Abstract
Biophysics in Waseda University was started in 1965 as one of the three key research areas that constitute the Physics Department. In the biophysics group, one theoretical lab and two experimental labs are now working on the cutting-edge themes on biophysics, disseminating the ideas and knowledge of biophysics to undergraduate and graduate students from the viewpoint of physics.
Keywords: Molecular machine, Polymorphism, Allostery, Bioinformatics, Computational biology, Myosin, Actin, Cofilin, On-chip cellomics, Community effect of cells, “Algebraic” system, “Geometric” system
What is Waseda University?
Waseda University is one of the leading Japanese private research universities located in Shinjuku, the center of Tokyo Metropolis, with about 40,000 undergraduate students and 9,000 postgraduate students. The University was established as Tokyo Senmon Gakko (Tokyo College) on October 21, 1882 by Marquess Shigenobu Ōkuma, the 5th Prime Minister of Japan, and was formally renamed to Waseda University in 1902 after the location of the founder’s villa in Waseda Village. The University started from three departments, political science and economics, law, and physical science, and is now organized into ten divisions covering 13 undergraduate schools, 24 graduate schools, 21 research institutes, and 9 affiliated institutes at the central campus in Shinjuku, and other branch campuses in Chūō, Nishitōkyō, Tokorozawa, Honjō, and Kitakyūshū.
Mission statement of the University was proclaimed by President Shigenobu Ōkuma on Waseda University’s 30th anniversary in October 1913 as “Waseda University holds as its founding principles the preservation of the independence of scholarship, the promotion of the practical application of scholarship, and the fostering of good citizens. Holding the independence of scholarship as acentral principle, Waseda University pledges to contribute to the scholarship of the world by regarding freedom of research as essential and devoting itself constantly to original research. Holding the practical application of scholarship as a central principle, Waseda University pledges to contribute to the progress of the times by establishing a path for the practical use of scholarship as well as pursuing theoretical research for its own sake. Holding the fostering of good citizens as a central principle, Waseda University pledges to cultivate people of character who can respect individuality, develop themselves and their families, benefit the nation and society, and be active in the world at large” (Ōkuma 1913). Especially, based on the fundamental statement of its educational policy “independence of scholarship,” the University has promoted scientific education and research without bending to authority or temporal fashion. As a result, the University is considered to be one of the most prestigious universities in Japan, consistently ranking among the top universities in Japan (Fig. 1).
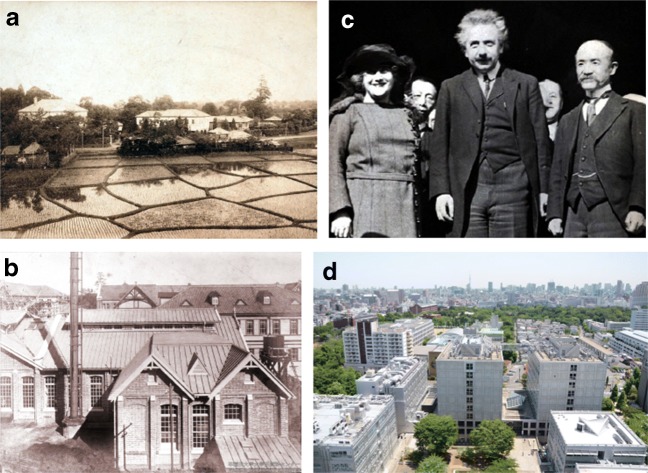
Fig. 1.
Photo retrospectives of Waseda University. a A panoramic view of Waseda Univeristy in 1890. b Science and Engineering Department in early 1900s. c Albert Einstein visited Waseda University to give a lecture (Nov. 29, 1922). d Faculty of Science and Engineering at present. (Source: (a),(b),(c), Waseda University Archives; (d), Office of Information & Public Relations, Waseda University.)
