In the 57th Annual Meeting of the Biophysical Society of Japan held in Miyazaki, we organized a symposium session entitled “Physics of chromatin dynamics—towards understanding the regulation of gene expression” on the morning of September 24, 2019. The key questions asked in the session were (i) how the structure and dynamics of chromatin can be described in physics terms, and (ii) how the methods and viewpoints of physics contribute to the understanding of the regulation of gene expression. In eukaryotic cells, genomic DNA is packed into the nucleus as a highly organized chromatin structure. Recent studies have revealed that the physical properties of the structure and dynamics of chromatin are important for the regulation of gene expression (Misteli 2007; Levens et al. 2016). Eight speakers, including three selected from the poster presenters, introduced their latest studies that utilized various experimental and theoretical approaches to capture the essential physics of chromatin dynamics.
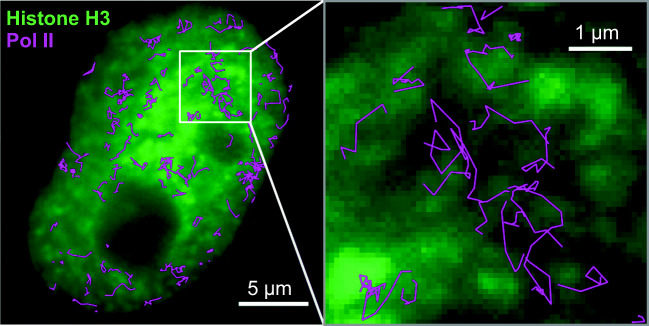
Yuma Ito (Tokyo Institute of Technology) started the session by reporting his single-molecule and super-resolution imaging of RNA polymerase II (Pol II) and histone variants in living cells (Fig. 1). A subtrajectory analysis developed by his team (Ito et al. 2017) revealed the distinct mobility of Pol II in different chromatin regions.
Fig. 1.
Dual-color single-molecule imaging of HeLa cell nucleus. Single-molecule trajectories of Pol II were superimposed on the fluorescence microscopy image of a HeLa cell nucleus stained for histone H3. Image credit: Drs. Ito Y and Tokunaga M
Kayo Hibino (National Institute of Genetics and the Graduate University for Advanced Studies, SOKENDAI) reported her single nucleosome imaging analysis (Nagashima et al. 2019) on mitotic chromosomes. Their quantification, in combination with knockdown experiments of condensin molecules, provided new insights into the mechanism how mitotic chromosomes are organized.
Tatsuo Akitaya (Asahikawa Med. Univ.) reported how DNA is compacted in vitro using short oligopeptide octamers. They evaluated how the order of cationic and polar nonionic residues in the octamers influenced DNA compaction efficiency and the mechanism of DNA compaction, which can be considered as either discrete coil-globules transition or as a continuous DNA collapse (Zinchenko et al. 2019).
Akinori Awazu (Hiroshima Univ.) reported how nucleosome-excluding non-looping insulator sequences (NENLIS) function as insulators (Matsushima et al. 2019). Using coarse-grained molecular dynamics simulations, they showed that NENLIS can hinder the associations of two flanking nucleosome-rich regions. Furthermore, epigenome data analysis of human cells supported the notion that NENLIS function as insulators in vivo.
Giovanni Brandani (Kyoto Univ.) reported application of his molecular dynamics simulations (Brandani et al. 2018; Brandani and Takada 2018) on nucleosome assembly. He and his colleagues revealed that the bending properties of DNA depends on the sequence of DNA and affects the efficiency of nucleosome assembly. His informatics analyses on yeast promoter sequences indicated that such mechanical effects influence nucleosome occupancy and gene expression.
Kyosuke Adachi (RIKEN Center for Biosystems Dynamics Research [BDR] and RIKEN Interdisciplinary Theoretical and Mathematical Sciences Program [iTHEMS]) and his colleague constructed a polymer model to explain the megabase-scale change in both the chromatin spatial structure and the histone modification profile, often observed during differentiation processes. Their model showed a discontinuous phase transition, suggesting that such transition is the physical origin of the large-scale change in chromatin (Adachi and Kawaguchi 2019).
Tetsuya Yamamoto (Nagoya Univ.) reported a soft matter physics approach on chromatin structure and gene transcription. Using a DNA brush as a model system of chromatin, they provided theories behind the transcriptional activities and phase separation observed in DNA brush structures (Yamamoto and Safran 2015; Yamamoto and Schiessel 2016, 2017).
