Main text
Biomolecules, such as proteins and nucleic acids, are nano-sized materials. Therefore, it is important to understand how biomolecules interact with other biomolecules and their surrounding environment in nano-space. Recent technologies have opened the door to observe the phenomenon more precisely. Moreover, synthetic approaches now allow us to design nano-sized materials and nano-environment rationally, enabling the approach of “understanding by creation” in many phenomena. Here, to combine these two approaches and to boost research of “biophysics in nano-space,” we invited eight researchers to a Biophysical meeting of Japan on the morning of September 26, 2019 in Miyazaki, and shared the cutting-edge achievements.
Hisashi Tadakuma (Osaka University, now ShanghaiTech University) presented their recent achievements regarding the transcription nano-chip, where RNA polymerase and the target gene were integrated on DNA origami scaffold (Masubuchi et al. 2018). DNA origami allowed them to precisely control the molecular layout with nano-meter resolution (Fig. 1), which enables the design of biochemical reactions by changing the intermolecular distances between the enzymes and the substrates, thus affecting the collision efficiency and subsequent reactions. Moreover, Tadakuma found that the biochemical factors (e.g., Km and Vmax) of the transcription on DNA origami are different from those in solution, presumably due to local environmental factors (e.g., pH and charge), thus shedding light on the effect of nano-environment on biochemical reactions. DNA origami is a versatile method to integrate vast kinds of molecules, which are not limited to biomolecules, and also allows researchers to integrate varieties of materials. These versatility of DNA origami, in combination with the structure design ability, allows nano-environments to be more easily constructed. Therefore, designing nano-reaction fields using DNA origami should be a powerful tool to understand the molecular mechanisms in nano-space and provide solutions to important bioengineering problems.
Fig. 1.
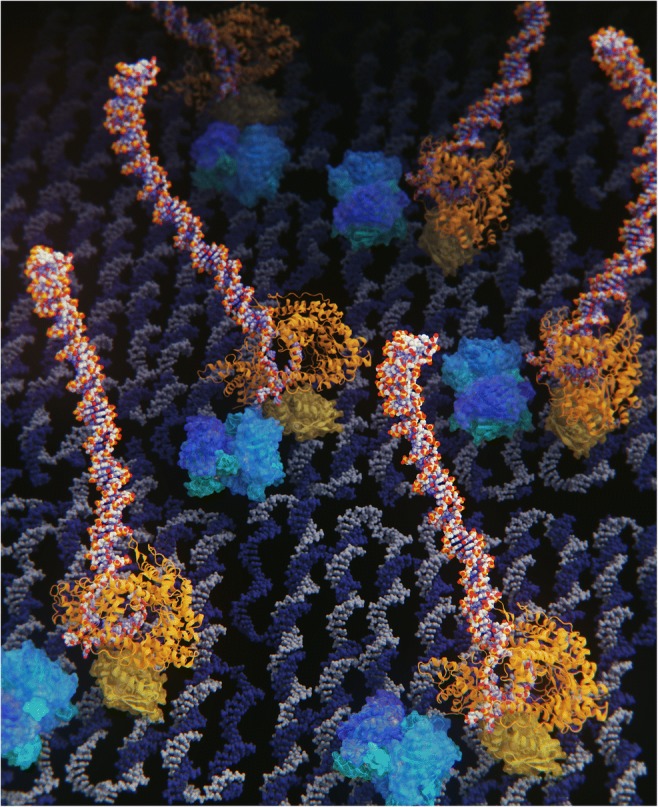
DNA origami allows us to rationally design the “nano-space.” DNA origami allowed researchers to precisely control the molecular layout with nano-meter resolution, which enables the design of biochemical reactions by changing the intermolecular distances between the enzymes and the substrates; thus, it would be helpful for a better understanding of biophysics in “nano-space.” 3DCG by SCIEMENT, Inc. (Director: Hirofumi Seo; used structures; PDB 1S77 (T7 RNA polymerase, orange), 1STP (StreptAvidin, blue), 2YMF (DNA origami, gray and dark blue), 3KZZ(SNAP-tag protein, yellow)
Masayuki Endo (Kyoto University) and his colleagues have developed new assays to explore the mechanical unfolding of the nucleic acid structures: G-quadruplex (GQ) and i-motif (iM) (Shrestha et al. 2017; Jonchhe et al. 2018). Using DNA origami, they made artificial nano-space environments, termed nano-cages (inside dimension sizes are from 6 to 15 nm). Using optical tweezers, they compared the mechanical unfolding of GQ and iM in the nano-cages and in solution. They found that both GQ and iM were quite stabilized inside the nano-cages, enhancing the folding rate much faster (> 100 times in the case of GQ). Using four different sizes of nano-cages, they found that the stability of GQ and iM increases as nano-cage size decreases. These phenomena are attributed to water activity reduction inside the nano-cages, showing the importance of nano-environments in nucleic acid folding.