History of biophysics in Waseda
Biophysics research was started when the Physics Department was established in 1965. Prior to its establishment, the Applied Physics Department was established in 1949 and covered traditional and major fields of physics and applied physics. In 1958, nuclear physics lab was also started in Waseda Research Institute for Science and Engineering. These institutes provided a good base for the eventual establishment of a dedicated physics department. To commemorate the 80th anniversary of the foundation of the University, Waseda decided to establish a new department, Physics Department, to cover three frontier fields in physics, namely nuclear and particle physics, condensed, matter physics, and biophysics. In the prospectus for establishment of the Physics Department, the mission of Physics Department was not only for the reinforcement of the field of physics in Waseda but also for providing a firm physical foundation for all the members and students in the Faculty of Science and Engineering. Establishment of biophysics research in 1960s was pioneering in Japan, and hence, Waseda research groups and those alumni became forerunners of Biophysics in Japan. Prof. Nobuhiko Saitô of Applied Physics Department, who was committed to the establishment of the Physics Department and is known worldwide as the physicist of statistical mechanics in polymers, commented, “Biophysics is to find the new approaches and methods to understand living systems exploiting the idea and methods of physics, and their methods and outcomes will finally become a part of life science itself.” The mission of the biophysics research in the Department was and is to contribute and lead the research and education in biophysics in Japan. Now, the biophysics group covers complementary fields by three independent laboratories, i.e., theoretical/computational biophysics, molecular biophysics, and experimental biophysics. The biophysics laboratories also have a good correlation with other laboratories in the department such as statistical physics, soft matter physics, mathematical physics, and physics-based engineering. During the last 54 years of biophysics researches in the Department, Professors Nobuhiko Saitô, Tsutomu Onisi, Hideo Suzuki, Hiroshi Asai, Shin’ichi Ishiwata, Takashi Funatsu, and Kazuhiko Kinoshita, Jr., contributed to the progress of biophysics before their retirements. Among a number of their pioneering works, the studies in single molecule observation and manipulation of motor proteins such as myosin, actin, and F1Fo ATP synthase are perhaps best known.
Highlights of biophysics studies in current Waseda
Computational biophysics—toward understanding the physical principle of living systems from the microscopic viewpoint
All living systems on earth are composed of atoms, but they are more than mere assemblies of atoms, utilizing water-soluble polymers. Polymers can provide two great benefits for living organisms: one is the spatiotemporal polymorphism, including structural flexibility and plasticity, which is the natural consequence of the large number of the internal degrees of freedom in a single polymer. Due to this spatiotemporal polymorphism, a protein molecule can exhibit so-called “allostery,” which is a vital physical property of a protein molecule, enabling proteins to respond to external inputs with high sensitivity, regulate interactions with other molecules autonomously, and execute biological functions as molecular machines. The other benefit comes from the fact that a polymer molecule can retain “information” in the form of 1D sequence of the constituent monomers. Obviously, the information encoded in the sequence prescribes the spatiotemporal polymorphism, including the 3D structure formation of protein that Prof. Saitô studied, so these two benefits of polymer are inseparably related with each other. The current research focuses of the computational biophysics group in Takano laboratory are mainly on the former, i.e., unveiling the principles that underlie allostery in the molecular machines which generate mechanical forces from the energy of ATP hydrolysis, regulate cytoskeletal dynamics through sensing mechanical and electrostatic inputs, synthesize ATP using electrochemical potential of proton across the membrane, and deliver high-energy electrons selectively to downstream enzymes. Some recent works are summarized in a review (Takano 2018). The basic questions are as follows: “Why is the thermodynamic stability of molecular machines only marginal, with an abundance of intrinsically disordered proteins?” The marginal stability of molecular machines is in marked contrast with human-made machines. Such an observation raises further questions—“Is there any relationship between the marginal stability and the biological functions?”; “How can small amounts of energy (such as provided by room-temperature thermal energy) be utilized and handled by molecular machines with high efficiency?”; “Is there any common principle in the Coulombic interaction network in our living system?”; “Is water indispensable for living systems and also what physical role does water play?”; “How can proteins, as ‘molecular devices’ in the large-scale integrated molecular network of living systems, retain and utilize physical information?” To address these questions, the second beneficial aspect of polymers, i.e., the informational aspect, should be of high value as well. Some of the contribution in this respect is reviewed in the next section.