Takahiro Sakaue (Aoyama Gakuin Univ. and Japan Science and Technology Agency [JST]) concluded the session by providing a polymer physics viewpoint on the structure and dynamics of chromatin. He talked about the topological effect of long chromatin polymers (Sakaue 2018) on their conformation and organization, and the anomalous dynamics of genetic loci (Sakaue and Saito 2016; Put et al. 2019).
Acknowledgements
The authors thank all the speakers of the session and Hiroshi Kimura (Tokyo Institute of Technology) for their comments on the manuscript.
Funding information
This session was co-sponsored by Grant-in-Aid for Scientific Research on Innovative Areas “Chromatin Potential” (JP18H05526, JP18H05527, and JP18H05529) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adachi K, Kawaguchi K (2019) Chromatin state switching in a polymer model with mark-conformation coupling. Phys Rev E 100, 060401(R) [DOI] [PubMed]
- Brandani GB, Takada S. Chromatin remodelers couple inchworm motion with twist-defect formation to slide nucleosomal DNA. PLoS Comput Biol. 2018;14:e1006512. doi: 10.1371/journal.pcbi.1006512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandani GB, Niina T, Tan C, Takada S. DNA sliding in nucleosomes via twist defect propagation revealed by molecular simulations. Nucleic Acids Res. 2018;46:2788–2801. doi: 10.1093/nar/gky158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Sakata-Sogawa K, Tokunaga M. Multi-color single-molecule tracking and subtrajectory analysis for quantification of spatiotemporal dynamics and kinetics upon T cell activation. Sci Rep. 2017;7:6994. doi: 10.1038/s41598-017-06960-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens D, Baranello L, Kouzine F. Controlling gene expression by DNA mechanics: emerging insights and challenges. Biophys Rev. 2016;8:259–268. doi: 10.1007/s12551-016-0216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima Y, Sakamoto N, Awazu A. Insulator activities of nucleosome-excluding DNA sequences without bound chromatin looping proteins. J Phys Chem B. 2019;123:1035–1043. doi: 10.1021/acs.jpcb.8b10518. [DOI] [PubMed] [Google Scholar]
- Misteli T. Beyond the sequence: cellular organization of genome function. Cell. 2007;128:787–800. doi: 10.1016/j.cell.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Nagashima R, Hibino K, Ashwin SS, Babokhov M, Fujishiro S, Imai R, Nozaki T, Tamura S, Tani T, Kimura H, Shribak M, Kanemaki MT, Sasai M, Maeshima K. Single nucleosome imaging reveals loose genome chromatin networks via active RNA polymerase II. J Cell Biol. 2019;218:1511–1530. doi: 10.1083/jcb.201811090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Put S, Sakaue T, Vanderzande C. Active dynamics and spatially coherent motion in chromosomes subject to enzymatic force dipoles. Phys Rev E. 2019;99:032421. doi: 10.1103/PhysRevE.99.032421. [DOI] [PubMed] [Google Scholar]
- Sakaue T. Topological free volume and quasi-glassy dynamics in the melt of ring polymers. Soft Matter. 2018;14:7507–7515. doi: 10.1039/C8SM00968F. [DOI] [PubMed] [Google Scholar]
- Sakaue T, Saito T. Active diffusion of model chromosomal loci driven by athermal noise. Soft Matter. 2016;13:81–87. doi: 10.1039/C6SM00775A. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Safran SA. Transcription rates in DNA brushes. Soft Matter. 2015;11:3017–3021. doi: 10.1039/C4SM02871F. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Schiessel H. Transcription driven phase separation in chromatin brush. Langmuir. 2016;32:3036–3044. doi: 10.1021/acs.langmuir.6b00442. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Schiessel H. Transcription dynamics stabilizes nucleus-like layer structure in chromatin brush. Soft Matter. 2017;13:5307–5316. doi: 10.1039/C7SM00239D. [DOI] [PubMed] [Google Scholar]
- Zinchenko A, Hiramatsu H, Yamaguchi H, Kubo K, Murata S, Kanbe T, Hazemoto N, Yoshikawa K, Akitaya T. Amino acid sequence of oligopeptide causes marked difference in DNA compaction and transcription. Biophys J. 2019;116:1836–1844. doi: 10.1016/j.bpj.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]