Abstract
Endometriosis is a benign disease characterized by endometrial glands and stroma outside the endometrial cavity. We reported two cases of endometriosis of the abdominal wall, with subcutaneous and intramuscular localization, that became symptomatic a few years after a cesarean intervention. These cases have a clinical pattern quite similar to cutaneous endometriosis, but they are more difficult to diagnose through physical examination because they are barely palpable. In this sense, coupled with suggestive symptoms, ultrasound examination can confirm the clinical suspicion of endometriosis without the use of computed tomography and/or magnetic resonance imaging.
Keywords: Endometriosis, Abdominal wall, Non-palpable mass, Cesarean scar, Ultrasound
Introduction
Endometriosis is defined as the presence of endometrial glands and stroma outside the endometrial cavity. The ectopic tissue is responsive to ovarian hormonal stimulation and proliferates when stimulated by cyclic estrogens, which makes it appear to “menstruate.” Endometriosis was first described by Carl von Rokitansky [1]. This condition has a prevalence of 10–20%, with an estimated prevalence of 5–10% in the general female population [2]. It generally occurs in pelvic sites, such as the ovaries, bowel, or pelvic peritoneum, but it can rarely arise in extrapelvic sites [1]. Major sites for extrapelvic endometriosis include the lungs, pleura, kidneys, bladder, omentum, bowels, lymph nodes, appendix, and skin (frequently on scar tissue) [3]. Subcutaneous and intramuscular endometriosis is often difficult to diagnose through only physical examination because the small nodules can be barely palpable. This condition is highly associated with previous abdominal surgery [4]. The occurrence of symptoms and the growth of the endometriosis depend on estrogen stimulation; in this sense, periodic increases in pain intensity associated with menstruation can occur [1, 3, 4]. Abdominal wall endometriosis (AWE) is often misdiagnosed as one of several other pathological conditions such as dermoid cyst, lymphoma, hernia, metastatic carcinoma, sarcoma, and hematoma [5, 6]. The average latency period between the [7] first appearance of symptoms and the definitive diagnosis has been estimated at around 10 years [8]. This diagnostic delay leads to patient psychological strain, worse quality of life, higher healthcare costs, and unnecessary diagnostic and therapeutic procedures [8, 9].
Case description
Case 1
A 40-year-old woman was referred to our clinic because of focal tenderness in the right inguinal area and dyspareunia, with no local swelling or palpable mass. She had two pregnancies, both delivered by cesarean sections, with the last one occurring 5 years before. Her past medical history was not suggestive of any systemic disease. During the physical examination, no abnormalities of the cesarean scar were detected and no palpable mass at the right side of the incision was identified. The abdominal pain first occurred two years after the last cesarean section; the pain pattern was intermittent and strongly correlated with her menstrual cycle.
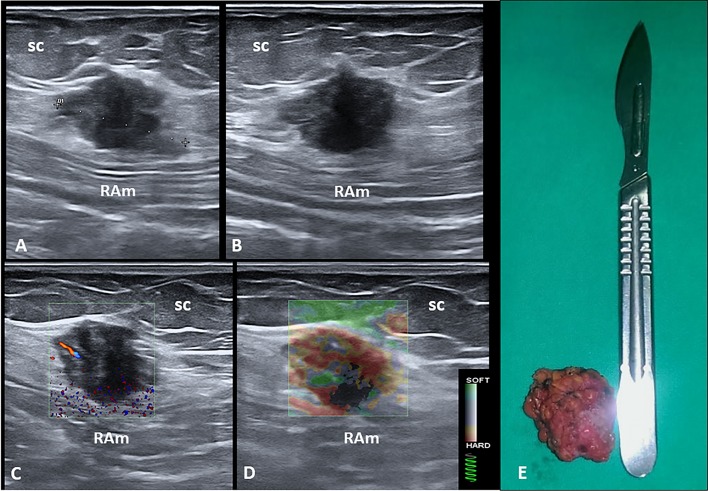
Sonographic examination of the abdominal wall revealed, in the right inguinal region and at the level of the subcutaneous tissue, a heterogeneous hypoechoic mass with echogenic intralesional spots (Fig. 1). The nodule was precisely located beneath the cesarean surgery scar and superficial to the rectus abdominis muscle fascia. Color Doppler examination showed the presence of intralesional vascular spots, and strain elastosonography revealed a hard pattern compared to the surrounding tissues (Fig. 1).
