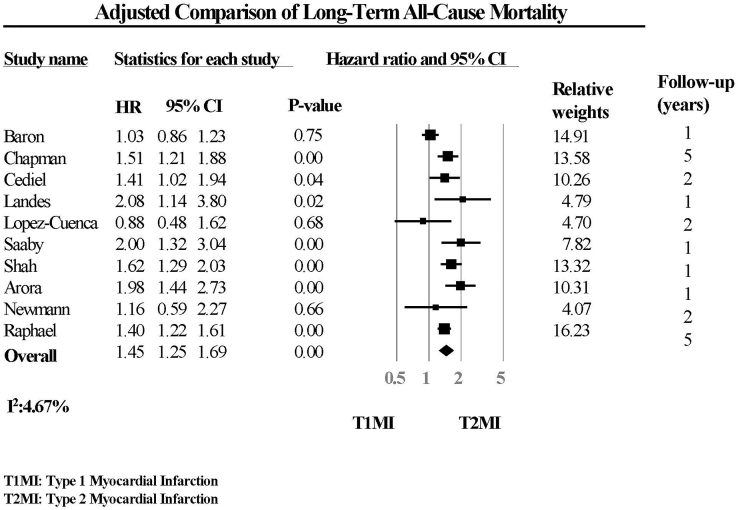
wPatients enrolled in the RCTs had the best unadjusted long-term survival compared with patients with T1MI and T2MI in the observational studies. Compared with patients with T1MI, patients with T2MI had 2.5-fold increase in unadjusted long-term mortality (Table 3 and Fig. 2) and an approximately 45% increase in adjusted long-term mortality (odds ratio, 1.45; 95% confidence interval, 1.25-1.69; P < 0.0001) (Table 3 and Fig. 3). The comparisons of long-term mortality between T2MI and T1MI were generally adjusted for age, sex, baseline characteristics, and comorbidities, except for the study by Newman et al.,15 in which long-term mortality was adjusted only for age, sex, and prior coronary artery disease.
Figure 2.
Unadjusted comparison of long-term all-cause mortality. CI, confidence interval; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction.
Figure 3.
Adjusted comparison of long-term all-cause mortality. CI, confidence interval; HR, hazard ratio; T1MI, type 1 myocardial infarction; T2MI, type 2 myocardial infarction.
The precipitating factors of T2MI were reported in 7 studies.7, 8, 9,12,14,21, 22 Arrhythmia, anemia/bleeding, respiratory diseases, heart failure, and infection/sepsis were the most common reported precipitating factors for T2MI (Table 4). There was no obvious publication bias detected as the funnel plot appeared to be symmetrical (Fig. 4).
Table 4.
Triggers of type 2 myocardial infarction
| Triggers of type 2 myocardial infarction | No. of studies (No. of patients) | Weighed mean, % (95% CIs) |
|---|---|---|
| Arrhythmia | 9 (36,592) | 22.4 (16.1-30.3) |
| Anemia/bleeding | 8 (35,044) | 15.9 (11.6-21.4) |
| Respiratory diseases | 5 (12,682) | 13.7 (8.3-21.8) |
| Heart failure | 4 (25,066) | 13.7 (8.3-21.8) |
| Hypertensive crisis | 6 (11,204) | 11.5 (6.6-19.2) |
| Sepsis/infection | 5 (24,387) | 10.1 (5.2-18.8) |
CI, confidence interval.
Figure 4.
Funnel plot of adjusted comparison of all-cause mortality.
Discussion
Our meta-analysis of observational studies showed that compared with patients with T1MI, patients with T2MI were older and more often female, had more hypertension, and had diabetes mellitus. Compared with patients with T1MI, patients with T2MI were 90% less likely to be treated with DAPT and 80% less likely to undergo percutaneous coronary intervention. Patients with T2MI had approximately 45% increase in adjusted odds of all-cause long-term mortality compared with patients with T1MI. The efficacy, effectiveness, and safety of DAPT have never been formally appraised in RCTs or observational studies.