Computational biophysics—from the viewpoint of information
Professor Nobuhiko Saitô was a core initial member of biophysics research at Waseda University where he studied the protein folding problem up to 1988 when he retired (Yura 2016). The main topic of the Saitô laboratory was the elucidation of the protein folding pathway and the prediction of the secondary/tertiary structure from amino acid sequence only with computational tools and databases (Saitô et al. 1988). Many students have graduated from the lab and have academic positions in universities now. The topic of their study has since diverged from the original folding problem to protein evolution, protein design, protein dynamics, and protein function prediction and integrated database building for genome and proteome (Gojobori et al. 2016). Although the topic has diverged and the number of application-oriented research has increased, the spirit of biophysics research in Waseda University has remained the same, which is to find physical principles in biological systems. When research inclines to the prediction of the output of the phenomenon, it is quite common to find the attitude of improving only the prediction results, which may sometimes introduce arbitrary parameters into the prediction system. The spirit of biophysics research in Waseda University never allows that approach and always stands on the principle that can be explained by the rules of physics. Hence, deep and sometimes unique analyses of the experimental data stored in databases to achieve the fundamental understanding are what tends to be found in the research published from the computational biophysics group at Waseda University. Figure 2 shows just one example of the integrated data analyses. This single figure contains data from three different databases which are integrated over the dynamic conformation of human ATP-binding cassette transporter type G (ABCG) dimer protein. The method of drawing this figure was initially presented by Prof. Nobuhiko Saitô (Nishikawa et al. 1972). The figure is an attempt to understand the physical principle of the evolutionarily fixed nucleotide mutations on the dynamics of protein structure (Sakamoto et al. 2019). The differential map in Fig. 2 clarifies the conformational change of human ABCG protein comparing the apo form [PDB ID: 5DO7] and ATP-binding form [PDB ID: 6HBU] without conducting molecular dynamics simulation. The blue square area in the center of the triangle tells that each subunit gets closer upon ATP-binding, but many red areas in the blue square tells that each subunit has some regions with conformational changes that increase the distance between the parts of the subunits. The distortion in the subunit structure is somewhat focused around the domain boundary, namely the linker region between NBD (nucleotide-binding domain) and TM (transmembrane) domain where allosteric interactions may govern these conformational changes. The detail of the discussion can be found in Sakamoto et al. (2019).
Fig. 2.

One example of the integrated data analyses in computational biophysics research. A differential map depicting the conformational change of ABCG protein upon ATP-binding. The map describes the difference in distances between amino acid residue i and j. Two different conformations of ABCG protein were retrieved from Protein Data Bank (PDB) and compared. A residue pair with reduction in the distances upon ATP-binding is colored in blue, and a pair with increasing distance in red. The box on the diagonal axis represents a domain organization of the protein, a red dot on the diagonal axis is the location of pathogenic mutation on the gene, a blue dot is the location of benign mutation on the gene, and a small black dot is the ATP-binding residue. This figure is reproduced from Sakamoto et al. (2019)
Structural polymorphism and functional differentiation of actin filaments
Actin is an exciting target of biophysical research, not only because it is an exceptionally conservative protein (and yet, the double-helical actin filaments are highly polymorphic) but also because, in most eukaryotic cells, actin filaments of a single isoform can concurrently interact with different actin-binding proteins (ABPs) and perform different tasks at different sites within one cell. We hypothesized that cooperative structural polymorphism of actin filaments is involved in the selection of the ABP leading to functional differentiation, and have been taking a multi-faceted approach to examine the idea experimentally. One novel and fruitful approach has been to use high-speed atomic force microscopy (HS-AFM), which was developed by Prof. Toshio Ando, now at Kanazawa University but also an alumnus of the Waseda biophysics laboratory. Ando and his colleagues redesigned conventional AFM and sped up the scanning speed by three orders, to the level that conformational changes of protein molecules can be directly imaged at the nm-resolution. The HS-AFM was first used to image “walking” of individual myosin V molecules along actin filaments (Kodera et al. 2010), a milestone achievement to visualize working protein molecules. We are collaborating with Prof. Ando and his colleagues to visualize specific cooperative conformational changes of actin filaments induced by binding of ABPs. It had been demonstrated that cofilin binds actin filaments cooperatively and form cofilin clusters along the filaments, and that the filaments in the clusters are supertwisted by 25% when compared with normal filaments (reviewed in Galkin et al. (2012)). We thus followed the process of the formation of cofilin clusters using HS-AFM (Ngo et al. 2016), and discovered that the conformational changes of a filament that occurred in the cofilin cluster are propagated to neighboring bare zones of the filament. Moreover, the cooperative conformational changes were propagated only towards the pointed ends of the polar actin filaments (Fig. 3). Such cooperative conformational changes of actin filaments would drive sorting of ABPs along and among actin filaments, since the cofilin-induced conformation of the filaments does not allow binding of myosin, while a different conformation of the filaments induced by myosin binding strongly inhibits cofilin binding (Ngo et al. 2016). We believe that this mutually exclusive cooperative binding of cofilin and myosin to actin filaments, mediated by cooperative conformational changes of the filaments and observed in vitro, is physiologically relevant, since intracellular localization of cofilin and myosin are also mutually exclusive. We have preliminary evidence that actin filaments in cells are also polymorphic, as indicated by observation of microinjected actin molecules with two fluorescent dyes that allow FRET-based detection of conformational changes.