Fig. 1.
Long (a) and short (b) axis B-mode ultrasound (US) images show the hypoechoic nodule located between the subcutaneous tissue (sc) and the muscular plane of the abdominal wall. Fine intralesional vascular spots are depicted using the color-power Doppler mode (c), and a hard pattern of the mass is clearly identified with the strain elastosonography modality (d). The macroscopic appearance of the corresponding surgical specimen after complete excision (e). RAm rectus abdominis muscle
Case 2
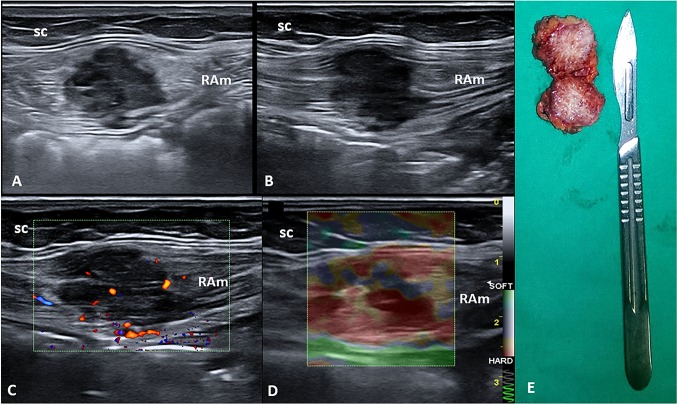
A 43-year-old woman was evaluated in our clinic complaining of pain over the hypogastric area. The obstetric history was characterized by multiple pregnancies with a cesarean section 3 years before. No previously diagnosed systemic disease was reported. The abdominal pain began two years after the last cesarean section, with a cyclic pattern strongly related to her menstrual cycle. During the physical examination, no abnormalities of the cesarean scar were detected and no palpable mass was identified. Ultrasound (US) imaging, using a multifrequency linear probe, showed a well-defined hypoechoic mass measuring 3 × 2.7 cm located on the right side of the Pfannenstiel scar and inside the rectus abdominis muscle (Fig. 2). Color Doppler modality revealed the presence of intralesional vascular spots, and strain elastosonography clearly showed a hard pattern compared to the surrounding tissues (Fig. 2).
Fig. 2.
Short (a) and long (b) axis B-mode ultrasound (US) images show the hypoechoic nodule located inside the rectus abdominis muscle (RAm). Fine intralesional vascular spots are depicted using the color-power Doppler mode (c), and a hard pattern of the mass is identified with the strain elastosonography modality (d). The macroscopic bilobed appearance of the corresponding surgical specimen after complete excision (e). sc subcutaneous tissue
Surgical treatment and histological diagnosis
Patients were referred for surgical treatment with complete excision of the masses (Figs. 1 and 2). The histological examination of the surgical specimens revealed benign endometrial glands and stromal tissues, thus confirming the diagnosis of endometriosis. Free surgical margins of 1 cm were histologically demonstrated in order to prevent local recurrence of the pathology. Note that the histopathological pattern also showed multiple widespread hemorrhagic foci inside the mass. The patients were discharged after uneventful hospital courses.
Discussion
The pathophysiological processes underlying endometriosis are unclear. Three theories (tubal regurgitation, coelomic metaplasia, and vascular spread) have been postulated to explain it [10].
