Abstract
Recently, the important role of microphase separation in living cells has been attracting considerable interest in relation to cell organization and function. For example, many studies have focused on liquid-liquid phase separation (LLPS) as a very plausible mechanism for the presence of membraneless organelles. To confirm the role of phase separation in living cells, experimental studies on models and/or reconstructed systems are needed. In this short review, we discuss current paradigms of LLPS and provide some example “review data” to demonstrate particular points relating to the specific localization of biological macromolecules like DNAs and actin proteins with spontaneous domain formation in microdroplets emerging in an aqueous two-phase system (ATPS) (we use polyethylene glycol (PEG)/dextran (DEX)—a binary polymer solution). We also suggest that phase separation and transition may play basic roles in regulation of the biochemical reactivity of individual long genomic DNAs.
Keywords: Phase transition, Macromolecular crowding, Depletion effect, DNA condensation, Microcompartmentalization, Aqueous two-phase system
Introduction
Simple physicochemical approaches are thought to be strong enough to reveal the intrinsic natures of ordinary molecular systems involved in biological phenomena. However, molecular systems in this setting are usually composed of various macromolecules and/or large amounts of self-assembled amphipathic lipids, and the complicated and crowded conditions could cause inhomogeneity in the intra- and extracellular environments (Ellis 2001; Rivas and Minton 2016), which might lead to nested microstructures typical of living systems. The cell itself is a compartment segregated from the extracellular environment, and various microcompartments, i.e., organelles, are observed inside cells. Organelles are essential for biological processes, and compartmentalization could be necessary for the well-regulated expression of their unique biological functions. While compartmentalized intracellular spaces are generally enclosed by phospholipid bilayer membranes, as with nuclei, endoplasmic reticuli, endosomes, mitochondria, etc., there are also membraneless organelles, such as nucleoli, some stress and granules, (Courchaine et al. 2016; Uversky 2017; Poudyal et al. 2018), in addition to other non-membranous organelles such as cytoskeletal systems including centrioles and transcription/translation systems with ribosomes.
An understanding of the molecular biology of cells can be based on an analysis of their components or genes. On the other hand, it is important to also investigate cell behavior based on structural relationships observed in biochemical systems (Mitrea et al. 2018). Thus, a biophysical approach, especially when combined with simple model experiments, is effective for revealing principles that are fundamental for living molecular systems. To explore the effects of microcompartments, the condensation and/or segregation of biochemical components is performed within confined phases using micelles, lipid droplets, reversed aqueous droplets, lipid vesicles (liposomes), and coacervates, which are regarded as model systems resembling cells and organelles (Dubuc et al. 2019). Microcompartments enclosed within lipid membranes have attracted attention for several decades and are usually modeled by vesicles and aqueous droplets, while membraneless organelles have been investigated through the use of various systems due to a lack of standard methods and an insufficient understanding of their formation mechanism (Boeynaems et al. 2018). Membraneless organelles exhibit fluidic morphologies and are able to partition hydrophilic biopolymers even without hydrophobic barriers (Nott et al. 2016).
Over the past decade, several research groups have suggested that the mechanism of formation of membraneless organelles can be explained in terms of phase separation (liquid-liquid phase separation, LLPS) (Jacobs and Frenkel 2017; Schuster et al. 2018; Alberti et al. 2019; Shakya et al. 2020; Klosin et al. 2020). In the cytosol, interactions can also occur among specific proteinous and nucleic acid polymers, and thus it is worth examining which domains of both molecules are important for the generation of microdroplets segregated from their surroundings upon LLPS. Meanwhile, the application of simple theoretical considerations to interpret the behavior of actual living matter from a physicochemical perspective can also be useful for further exploring the universality of such phenomena (Hyman et al. 2014).
Phenomena such as LLPS may account for how complicated molecular systems can avoid adverse biochemical crosstalk even in a highly crowded cytoplasm. Generally, phase separation/phase transition can exhibit steep spatial/temporal discontinuity, which may be associated with the robust regulation of reactivity in situ and flexible structures with clear interfaces (Sakuta et al. 2019; de Gennes et al. 2004). As a chemical phase is defined as a region of uniform chemical composition in relation to a characteristic length scale. As the characteristic length scale decreases, the phenomenon of microheterogeneity becomes extant. Phase separation in biology may occur on various size scales, implying that phase behavior can play a role in multiscale states, possibly related to the emergence of hierarchical structures in living systems.
