Abstract
Transcatheter mitral valve repair using the MitraClip (Abbott Vascular, Santa Clara, CA) is a reasonable option for the treatment of patients with severe symptomatic degenerative mitral regurgitation (MR) who are at prohibitive surgical risk. The occurrence of recurrent severe MR after initial successful MitraClip repair is uncommon. Data are sparse on the management of recurrent severe MR after initial successful repair using the MitraClip. We describe a successful case of redo MitraClip repair for late recurrent severe MR secondary to progressive degenerative mitral valve disease after a successful initial MitraClip procedure and review the literature.
Résumé
La réparation transcathéter de la valve mitrale au moyen d’un dispositif MitraClip (Abbott Vascular, Santa Clara, CA) constitue une bonne option pour le traitement de l’insuffisance mitrale (IM) dégénérative symptomatique grave lorsque la chirurgie représente un risque prohibitif pour le patient. Il est rare qu’une IM grave récurrente survienne après l’implantation réussie d’un dispositif MitraClip. On dispose de très peu de données sur la prise en charge de l’IM grave récurrente après une première réparation au moyen d’un dispositif MitraClip. Nous présentons le cas d’une seconde réparation au moyen d’un dispositif MitraClip pour remédier à une IM grave récurrente tardive secondaire à une atteinte dégénérative évolutive de la valve mitrale survenue après une première intervention efficace au moyen d’un dispositif MitraClip, et nous passons en revue les publications portant sur cette question.
Novel Teaching Points.
-
•
The repeat MitraClip procedure is a potential option for patients with recurrent severe MR after an initial successful MitraClip procedure when the anatomy is suitable.
Transcatheter mitral valve repair using the MitraClip (Abbott Vascular, Santa Clara, CA) is a reasonable option for the treatment of patients with severe, symptomatic, degenerative mitral regurgitation (MR) who are at prohibitive surgical risk.1 The occurrence of recurrent severe MR after initial successful MitraClip