Abstract
Background
Current guidelines are relatively general regarding the type of patient with heart failure (HF) who should be considered for catheter ablation (CA) of atrial fibrillation (AF). The aim of the present study was to identify clinical predictors and sex differences for treatment with CA in the AF-HF population.
Methods
A population-based AF-HF cohort was created using the Quebec administrative data (2000-2017). Patients were followed from the date of diagnosis of both diseases to the date of CA or death. Predictors for CA, represented by time-varying covariates, were assessed in a multivariable Cox model that accounted for the competing risk of death.
Results
Among 101,931 patients with AF-HF with medication information (median age, 80.7 years; interquartile range [IQR], 73.9-86.3; 51.4% were female, median CHA2DS2-VASc, 4; IQR, 3-4), only 432 (0.4%) underwent CA after a median of 0.8 years (IQR, 0.1-2.7). Independent of multiple comorbidities and advanced age, which were associated with a lower likelihood of CA, women were approximately half as likely to undergo a CA (26% were women; adjusted hazard ratio, 0.6; 95% confidence interval, 0.4-0.7). Prior use of direct-acting oral anticoagulants and antiarrhythmics, and the presence of an implantable cardioverter-defibrillator were also predictors for CA treatment (P < 0.05 for all).
Conclusion
In a real-world population, CA was infrequently used to treat AF among patients with HF, and the likelihood of CA was further reduced in women. Because patients with CA had few comorbidities, future studies need to be conducted to determine whether CA can be beneficial in subjects whose clinical characteristics are more representative of the AF-HF population.
Résumé
Contexte
Les lignes directrices actuelles abordent de façon relativement générale les cas d’insuffisance cardiaque (IC) où les patients devraient être considérés comme des candidats à l’ablation par cathéter (AC) pour le traitement de la fibrillation auriculaire (FA). La présente étude visait à cerner les prédicteurs cliniques et les différences entre les sexes dans le contexte de l’AC au sein de la population atteinte de FA et d’IC.
Méthodologie
Une cohorte populationnelle de patients atteints de FA et d’IC a été constituée à partir de données administratives du Québec (2000-2017). Le suivi des patients allait de la date du diagnostic des deux affections à la date de l’AC ou du décès. Les prédicteurs d’AC, représentés par des covariables temporalisées, ont été évalués dans un modèle de Cox multivarié tenant compte du risque concurrent de décès.
Résultats
Sur 101 931 patients atteints de FA et d’IC dont la médication était documentée (âge médian : 80,7 ans; intervalle interquartile [IIQ] : 73,9-86,3; proportion de patients de sexe féminin : 51,4 %; score CHA2DS2-VASc médian : 4; IIQ : 3-4), seulement 432 (0,4 %) ont subi une AC au bout d’un laps de temps médian de 0,8 an (IIQ : 0,1-2,7). Indépendamment des maladies concomitantes multiples et de l’âge avancé, associés à une moindre probabilité d’AC, les femmes étaient environ deux fois moins susceptibles de subir une AC (proportion de patients de sexe féminin : 26 %; rapport des risques instantanés corrigé : 0,6; intervalle de confiance à 95 % : de 0,4 à 0,7). Les antécédents de traitement par des anticoagulants oraux à action directe et des antiarythmiques, ainsi que la présence d’un défibrillateur cardioverteur implantable étaient également des prédicteurs d’AC (p < 0,05 dans tous les cas).
Conclusion
Au sein d’une population en contexte réel, l’AC a été rarement pratiquée pour traiter la FA chez des patients atteints d’IC. En outre, la probabilité d’une AC était moindre chez les femmes. Étant donné que les patients ayant subi une AC présentaient peu de maladies concomitantes, d’autres études devront être menées pour déterminer si l’AC peut être salutaire chez les personnes présentant des caractéristiques cliniques plus représentatives de la population atteinte de FA et d’IC.