There were marked differences in unadjusted short- and long-term mortality rates among the 3 groups of patients (patients with ACS enrolled in the RCTs, T1MI, and T2MI in the observational cohorts). Although both short- and long-term mortality were less than 5% in patients with ACS enrolled in the RCTs that evaluated DAPT, unadjusted short- and long-term mortality were 7% and 11%, respectively, for patients with T1MI in the observational studies. The higher unadjusted short- and long-term mortality of patients in the observational studies likely would be due to the enrollment of patients without MI, with younger age, and with fewer comorbid conditions in the RCTs than patients in the observational studies.78
The lack of significant heterogeneity in our random-effects model of adjusted odds was markedly in contrast to the heterogeneity observed in the random-effects model of unadjusted odds of long-term mortality. By using adjusted odds, we were able to make the populations more comparable between studies and between patients with T1MI and T2MI. Therefore, our estimate suggested a true increase in odd of long-term mortality in patients with T2MI compared with patients with T1MI, adjusted for the increased age and higher comorbidities of patients with T2MI.
In a meta-analysis of 9 observational studies, Gupta et al.79 reported a 3-fold increase in short and intermediate-term mortalities in patients with T2MI compared with patients with T1MI. Compared with the previous meta-analysis,79 our study provided more long-term information with 7 studies reporting data beyond 1-year follow-up. Most important, our adjusted estimate for long-term mortality may be less confounded by differences in clinical characteristics between patients with T2MI and T1MI.
Short- and long-term mortality were high in patients with T2MI with weighted mean rates of 15% and 30%, respectively. This finding implied that approximately 1 in 3 patients with T2MI may die beyond 1 year after the index event. Even after adjustment for their increased age and comorbidities, patients with T2MI remained at higher risk of long-term all-cause mortality compared with patients with T1MI. Although the increased mortality of a T2MI may not be entirely due to cardiovascular diseases, its occurrence indicates worse outcome that would justify close follow-up of these patients.
Recognizing the triggers of T2MI is imperative to prevent its occurrence. The most frequently reported condition associated with T2MI was arrhythmia, which could be tachyarrhythmia or bradyarrhythmia. Although clinicians may be aware that tachyarrhythmia can increase myocardial oxygen demand,1,2 it is not always recognized that severe bradyarrhythmia might precipitate T2MI because of a reduction in myocardial oxygen supply. Anemia and bleeding were also common precipitating factors of T2MI. Expedient control of bleedings or transfusion of blood products may be valuable to prevent T2MI in susceptible patients. Because we excluded studies evaluating exclusively postoperative myocardial injury, we could not examine the frequency of its occurrence in T2MI.
At present, there is a lack of contemporary management guidelines for patients with T2MI. Beyond control of the underlying conditions, the efficacy of DAPT had never been formally evaluated for patients with T2MI in RCTs. Kidd et al.5 demonstrated a reduction of T2MI with vorapaxar in patients with T2MI, suggesting that antiplatelets may reduce the occurrence of T2MI in patients at risk. Nevertheless, the benefits observed with vorapaxar may not be able to be replicated with direct and indirect P2Y12 receptor inhibitors because of their different mechanisms of actions.
The incidence of T2MI will likely escalate with the increasing use of high-sensitivity troponin assays. Although our detection of T2MI may be enhanced, knowledge gaps concerning the optimal management of these patients persist. The high mortality rates of these patients underlined the need for future research evaluating the role of conventional ACS therapy (eg, DAPT and coronary intervention) in patients with T2MI.80
Limitations
Our systematic reviews had a few noteworthy limitations. First, the lack of patient-level data precluded us from computing adjusted odds ratios for short-term/intermediate mortality and reinfarction. Second, our adjusted comparison of long-term mortality may still be flawed by residual confounders that may not have been accounted for in the individual studies. Third, we could not compare the risk of cardiovascular mortality in patients with T2MI with that of patients with T1MI because only 3 studies reported cardiovascular mortality.19,26,27 Fourth, because we excluded studies of myocardial injury after surgeries, our summary estimates of T2MI could not be extrapolated to patients with postoperative T2MI. Finally, it was possible that some patients with T2MI might have only myocardial injury without actual myocardial necrosis. Because patients with myocardial injury generally had better outcomes than patients with myocardial infarction,79,81 our evaluations of odds of mortality may be underestimated because of the potential inclusion of patients with myocardial injury.