repair is uncommon. Data are sparse on the management of recurrent severe MR after initial successful MitraClip repair.2 We describe a successful case of redo MitraClip repair for late recurrent severe MR secondary to progressive degenerative mitral valve disease after a successful initial MitraClip procedure and review the literature on this topic.
Case Presentation
History
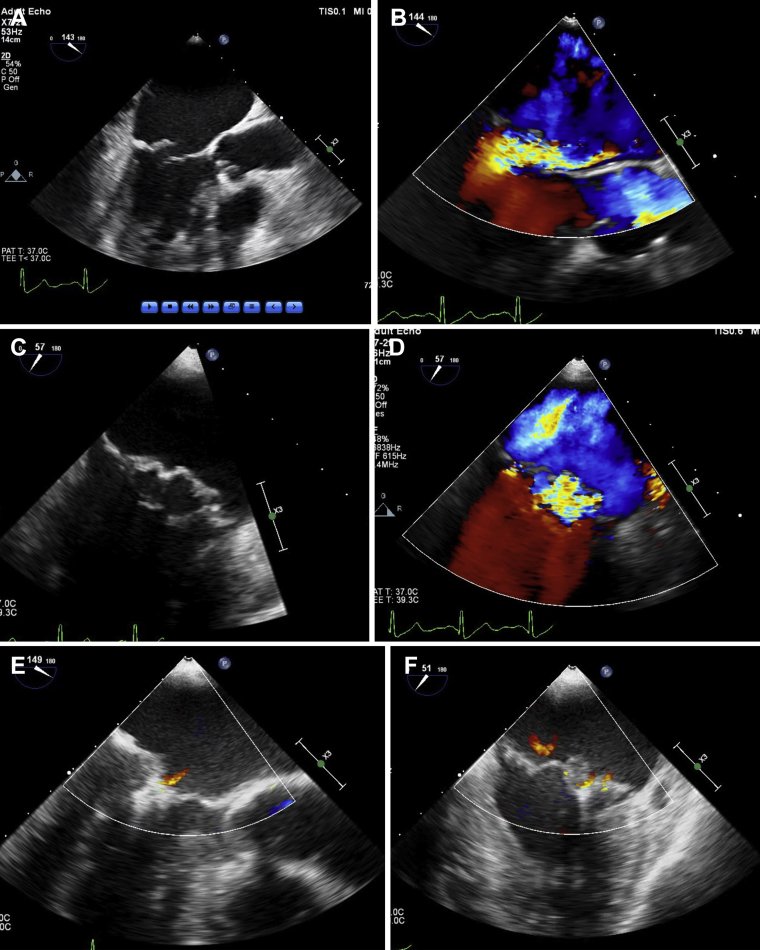
An 84-year-old man presented with symptoms of worsening dyspnea on exertion for 3 months. He could not walk more than 1 block without having to stop because of dyspnea (New York Heart Association class III). Eighteen months before the current presentation, he underwent successful transcatheter mitral valve repair with 1 MitraClip (first-generation or classic MitraClip) for severe degenerative MR secondary to flail posterior mitral valve leaflet (lateral aspect of P2 scallop) (Fig. 1A-D; Videos 1A-E
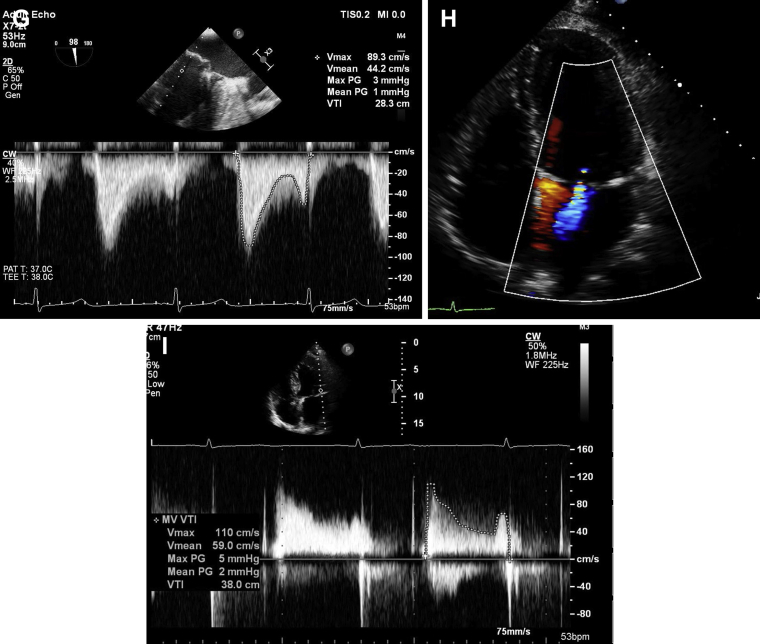
 , view videos online), resulting in trivial to mild residual MR and 1 to 2 mm Hg mean mitral valve gradient (Fig. 1E-G; Videos 2A and 2B
, view videos online), resulting in trivial to mild residual MR and 1 to 2 mm Hg mean mitral valve gradient (Fig. 1E-G; Videos 2A and 2B
 , view videos online) with sustained good results on 30-day follow-up (Fig. 1H and I; Video 3
, view videos online) with sustained good results on 30-day follow-up (Fig. 1H and I; Video 3
 , view video online). His medical history also included hypertension, hyperlipidemia, chronic kidney disease, stroke without residual neurologic deficit, severe coronary artery disease with history of coronary artery bypass grafting, and mild aortic regurgitation. The patient had excellent clinical results after the initial MitraClip procedure and could walk 2 miles or more each day without dyspnea. His symptoms then proceeded to be similar to those occurring before the initial MitraClip procedure. He was receiving appropriate medical therapy. Physical examination revealed normal blood pressure and III/VI holosystolic murmur at the apex consistent with severe MR.