In this short review, which chiefly discusses our previous work, we consider the role of phase behavior in model experiments with both multicomponent polymer solutions and single polymer chains. We attempt to describe and relate the nonspecific characteristics of macromolecular phase separation to actual specific functions, like switching of gene expression and intracellular compartmentalization.
Simple models for chromosomes: phase behavior in a single DNA chain
In both eukaryotes and bacteria, relatively long chromosomal DNAs do not assume a random coiled form, but rather exhibit a folded conformation through interactions between polycationic DNA-binding proteins like histones (Schiessel 2003; Woodcock 2005). In the classical understanding of the storage of large DNA molecules in compact spaces, it is considered that some DNA-binding proteins as well as proteinous cofactors contribute to such packaging in a stepwise manner (Ozer et al. 2015). In the systematic packaging of genomic DNAs, it has been well established that there are domains with different packing densities along chromosomes, and these domains form functional regimes, e.g., even inside eukaryotic nuclei during interphase (Lieberman-Aiden et al. 2009). Though the behavior could possibly be attributed to the mechanical accumulation of block units of proteinous factors, a number of research groups have recently advocated that the presence of such inhomogeneous subsets in actual chromosomes can be interpreted as microphase separation (Larson et al. 2017; Strom et al. 2017). If so, how does microphase separation occur? To understand the mechanism, we must revisit the nature of the physicochemical behavior of long DNA molecules, which our groups have explored for several decades (Yoshikawa 2001; Zinchenko et al. 2008; Nishio et al. 2018; Nishio et al. 2019).
Discrete transition in the higher-ordered structure of a single DNA
Since double-stranded DNAs are semiflexible with negative charges aligned along their sugar-phosphate backbones, they are apt to spatially expand their conformation in aqueous conditions. Counter cations like group I and II metals from the periodic table condense around the aligned phosphate moieties, resulting in the shrinkage of DNA chains; however, shrinkage into a tight form cannot be achieved by alkaline metal (+1) (Bloomfield 1996). In the presence of other compounds such as alcohol or polyethylene glycol (PEG), DNAs can be tightly compacted with these metal ions consumed as counter ions against the phosphates (Bloomfield 1996): this phenomenon is well known as ethanol or PEG precipitation. As described in the literature by Yoshikawa and colleagues, when this DNA condensation is examined more closely using the direct observation of single DNA chains by fluorescence microscopy (Minagawa et al. 1994), it is clear that this phase transition behavior is almost discontinuous for long dsDNA and can be reduced to a discrete, or first order, phase transition with a change in conformation (Vasilevskaya et al. 1995; Ueda and Yoshikawa 1996; Yoshikawa et al. 1996), with the driving force being a change in the translational entropy of electrolytes in the system (Mayama et al. 2000).
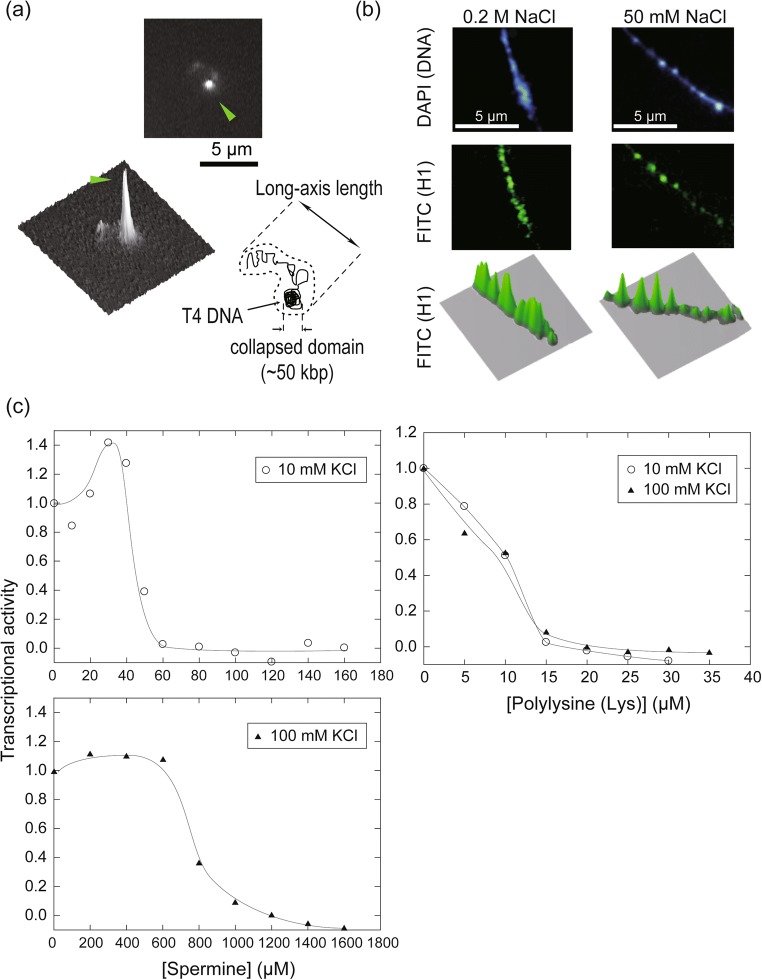