Fig. 3.
Unidirectional propagation of cofilin-induced conformational changes of an actin filament, as visualized by HS-AFM. a Time lapse HS-AFM images of a portion of an actin filament, gradually decorated by cofilin over time. Left: transient binding of two S1 molecules enabled us to identify that the pointed end of this filament is on the left of this image. At time zero, pointed end section of one cofilin cluster and barbed end section of another cluster are visible. Red arrows indicate crossover points, where the two protofilaments are aligned vertically, in the clusters. Crossover points in the cofilin clusters appear brighter (i.e., taller) and thicker (due to decoration with cofilin), and the distance between the crossover points (i.e., half helical pitch) in the clusters are shorter (due to supertwisting of the helix). The pointed end border of the right cluster steadily grew to the left (i.e., to the pointed end), while the barbed end border of the left cluster did not grow. At 72.6 s, the two clusters merged into one. Detailed analysis demonstrated that the bare section of the filament on the pointed end side of the cluster is supertwisted, while that on the barbed end side of the cluster has normal helical pitch. b Schematic representation of the cofilin-induced conformational change of an actin filament and the propagation to the bare section on the pointed end side (orange). The supertwisted bare zone is hypothesized to have an elevated affinity for cofilin in solution, resulting in unidirectional growth of the cluster to the pointed end direction. Reproduced from (Ngo et al. 2015)
It is also noteworthy that the structure of actin filaments are mechanosensitive. Mechanically stretched actin filaments have a higher affinity for myosin (Uyeda et al. 2011), but a lower affinity for cofilin (Hayakawa et al. 2011). We thus speculate that binding of ABPs and mechanical tension collaborate to modulate the structure of actin filaments, which in turn regulates binding of the same and other ABPs, leading to functional differentiation of the filaments. When tension-mediated conformational change of actin filaments recruits more myosin binding, this would increase active force generation, forming a tension-mediated positive feedback loop. It is tempting to speculate that those multiple cooperative conformational changes in actin filaments depend on intricate networks of intramolecular and intermolecular allostery, and that is the reason why actin is so conservative; actin is such a highly sophisticated molecular machine that has evolved to the level that there is little room to change. Takano’s group is currently examining this possibility using molecular dynamics simulations.
On-chip differential screening of isolated single E. coli for algebraic understanding of epigenetic information
A series of studies aimed at developing methods and technologies for analyzing epigenetic information in cells and in those networks, as well as that of genetic information, was examined to expand our understanding of how the dynamical behavior of living systems are determined by such genetic and epigenetic information. Technology for analyzing such epigenetic information was developed starting from the twin complementary viewpoints of cell regulation as an “algebraic” system (emphasis on temporal aspects) and as a “geometric” system (emphasis on spatial aspects)(Figs. 4a and 5a). Exploiting the combination of latest microfabrication technologies and measurement technologies, which we call on-chip cellomics, we can select, control, and re-construct the environments, interaction of single cells, and cell networks from “algebraic” and “geometric” viewpoints.