, view video online). His medical history also included hypertension, hyperlipidemia, chronic kidney disease, stroke without residual neurologic deficit, severe coronary artery disease with history of coronary artery bypass grafting, and mild aortic regurgitation. The patient had excellent clinical results after the initial MitraClip procedure and could walk 2 miles or more each day without dyspnea. His symptoms then proceeded to be similar to those occurring before the initial MitraClip procedure. He was receiving appropriate medical therapy. Physical examination revealed normal blood pressure and III/VI holosystolic murmur at the apex consistent with severe MR.
Figure 1.
Long-axis (A, B) and intercommissural (C, D) transesophageal echocardiography (TEE) image demonstrating severe mitral regurgitation (MR) secondary to flail P2 scallop of mitral valve before the initial MitraClip (Abbott Vascular, Santa Clara, CA) repair. Long-axis (E) and intercommissural (F) intraprocedural TEE image demonstrating mild residual MR after the initial MitraClip procedure with a mean gradient of 1 mm Hg (G) across the mitral valve. Mild residual MR (H) and mean mitral valve gradient of 2 mm Hg (I) on 30-day follow-up transthoracic echocardiogram (TTE) after the initial MitraClip procedure.
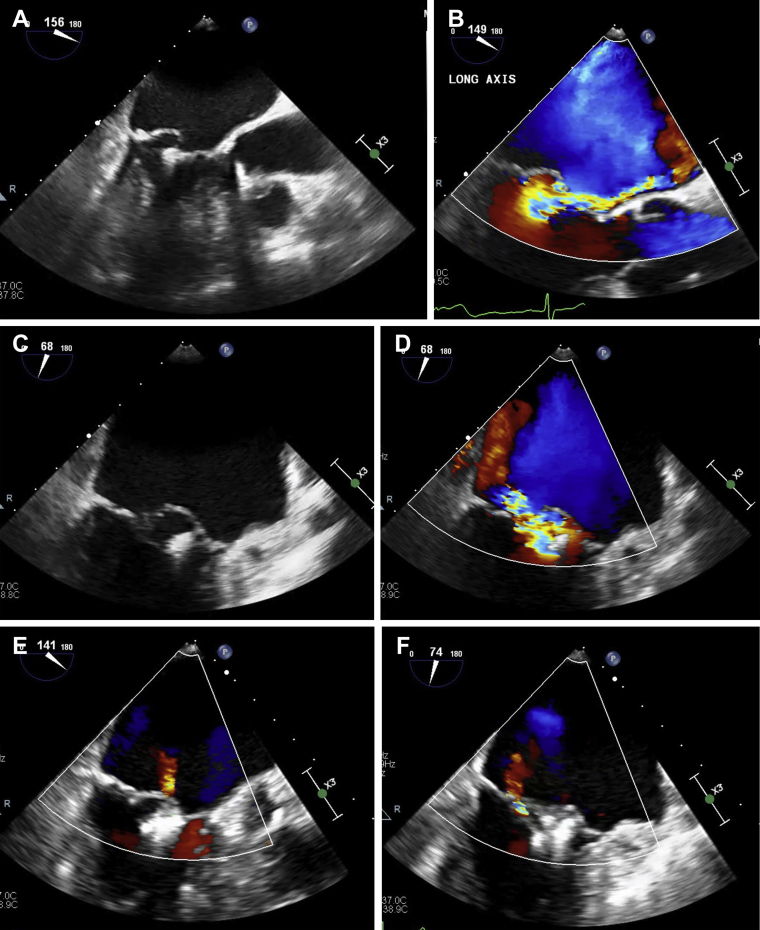
Transthoracic echocardiogram (TTE) showed preserved left ventricular ejection fraction of 60% to 65%, a well-affixed MitraClip, and severe MR. Transesophageal echocardiogram (TEE) confirmed the presence of severe MR, with its origin medial to the previously placed MitraClip on the A lateral aspect of A2-P2 scallops with development of flail P2 scallop medial to the MitraClip (Fig. 2A-D; Videos 4A-E
 , view videos online) and 2 mm Hg mean mitral valve gradient. The pre-MitraClip mitral valve area was 4.1 sq cm. It was thought that the patient developed recurrent severe MR secondary to progressive degenerative mitral valve disease with torn chordae in the P2 scallop region, medial to the first MitraClip. There was no evidence of leaflet perforation, partial clip detachment (PCD), or single leaflet device attachment (SLDA). The iatrogenic atrial septal defect (iASD) from the initial MitraClip procedure had spontaneously closed.