Atrial fibrillation (AF) and heart failure (HF) frequently coexist with AF, affecting approximately 15% to 30% of patients with clinically overt HF.1 The presence of both diseases substantially increases the risk of all-cause mortality,2 HF hospitalization,1 and thromboembolism.3
Treatment of this high-risk population is challenging with little consensus on an effective management strategy.1,4 Pharmacological rhythm-control strategies have failed to show a reduction in cardiovascular mortality, all-cause mortality, and stroke in large randomized trials, with an indication that antiarrhythmic drugs (AADs) may increase HF hospitalizations.5
In the absence of effective pharmacological rhythm-control options for patients with AF and HF, catheter ablation (CA) for AF has emerged as a treatment option. Randomized trials, including the Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE-AF) and the Ablation vs Amiodarone for Treatment of Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted ICD/CRTD (AATAC) trials, have shown a reduction in HF hospitalizations in patients with AF-HF with reduced ejection fraction treated with CA compared with medical therapy.6, 7, 8 CASTLE-AF also showed a statistically significant reduction in all-cause mortality,7 where a mortality benefit was further supported in a subgroup analysis of the Catheter Ablation vs Anti-Arrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial.9 Although the results of randomized trials are encouraging, subjects who meet the strict inclusion and exclusion criteria may not reflect the AF-HF population encountered in clinical practice. Further, only 15% to 25% of study subjects included in these trials were women, and it is unclear if this CA treatment selection pattern persists in the real-world population with AF-HF.6,7
Canadian clinical guidelines recommend CA as a second-line treatment option for AF and do not have a specific recommendation for patients with comorbid HF (moderate quality of evidence).10 American guidelines suggest referral may be reasonable in patients with AF-HF with reduced ejection fraction with weak evidence of a benefit (level IIb).11 In both sets of guidelines, however, no patient-specific inclusions/exclusions are recommended for patients with HF.10,11 Thus, criteria to select patients with AF-HF for CA are based on the electrophysiologists’ expert opinion and the real-world CA treatment pattern is unknown. The objective of the present study was to characterize the real-world patterns of CA use in patients with HF by identifying clinical predictors and sex differences.
Methods
Study design
A population-based cohort of patients with AF and HF was assembled using administrative databases to identify predictors for CA in Quebec, Canada, between April 1, 2000, and March 31, 2017. The study received institutional review board approval from the McGill University Faculty of Medicine (A05-M79-08B).
Data sources and study population
First, the Quebec AF cohort was created from linked hospital discharge summary and physician claims databases, Maintenance et Exploitation des Données pour l’Étude de la Clientèle Hospitalière (MED-ECHO) and la Régie de l’assurance maladie du Québec (RAMQ), respectively, as described previously.12, 13, 14 Recent years of data, until 2017, were added.
To create the AF-HF cohort, only patients with a primary or secondary diagnosis of HF at hospitalization were included (International Classification of Diseases 9th and 10th Revisions codes 428.0-4, 428.9/I50.1-4,8,9). Patients entered the cohort on the first date they had both diseases diagnosed. Patients with a CA before the cohort entry were excluded.
The main cohort was limited to a subset of patients with AF-HF who had government prescription insurance coverage (medication cohort). In Quebec, all patients 65 years and older, and approximately half of patients 65 years and younger (without private coverage) are covered by the government prescription insurance and have medication prescriptions captured in RAMQ. In sensitivity analysis, we included all patients with AF and HF, regardless of medication insurance coverage (overall cohort). Considering the first study on CA in patients with AF-HF was published in 2008, an additional sensitivity analysis limiting the cohort duration from 2009 to 2017 was also conducted.
Outcome ascertainment
A provincial physician billing code for percutaneous AF ablation (RAMQ code 291) was used to identify CA. The date of CA was defined as the date of the procedure as billed in RAMQ. To exclude complex ablations for congenital heart disease or ventricular tachycardia (also billed under RAMQ code 291), the date of CA was matched to date of AF admission (International Classification of Diseases 9th and 10th Revisions codes for AF) or a diagnosis code linked with CA in RAMQ. Patients with any diagnosis of congenital heart disease or a primary or major secondary diagnosis for ventricular tachycardia were also excluded.
Potential predictors
Potential predictors for CA therapy, considered in our analyses, include patient and procedure-specific factors of CA and variables that may act as markers of AF and HF disease severity (listed in Table 1). The presence of potential predictors at cohort entry was identified from comorbidities listed at hospital admissions within the 1-year period before cohort entry. For patients who did not undergo CA on the date of cohort entry (date of diagnosis of AF and HF), comorbidities acquired during follow-up were represented in the prediction models as time-varying covariates. Because we investigated comorbidities corresponding to chronic diseases, a patient was considered exposed from the date of the first hospitalization that indicated the relevant diagnosis to the end of the follow-up period (comorbidities were listed as any diagnosis at admission). Implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT) acquired during follow-up were also incorporated as time-varying covariates.
Table 1.