In addition, the compaction of long DNAs with polycations like polyamines (spermine and spermidine) and histone can be observed at the level of individual chains as a first-order phase transition, which is believed to be caused by the exchange of counter ions that have accumulated around DNAs (Takahashi et al. 1997; Murayama and Yoshikawa 1999). The folding transition occurs essentially in an all-or-none (intermolecular) manner, i.e., each DNA molecule can assume a folded (compacted) or unfolded morphology depending on the polycation concentration (Takahashi et al. 1997; Tongu et al. 2016). Interestingly, the distance within which the phase transition can propagate along the chain can be modulated by the concentration ratio of polyvalent to mono-/di-valent salts, since a coexisting salt with a lower valency can alter the free energy of the system, or apparently shield electrostatic interaction by competing for binding sites on DNAs with polycations (Yoshikawa 2001). For example, while long DNAs from bacteriophage T4 undergo an intermolecular folding transition at a low 1:1 salt concentration, in the presence of 1:1 salt near a physiological concentration, they exhibit intramolecular phase segregation at a single-chain level with some domains folded and others unfolded concurrently (Takagi et al. 2001) (Fig. 1a). This behavior is explained in terms of the limitation of the effective reach of a phase transition in the microsystem, i.e., microphase separation generates compacted as well as loosened domains along a DNA chain, which could be similarly modeled as a primitive chromosome with alternating low and high densities. This trend observed in naked DNAs is also seen in reconstituted artificial chromatins which can show a first-order phase transition at an intra-chain level between dense and dispersed states (Yoshikawa et al. 2001; Nakai et al. 2005) (Fig. 1b).
Fig. 1.
Phase transition in higher-ordered conformation and biochemical reactivity in long DNAs of bacteriophage T4. a Intramolecular phase segregation in a single T4 DNA chain (166 kbp) in the presence of 1.5 mM spermidine as a DNA-condensing agent with a buffer (10 mM Tris-HCl, 100 mM NaCl). A T4 DNA chain stained by DAPI (4′,6-diamidino-2-phenylindole), a specific fluorescent dye that binds to the minor groove, is shown in a typical image obtained by fluorescence microscopy, with a schematic illustration and a fluorescence profile. The bright spot indicated by a green arrow head is a partially condensed phase on a single chain (for more details, see Takagi et al. 2001). b Typical fluorescence images of the self-assembled pearling structure of long T4 DNAs with histone H1 proteins with various ionic strengths. T4 DNA and H1 protein were stained by DAPI and a set of antibodies (mouse anti-histone H1 IgG as the primary antibody and FITC-conjugated goat anti-mouse IgG as the secondary antibody). The extent to which DNA was condensed by H1 could be modulated by altering the 1:1 salt (NaCl) concentration. The panel is rearranged from that originally published in our previous work (Yoshikawa et al. 2001), with permission. For more details, see Yoshikawa et al. 2001. c Steep and gradual changes in the transcriptional activity of T4 DNA as templates for E. coli RNA polymerase holoenzyme in the presence of the condensing agents spermine and polylysine. DNA condensation proceeding, transcriptional activity decreased steeply and gradually with spermine and polylysine, respectively. The profile of transcriptional activity could be shifted sensitively by adding KCl, unlike in the case of polylysine, where KCl did not affect the profile. This reflects the difference in behavior of small and large condensing agents. The relative transcriptional activity was calculated from the increase in intensity of the fluorescence of uridine 5′-triphospate, P3-(5-sulfo-1-naphthylamide), tetra(triethylammonium) salt (UTP γ-AmNS; Molecular Probes). The UTP analogue became fluorescent upon being consumed by E. coli RNA polymerase. The panel in c shows unpublished data