, view videos online) and 2 mm Hg mean mitral valve gradient. The pre-MitraClip mitral valve area was 4.1 sq cm. It was thought that the patient developed recurrent severe MR secondary to progressive degenerative mitral valve disease with torn chordae in the P2 scallop region, medial to the first MitraClip. There was no evidence of leaflet perforation, partial clip detachment (PCD), or single leaflet device attachment (SLDA). The iatrogenic atrial septal defect (iASD) from the initial MitraClip procedure had spontaneously closed.
Figure 2.
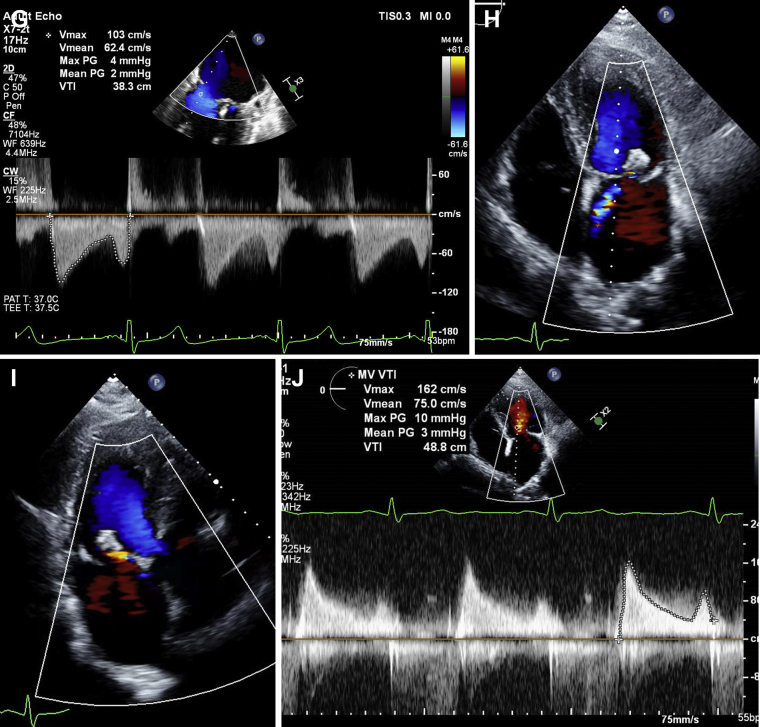
Long-axis (A, B) and intercommissural (C, D) TEE image demonstrating severe MR secondary to flail P2 scallop of the mitral valve, medial to the initial MitraClip, before redo MitraClip repair. Long-axis (E) and intercommissural (F) TEE image demonstrating mild residual MR after redo MitraClip repair with a mean gradient of 2 mm Hg (G) across the mitral valve. Four-chamber (H) and long-axis (I) TTE image demonstrating mild residual MR with mean mitral gradient of 3 mm Hg (J) on 12-month TTE after the redo MitraClip procedure.
The patient’s Society of Thoracic Surgeons Predicted Risk of Mortality score was 6% for mitral valve repair and 9.1% for mitral valve replacement. After discussion with the multidisciplinary heart team, it was decided to proceed with redo MitraClip repair, with the goal of placing 1 or more MitraClips medial to the previously placed clip to reduce the severity of MR.