Baseline characteristics
| All patients with available medication information (N = 101,931) N (%) |
|
|---|---|
| Median age (IQR), y | 80.7 (73.9-82.3) |
| <65 | 6724 (6.6) |
| 65-75 | 22,126 (21.7) |
| ≥75 | 73,081 (71.7) |
| Women | 52,402 (51.4) |
| Hypertension | 32,578 (32.0) |
| Diabetes mellitus | 16,832 (16.5) |
| Coronary artery disease | 27,323 (26.8) |
| Prior myocardial infarction | 11,464 (11.2) |
| Valvular disease | 27,831 (27.3) |
| Valve replacement | 2847 (2.8) |
| Chronic obstructive pulmonary disease | 16,505 (16.2) |
| Chronic renal failure | 14,456 (14.2) |
| Prior stroke (including transient ischemic attack) | 2095 (2.1) |
| Liver disease | 2241 (2.2) |
| Vascular disease | 11,996 (11.8) |
| Prior major bleeding | 4155 (4.1) |
| Implantable cardioverter defibrillator | 2566 (2.5) |
| Cardiac resynchronization therapy | 9608 (9.4) |
| Median CHA2DS2-VASc score | 4 (3-4) |
| CHA2DS2-VASc score ≥2 | 98,939 (97.1) |
| Median HAS-BLED score | 1 (1-2) |
| Medications | |
| OAC | 55,576 (54.5) |
| Warfarin | 48,547 (47.6) |
| DOAC | 8607 (8.4) |
| Dabigatran | 2999 (2.9) |
| Rivaroxaban | 3105 (3.0) |
| Apixaban | 3050 (3.0) |
| Antiarrhythmic medication | 15,018 (14.8) |
| Amiodarone | 10,152 (10.0) |
| Sotalol | 3333 (3.3) |
| Class 1 antiarrhythmic | 2443 (2.4) |
| Digoxin | 25,140 (24.7) |
| β-Blocker | 50,766 (49.8) |
| Angiotensin-converting enzyme inhibitor | 40,462 (39.7) |
| Angiotensin II receptor blockers | 18,324 (18.0) |
| Calcium channel blocker | 17,646 (17.3) |
| Diuretic | 70,839 (69.5) |
The distribution of patient characteristics at cohort entry is shown and does not include the comorbidities, devices, and medications acquired during the follow-up period that are included in the analysis. Prevalence of patient characteristics is lower because they are measured at the time of initial AF-HF disease diagnosis, and more patients develop the comorbidities over time in the cohort.
DOAC, direct oral anticoagulant; IQR, interquartile range; OAC, oral anticoagulant.
Any exposure to pertinent medications was also assessed using time-varying covariates described earlier. Although a patient may not have been taking a medication throughout the follow-up, prior use of medications may predict CA, for example, patients who failed pharmacologic rhythm therapy may be referred for CA, as per clinical guidelines.10,11
In Quebec, the waiting period for CA (the date CA was requested to date CA was performed) is approximately 3 to 6 months. Therefore, predictors first captured within less than 3 months before CA were excluded, based on the assumption that the decision to perform the CA for the patient likely had already been taken. A sensitivity analysis with a blanking period of 6 months before CA was also conducted.
Statistical analysis
In descriptive analyses, distributions of continuous variables were summarized with medians and interquartile ranges (IQRs), and categorical variables were described by frequency and percentages. To assess sex differences in the AF-HF cohort among patients who underwent CA, differences in the distribution of continuous and categorical variables between men and women were compared by the Wilcoxon rank-sum test and chi-square test, respectively. CHA2DS2-Vasc (1 point each for congestive heart failure/left ventricular dysfunction, hypertension, diabetes mellitus, vascular disease, age 65-75 years, and female sex; 2 points for each of age ≥75 years, or previous stroke/TIA) and modified HAS-Bled (1 point each for hypertension, abnormal renal and/liver function, previous stroke, bleeding history, and age ≥65 years) scores were calculated at cohort entry.
Predictors of CA were identified using multivariable Cox proportional hazards models, extended to account for the competing risk of all-cause mortality using the Lunn and McNeil approach.15 In addition to age at cohort entry and sex, time-varying covariates were included in the multivariable model as potential predictors after potential collinearity between predictors was investigated. Predictors were identified as variables that were statistically significant (P ≤ 0.05) and clinically significant (P ≤ 0.1) in the multivariable Cox model. Backwards elimination was also performed to verify results from prediction model (P ≤ 0.05). All analyses were first conducted in the medication cohort (main analysis) and then, in sensitivity analysis, were replicated for all study subjects including patients who had no data on medication use. An additional evaluation of predictors was conducted limiting the cohort to 2009-2017 and a comparison of baseline characteristics between patients who underwent CAs between 2000-2014 and 2015-2017 was also performed (Supplementary Material). SAS software version 9.4 (SAS Institute, Cary, NC) was used for all analyses.
Results
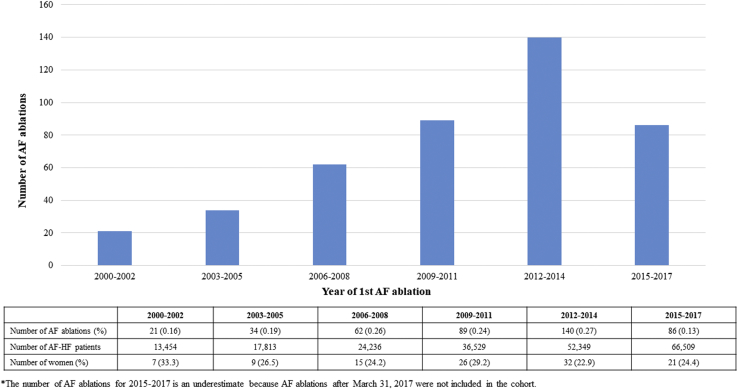
Among the 101,931 patients with AF-HF with available medication information, 432 (0.4%) underwent CA within a median of 0.8 years (IQR, 0.1-2.7) after cohort entry. The number of CAs performed per year increased; however, the low rate of CAs was relatively constant (P value for trend = 0.38) (Fig. 1; Supplementary Material).