Conclusion
Even after accounting for their increased comorbidities, patients with T2MI still have higher all-cause long-term mortality compared with patients with T1MI. The efficacy, effectiveness, and safety of DAPT in T2MI have not been formally appraised in any RCTs or observational study. Therefore, the role of DAPT in T2MI remains undefined. This knowledge gap underscores the need for future studies evaluating DAPT in patients with T2MI to optimize the management of these high-risk patients.
Funding Sources
There are no funding sources to declare.
Disclosures
Andrew Yan received research grant support from AstraZeneca. Jean-Francois Tanguay received research grant support and speaker/consulting honoraria from Abbott Vascular, Amgen, AstraZeneca, Bayer, Biosensors, Idorsia, Medtronic, Sanofi, and Servier. Shamir Mehta received institutional research grant support from AstraZeneca, Abbott, Boston Scientific, and Sanofi. Shaun Goodman received research grant support and speaker/consulting honoraria from AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi-Sankyo, Eli Lilly, Merck, and Sanofi. Thao Huynh received research grant support and speaker/consulting honoraria from AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi-Sankyo, Eli Lilly, Merck, and Sanofi.
Footnotes
Ethics Statement: The research reported has adhered to the relevant ethical guidelines.
See page 125 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.02.005.
Supplementary Material
References
- 1.Thygesen K., Alpert J.S., White H.D. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K., Alpert J.S., Jaffe A.S. and the Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. J Am Coll Cardiol. 2018;72:2231–2264. [Google Scholar]
- 3.Valgimigli M., Bueno H., Byrne R.A. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery. Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 4.Mehta S., Armstrong P., Cantor W. 2018 Canadian Cardiovascular Society/Canadian Association of interventional cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol. 2018;34:214–233. doi: 10.1016/j.cjca.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Kidd S.K., Bonaca M.P., Braunwald E. Universal classification system type of incident myocardial infarction in patients with stable atherosclerosis: observations from thrombin receptor antagonist in secondary prevention of atherothrombotic ischemic events (TRA 2oP) – TIMI 50. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 7.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.Arora S., Strassle P.D., Qamar A. Impact of type 2 myocardial infarction on hospital-level MI outcomes: implications for quality and public reporting. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron T., Hambraeus K., Sundström J. Type 2 myocardial infarction in clinical practice. Heart. 2015;101:101–106. doi: 10.1136/heartjnl-2014-306093. [DOI] [PubMed] [Google Scholar]
- 10.Cediel G., Gonzalez-del-Hoyo M., Carrasquer A. Outcomes with type 2 myocardial infarction compared with non-ischaemic myocardial injury. Heart. 2017;103:616–622. doi: 10.1136/heartjnl-2016-310243. [DOI] [PubMed] [Google Scholar]
- 11.Chapman A.R., Shah A.S.V., Lee K.K. Long-term outcomes in patients with type 2 myocardial infarction and myocardial injury. Circulation. 2018;137:1236–1245. doi: 10.1161/CIRCULATIONAHA.117.031806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez M.A., Eilen D.J., Marzouq R.A. The universal classification is an independent predictor of long-term outcomes in acute myocardial infarction. Cardiovasc Revasc Med. 2011;12:35–40. doi: 10.1016/j.carrev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Greenslade J.H., Adikari T., Mueller C. Characteristics and occurrence of type 2 myocardial infarction in emergency department patients: a prospective study. Emerg Med J. 2018;35:169–175. doi: 10.1136/emermed-2017-206869. [DOI] [PubMed] [Google Scholar]
- 14.Nestelberger T., Boeddinghaus J., Badertscher P. Effect of definition on incidence and prognosis of type 2 myocardial infarction. J Am Coll Cardiol. 2017;70:1558–1568. doi: 10.1016/j.jacc.2017.07.774. [DOI] [PubMed] [Google Scholar]