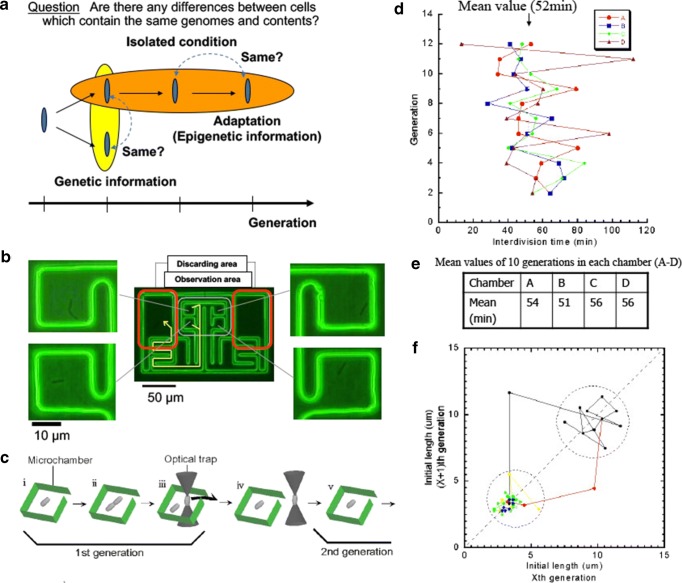
Fig. 4.
On-chip “algibraic” single-cell analysis. a Schematic illustration of the concept of temporal aspect of epigenetic information. b On-chip single E. coli cultivation assay. Isolated single E. colis were cultivated in the cultivation area in the microfabricated chip. c Procedure to maintain the single cell within each of cultivation chamber. When the cell divided into two daughter cells, one of the two daughter cells were excluded from the chamber with optical tweezers. d Time-course change of cell division intervals of four direct descendant cells under isolated conditions. Their division time were fluctuated more than 20%. e The averaged mean division time in 12 generations were almost same around 54 min, whereas their fluctuation were large as shown in Fig. panel (d). f Logistic map of neighboring generation of their division time. Their fluctuation was within an area; however, once the cell division error was occurred, the longer cells fluctuated within another area as another size phenotype with faster cell cycles, i.e., when the cell size was increased over the critical size, their longer shape was maintained as the epigenetic memory of phenotype
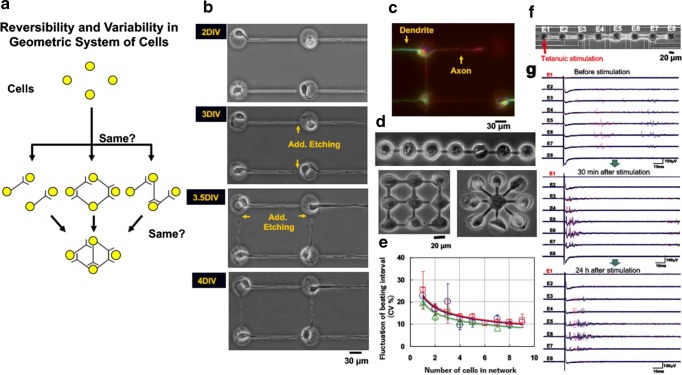
Fig. 5.
On-chip “geometric” cell network analysis. a Schematic illustration of the concept of spatial aspect of epigenetic information. b Stepwise microfabrication of agarose microstructures during cell cultivation. Single neurons were cultivated in each isolated microchamber with a single tunnel to introduce axon into it. Once axon was elongated to the desired pathway, additional melting of a portion of the agarose layer stepwisely connects the neurons with direction controlled manner. c Fluorescence micrograph of neuronal network showing the direction control of axon and dendrite was successful. d Another example of agarose microfabrication of geometric spatial arrangement control of cardiomyocyte network: straight lined-up, grid, and star shapes of nine cells. e The graph shows fluctuation of beating intervals decreased according to the increase of cell number regardless of their geometry. f Straight eight neuron network on the eight micro- electrodes surrounded in the agarose microstructure. g Time-course change of external field potentials of neurons in Fig. panel (g). Upper, before tetanuic stimulation. Middle, 30 min after stimulation. Lower, 24 h after stimulation. Even simple eight neuronal network can provide the long-term potentiation and its disappearance in 24 h after stimulation as a characteristics of neuronal networks’ epigenetic information
Figure 4 shows an example of “algebraic” perspectives of epigenetic information in isolated single cells of E. coli. Exploiting the microfabrication technology and optical tweezers, we cultivate and observe cells under fully controlled conditions (e.g., cell population or nutrient conditions) in an on-chip single-cell cultivation chip. Time-course change of their growth and cell division time over generations were analyzed and we found that (1) their fluctuation of cell division time were more than 20% even when they are sister cells; however, the mean value of their cell division time were strictly the same without any hysteresis of former generation, and (2) the epigenetic information of cell division time was memorized in their cell shapes, which were memorized as an inheritable epigenetic information even after cell division (Inoue et al. 2001a, b; Umehara et al. 2007a, b; Wakamoto et al. 2001, 2003, 2005; Wakamoto and Yasuda2006).