A widely accepted explanation for the presence of endometriosis in unusual sites (e.g., lungs, brain, and incisional scars) is that endometrial cells are transported through hematogenous, lymphatic, or iatrogenic routes [11]. Some authors have suggested that natural killer activity and/or altered peritoneal macrophage maturation may play a role in its pathogenesis [12].
Today, surgical scar endometriosis following obstetric and gynecological procedures is more common due to an increasing number of cesarean sections worldwide [13]. Health care providers should suspect cutaneous endometriosis in any women with pain and a lump in the incisional scar after pelvic surgery [8]. Cesarean section (CS) is the most common surgery performed around the world; the World Health Organization (WHO) suggests a cesarean rate from 5 to 15%, but the worldwide percentage is higher [14]. An alarming increase in the rate of CS has been observed in the last decade; CS prevalence had an estimate of 17.6% in 2010. Women’s motivations for the choice of CS include fear of vaginal delivery, preservation of coital function, relief from the pain of labor, and to obtain a tubal ligation [13, 14]. Generally, abdominal wall endometriosis is confined to the peritoneal surface and is mainly associated with cesarean section (incidence 1–2%), but it may also result from a previous surgical procedure [15]. Several studies have estimated the time interval between surgery and clinical presentation to range from 3 months to 10 years [15].
The pathogenesis of endometriosis is complex. AWE is believed to result from a mechanical iatrogenic implantation with endometrial cells during surgical intervention through the direct inoculation of the abdominal fascia and/or subcutaneous tissue. Under estrogen stimulation, these cells become active and expand [10]. Some authors have examined the factors contributing to CSE and defined possible causes, including: the easy separation and transport of endometrial cells by the amniotic fluid flow into the pelvic cavity after hysterotomy; the large number of endometrial cells that spread into the pelvis before hysterotomy closure, which can potentially be trapped in the wound; and the nurturing role of blood and hormones after inoculation of the cells, which allows them to grow and to develop into subcutaneous masses [16]. It is important to highlight that a higher incidence is reported after early hysterotomy (at the end of the second or the beginning of the third trimester), as early decidua seems to have more pluripotential capabilities and can result in enhanced cellular replication, producing endometriosis [15, 16]. Endometriosis guidelines report that only histological examination can provide definitive confirmation of the diagnosis [17]. However, medical history combined with a gynecological examination has a combined sensitivity of around 80% for diagnosing endometriosis [18]. As illustrated in our cases, patients are referred for medical examination due to the presence of abdominal/pelvic pain that often does not present clear and immediate anatomical and pathological explanations; therefore, it is often misdiagnosed as an irritable bowel or a functional disorder. As previously mentioned, the location of endometriosis can be variable and widespread. The qualitative assessment of pain often shows a close relationship with the menstrual cycle, and this represents the main clue for the diagnosis of endometriosis [17, 18].
In this setting, US (transabdominal and transvaginal) is routinely employed to detect ovarian endometrial cysts, uterine adenomyosis, and adhesions in the inner genital region, as well as to evaluate uterine motility status and any thickening of the intestinal wall (bowel localization) [5, 9]. Sometimes, US kidney evaluation can be performed to exclude asymptomatic hydronephrosis caused by deep infiltrating endometriosis affecting the ureters. In the case of deep infiltrating endometriosis, magnetic resonance imaging is the method of choice to determine the extent of the disease [19].
In our patients, transabdominal US allowed the identification of pathological masses not otherwise detectable with a normal physical examination. Also, tenderness in the site of the probe’s pressure and the location of the masses in relation to the surgical scar directed the diagnostic suspicion toward extrauterine endometriosis. Specifically, US examination showed hypoechoic masses with well-defined boundaries (benignity criterion), but with intralesional vascularization (malignancy criterion). Moreover, strain elastography produced a different echostructural pattern with respect to the surrounding tissue, thus confirming the ectopic nature of the masses.