Figure 1.
Number of atrial fibrillation (AF) ablations over time (N = 432).
Baseline characteristics
At cohort entry, patients with available medication information had a median age of 80.7 years (73.9-82.3) and a high median CHA2DS2-VASc score of 4 (3-4), and 51.4% were women. Only 2.5% of the population had an ICD, and 9.4% had a CRT device. Warfarin was the most frequently prescribed oral anticoagulant (OAC) (47.6%), and amiodarone was the most frequent AAD (10.0%). Diuretics were prescribed in 69.5% of the population (Table 1).
Sex differences
Although more than half (51.4%) of the population with AF-HF were women, only 25.6% of patients receiving CA were women. In the AF-HF cohort, the presence of most comorbidities, ICDs, and CRTs, and the use of medications were less frequent in women than in men, whereas men were younger and had less hypertension, valve disease, and prior stroke (Table 2; P values < 0.05 for all comparisons). Despite the differences between men and women in the full cohort of patients with AF and HF, in the relatively small CA subpopulation, there were no statistically significant differences in most patient characteristics except that women had less coronary artery disease, chronic renal failure, ICDs, and CRTs (Table 2). Overall, the shape of the distribution of CHA2DS2-VASc scores for patients with and without CA was similar between men and women, except the distribution was shifted to the right because of an extra point assignment for female sex (Fig. 2A). The distribution of HAS-BLED scores was similar between the sexes for patients with CA and patients without CA (Fig. 2B). Independent of multiple comorbidities and advanced age, women were approximately half as likely to undergo CA (adjusted hazard ratio [aHR], 0.6; 95% CI, 0.4-0.7).
Table 2.
Sex differences in patients with AF-HF with and without CA
| All patients with AF-HF (N = 101,931) N (%) |
Patients with CA (N = 432) N (%) |
|||
|---|---|---|---|---|
| Male 49,528 (48.6) |
Female 52,403 (51.4) |
Male 322 (74.4) |
Female 110 (25.6)∗ |
|
| Median age (IQR), y | 78.5 (71.8-84.3) | 82.6 (76.3-87.8)∗ | 66.5 (58.4-71.6) | 65.0 (60.6-73.0) |
| <65 | 4385 (9.0) | 2339 (4.5) | 140 (43.5) | 54 (49.1) |
| 65-75 | 13,350 (27.0) | 8776 (16.8) | 143 (44.4) | 36 (32.7) |
| ≥75 | 31,793 (64.1) | 41,288 (78.8) | 39 (12.1) | 20 (18.2) |
| Hypertension | 14,922 (30.1) | 17,655 (33.7)∗ | 83 (25.5) | 27 (24.6) |
| Diabetes mellitus | 8820 (17.8) | 8012 (15.3)∗ | 51 (15.8) | 16 (14.5) |
| Coronary artery disease | 14,905 (30.1) | 12,418 (23.7)∗ | 74 (22.7) | 15 (13.6)∗ |
| Prior myocardial infarction | 6637 (13.4) | 4827 (9.2)∗ | 42 (13.0) | 7 (6.4) |
| Valvular disease | 12,029 (24.3) | 15,802 (30.2)∗ | 98 (30.3) | 40 (36.0) |
| Valve replacement | 1439 (2.9) | 1408 (2.7)∗ | 5 (1.6) | 2 (1.8) |
| Chronic obstructive pulmonary disease | 9071 (18.3) | 7434 (14.2)∗ | 29 (9.0) | 4 (3.6) |
| Chronic renal failure | 7679 (15.5) | 6777 (12.9)∗ | 28 (8.7) | 3 (2.7)∗ |
| Prior stroke (including TIA) | 906 (1.8) | 1189 (2.3)∗ | 1 (0.3) | 0 (0.0) |
| Liver disease | 1258 (2.5) | 983 (1.9)∗ | 7 (2.2) | 0 (0.0) |
| Vascular disease | 6662 (13.5) | 5334 (10.2)∗ | 17 (5.3) | 4 (3.6) |
| Prior major bleeding | 2514 (5.1) | 1641 (3.1)∗ | 6 (1.9) | 2 (1.8) |
| Implantable cardioverter defibrillator | 2091 (4.2) | 475 (0.9)∗ | 70 (21.7) | 6 (5.5)∗ |
| Cardiac resynchronization therapy | 5447 (11.0) | 4161 (7.9)∗ | 73 (22.7) | 10 (9.1)∗ |
| Median CHA2DS2-VASc score | 3 (3-4) | 4 (4-5)∗ | 2 (1-3) | 3 (2-4)∗ |
| Median HAS-BLED score | 1 (1-2) | 1 (1-2)∗ | 1 (0-1) | 1 (0-1) |
The baseline table presents the distribution of patient characteristics at cohort entry and does not include the comorbidities and devices acquired during the follow-up period, which is included in the analysis.