On-chip cell network of neurons and cardiomyocytes to understand the epigenetic information in their geometry
The spatial viewpoint is complementary to the temporal aspect of epigenetic information. We thus commenced a series of studies to analyze epigenetic information in the spatial structures of a cell network in order to expand our understanding of how the fates of living systems are determined. As shown in Fig. 5, we cultivated and observed cell networks using an on-chip agarose microchamber system exploiting photo-thermal etching technology, which can control the microstructure of microchambers even during cell cultivation, and their electrophysiological responses were recorded with single-cell size multi-electrode array analysis system (Hattori et al., 2004; Hayashi et al.2017; Kaneko et al. 2007a, b; Kaneko et al. 2014; Kojima et al. 2003, 2006; Moriguchi et al. 2002; Sugio et al. 2004; Suzuki et al. 2005; Suzuki and Yasuda 2007). The advantage of our on-chip cellomics approach is that as it is a re-constructive approach of the simplified artificial minimum cell network model on a chip, it removes the complexity in underlying physicochemical reactions that are not always completely understood and for which most of the necessary variables cannot be measured. Moreover, this approach shifts the view of cell regulatory processes from a basic chemical ground to a paradigm of the cell as an information-processing unit working as an intelligent machine capable of adapting to changing environmental and internal conditions. This is an alternative representation of the cell and can bring new insights into cellular processes. Thus, models derived from such a viewpoint can directly help in more traditional biochemical and molecular biological analyses that assist in our understanding of control in cells. We have used on-chip cellomics technologies to extend ideas from the genetic to the genetic-epigenetic network in investigating topics like the population effect and the community effect of cells, and network pattern formation in cell groups. After sufficient experimental observations, we can understand the role of epigenetic information in modeling more complex signaling cascades. This field has almost been entirely monopolized by physicochemical models, which provide a good standard for comparison, evaluation, and development with our approach. The ultimate aim of our study is to provide a comprehensive understanding of living systems as products of both genetic and epigenetic information. It would permit us to describe the phenomena occurring in cell systems sufficiently well to be able to interpret and control them, and moreover increase confidence associated with the use of cell models in practical applications such as predictive drug screening (Asahi et al. 2018, 2019).
Message to readers
We hope that this short descriptive history of biophysical research at Waseda University has helped to stir the interest of readers of this Special Issue. We welcome contact from students, potential overseas collaborators, and foreign and domestic scientists who are interested in working with us directly at Waseda in the exciting and diverse area of modern biophysics.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Asahi Y, Hamada T, Hattori A, Matsuura K, Odaka M, Nomura F, Kaneko T, Abe Y, Takasuna K, Sanbuissho A, Yasuda K (2018) On-chip spatiotemporal electrophysiological analysis of human stem cell derived cardiomyocytes enables quantitative assessment of proarrhythmia in drug development. Sci Rep 8(1), 10.1038/s41598-018-32921-1 [DOI] [PMC free article] [PubMed]
- Asahi Y, Nomura F, Abe Y, Doi M, Sakakura T, Takasuna K, Yasuda K. Electrophysiological evaluation of pentamidine and 17-AAG in human stem cell-derived cardiomyocytes for safety assessment. Eur J Pharmacol. 2019;842:221–230. doi: 10.1016/j.ejphar.2018.10.046. [DOI] [PubMed] [Google Scholar]