Conformational transition functions as a switch in biochemical reactions
The physical boundaries of microdomains generated by microphase separation can qualitatively distinguish domains with different biochemical activities, as implied by the alternating states of gene expression among condensed and decondensed chromatins in actual cells (Solovei et al. 2016). Such behavior can be reproduced in a simple model experiment using long DNAs as substrates for transcription and translation (Yamada et al. 2005; Kanemura et al. 2018). Generally, the first-order phase transition in the conformation of dsDNA is only observed when the DNA chains are much longer than the persistence length (~ 150 bp) or Kuhn length (~ 300 bp) of dsDNA (Yoshikawa 2001). Thus, if the T7 promoter is included, DNAs from λ phage can undergo a discrete phase transition, and their availability as templates for T7 RNA polymerase is clearly reduced, accompanied by a folding transition of long DNA induced by the addition of a polyamine such as spermine and a macromolecular crowding polymer like PEG (Tsumoto et al. 2003; Luckel et al. 2005). Steep switching was not detected with short dsDNA templates of size less than the persistence length, which suggests that the behavior is due to a phase transition that originates on the basis of the DNA length.
Multivalent cations with medium molecular mass, such as polyamine, are considered to bind to DNAs nonspecifically thereby permitting the dynamic association/dissociation of polycations around the sugar-phosphate backbone (Nishio et al. 2019). Since a discrete transition can clearly demarcate the boundaries of active and inactive regions on DNAs, strict switching can be realized; interestingly, it has been shown that this strict regulation in conformation is rather typical in the presence of small condensing agents with nonspecific functions (Zinchenko et al. 2019). With the native combination of the substrate (T4 phage DNA) and the polymerase (E.coli holoenzyme), a similar trend that is observed in the case of T7 RNA polymerase (Tsumoto et al. 2003; Luckel et al. 2005) is seen, in which the transcription activity is switched steeply or gradually by the addition of a small polyamine like spermine or a polycationic peptide like polylysine, respectively, as shown in Fig. 1c.
Very recently, we demonstrated that the microphase separation of intra-chain conformation can be finely resolved by the addition of polyamine in a cell-free gene expression system, where plasmid vectors assumed a bouquet-like shape with condensed domains as well as a partly decondensed aligned region (possibly related to the steep enhancement of gene expression just prior to polyamine-induced inactivation). These results suggested that hierarchical structures with different genetic activities can be realized by microphase segregation in a simple system without proteinous factors that bind to DNAs specifically (Kanemura et al. 2018). As reviewed above, protocols for regulation and protection of gene materials can be designed artificially with ease using such intrinsic properties of long DNAs (Estévez-Torres and Baigl 2011; Venancio-Marques et al. 2014).