The procedure was performed with the patient under general anaesthesia, using right femoral venous access. Trans-septal puncture was performed in the usual fashion at a high and posterior location achieving a puncture height of 4.3 cm from the mitral valve given that our target was in the center, that is, the A2-P2 scallop region rather than toward the commissures. By using 3-dimensional TEE guidance and fluoroscopy, a MitraClip NT was advanced and used to grasp the A2 and P2 scallops of the mitral valve, medial to the previously placed MitraClip, with reduction in MR from severe to mild, with mean mitral valve gradient of 2 mm Hg (Fig. 2E-G; Videos 5A-C
 , view videos online). We decided to conclude the procedure, given the successful result with acceptable residual MR and gradient. The iASD demonstrated left to right shunt and was left alone without closure. The patient was discharged home the next day without any periprocedural complications.
, view videos online). We decided to conclude the procedure, given the successful result with acceptable residual MR and gradient. The iASD demonstrated left to right shunt and was left alone without closure. The patient was discharged home the next day without any periprocedural complications.
The patient was followed up at 1, 3, 6, and 12 months in an outpatient clinic. He continues to feel well and is able to walk 2 miles or more each day without symptoms of dyspnea on exertion. The most recent TTE at 12 months demonstrated left ventricular ejection fraction of 60%, 2 well-affixed MitraClips, with mild residual MR and mean mitral valve gradient of 3 mm Hg (Fig. 2H-J; Videos 6A and 6B
 , view videos online).
, view videos online).
Discussion
Transcatheter mitral valve repair using the MitraClip is a reasonable option for the treatment of patients with severe symptomatic degenerative MR who are at prohibitive surgical risk.1 Since commercial approval of the MitraClip by the Food and Drug Administration, more than 12,000 patients have undergone transcatheter mitral valve repair at 275 centers in the United States. The occurrence of recurrent severe MR requiring repeat MitraClip repair after the initial successful MitraClip repair is uncommon (2%-4%).2, 3, 4 Data are sparse on the management of recurrent severe MR after initial successful MitraClip placement. In the Endovascular Valve Edge-to-Edge Repair Study (EVEREST) trial, 5 of 184 patients (2.7%) underwent a redo MitraClip procedure; 4 of the 5 patients were successfully treated within 12 months, and 1 patient who did not have a successful second intervention received an additional procedure to place a second MitraClip between 12 months and 4 years of follow-up.5 Patients undergoing MitraClip repair are prohibitive surgical risk candidates to begin with, and the option of surgery for recurrent severe symptomatic MR usually does not exist. A redo MitraClip procedure, if anatomically feasible, is an attractive option in such patients.
Our patient underwent the first MitraClip procedure with the classic or the first generation of the device with good results as demonstrated by immediate postprocedural TEE and 30-day TTE in addition to improvement in symptoms. Upon reviewing the echocardiography images from the initial implant, we retrospectively thought that 2 MitraClips would have possibly provided a more durable long-term result given that the flail width was somewhat wide; however, given the successful result with 1 MitraClip at that time, we did not think this was necessary. It is well known that the risk/benefit ratio in terms of further reductions in MR must be balanced with a reduction in mitral valve area and the risk of creating mitral stenosis. We had achieved trivial to mild MR with an initial single MitraClip with excellent leaflet insertion. Upon reviewing the TEE at the patient’s second presentation with recurrent symptoms, we found that the original MitraClip was well affixed on the lateral aspect of A2-P2 scallops; however, severe MR was observed medial to the MitraClip. We think that the patient underwent progressive degeneration of the myxomatous valve and developed torn chordae in the medial aspect of the P2 scallop, resulting in recurrence of severe MR. However, it is possible that insufficient flail segment was contained in the first MitraClip to hold enough leaflet for more than the given time period to prevent recurrent MR, especially in a myxomatous valve. Ongoing stress on the valve created by residual billowing of the leaflets could have led to incomplete or