- Galkin V, Orlova A, Egelman E. Actin filaments as tension sensors. Curr Biol. 2012;22(3):R96–101. doi: 10.1016/j.cub.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gojobori T, Ikeo K, Katayama Y, Kawabata T, Kinjo AR, Kinoshita K, Kwon Y, Migita O, Mizutani H, Muraoka M, Nagata K, Omori S, Sugawara H, Yamada D, Yura K. VaProS: a database-integration approach for protein/genome information retrieval. J Struct Funct Genomics. 2016;17(4):69–81. doi: 10.1007/s10969-016-9211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A, Moriguchi H, Ishiwata S, Yasuda K. A 1480/1064 nm dual wavelength photo-thermal etching system for non-contact three-dimensional microstructure generation into agar microculture chip. Sensors and Actuators B: Chemical. 2004;100(3):455–462. doi: 10.1016/j.snb.2003.11.041. [DOI] [Google Scholar]
- Hayakawa K, Tatsumi H, Sokabe M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J Cell Biol. 2011;195(5):721–727. doi: 10.1083/jcb.201102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Tokihiro T, Kurihara H, Yasuda K. Community effect of cardiomyocytes in beating rhythms is determined by stable cells. Sci Rep. 2017;7(1):15450. doi: 10.1038/s41598-017-15727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I, Wakamoto Y, Moriguchi H, Okano K, Yasuda K. On-chip culture system for observation of isolated individual cells. Lab Chip. 2001;1(1):50–55. doi: 10.1039/b103931h[doi]. [DOI] [PubMed] [Google Scholar]
- Inoue I, Wakamoto Y, Yasuda K. Non-genetic variability of division cycle and growth of isolated individual cells in on-chip culture system. Proceedings of the Japan Academy Ser B, Physical and Biological Sciences. 2001;77(8):145–150. doi: 10.2183/pjab.77.145. [DOI] [Google Scholar]
- Kaneko T, Kojima K, Yasuda K. An on-chip cardiomyocyte cell network assay for stable drug screening regarding community effect of cell network size. Analyst. 2007;132(9):892–898. doi: 10.1039/b704961g. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Kojima K, Yasuda K. Dependence of the community effect of cultured cardiomyocytes on the cell network pattern. Biochem Biophys Res Commun. 2007;356(2):494–498. doi: 10.1016/j.bbrc.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Nomura F, Hamada T, Abe Y, Takamori H, Sakakura T, Takasuna K, Sanbuissho A, Hyllner J, Sartipy P, Yasuda K. On-chip in vitro cell-network pre-clinical cardiac toxicity using spatiotemporal human cardiomyocyte measurement on a chip. Sci Rep. 2014;4:4670. doi: 10.1038/srep04670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera N, Yamamoto D, Ishikawa R, Ando T. Video imaging of walking myosin V by high-speed atomic force microscopy. Nature. 2010;468(7320):72–76. doi: 10.1038/nature09450. [DOI] [PubMed] [Google Scholar]
- Kojima K, Moriguchi H, Hattori A, Kaneko T, Yasuda K. Two-dimensional network formation of cardiac myocytes in agar microculture chip with 1480 nm infrared laser photo-thermal etching. Lab Chip. 2003;3(4):292–296. doi: 10.1039/b304652d. [DOI] [PubMed] [Google Scholar]
- Kojima K, Kaneko T, Yasuda K. Role of the community effect of cardiomyocyte in the entrainment and reestablishment of stable beating rhythms. Biochem Biophys Res Commun. 2006;351(1):209–215. doi: 10.1016/j.bbrc.2006.10.037. [DOI] [PubMed] [Google Scholar]
- Ōkuma S. Mission statement of Waseda University. Waseda Gakuhou (in Japanese) 1913;225:8–10. [Google Scholar]
- Moriguchi H, Wakamoto Y, Sugio Y, Takahashi K, Inoue I, Yasuda K. An agar-microchamber cell-cultivation system: flexible change of microchamber shapes during cultivation by photo-thermal etching. Lab Chip. 2002;2(2):125–132. doi: 10.1039/b202569h[doi]. [DOI] [PubMed] [Google Scholar]
- Ngo K, Umeki N, Kijima S, Kodera N, Ueno H, Furutani-Umezu N, et al. Allosteric regulation by cooperative conformational changes of actin filaments drives mutually exclusive binding with cofilin and myosin. Sci Rep. 2016;6:35449. doi: 10.1038/srep35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo KX, Kodera N, Katayama E, Ando T, Uyeda TQP (2015) Cofilin-induced unidirectional cooperative conformational changes in actin filaments revealed by high-speed atomic force microscopy. 10.7554/eLife.04806 [DOI] [PMC free article] [PubMed]