Kotaro Tsuboyama (The University of Tokyo) presented his recent finding on the new Hero protein family (Tsuboyama et al. 2019). From the classical point of view, folding is a fundamental feature of the “protein.” For most proteins, heating induces protein unfolding, and the exposure of hydrophobic parts results in protein aggregation. Some extremophiles have highly disordered and heat-resistant proteins, which contribute to tolerate extreme conditions such as drying, but these strange proteins have only been identified in extremophiles. A turning point in this field came from serendipity. Yukihide Tomari and Shintaro Iwasaki (The University of Tokyo) were attempting to identify factors that contribute to the reconstitution of RNA-induced silencing complex (RISC) function in vitro (Iwasaki et al. 2015). RISC is made of an Ago protein and small non-coding RNA, which is the heart of RNA silencing (RNAi). In vitro, Ago sticks to the purification beads. From the boiled supernatants of Drosophila cell lysates, they isolated a strange protein, large but unfolded, which increases the elution efficiency of Ago from the beads, and it was termed the Herohero-kun or Mr. Herohero (Japanese for “flimsy and flexible”). Tsuboyama further extended these experiments. From the supernatants of boiled Drosophila and human cell lysates, he selected six Hero-proteins (HEat-Resistant Obscure proteins) and identified their characteristics. Hero-proteins are hydrophilic and highly charged, and function to stabilize various “client” proteins, protecting them from denaturation even under stress conditions such as heat shock, desiccation, and exposure to organic solvents. Tsuboyama also found that the overexpression of Hero-proteins extends the lifespan of Drosophila. These results suggest that organisms naturally use Hero-proteins as molecular shields to stabilize protein functions, highlighting the importance of protein interaction with unstructured polymers in nano-space.
Shin-ichiro Nomura’s (Tohoku University) research focused on the reaction in the nano-space, especially in the space encapsulated by lipid vesicles. Mimicking a natural cell, he encapsulated proteins and nucleic acids, and demonstrated the deformation of the artificial cells (Sato et al. 2017). Inside the artificial cells, he put an actuator system composed of kinesin motor proteins, microtubule rails, and actuator-controlling device (clutch) made with DNA. The motor-rail system generated force, and the clutch transmitted the force to the lipid membrane, deforming the membrane shape. At current, the duration of deformation reaction, the functional lifespan of artificial cells, prolonged up to 80 min. He found that the limitation is not the exhaustion of energy source (e.g., ATP) but the degradation of microtubule rails. The next step in his research was investigating communication between these artificial cells. DNA was employed as a signaling molecule as DNA contains information and can also act as a template for signal amplification. But poor signal amplification efficiency inside the artificial cells is the problem. Nomura tackled this problem by using a DNA polymerase and a nickase in isothermal conditions, achieving 5000 times amplification within 30 min (Sato et al. 2019). The last topic to be investigated was the effects of lipid encapsulation on DNA origami folding (Watanabe et al. 2020). Nomura found that the refolding of heat-denatured DNA origami was enhanced inside the lipid membrane, presumably because of nucleation process affected by DNA–lipid interactions, highlighting the importance of DNA–lipid interaction in nano-space.
Yoshihiro Sasaki (Kyoto University) talked about their nano-gel method to deliver exosomes into the target cells (Sawada et al. 2020). Exosomes carry RNAs that can act to direct cell fate control. However, the poor uptake efficiency of exosomes has been a major problem. To overcome these issues, Sasaki prepared a hybrid of exosomes and magnetic nanoparticles, enabling their remote targeting using magnetic field. Uniquely, after targeting and delivery to the cell, the nano-gel can be removed from the cell using a magnetic field. Using this technique, they succeeded in differentiating stem cells from neuron-like cells, indicating that the delivered exosomes maintained their physiological functions.