Few previous works have investigated the role of elastography in endometriosis, and some authors have demonstrated that sonoelastography has high sensitivity and specificity for distinguishing endometrioma from hemorrhagic ovarian cysts [20]. Previously published papers have also evaluated elastography in diagnosing cesarean section scar endometrioma and have shown that the endometrioma presents a typical blue–green–red appearance with clearly defined outer borders (i.e., red and green areas correspond to the central hypoechoic soft areas) [21].
In our opinion, the two clinical cases demonstrate how a simple and repeatable method such as US allowed us to diagnose the abdominal wall masses that would not have been detected with a simple physical examination. Furthermore, the US study performed with a high-resolution linear probe allowed us to examine all the characteristics of the masses (structure, margins, ratios, and vascularization), avoiding the use of advanced imaging methods such as CT and magnetic resonance, which have known side effects [22]. The use of strain elastosonography allowed a qualitative estimate of the stiffness of the ectopic tissue. The main limitation of US, and of all imaging methods, is that the examination is not conclusive enough for a specific histopathological diagnosis. Therefore, since these US methods have the same diagnostic accuracy with lower cost and time requirements and fewer side effects, they seem to be the method of choice for the diagnosis of endometriotic pathology in the abdominal wall, in combination with an accurate past medical history (cyclic abdominal pain and specific pain localization next to the CS scar).
Conclusion
The endometriosis of the abdominal wall following an obstetric and gynecological procedure is becoming more frequently diagnosed due to the increasing number of cesarean sections worldwide. Likewise, subcutaneous and intramuscular endometrioses of the abdominal wall are not rare gynecological conditions, but they are quite difficult to clinically diagnose if the mass is not easily palpable.
Ultrasound imaging in the clinical setting, with a high-frequency linear probe, is a valuable tool to promptly identify endometriotic foci inside the superficial tissues of the abdominal wall. Furthermore, color-power Doppler and strain elastosonography can provide additional information regarding the vascular pattern and physical features (e.g., soft or hard) of the mass. To the best of our knowledge, our report is the first on the use of elastosonography in the diagnosis of extrauterine endometriotic lesions. Future studies are needed on the elastosonographic features of endometriotic masses, both with strain and shear wave techniques. It would be interesting to conduct, in concert with the menstrual cycle, an ultrasound screening of the abdominal wall, in the neighboring sites, underlying the surgical scar to highlight small non-palpable endometriotic foci. (In some cases, the ultrasound could precede the clinical diagnosis.) Only in doubtful cases would level II diagnostic methods be used. In selected cases, cytological analysis using ultrasound-guided aspirate would be possible.
Compliance with ethical standards
Conflict of interest
Authors declare no conflicts of interest.
Research involving human participants and/or animals
Not applicable.
Informed consent
Written informed consent was obtained by both patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rokitansky K. U ¨ ber Uterusdru ¨sen-Neubildung. Z Gesellschaft Aerzte (Wien) 1860;16:577–581. [Google Scholar]
- 2.Fuldeore MJ, Soliman AM. Prevalence and symptomatic burden of diagnosed endometriosis in the United States: national estimates from a cross-sectional survey of 59,411 women. Gynecol Obstet Invest. 2017;82:453–461. doi: 10.1159/000452660. [DOI] [PubMed] [Google Scholar]
- 3.Davis AC, Goldberg JM. Extrapelvic endometriosis. Semin Reprod Med. 2017;35:98–101. doi: 10.1055/s-0036-1597122. [DOI] [PubMed] [Google Scholar]