- 15.Neumann J.T., Sorensen N.A., Rubsamen N. Discrimination of patients with type 2 myocardial infarction. Eur Heart J. 2017;38:3514–3520. doi: 10.1093/eurheartj/ehx457. [DOI] [PubMed] [Google Scholar]
- 16.Javed U., Aftab W., Ambrose J.A. Frequency of elevated troponin I and diagnosis of acute myocardial infarction. Am J Cardiol. 2009;104:9–13. doi: 10.1016/j.amjcard.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Cuenca A., Gomez-Molina M., Flores-Blanco P.J. Comparison between type-2 and type-1 myocardial infarction: clinical features, treatment strategies, and outcomes. J Geriatr Cardiol. 2016;13:15–22. doi: 10.11909/j.issn.1671-5411.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radovanovic D., Pilgrim T., Seifert B. Type 2 myocardial infarction: incidence, presentation, treatment, and outcome in routine clinical practice. J Cardiovasc Med. 2017;18:341–347. doi: 10.2459/JCM.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 19.Lambrecht S., Sarkisian L., Saaby L. Different causes of death in patients with myocardial infarction type 1, type 2, and myocardial injury. Am J Med. 2018;131:548–554. doi: 10.1016/j.amjmed.2017.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Sandoval Y., Smith S.W., Schulz K.M. Diagnosis of type 1 and type 2 myocardial infarction using a high-sensitivity cardiac troponin I assay with sex-specific 99th percentiles based on the third universal definition of myocardial infarction classification system. Clin Chem. 2015;61:657–663. doi: 10.1373/clinchem.2014.236638. [DOI] [PubMed] [Google Scholar]
- 21.Sandoval Y., Smith S.W., Sexter A. Type 1 and 2 myocardial infarction and myocardial injury: clinical transition to high-sensitivity cardiac troponin I. Am J Med. 2017;130 doi: 10.1016/j.amjmed.2017.05.049. 1431-9.e4. [DOI] [PubMed] [Google Scholar]
- 22.Shah A.S.V., McAllister D.A., Mills R. Sensitive troponin assay and the classification of myocardial infarction. Am J Med. 2015;128:493–501.e3. doi: 10.1016/j.amjmed.2014.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein G.Y., Herscovici G., Korenfeld R. Type-II myocardial infarction--patient characteristics, management, and outcomes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smilowitz N.R., Subramanyam P., Gianos E. Treatment and outcomes of type 2 myocardial infarction and myocardial injury compared with type 1 myocardial infarction. Coron Artery Dis. 2018;29:46–52. doi: 10.1097/MCA.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saaby L., Poulsen T.S., Diederichsen A.C.P. Mortality rate in type 2 myocardial infarction: observations from an unselected hospital cohort. Am J Med. 2014;127:295–302. doi: 10.1016/j.amjmed.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Raphael C.E., Roger V.L., Sandoval Y. Incidence, trends and outcomes of type 2 myocardial infarction in a community cohort. Circulation. 2020;141:454–463. doi: 10.1161/CIRCULATIONAHA.119.043100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landes U., Bental T., Orvin K. Type 2 myocardial infarction: a descriptive analysis and comparison with type 1 myocardial infarction. J Cardiol. 2016;67:51–56. doi: 10.1016/j.jjcc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Abuzahra M., Pillai M., Caldera A. Comparison of higher clopidogrel loading and maintenance dose to standard dose on platelet function and outcomes after percutaneous coronary intervention using drug-eluting stents. Am J Cardiol. 2008;102:401–403. doi: 10.1016/j.amjcard.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 29.Akbulut M., Kutlu M., Ozbay Y. Efficacy of clopidogrel on reperfusion and high-sensitivity C-reactive protein in patients with acute myocardial infarction. Mediators Inflamm. 2009;2009:932515. doi: 10.1155/2009/932515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexopoulos D., Galati A., Xanthopoulou I. Ticagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: a pharmacodynamic study. J Am Coll Cardiol. 2012;60:193–199. doi: 10.1016/j.jacc.2012.03.050. [DOI] [PubMed] [Google Scholar]