Jocelyn Kishi (Harvard University) presented her SABER, signal amplification by exchange reaction, technology-based imaging methods (Kishi et al. 2019; Saka et al. 2019). Fluorescent in situ hybridization (FISH) and immunofluorescence (IF) techniques provide quantitative information about the localizations and quantities of molecular species in fixed cells and tissues. However, several drawbacks such as high tissue autofluorescence and slow imaging speed hinder the versatility of these techniques to map large tissues efficiently. Moreover, only a few targets can be easily imaged simultaneously with standard methods. Kishi took advantages of DNA nanotechnology (i.e., rational kinetic design through probe sequence design) to carry out quantitative and orthogonal imaging of multiple targets with high amplification efficiency. Using these newly developed techniques, they succeeded in imaging at least 17 targets (nucleic acids and proteins) simultaneously inside fixed tissues. SABER may also be useful for the DNA microscopy (Weinstein et al. 2019).
Atsuko Iwane talked about her group’s recent achievements in 3D tissue reconstruction using focused ion beam-scanning electron microscopy (FIB-SEM) (Ichinose and Iwane 2017; Ichinose et al. 2019). The FIB-SEM is a cutting-edge technology that combines FIB and SEM, which allows for the precise 3D localization of the target biomolecules (proteins and nucleic acids etc.). Using ion beam etching, one can image the sample layer-by-layer using SEM. Thus, the 3D deep-tissue imaging can be achieved. Iwane’s work focused on collagen crystals (actinotrichia), which are believed to play a central role in zebrafish fin formation. Iwane solved many problems such as charging up of the samples, which is an intrinsic problem with electron microscopy imaging, and succeeded in precise 3D reconstitution of their sample. Although ion beam etching is time-consuming with the current technology, due to its high spatial resolution, FIB-SEM should be a promising technology to understand the localization and function of biomolecules in nano-space.
Daiju Kitagawa presented his recent finding of the molecular mechanisms that determine a single site for centriole duplication, a long-standing question of the cell biology (Yamamoto and Kitagawa 2019; Takao et al. 2019). Kitagawa beautifully showed that intrinsic self-organization of Plk4 is the key for single-site determination process. By biochemical and cell biological analyses, Kitagawa revealed that Plk4 has the ability to phase-separate into condensates via an intrinsically disordered linker, regulated by the autophosphorylation of Plk4. Super-resolution imaging revealed that scaffold protein CEP152 is symmetrically distributed around the mother centriole. In contrast, autophophorylated Plk4 is distributed asymmetrically, making a single spot around the mother centriole before the initiation of procentriole formation. Furthermore, their new model based on the Turing model can explain the whole site-determination process. Kitagawa’s data showed that the new technology clarifies the phenomena in more detail, allowing researchers to achieve a better understanding of biophysics at the nano-space level.
In summary, we hope that new technologies further drive the expansion of the research field of “biophysics in nano-space.”
Funding information
This work was partially supported by a Grant-in-Aid for Scientific Research (B) (to H.T. no. 19H03197) from the Japan Society for the Promotion of Science.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hisashi Tadakuma, Email: tadakumahisashi@shanghaitech.edu.cn.
Daiju Kitagawa, Email: dkitagawa@mol.f.u-tokyo.ac.jp.