- 4.Bozkurt M, Çil AS, Bozkurt DK. Intramuscular abdominal wall endometriosis treated by ultrasound-guided ethanol injection. Clin Med Res. 2014;12:160–165. doi: 10.3121/cmr.2013.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van den Bosch T, Van Schoubroeck D. Ultrasound diagnosis of endometriosis and adenomyosis: state of the art. Best Pract Res Clin Obstet Gynaecol. 2018;51:16–24. doi: 10.1016/j.bpobgyn.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Cocco G, Boccatonda A, D’Ardes D, et al. Mantle cell lymphoma: from ultrasound examination to histological diagnosis. J Ultrasound. 2018;21:339. doi: 10.1007/s40477-018-0318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alnafisah F, Dawa SK, Alalfy S. Skin endometriosis at the caesarean section scar: a case report and review of the literature. Cureus. 2018;10:e2063. doi: 10.7759/cureus.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mistrangelo M, Gilbo N, Cassoni P, et al. Surgical scar endometriosis. Surg Today. 2014;44:767–772. doi: 10.1007/s00595-012-0459-3. [DOI] [PubMed] [Google Scholar]
- 9.Burghaus S, Hildebrandt T, Fahlbusch C, et al. Standards used by a clinical and scientific endometriosis center for the diagnosis and therapy of patients with endometriosis. Geburtshilfe Frauenheilkd. 2019;79:487–497. doi: 10.1055/a-0813-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang PH, Juang CM, Chao HT, et al. Wound endometriosis: risk factor evaluation and treatment. J Chin Med Assoc. 2003;66:113–119. [PubMed] [Google Scholar]
- 12.Oosterlynck DJ, Cornillie FJ, Waer M, Vandeputte M, Koninckx PR. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil Steril. 1991;56:45–51. doi: 10.1016/S0015-0282(16)54414-8. [DOI] [PubMed] [Google Scholar]
- 13.Betrán AP, Ye J, Moller AB, et al. The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS ONE. 2016;11:e0148343. doi: 10.1371/journal.pone.0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Zhang J, Zamora J, et al. Increases in caesarean delivery rates and change of perinatal outcomes in low- and middle-income countries: a hospital-level analysis of two WHO surveys. Paediatr Perinat Epidemiol. 2017;31:251–262. doi: 10.1111/ppe.12363. [DOI] [PubMed] [Google Scholar]
- 15.Wicherek L, Klimek M, Skret-Magierlo J, et al. The obstetrical history in patients with Pfannenstiel scar endometriomas—an analysis of 81 patients. Gynecol Obstet Invest. 2007;63:107–113. doi: 10.1159/000096083. [DOI] [PubMed] [Google Scholar]
- 16.Hufnagel D, Li F, Cosar E, et al. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin Reprod Med. 2015;33:333–340. doi: 10.1055/s-0035-1564609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch M, Begum MR, Paniz É, et al. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. BJOG. 2018;125:556–564. doi: 10.1111/1471-0528.14838. [DOI] [PubMed] [Google Scholar]
- 18.Kavoussi SK, Lim CS, Skinner BD, et al. New paradigms in the diagnosis and management of endometriosis. Curr Opin Obstet Gynecol. 2016;28:267–276. doi: 10.1097/GCO.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 19.Guerriero S, Saba L, Pascual MA, et al. Transvaginal ultrasound vs. magnetic resonance imaging for diagnosing deep infiltrating endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51:586–595. doi: 10.1002/uog.18961. [DOI] [PubMed] [Google Scholar]
- 20.Batur A, Yavuz A, Ozgokce M, et al. The utility of ultrasound elastography in differentiation of endometriomas and hemorrhagic ovarian cysts. J Med Ultrason. 2016;43:395–400. doi: 10.1007/s10396-016-0701-5. [DOI] [PubMed] [Google Scholar]
- 21.Fawzy M, Amer T. Efficacy of transabdominal sonoelastography in the diagnosis of caesarean section scar endometrioma: a pilot study. J Obstet Gynaecol. 2015;35:832–834. doi: 10.3109/01443615.2015.1011107. [DOI] [PubMed] [Google Scholar]
- 22.Savelli L, Manuzzi L, Di Donato N, et al. Endometriosis of the abdominal wall: ultrasonographic and Doppler characteristics. Ultrasound Obstet Gynecol. 2012;39:336–340. doi: 10.1002/uog.10052. [DOI] [PubMed] [Google Scholar]