AF, atrial fibrillation; CA, catheter ablation; HF, heart failure; TIA, transient ischemic attack.
P values of < 0.05 are considered statistically significant. P values compare male and female patients.
Figure 2.
(A) Distribution of CHADS2 scores by sex. (B) Distribution of HAS-BLED scores by sex.
Other predictors for CA
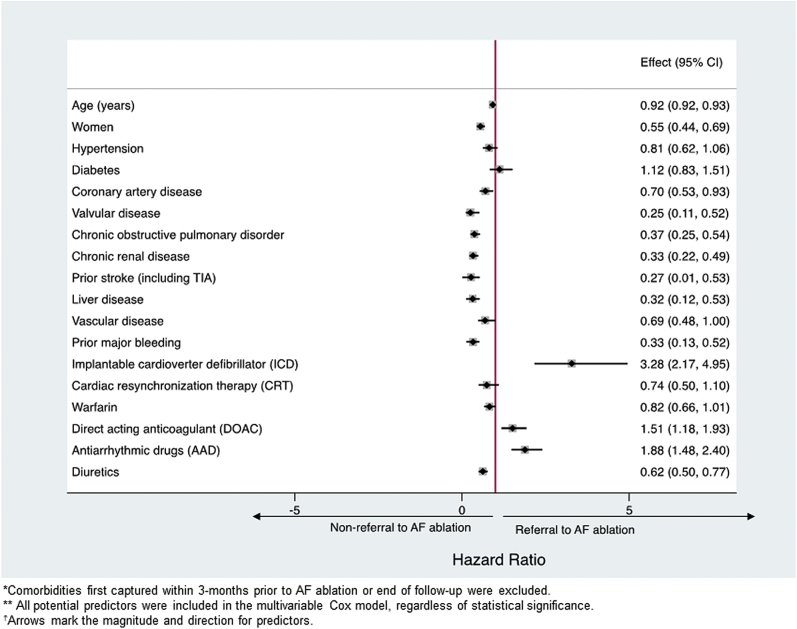
Presence of an ICD (aHR, 3.3; 95% CI, 2.2-5.0) and a prior prescription for a direct oral anticoagulant (DOAC) (aHR, 1.5; 95% CI, 1.2-1.9) and AADs (aHR, 1.9; 95% CI, 1.5-2.4) during follow-up were associated with higher likelihood of CA (Fig. 3). On the other hand, advanced age, female sex, chronic obstructive pulmonary disorder, liver disease, renal disease, prior stroke (including transient ischemic attacks), valve disease, coronary artery disease, prior bleeding, and diuretic use were associated with a lower probability of CA (Fig. 3, P < 0.05 for all). Predictors remained the same after comorbidities, medications, and devices first captured within 6 months before CA were excluded (not exposed) (Supplemental Fig. S1).
Figure 3.
Predictors for treatment with AF ablation.
Sensitivity analyses
In the overall cohort of patients with AF-HF, including those without medication information (N = 112,955), 700 (0.6%) underwent CA (Supplemental Table S1; Supplemental Fig. S2). Comorbidities and procedures identified as predictors of CA were the same as in the medication cohort, except for the presence of a CRT. CRT was associated with a nonsignificant reduction in CA use in the medication cohort (aHR, 0.74; 95% CI, 0.50-1.10) (Fig. 3), and the association was statistically significant in the overall cohort (aHR, 0.72; 95% CI, 0.53-0.97) (Supplemental Fig. S3). Model diagnostics (predictive values) for the main model and sensitivity analyses are presented in Supplemental Table S2.
Predictors for referral were the same in the cohort limited to 2009-2017 except for hypertension, which was a statistically significant predictor for nonreferral to CA (aHR, 0.73; 95% CI, 0.54-0.98) (Supplemental Fig. S4). In addition, baseline characteristics between patients who underwent CA before and after (including) 2015 demonstrated that there was only a statistically significant difference in the proportion of patients undergoing CA with renal disease, valvular disease, prior use of specific OAC therapy, and angiotensin II receptor blockers (Supplemental Table S3). In an additional sensitivity analysis excluding patients with a left ventricular assist device or heart transplant (25 [0.02%] patients excluded), the HRs were also similar to those of the overall cohort (Supplemental Fig. S5).
Discussion
In this population-level assessment of CA in patients with AF-HF, we demonstrated that (1) CA is infrequently used to treat the AF-HF population (0.4%); (2) patients undergoing CA had few comorbidities; (3) patients were approximately half as likely to be women; and (4) patients were more likely to have had an ICD and been prescribed an AAD or DOAC.