Simple models for biological compartments: phase separation of aqueous microdroplets
Microphase segregation is a characteristic phenomenon that long semiflexible polymers like chromosomal DNAs exhibit in nature. On the other hand, phase behavior is also generally observed inside cells, since the cytoplasm is a multicomponent aqueous system with high polymer concentrations. Some researchers have recently argued that microphase behavior could be the origin of intracellular membraneless organelles like nucleoli (Brangwynne et al. 2009; Brangwynne et al. 2011; Feric et al. 2016; Strom and Brangwynne 2019; Courchaine et al. 2016; Uversky 2017; Poudyal et al. 2018). For modeling membraneless microcompartments, LLPS systems that can be reconstituted in in vitro experiments are greatly desired (Aumiller and Keating 2017). Although various mechanisms like coacervation first described by Oparin may be involved in LLPS, we would here like to consider one of the simplest binary polymer systems consisting of popular macromolecular crowders, polyethylene glycol (PEG), and dextran (DEX); the system referred to as an aqueous two-phase system (ATPS) shows very interesting biomimetic behavior resembling that of mesoscale ordered structures. As established by Albertsson about 50 years ago, the ATPS is a well-known tool for effective bioseparation (Albertsson 1971). The surface free energy of the interface between such polymers is relatively weak, but not negligible, and so homogeneous mixing can be avoided to form aqueous microdroplets under a two-phase regime in the phase diagram (Atefi et al. 2014). In addition, if a condition is set at the side of the two-phase regime very near a binodal line, we are able to obtain relatively stable droplets, the size of which is comparable to cell sizes (~ 1–100 μm in diameter). Originally, the ATPS was used for biomolecular separation, and in this role it is effective for sharply partitioning biological macromolecules. As such this system is attractive for reconstituting water/water micro reactors for modeling biological environments (Esquena 2016; Chao and Shum 2020). Interestingly, behavior of biomacromolecules is expected to be further affected under confined conditions as in actual cells (Shew 2003; Iwaki et al. 2005; Hamada et al. 2015).
Research to mimic intracellular structures
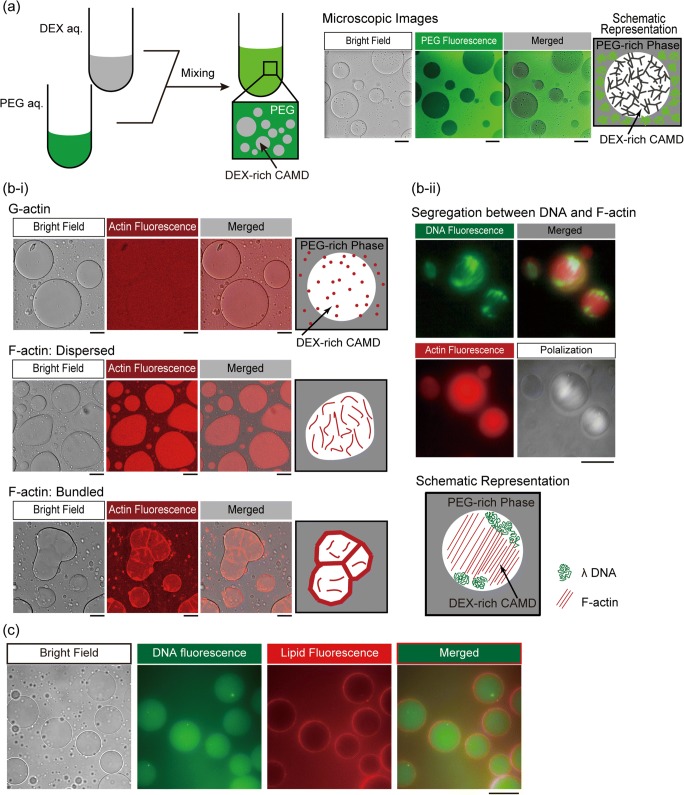
From the point of view of chemical engineering, the encapsulation of biomolecules inside droplets has been actively studied for several decades. One of the most successful examples is the lipid (water/oil/water) vesicle (liposome), giant liposomes or giant unilamellar vesicles (GUVs) which can have often been used to prepare model cells by encapsulating various cell-free biochemical systems (Liu et al. 2013; Rideau et al. 2018). As mentioned above, water/water droplets can be used for such studies. When the mass ratio of PEG to DEX is large, DEX-rich microdroplets may appear and are surrounded by PEG-rich solution, indicating that the interior of the droplet is large enough to harbor bulky molecules like dsDNAs compared with the PEG-rich exterior, which is more flexible than DEX, i.e., bulky polymers are excluded by the depletion effect into the inner space of DEX-rich droplets without electrostatic attraction (Fig.2 (a)) (Nakatani et al. 2018).
Fig. 2.