- 31.Alexopoulos D., Xanthopoulou I., Plakomyti T.-E. Pharmacodynamic effect of prasugrel 5 mg vs clopidogrel 150 mg in elderly patients with high on-clopidogrel platelet reactivity. Am Heart J. 2013;165:73–79. doi: 10.1016/j.ahj.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Angiolillo D.J., Saucedo J.F., Deraad R. Increased platelet inhibition after switching from maintenance clopidogrel to prasugrel in patients with acute coronary syndromes: results of the SWAP (SWitching Anti Platelet) study. J Am Coll Cardiol. 2010;56:1017–1023. doi: 10.1016/j.jacc.2010.02.072. [DOI] [PubMed] [Google Scholar]
- 33.Bernardi V., Szarfer J., Summay G. Long-term versus short-term clopidogrel therapy in patients undergoing coronary stenting (from the Randomized Argentine Clopidogrel Stent [RACS] trial) Am J Cardiol. 2007;99:349–352. doi: 10.1016/j.amjcard.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Bonaca M.P., Bhatt D.L., Cohen M. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372:1791–1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 35.Bonello L., Camoin-Jau L., Armero S. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. Am J Cardiol. 2009;103:5–10. doi: 10.1016/j.amjcard.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 36.Bonello L., Camoin-Jau L., Arques S. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol. 2008;51:1404–1411. doi: 10.1016/j.jacc.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 37.Brener S.J., Oldroyd K.G., Maehara A. Outcomes in patients with ST-segment elevation acute myocardial infarction treated with clopidogrel versus prasugrel (from the INFUSE-AMI trial) Am J Cardiol. 2014;113:1457–1460. doi: 10.1016/j.amjcard.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 38.Caiazzo G., De Rosa S., Torella D. Administration of a loading dose has no additive effect on platelet aggregation during the switch from ongoing clopidogrel treatment to ticagrelor in patients with acute coronary syndrome. Circ Cardiovasc Interv. 2014;7:104–112. doi: 10.1161/CIRCINTERVENTIONS.113.000512. [DOI] [PubMed] [Google Scholar]
- 39.Cannon C.P., Harrington R.A., James S. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet. 2010;375:283–293. doi: 10.1016/S0140-6736(09)62191-7. [DOI] [PubMed] [Google Scholar]
- 40.Cannon C.P., Husted S., Harrington R.A. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 tri. J Am Coll Cardiol. 2007;50:1844–1851. doi: 10.1016/j.jacc.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 41.Chen Z.M., Jiang L.X., Chen Y.P. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1607–1621. doi: 10.1016/S0140-6736(05)67660-X. [DOI] [PubMed] [Google Scholar]
- 42.Cuisset T., Frere C., Quilici J. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol. 2006;48:1339–1345. doi: 10.1016/j.jacc.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 43.Dangas G., Mehran R., Guagliumi G. Role of clopidogrel loading dose in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: results from the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trial. J Am Coll Cardiol. 2009;54:1438–1446. doi: 10.1016/j.jacc.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Diodati J.G., Saucedo J.F., French J.K. Effect on platelet reactivity from a prasugrel loading dose after a clopidogrel loading dose compared with a prasugrel loading dose alone: Transferring From Clopidogrel Loading Dose to Prasugrel Loading Dose in Acute Coronary Syndrome Patients (TRIPLET) Circ Cardiovasc Interv. 2013;6:567–574. doi: 10.1161/CIRCINTERVENTIONS.112.000063. [DOI] [PubMed] [Google Scholar]
- 45.Dogan A., Ozgul M., Ozaydin M. Effect of clopidogrel plus aspirin on tissue perfusion and coronary flow in patients with ST-segment elevation myocardial infarction: a new reperfusion strategy. Am Heart J. 2005;149:1037–1042. doi: 10.1016/j.ahj.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 46.Dridi N.P., Johansson P.I., Clemmensen P. Prasugrel or double-dose clopidogrel to overcome clopidogrel low-response – The TAILOR (Thrombocytes And IndividuaLization of ORal antiplatelet therapy in percutaneous coronary intervention) randomized trial. Platelets. 2014;25:506–512. doi: 10.3109/09537104.2013.845874. [DOI] [PubMed] [Google Scholar]