- Nishikawa K, Ooi T, Isogai Y, Saitô N. Tertiary structure of proteins. I. Representation and computation of the conformations. J Phys Soc Jpn. 1972;32:1331–1337. doi: 10.1143/JPSJ.32.1331. [DOI] [Google Scholar]
- Saitô N, Shigaki T, Kobayashi Y, Yamamoto M. Mechanism of protein folding: I. General considerations and refolding of myoglobin. Proteins: Structure, Function, and Bioinformatics. 1988;3(3):199–207. doi: 10.1002/prot.340030308. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Suzuki H, Yura K. Relationship between conformation shift and disease related variation sites in ATP-binding cassette transporter proteins. Biophysics and Physicobiology. 2019;16:68–79. doi: 10.2142/biophysico.16.0_68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio Y, Kojima K, Moriguchi H, Takahashi K, Kaneko T, Yasuda K. An agar-based on-chip neural-cell-cultivation system for stepwise control of network pattern generation during cultivation. Sensors and Actuators B: Chemical. 2004;99(1):156–162. doi: 10.1016/S0925-4005(03)00550-1. [DOI] [Google Scholar]
- Suzuki I, Yasuda K. Detection of tetanus-induced effects in linearly lined-up micropatterned neuronal networks: application of a multi-electrode array chip combined with agarose microstructures. Biochem Biophys Res Commun. 2007;356(2):470–475. doi: 10.1016/j.bbrc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Sugio Y, Jimbo Y, Yasuda K. Stepwise pattern modification of neuronal network in photo-thermally-etched agarose architecture on multi-electrode array chip for individual-cell-based electrophysiological measurement. Lab Chip. 2005;5(3):241–247. doi: 10.1039/b406885h[doi]. [DOI] [PubMed] [Google Scholar]
- Takano Mitsunori. The Role of Water in ATP Hydrolysis Energy Transduction by Protein Machinery. Singapore: Springer Singapore; 2018. Orchestrated Electrostatic Interactions Among Myosin, Actin, ATP, and Water; pp. 113–122. [Google Scholar]
- Umehara S, Hattori A, Inoue I, Yasuda K. Asynchrony in the growth and motility responses to environmental changes by individual bacterial cells. Biochem Biophys Res Commun. 2007;356(2):464–469. doi: 10.1016/j.bbrc.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Umehara S, Inoue I, Wakamoto Y, Yasuda K. Origin of individuality of two daughter cells during the division process examined by the simultaneous measurement of growth and swimming property using an on-chip single-cell cultivation system. Biophysical Journal. 2007;93(3):1061–1067. doi: 10.1529/biophysj.106.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda T, Iwadate Y, Umeki N, Nagasaki A, Yunuma S. Stretching actin filaments within cells enhances their affinity for the myosin II motor domain. PLoS One. 2011;6(10):e26200. doi: 10.1371/journal.pone.0026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamoto Y, Yasuda K. Quantitative evaluation of cell-to-cell communication effects in cell group class using on-chip individual-cell-based cultivation system. Biochem Biophys Res Commun. 2006;349(3):1130–1138. doi: 10.1016/j.bbrc.2006.08.149. [DOI] [PubMed] [Google Scholar]
- Wakamoto Y, Inoue I, Moriguchi H, Yasuda K. Analysis of single-cell differences by use of an on-chip microculture system and optical trapping. Fresenius J Anal Chem. 2001;371(2):276–281. doi: 10.1007/s002160100999. [DOI] [PubMed] [Google Scholar]
- Wakamoto Y, Umehara S, Matsumura K, Inoue I, Yasuda K. Development of non-destructive, non-contact single-cell based differential cell assay using on-chip microcultivation and optical tweezers. Sensors and Actuators B: Chemical. 2003;96(3):693–700. doi: 10.1016/S0925-4005(03)00549-5. [DOI] [Google Scholar]
- Wakamoto Y, Ramsden J, Yasuda K. Single-cell growth and division dynamics showing epigenetic correlations. Analyst. 2005;130(3):311–317. doi: 10.1039/b409860a. [DOI] [PubMed] [Google Scholar]
- Yura K. Obituary: Nobuhiko Saitô, a man who understood protein folding in his own way. Biophysics and Physicobiology. 2016;13:245–247. doi: 10.2142/biophysico.13.0_245. [DOI] [PMC free article] [PubMed] [Google Scholar]