Spontaneous appearance of the cell-like structure in a cell-sized aqueous/aqueous microdroplet (CAMD) through the phase segregation of PEG and DEX. (a) Emergence of a cell-sized microdroplet upon mixing of PEG and DEX with a composition near the binodal line (each 5%). As shown in the fluorescent microscopic images, the interior and exterior of droplets were occupied by DEX and PEG. Scale bar 100 μm. (b-i) Localization of actin depending on the polymerization state. Monomeric G-actin was distributed homogeneously (upper). Polymerized dispersed F-actin (middle, KCl 40 mM) and bundled F-actin (bottom, MgCl2 2.0 mM) were localized in the DEX-rich droplet. Bundled F-actin was located on the surface of droplets, in contrast to dispersed F-actin, which was distributed uniformly in the droplet. Scale bar 50 μm. (b-ii) Cooperative behavior of F-actin and long DNA through spontaneous localization in the droplet. Dispersed F-actin (b-ii middle) and λ DNA (49 kbp) were localized inside of droplets, and caused segregation between pairs of different biopolymers. By polarization microscopy (as in the lower-right panel), F-actin was assembled in a nematic liquid-crystal state and DNA was compressed to both poles as shown in the schematic representation. Scale bar 100 μm. Panels (a) and (b) are rearranged from those originally published in our previous study (Nakatani et al. 2018) and reprinted here under CC BY-NC 4.0 (https://creativecommons.org/licenses/by-nc/4.0/). (c) Spontaneous composition of phospholipid membranous structure and encapsulation of double-stranded DNA. Salmon sperm DNA (150 μM in nucleotide units, labeled with GelGreen) was entrapped in the droplet, and phospholipid from soy bean lecithin labeled by the addition of Rhodamine-DHPE was accumulated on the surface of the DEX-rich droplet (PEG:DEX = 6.5%:2%). The panel contains unpublished data
We previously found that DNA aggregates which partitioned into DEX droplets were localized robustly so that they could not be pulled from the droplet to the exterior through manipulation with optical tweezers (Toyama et al. 2008; Tsumoto et al. 2015). As shown in the literature, the interface of droplets of PEG/DEX ATPS can serve as a filter to distinguish DNAs in long semiflexible (stiffer) forms from single-stranded short DNAs (Nakatani et al. 2018), although partition coefficients are affected by coexisting salt species (Albertsson 1971).
Proteinous molecules also undergo selective localization due to their wide variety in the three-dimensional structures (Song et al. 2018). We have revealed switching behavior in the localization of the cytoskeletal protein actin (Nakatani et al. 2018) (Fig. 2 (b-i)). Generally, actin proteins take monomeric (G-actin) and polymeric (F-actin) forms, and the dominant form is determined depending on the salt condition in in vitro experiments (Kasai et al. 1962; Hanson 1973). When monomeric G-actin is traced by fluorescent labeling (Fujiwara et al. 2002), it is observed that the molecules are distributed homogeneously inside and outside droplets. However, when the salt concentration is changed to induce their polymerization into filamentous F-actin, they are entrapped inside DEX-rich droplets, which are clearly visualized by high-contrast illumination specific to the interior of the droplet where F-actin proteins are distributed evenly. Under the addition of magnesium ion, bundles of F-actin are localized with a clear preference just beneath the droplet interfaces. Using a simple model experiment, we found that molecular localization may possibly be switched in a specific manner in such crowded conditions even without other proteinous factors. It should also be noted that crowded environments promote polymerization/bundling of actin proteins comparable with the behavior observed in actual cells (Suzuki et al. 1989; Peterson et al. 2004).
In simple model systems, multiple components can be entrapped into microcompartments simultaneously, as in actual cells. When crowded by various polymers, the interiors of the droplets serve as containers where inhomogeneous fine structures can develop. Indeed, when F-actin proteins are entrapped in droplets with λ DNAs, F-actin filaments aligned in parallel exclude DNAs to form subdomains, which implies that biomacromolecules generally tend to form different phases under crowded conditions (Fig. 2 (b-ii)) (Nakatani et al. 2018). When DNAs and phospholipids coexist, dsDNA can enter the interior, accompanied by the spontaneous accumulation of membranous lipids over the interface of the droplets (Fig. 2 (c)). Dewey et al. (2014) also observed droplets which interfaces were stabilized by small liposomes prepared in advance; in Fig. 2 (c), lipid components and the route for preparation that we used were different, and we now try to evaluate the extent to which the interface may function as a barrier for permeation of hydrophilic molecules. It should be noted that the localization of these molecules in a model experiment was morphologically similar to that observed in living cells (Alberts et al. 2010).