- 47.Ducci K., Grotti S., Falsini G. Comparison of pre-hospital 600 mg or 900 mg vs. peri-interventional 300 mg clopidogrel in patients with ST-elevation myocardial infarction undergoing primary coronary angioplasty. The Load&Go randomized trial. Int J Cardiol. 2013;168:4814–4816. doi: 10.1016/j.ijcard.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Ebrahimi R., Dyke C., Mehran R. Outcomes following pre-operative clopidogrel administration in patients with acute coronary syndromes undergoing coronary artery bypass surgery: the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. J Am Coll Cardiol. 2009;53:1965–1972. doi: 10.1016/j.jacc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 49.Fiedler K.A., Mehilli J., Kufner S. Randomised, double-blind trial on the value of tapered discontinuation of clopidogrel maintenance therapy after drug-eluting stent implantation. Intracoronary Stenting and Antithrombotic Regimen: CAUTION in Discontinuing Clopidogrel Therapy--ISAR-CAUTION. Thromb Haemost. 2014;111:1041–1049. doi: 10.1160/TH13-11-0900. [DOI] [PubMed] [Google Scholar]
- 50.Mauri L., Kereiakes D.J., Yeh R.W. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mehta S.R., Tanguay J.-F., Eikelboom J.W. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet. 2010;376:1233–1243. doi: 10.1016/S0140-6736(10)61088-4. [DOI] [PubMed] [Google Scholar]
- 52.Montalescot G., Bolognese L., Dudek D. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med. 2013;369:999–1010. doi: 10.1056/NEJMoa1308075. [DOI] [PubMed] [Google Scholar]
- 53.Montalescot G., Sideris G., Meuleman C. A randomized comparison of high clopidogrel loading doses in patients with non-ST-segment elevation acute coronary syndromes: the ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) J Am Coll Cardiol. 2006;48:931–938. doi: 10.1016/j.jacc.2006.04.090. [DOI] [PubMed] [Google Scholar]
- 54.Montalescot G., Sideris G., Cohen R. Prasugrel compared with high-dose clopidogrel in acute coronary syndrome. The randomised, double-blind ACAPULCO study. Thromb Haemost. 2010;103:213–223. doi: 10.1160/TH09-07-0482. [DOI] [PubMed] [Google Scholar]
- 55.Montalescot G., van’t Hof A.W., Lapostolle F. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. 2014;371:1016–1027. doi: 10.1056/NEJMoa1407024. [DOI] [PubMed] [Google Scholar]
- 56.Muller C., Buttner H.J., Petersen J., Roskamm H. A randomized comparison of clopidogrel and aspirin versus ticlopidine and aspirin after the placement of coronary-artery stents. Circulation. 2000;101:590–593. doi: 10.1161/01.cir.101.6.590. [DOI] [PubMed] [Google Scholar]
- 57.Parodi G., Bellandi B., Valenti R. Comparison of double (360 mg) ticagrelor loading dose with standard (60 mg) prasugrel loading dose in ST-elevation myocardial infarction patients: the Rapid Activity of Platelet Inhibitor Drugs (RAPID) primary PCI 2 study. Am Heart J. 2014;167:909–914. doi: 10.1016/j.ahj.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Parodi G., Sciagrà R., Migliorini A. A randomized trial comparing clopidogrel versus ticlopidine therapy in patients undergoing infarct artery stenting for acute myocardial infarction with abciximab as adjunctive therapy. Am Heart J. 2005;150:220. doi: 10.1016/j.ahj.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Parodi G., Valenti R., Bellandi B. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol. 2013;61:1601–1606. doi: 10.1016/j.jacc.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 60.Patti G., Bárczi G., Orlic D. Outcome comparison of 600- and 300-mg loading doses of clopidogrel in patients undergoing primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: results from the ARMYDA-6 MI (Antiplatelet therapy for Reduction of MYocar. J Am Coll Cardiol. 2011;58:1592–1599. doi: 10.1016/j.jacc.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 61.Patti G., Pasceri V., Mangiacapra F. Efficacy of clopidogrel reloading in patients with acute coronary syndrome undergoing percutaneous coronary intervention during chronic clopidogrel therapy (from the Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty [ARMYDA-8 RELO. Am J Cardiol. 2013;112:162–168. doi: 10.1016/j.amjcard.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 62.Price M.J., Berger P.B., Teirstein P.S. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 63.Rabbani L.E., Iyengar S., Dangas G.D. Impact of thienopyridine administration prior to primary stenting in acute myocardial infarction. J Interv Cardiol. 2009;22:378–384. doi: 10.1111/j.1540-8183.2009.00474.x. [DOI] [PubMed] [Google Scholar]