partial pulling out of the leaflet from between the arms and the grippers over time. In general, an additional MitraClip can be placed if the native mitral valve area is > 4 sq cm and transmitral gradient is < 5 mm Hg after placement of 1 MitraClip with a low risk of creating significant mitral stenosis. Given the low mean mitral gradient of 2 mm Hg in our patient, initial pre-MitraClip mitral valve area of 4.1 sq cm, and adequate room medial to the MitraClip, we decided to proceed with the redo MitraClip procedure. We achieved a satisfactory result with the placement of the MitraClip NT medial to the original MitraClip. The latest iterations of the MitraClip device, the MitraClip NTR or XTR, were introduced in mid-2018 and were not available at the time of the redo MitraClip procedure; thus, we used the MitraClip NT. It is possible that the problem highlighted by our case and other reports discussed next may become obsolete, because the XTR device, characterized by extended clip arms and longer grippers, may facilitate more effective leaflet approximation with reduction in stress exerted on the flail segment over time, although this remains to be proven in ongoing and future investigations. There are few data on which iASDs should be closed after trans-septal mitral procedures. In general, in our practice we consider closing iASDs that are large (> 8 mm) or associated with large left to right shunt, right to left shunt with hypoxemia, severe right ventricular dysfunction, and pulmonary hypertension.
The largest published experience on a repeat MitraClip procedure for recurrent severe MR consists of 21 of 410 patients (5.1%) treated at a single center in Germany.2 Mechanisms described for recurrence of MR include progression of underlying pathology (functional or degenerative), loss of leaflet insertion (LLI), leaflet perforation, and PCD or SLDA. Repeat MitraClip interventions occurred at a median of 6.3 months after the index procedure. In contrast, our patient developed late recurrence at 1.5 years from the index procedure. Of the 21 patients who underwent a repeat MitraClip procedure, 8 had LLI, of whom 3 met criteria for PCD or SLDA. The procedural success rate of repeat MitraClip procedure was only 62% (13/21 cases) in contrast to > 90% for index procedure. In addition, LLI at the time of repeat intervention was strongly associated with reduced success; only 25% of repeat procedures could be successfully performed when LLI was present, as opposed to 85% repeat interventional success when leaflet insertion was adequate. None of the patients with PCD could be treated successfully. At follow-up, 4 patients (3 with LLI, of whom 1 had PCD and 1 had adequate leaflet insertion) underwent mitral valve surgery at a median of 20 days after the failed repeat intervention, and 1 patient underwent mitral valve surgery for recurrence of significant MR 43 days after the initially successful repeat procedure. Thirteen patients (62%) died at a median of 3 months after repeat MitraClip intervention, including 3 (with functional MR) of the 5 patients who underwent surgery. The 2 patients with degenerative MR who underwent surgery were alive at 13 and 32 months after the repeat intervention. This study shows that the repeat MitraClip procedure is feasible particularly in patients with adequate leaflet insertion at the time of repeat intervention. In contrast to the study by Kreidel et al.,2 successful treatment of recurrent MR secondary to early PCD (within 3 days of the index procedure) in 4 patients with a repeat MitraClip procedure was recently reported by Hachinohe et al.6 Given sparse data, it is difficult to make recommendations regarding management strategies for recurrent severe MR after an initial successful MitraClip procedure. Individual decisions must be made after careful review of anatomy, suitability for redo MitraClip based on gradient, location, valve area, and patient-specific risk factors, especially surgical risk.
Conclusion
We describe a successful case of a redo MitraClip procedure for late recurrent severe MR secondary to progressive degenerative mitral valve disease after the successful initial MitraClip procedure. On the basis of the existing literature, we think that the redo MitraClip procedure should be considered in patients with a high prohibitive surgical risk and recurrent severe MR if the anatomy is suitable for a repeat procedure.
Funding Sources
There are no funding sources to declare.
Disclosures
Dr Goel is on the Speaker’s Bureau for Abbott Structural Heart.