Use of CA
On the basis of the promising results from recently published randomized trials, current clinical guidelines recommend the use of CA as second-line therapy to treat symptomatic AF in patients with comorbid HF;10,11 however, the present study demonstrated that < 1% of the Quebec population with AF-HF underwent CA in real-world practice. The use of CA in the AF-HF population is less than the rate of CA use in the general AF population (1.3%-3.9%).12,16,17 In addition, although the number of CAs increased over time in the AF-HF population, the trend was not statistically significant (P = 0.38).13,18 A trend toward increased use of CA is expected on the basis of the results of the CASTLE-AF and CABANA trials with updates to the clinical guidelines.6,7,10,11
Although the use of CA in the AF-HF population may increase, the scope of its use may be limited because of the additional comorbidities that accompany HF. It is estimated that 40% to 93% of patients with HF have 2 or more additional comorbidities.4 In our study, more than 97% of patients had a CHA2DS2-VASc score ≥ 2, but decreased to 75% in patients who underwent CA. Previous evaluations characterizing the profile of patients undergoing CA have determined that patients were often younger and had fewer comorbidities; however, patients with CHA2DS2-VASc scores ≤ 2 have increasingly been undergoing the procedure in recent years.13,18 Because a majority of patients with AF-HF had a CHA2DS2-VASc score ≥ 2, few patients are likely to qualify for CA with current CA practices.
Sex differences
Large epidemiologic studies of the AF population found that women with AF are often older at disease onset19 and have more hypertension,20 previous stroke,21 and valvular disease,20 whereas men have more diabetes,19,22 coronary artery disease,20,22 and chronic obstructive pulmonary disorder,20 all of which were mirrored in the present AF subpopulation with HF. Further, women with AF also have more adverse events from AADs,23 higher stroke risk,20,22 more disabling strokes,22 and a higher cardiovascular mortality compared with men.21 Despite the increased risk of events in women, which is further elevated with the addition of HF, only one-quarter of patients with CA were women. The disparity between sexes for treatment with CA is not unique to the AF-HF population, and several studies have shown that women are substantially less likely to have a CA in the general AF population.13,21,24 Among patients with AF treated with CA, it is estimated that < 30% were women.13,21,24 A similar trend of the unequal distribution of the sexes was demonstrated in randomized trials on CA in patients with AF-HF, in which 14.3% and 25.6% of patients enrolled in CASTLE-AF7 and AATAC6 were women, respectively.
It has been suggested that fewer women are treated with CA because of older age,25 presence of comorbidities that reflect a more diseased substrate,24,25 and 1.3- to 2.3-fold increased risk of procedural complications,26 including tamponade27 and vascular site complications.27 There is also evidence that the CA procedure in women is more difficult to perform because they tend to have more nonpulmonary vein triggers and atrial fibrosis.24,25 In addition, a study by Hoyt et al.26 suggests that women may have a higher rate of prolonged hospitalization after CA than men. Regardless, the women and men who underwent CA in the present study had similar patient characteristics, which may suggest that the strict criteria for CA may be based on the patient characteristics of men, who also comprise the majority of subjects enrolled in randomized trials,6,7 which the clinical guidelines are based on.10,11
Lower probability of CA with major comorbidities and advanced age
Elevated age, CHA2DS2-VASc scores, HAS-BLED scores, and the presence of chronic obstructive pulmonary disorder have all been identified as predictors for an increased risk of complications post-CA, all-cause mortality in the AF population, and hospitalizations in patients with HF.1,4 The high susceptibility for adverse events and outcomes may explain the reluctance to proceed with CA in this high-risk population.
Presence of cardiac electronic implantable devices as predictors
The probability of CA was also reduced with the presence of a CRT device when patients aged < 65 years were included in the cohort, and a similar trend existed in the medication cohort. It is possible that CRT was a marker for more advanced HF and more severe atrial disease, which may have deterred from consideration of CA. Furthermore, CRT may have been associated with atrial valve nodal ablation (rather than CA of AF) in some patients to ensure biventricular capture in patients with AF.28,29 In contrast, patients with an ICD (without CRT) may be more likely to undergo CA for AF because it is common practice to consider device upgrade to CRT before atrial valve node ablation.28,29
Medication use as predictors
Clinical guidelines recommend CA in symptomatic patients with AF refractory to at least 1 AAD.10,11 Although CA has increasingly been used as a first-line therapy in patients with paroxysmal AF,30 it remains likely that patients were prescribed an AAD before CA.10,11 In addition, studies evaluating AAD prescription patterns demonstrated that patients treated by cardiac electrophysiologists were more likely to be prescribed an AAD, who in turn may be more likely to proceed with CA.31,32
DOACs were prescribed in < 10% of the AF-HF population; however, 24% of patients who underwent CA had a prior prescription for DOACs. Given that DOACs are more likely to be prescribed by cardiologists,33 DOAC use may be associated with management by a cardiologist who may be more likely to refer a patient for CA compared with a nonspecialist. Patients taking DOACs may be less likely to have comorbidities, such as renal disease, in which the efficacy and safety of DOACs have not been established.10,11
The use of diuretics has been shown to be a marker of advanced HF and worse prognosis in patients with HF.34 Therefore, prior use of diuretics may be a surrogate marker for HF disease severity, and the results of the present study suggest that patients with more advanced HF (or diuretic use) were less likely to be treated with CA.