- 64.Roe M.T., Armstrong P.W., Fox K.A.A. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–1309. doi: 10.1056/NEJMoa1205512. [DOI] [PubMed] [Google Scholar]
- 65.Sabatine M.S., Cannon C.P., Gibson C.M. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 66.Schulz-Schüpke S., Byrne R.A., Ten Berg J.M. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. 2015;36:1252–1263. doi: 10.1093/eurheartj/ehu523. [DOI] [PubMed] [Google Scholar]
- 67.Schulz S., Richardt G., Laugwitz K.-L. Comparison of prasugrel and bivalirudin vs clopidogrel and heparin in patients with ST-segment elevation myocardial infarction: design and rationale of the Bavarian Reperfusion Alternatives Evaluation (BRAVE) 4 trial. Clin Cardiol. 2014;37:270–276. doi: 10.1002/clc.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Steinhubl S.R., Berger P.B., Mann J.T., III Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention. JAMA. 2002;288:2411. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 69.Taniuchi M., Kurz H.I., Lasala J.M. Randomized comparison of ticlopidine and clopidogrel after intracoronary stent implantation in a broad patient population. Circulation. 2001;104:539–543. doi: 10.1161/hc3001.093435. [DOI] [PubMed] [Google Scholar]
- 70.Valgimigli M., Campo G., Monti M. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–2026. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- 71.Winter J.L., Lindefjeld D.S., Veas N. Angiographic and electrocardiographic parameters of myocardial reperfusion in angioplasty of patients with ST elevation acute myocardial infarction loaded with ticagrelor or clopidogrel (MICAMI-TICLO trial) Cardiovasc Revasc Med. 2014;15:284–288. doi: 10.1016/j.carrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 72.Wiviott S.D., Antman E.M., Gibson C.M. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infar. Am Heart J. 2006;152:627–635. doi: 10.1016/j.ahj.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 73.Wiviott S.D., Antman E.M., Winters K.J. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation. 2005;111:3366–3373. doi: 10.1161/CIRCULATIONAHA.104.502815. [DOI] [PubMed] [Google Scholar]
- 74.Woodward M., Lowe G.D.O., Francis L.M.A., Rumley A., Cobbe S.M. A randomized comparison of the effects of aspirin and clopidogrel on thrombotic risk factors and C-reactive protein following myocardial infarction: the CADET trial. J Thromb Haemost. 2004;2:1934–1940. doi: 10.1111/j.1538-7836.2004.01017.x. [DOI] [PubMed] [Google Scholar]
- 75.Xie X., Ma Y.-T., Yang Y.-N. Personalized antiplatelet therapy according to CYP2C19 genotype after percutaneous coronary intervention: a randomized control trial. Int J Cardiol. 2013;168:3736–3740. doi: 10.1016/j.ijcard.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 76.Yong G., Rankin J., Ferguson L. Randomized trial comparing 600- with 300-mg loading dose of clopidogrel in patients with non-ST elevation acute coronary syndrome undergoing percutaneous coronary intervention: results of the Platelet Responsiveness to Aspirin and Clopidogrel and Troponin. Am Heart J. 2009;157:60.e1–60.e9. doi: 10.1016/j.ahj.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 77.Yusuf S., Zhao F., Mehta S.R. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 78.Gurwitz J.H., Col N.F., Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. JAMA. 1992;268:1417–1422. [PubMed] [Google Scholar]
- 79.Gupta S., Vaidya S.R., Arora S., Bahekar A., Devarapally S.R. Type 2 versus type 1 myocardial infarction: a comparison of clinical characteristics and outcomes with a meta-analysis of observational studies. Cardiovasc Diagn Ther. 2017;7:348–358. doi: 10.21037/cdt.2017.03.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeymer U., Arntz H.-R., Mark B. Efficacy and safety of a high loading dose of clopidogrel administered prehospitally to improve primary percutaneous coronary intervention in acute myocardial infarction: the randomized CIPAMI trial. Clin Res Cardiol. 2012;101:305–312. doi: 10.1007/s00392-011-0393-1. [DOI] [PubMed] [Google Scholar]
- 81.DeFillippis A., Chapman A.R., Mills N.L. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140:1161–1178. doi: 10.1161/CIRCULATIONAHA.119.040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.