References
- Ichinose TM, Iwane AH. Cytological analyses by advanced electron microscopy. Cyanidioschyzon merolae. 2017;9:129–151. doi: 10.1007/978-981-10-6101-1_9. [DOI] [Google Scholar]
- Ichinose TM, Itabashi T, Mori H, Kuroda J, Imayasu M, Kondo S, Iwane AH. 3D-structual modeling of differentiation and developmental process using advanced electron microscopy and light microscopy. Biophys J. 2019;116:572. doi: 10.1016/j.bpj.2018.11.3079. [DOI] [Google Scholar]
- Iwasaki S, Sasaki HM, Sakaguchi Y, Suzuki T, Tadakuma H, Tomari Y. Defining fundamental steps in the assembly of the Drosophila RNAi enzyme complex. Nature. 2015;521(7553):533–536. doi: 10.1038/nature14254. [DOI] [PubMed] [Google Scholar]
- Jonchhe S, Pandey S, Emura T, Hidaka K, Hossain MA, Shrestha P, Sugiyama H, Endo M, Mao H. Decreased water activity in nanoconfinement contributes to the folding of G-quadruplex and i-motif structures. Proc Natl Acad Sci U S A. 2018;115:9539–9544. doi: 10.1073/pnas.1805939115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi JY, Lapan SW, Beliveau BJ, West ER, Zhu A, Sasaki HM, Saka SK, Wang Y, Cepko CL, Yin P. SABER amplifies FISH: enhanced multiplexed imaging of RNA and DNA in cells and tissues. Nat Methods. 2019;16(6):533–544. doi: 10.1038/s41592-019-0404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi T, Endo M, Iizuka R, Iguchi A, Yoon DH, Sekiguchi T, Qi H, Iinuma R, Miyazono Y, Shoji S, Funatsu T, Sugiyama H, Harada Y, Ueda T, Tadakuma H. Construction of integrated gene logic-chip. Nat Nanotechnol. 2018;13(10):933–940. doi: 10.1038/s41565-018-0202-3. [DOI] [PubMed] [Google Scholar]
- Saka SK, Wang Y, Kishi JY, Zhu A, Zeng Y, Xie W, Kirli K, Yapp C, Cicconet M, Beliveau BJ, Lapan SW, Yin S, Lin M, Boyden ES, Kaeser PS, Pihan G, Church GM, Yin P. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues. Nat Biotechnol. 2019;37(9):1080–1090. doi: 10.1038/s41587-019-0207-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Hiratsuka Y, Kawamata I, Murata S, Nomura SM. Micrometer-sized molecular robot changes its shape in response to signal molecules. Sci Robot. 2017;2(4):aal3735. doi: 10.1126/scirobotics.aal3735. [DOI] [PubMed] [Google Scholar]
- Sato Y, Komiya K, Kawamata I, Murata S, Nomura SM. Isothermal amplification of specific DNA molecules inside giant unilamellar vesicles. Chem Commun. 2019;55:9084–9087. doi: 10.1039/C9CC03277K. [DOI] [PubMed] [Google Scholar]
- Sawada SI, Sato YT, Kawasaki R, Yasuoka JI, Mizuta R, Sasaki Y, Akiyoshi K. Nanogel hybrid assembly for exosome intracellular delivery: effects to endocytosis and fusion by exosome surface polymer engineering. Biomater Sci. 2020;8:619–630. doi: 10.1039/C9BM01232J. [DOI] [PubMed] [Google Scholar]
- Shrestha P, Jonchhe S, Emura T, Hidaka K, Endo M, Sugiyama H, Mao H. Confined space facilitates G-quadruplex formation. Nat Nanotechnol. 2017;12(6):582–588. doi: 10.1038/nnano.2017.29. [DOI] [PubMed] [Google Scholar]
- Takao D, Yamamoto S, Kitagawa D. A theory of centriole duplication based on self-organized spatial pattern formation. J Cell Biol. 2019;218(11):3537–3547. doi: 10.1083/jcb.201904156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboyama K, Osaki T, Suzuki-Matsuura E, Kozuka-Hata H, Okada Y, Oyama M, Ikeuchi Y, Iwasaki S, Tomari Y (2019) A widespread family of heat-resistant obscure (Hero) proteins protect against protein instability and aggregation. bioRxiv. 10.1101/816124 [DOI] [PMC free article] [PubMed]
- Watanabe T, Sato Y, Otaka H, Kawamata I, Murata S, Nomura SM. DNA origami “quick” refolding inside of a micron-sized compartment. Molecules. 2020;25(1):8. doi: 10.3390/molecules25010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JA, Regev A, Zhang F. DNA microscopy: optics-free spatio-genetic imaging by a stand-alone chemical reaction. Cell. 2019;178(1):229–241.e16. doi: 10.1016/j.cell.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Kitagawa D. Self-organization of Plk4 regulates symmetry breaking in centriole duplication. Nat Commun. 2019;10(1):1810. doi: 10.1038/s41467-019-09847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