Limitations
Given the nature of the administrative databases used to characterize the type CA patients in the AF-HF population, potential clinical predictors for CA were missing, such as the type of AF (paroxysmal, persistent, and permanent), New York Heart Association class, and left ventricular ejection fraction. To account for severity of disease, we used proxy confounders such as the use of diuretics and presence of a CRT.
Medication information was only present for a subset of the population (patients aged > 65 years or without alternate forms of drug insurance); therefore, the results may be less generalizable to the typically younger population treated with CA. In our study, we found that > 90% of patients with AF-HF had government prescription coverage, but only 65% of the patients treated with CA were covered. Although we could not investigate medications as predictors in the entire AF-HF population, the same patient characteristics were identified as predictors in the medication and overall cohorts. The present study accounts for waiting time until CA (date of referral to date of CA) by blanking comorbidities first captured within 3 or 6 months before CA; however, waiting times may be longer than 6 months before the date of CA.
Conclusion
In a real-world population, CA was infrequently used to treat AF among patients with comorbid HF, and the likelihood of CA was further reduced in women. However, the frequency of CA increased over time. Encouraging results from randomized trials and updates to clinical guidelines may increase the frequency of CA in the AF-HF population, but the additional comorbidities that commonly coexist in the AF-HF population may prevent the widespread use of CA. Future studies need to be conducted in subjects whose clinical characteristics are more representative of the real-world AF-HF population to determine if CA should be considered more frequently as a treatment option for patients with AF with comorbid HF.
Funding Sources
This study was supported by an operating grant from the Canadian Institutes of Health Research, a Clinical Research Scholar Award to Dr Essebag from Fonds de recherché du Quebec-Santé, and a Doctoral Training award to Michelle Samuel from Fonds de recherché du Quebec-Santé. Drs Pilote and Abrahamowicz hold James McGill chairs at McGill University.
Disclosures
Dr Essebag has received honoraria from Biosense Webster Inc., St. Jude Medical, Medtronic Inc., Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Servier. All other authors have nothing to disclose.
Footnotes
Ethics Statement: The present article adheres to ethical guidelines.
See page 92 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.01.004.
Supplementary Material
References
- 1.Skanes A.C., Tang A.S.L. Atrial fibrillation and heart failure: untangling a modern gordian knot. Can J Cardiol. 2018;34:1437–1448. doi: 10.1016/j.cjca.2018.07.483. [DOI] [PubMed] [Google Scholar]
- 2.Huang H.D., Waks J.W., Contreras-Valdes F.M. Incidence and risk factors for symptomatic heart failure after catheter ablation of atrial fibrillation and atrial flutter. Europace. 2016;18:521–530. doi: 10.1093/europace/euv215. [DOI] [PubMed] [Google Scholar]
- 3.Siller-Matula J.M., Pecen L., Patti G. Heart failure subtypes and thromboembolic risk in patients with atrial fibrillation: the PREFER in AF - HF substudy. Int J Cardiol. 2018;265:141–147. doi: 10.1016/j.ijcard.2018.04.093. [DOI] [PubMed] [Google Scholar]
- 4.Richter S., Di Biase L., Hindricks G. Atrial fibrillation ablation in heart failure. Eur Heart J. 2019;40:663–671. doi: 10.1093/eurheartj/ehy778. [DOI] [PubMed] [Google Scholar]
- 5.Roy D., Talajic M., Nattel S. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 6.Di Biase L., Mohanty P., Mohanty S. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC Multicenter Randomized Trial. Circulation. 2016;133:1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 7.Marrouche N.F., Brachmann J., Andresen D. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 8.AlTurki A., Proietti R., Dawas A. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2019;19:18. doi: 10.1186/s12872-019-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Packer D.L., Mark D.B., Robb R.A. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade J.G., Verma A., Mitchell L.B. 2018 Focused Update of the Canadian Cardiovascular Society Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2018;34:1371–1392. doi: 10.1016/j.cjca.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 11.January C.T., Wann L.S., Calkins H. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019:25873. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Joza J., Samuel M., Jackevicius C.A. Long-term risk of stroke and bleeding post-atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2018;29:1355–1362. doi: 10.1111/jce.13702. [DOI] [PubMed] [Google Scholar]