Footnotes
Ethics Statement: This work has adhered to the relevant ethical guidelines.
See page 185 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2020.01.009.
Supplementary Material
Long-axis (A and B) and intercommissural (C and D) transesophageal echocardiography (TEE) image demonstrating severe mitral regurgitation (MR) secondary to flail P2 scallop of the mitral valve before the initial MitraClip (Abbott Vascular, Santa Clara, CA) procedure. Three-dimensional TEE showing P2 flail (E).
Long-axis (A) and intercommissural (B) TEE image demonstrating mild residual MR after the initial MitraClip procedure.
Mild residual MR on 30-day follow-up transthoracic echocardiogram (TTE) after the initial MitraClip procedure.
Long-axis (A and B) and intercommissural (C and D) TEE image demonstrating severe MR secondary to flail P2 scallop of the mitral valve, medial to initial MitraClip. Three-dimensional TEE (E) showing flail P2 medial to the initial MitraClip procedure.
Long-axis (A) and intercommissural (B) TEE image demonstrating mild residual MR after the redo MitraClip procedure. Three-dimensional TEE (C) showing 2 MitraClips attached to A2P2 scallops of the mitral valve.
Four-chamber (A) and long-axis (B) TTE image demonstrating mild residual MR 12 months after the redo MitraClip procedure.
References
- 1.Nishimura R.A., Otto C.M., Bonow R.O. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 2.Kreidel F., Frerker C., Schluter M. Repeat MitraClip therapy for significant recurrent mitral regurgitation in high surgical risk patients: impact of loss of leaflet insertion. JACC Cardiovasc Interv. 2015;8:1480–1489. doi: 10.1016/j.jcin.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Tay E.L., Lim D.S., Yip J. Redo MitraClip mitral valve repair after a late single leaflet detachment. Catheter Cardiovasc Interv. 2014;84:160–163. doi: 10.1002/ccd.25337. [DOI] [PubMed] [Google Scholar]
- 4.DeBonis M., Lapenna E., Buzzatti N. Optimal results immediately after MitraClip therapy or surgical edge-to-edge repair for functional mitral regurgitation: are they really stable at 4 years? Eur J Cardiothorac Surg. 2016;50:488–494. doi: 10.1093/ejcts/ezw093. [DOI] [PubMed] [Google Scholar]
- 5.Mauri L., Foster E., Glower D.D. 4-year results of a randomized controlled trial of percutaneous repair versus surgery for mitral regurgitation. J Am Coll Cardiol. 2013;62:317–328. doi: 10.1016/j.jacc.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Hachinohe D., Latib A., Agricola E., Colombo A. Repeat MitraClip for early recurrent mitral regurgitation. Catheter Cardiovasc Interv. 2018;92:611–616. doi: 10.1002/ccd.27460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Long-axis (A and B) and intercommissural (C and D) transesophageal echocardiography (TEE) image demonstrating severe mitral regurgitation (MR) secondary to flail P2 scallop of the mitral valve before the initial MitraClip (Abbott Vascular, Santa Clara, CA) procedure. Three-dimensional TEE showing P2 flail (E).
Long-axis (A) and intercommissural (B) TEE image demonstrating mild residual MR after the initial MitraClip procedure.
Mild residual MR on 30-day follow-up transthoracic echocardiogram (TTE) after the initial MitraClip procedure.
Long-axis (A and B) and intercommissural (C and D) TEE image demonstrating severe MR secondary to flail P2 scallop of the mitral valve, medial to initial MitraClip. Three-dimensional TEE (E) showing flail P2 medial to the initial MitraClip procedure.
Long-axis (A) and intercommissural (B) TEE image demonstrating mild residual MR after the redo MitraClip procedure. Three-dimensional TEE (C) showing 2 MitraClips attached to A2P2 scallops of the mitral valve.
Four-chamber (A) and long-axis (B) TTE image demonstrating mild residual MR 12 months after the redo MitraClip procedure.