Toward higher hierarchical cell assembly
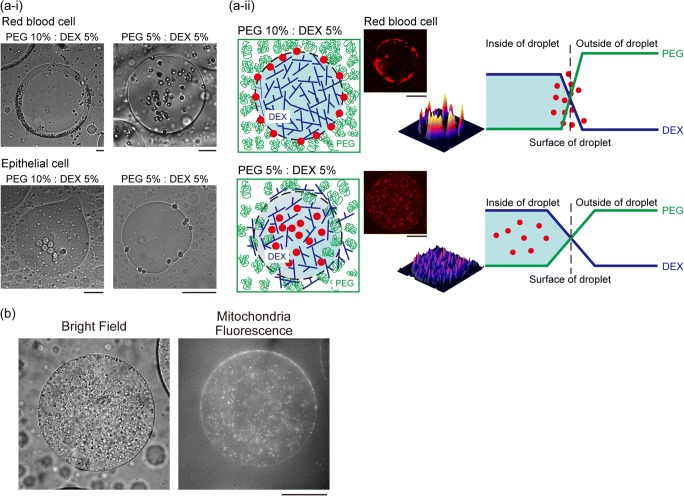
As shown in the above molecular systems, cells can be cooperatively well-organized into ordered assemblies through the use of macromolecular crowding. Yoshikawa and co-workers showed that, in dextran solution, mammalian cells can be firmly deposited with the desired arrangement using optical manipulation (Hashimoto et al. 2016; Yoshida et al. 2017; Yamazaki et al. 2019). Moderate crowding generated by dextran in the exterior provides an opportunity for rearrangement through which cell-cell association in the assembly can be reinforced. For highly organized cell spheroids, droplets in ATPSs are good containers for culturing cells (Atefi et al. 2015; Han et al. 2015; Agarwal et al. 2019). Interestingly, without external manipulation, and instead by simply adjusting the PEG/DEX ratio, it is possible to modulate the arrangement of cells in microdroplets (Fig. 3 (a)); especially, when different types of cells are incorporated, surface tension and the depletion effect influence their localization (Fig. 3 (a-i)). Red blood cells and epithelial cells exhibited opposite trends in localization in ATPS droplets, which can be interpreted in terms of the extent to which surfaces can be stabilized by cells and cells can be excluded by surrounding PEG molecules (Sakuta et al. 2019) (Fig. 3 (a-ii)). In addition, we have efficiently entrapped intact mitochondria inside DEX-rich droplets, where many mitochondria can be condensed within single droplets, albeit with some adsorption at the interface (Fig. 3 (b)); as shown in the case of cells (Sakuta et al. 2019), mitochondria exhibited specific localization inside microdroplets.
Fig. 3.
Specific localization of cells and intracellular organelles in an aqueous/aqueous microdroplet. (a-i) Specific distribution depending on the cell type and the concentrations of PEG and DEX. Upper panels: microscopic images of red blood cells (RBC) in microdroplets with the composition of PEG:DEX = 10%:5% and 5%:5%. Lower panels: microscopic images of mouse mammary gland epithelial cells (NMuMG cells) in microdroplets. Scale bar 50 μm. (a-ii) Schematic representation of the proposed mechanism of RBC distribution inside microdroplets generated by PEG and DEX. Scale bar 100 μm. The panels in (a) are rearranged from those originally published in our previous study (Sakuta et al. 2019) and reprinted here under CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). (b) Localization of mitochondria in the DEX-rich droplet (PEG:DEX = 5%:5%). Mitochondria was isolated from rat liver and labeled by rhodamine 123 (Ex.: 507 nm, Em.: 529 nm). H. Sakuta, K. Sadakane, Y. Shinohara, and K. Yoshikawa, to be submitted for publication
Conclusions
As discussed within this review, recent consideration of cell behavior has focused on phase separation and phase transition in the cellular environment. In our opinion, we should also revisit the simple question of how biological material exhibits such phase dynamics in nature. Here, we have discussed the phase-transition behavior of long semiflexible DNA chains. We have also discussed lipid bilayer membranes may be regarded as a water/oil/water (W/O/W) emulsion (phase separation). Throughout this review, we have also discussed how water/water (W/W) LLPS can also occur ubiquitously in biology, and may be important for various cell functions. Cell organization in higher hierarchical levels may be considered to be neatly regulated in mechanical systems; although at present, it is difficult to understand how living organisms may be described in such a mechanical fashion. Regarding cell assembly, the self-assembly of molecules could play an essential role, and it may be worthwhile to consider phase behavior when seeking to understand biological function at any hierarchical level.