- 13.Avgil Tsadok M., Gagnon J., Joza J. Temporal trends and sex differences in pulmonary vein isolation for patients with atrial fibrillation. Heart Rhythm. 2015;12:1979–1986. doi: 10.1016/j.hrthm.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 14.Samuel M., Avgil Tsadok M., Joza J. Catheter ablation for the treatment of atrial fibrillation is associated with a reduction in health care resource utilization. J Cardiovasc Electrophysiol. 2017;28:733–741. doi: 10.1111/jce.13225. [DOI] [PubMed] [Google Scholar]
- 15.Lunn M., McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 16.Reynolds M.R., Gunnarsson C.L., Hunter T.D. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5:171–181. doi: 10.1161/CIRCOUTCOMES.111.963108. [DOI] [PubMed] [Google Scholar]
- 17.Arora S., Lahewala S., Tripathi B. Causes and predictors of readmission in patients with atrial fibrillation undergoing catheter ablation: a national population-based cohort study. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karasoy D., Gislason G.H., Hansen J. Temporal changes in patient characteristics and prior pharmacotherapy in patients undergoing radiofrequency ablation of atrial fibrillation: a Danish nationwide cohort study. Europace. 2013;15:669–675. doi: 10.1093/europace/eus418. [DOI] [PubMed] [Google Scholar]
- 19.Magnussen C., Niiranen T.J., Ojeda F.M. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe) Circulation. 2017;136:1588–1597. doi: 10.1161/CIRCULATIONAHA.117.028981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lip G.Y., Laroche C., Boriani G. Sex-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Observational Research Programme Pilot survey on Atrial Fibrillation. Europace. 2015;17:24–31. doi: 10.1093/europace/euu155. [DOI] [PubMed] [Google Scholar]
- 21.Weberndörfer V., Beinart R., Ricciardi D. Sex differences in rate and rhythm control for atrial fibrillation. Europace. 2019;21:690–697. doi: 10.1093/europace/euy295. [DOI] [PubMed] [Google Scholar]
- 22.Fang M.C., Singer D.E., Chang Y. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–1691. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rienstra M., Van Veldhuisen D.J., Hagens V.E. Gender-related differences in rhythm control treatment in persistent atrial fibrillation: data of the Rate Control Versus Electrical Cardioversion (RACE) study. J Am Coll Cardiol. 2005;46:1298–1306. doi: 10.1016/j.jacc.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 24.Patel N., Deshmukh A., Thakkar B. Gender, race, and health insurance status in patients undergoing catheter ablation for atrial fibrillation. Am J Cardiol. 2016;117:1117–1126. doi: 10.1016/j.amjcard.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 25.Linde C., Bongiorni M.G., Birgersdotter-Green U. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. 2018;20:1565–ao. doi: 10.1093/europace/euy067. [DOI] [PubMed] [Google Scholar]
- 26.Hoyt H., Bhonsale A., Chilukuri K. Complications arising from catheter ablation of atrial fibrillation: temporal trends and predictors. Heart Rhythm. 2011;8:1869–1874. doi: 10.1016/j.hrthm.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 27.Michowitz Y., Rahkovich M., Oral H. Effects of sex on the incidence of cardiac tamponade after catheter ablation of atrial fibrillation: results from a worldwide survey in 34 943 atrial fibrillation ablation procedures. Circ Arrhythm Electrophysiol. 2014;7:274–280. doi: 10.1161/CIRCEP.113.000760. [DOI] [PubMed] [Google Scholar]
- 28.Yancy C.W., Jessup M., Bozkurt B. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 29.Parkash R., Philippon F., Shanks M. Canadian Cardiovascular Society guidelines on the use of cardiac resynchronization therapy: implementation. Can J Cardiol. 2013;29:1346–1360. doi: 10.1016/j.cjca.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Andrade J.G., Champagne J., Deyell M.W. A randomized clinical trial of early invasive intervention for atrial fibrillation (EARLY-AF) - methods and rationale. Am Heart J. 2018;206:94–104. doi: 10.1016/j.ahj.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Ionescu-Ittu R., Abrahamowicz M., Jackevicius C.A. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch Intern Med. 2012;172:997–1004. doi: 10.1001/archinternmed.2012.2266. [DOI] [PubMed] [Google Scholar]
- 32.Lip G.Y., Laroche C., Ioachim P.M. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry) Eur Heart J. 2014;35:3365–3376. doi: 10.1093/eurheartj/ehu374. [DOI] [PubMed] [Google Scholar]
- 33.Weitz J.I., Semchuk W., Turpie A.G. Trends in prescribing oral anticoagulants in Canada, 2008-2014. Clin Ther. 2015;37:2506–25014. e4. doi: 10.1016/j.clinthera.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Pellicori P., Cleland J.G., Zhang J. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther. 2016;30:599–609. doi: 10.1007/s10557-016-6697-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.