Acknowledgments
K. Tsumoto thanks Ms. Aya Matsumoto (Mie University) for her help with the experiment that produced the data shown in Fig. 1c.
Funding information
This work was supported in part by KAKENHI (JP15H02121 to K.Y.; JP18H04976 to K. Tsumoto).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agarwal R, Liu G, Tam NW, Gratzer PF, Frampton JP. Precision cell delivery in biphasic polymer systems enhances growth of keratinocytes in culture and promotes their attachment on acellular dermal matrices. J Tissue Eng Regen Med. 2019;13:997–1006. doi: 10.1002/term.2845. [DOI] [PubMed] [Google Scholar]
- Alberti S, Gladfelter A, Mittag T. Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell. 2019;176:419–434. doi: 10.1016/j.cell.2018.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts B, Bray D, Hopkin K, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Essential cell biology. 3. New York: Garland Science; 2010. [Google Scholar]
- Albertsson PÅ. Partition of cell particles and macromolecules. 2. New York: Wiley; 1971. [Google Scholar]
- Atefi E, Mann JA, Jr, Tavana H. Ultralow interfacial tensions of aqueous two-phase systems measured using drop shape. Langmuir. 2014;30:9691–9699. doi: 10.1021/la500930x. [DOI] [PubMed] [Google Scholar]
- Atefi E, Joshi R, Mann JA, Jr, Tavana H. Interfacial tension effect on cell partition in aqueous two-phase systems. ACS Appl Mater Interfaces. 2015;7:21305–21314. doi: 10.1021/acsami.5b05757. [DOI] [PubMed] [Google Scholar]
- Aumiller WM, Jr, Keating CD. Experimental models for dynamic compartmentalization of biomolecules in liquid organelles: reversible formation and partitioning in aqueous biphasic systems. Adv Colloid Interf Sci. 2017;239:75–87. doi: 10.1016/j.cis.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Bloomfield VA. DNA condensation. Curr Opin Struct Biol. 1996;6:334–341. doi: 10.1016/S0959-440X(96)80052-2. [DOI] [PubMed] [Google Scholar]
- Boeynaems S, Alberti S, Fawzi NL, et al. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 2018;28:420–435. doi: 10.1016/j.tcb.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, Gharakhani J, Jülicher F, Hyman AA. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- Brangwynne CP, Mitchison TJ, Hyman AA. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 2011;108:4334–4339. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Shum HC. Emerging aqueous two-phase systems: from fundamentals of interfaces to biomedical applications. Chem Soc Rev. 2020;49:114–142. doi: 10.1039/c9cs00466a. [DOI] [PubMed] [Google Scholar]
- Courchaine EM, Lu A, Neugebauer KM. Droplet organelles? EMBO J. 2016;35:1603–1612. doi: 10.15252/embj.201593517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gennes PG, Brochard-Wyart F, Quéré D. Capillarity and wetting phenomena. New York: Springer; 2004. [Google Scholar]
- Dewey DC, Strulson CA, Cacace DN, Bevilacqua PC, Keating CD. Bioreactor droplets from liposome-stabilized all-aqueous emulsions. Nat Commun. 2014;5:4670. doi: 10.1038/ncomms5670. [DOI] [PubMed] [Google Scholar]
- Dubuc E, Pieters PA, van der Linden AJ, van Hest JC, Huck WT, de Greef TF. Cell-free microcompartmentalised transcription-translation for the prototyping of synthetic communication networks. Curr Opin Biotechnol. 2019;58:72–80. doi: 10.1016/j.copbio.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ. Macromolecular crowding: an important but neglected aspect of the intracellular environment. Curr Opin Struct Biol. 2001;11:114–119. doi: 10.1016/S0959-440X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- Esquena J. Water-in-water (W/W) emulsions. Curr Opin Colloid Interface Sci. 2016;25:109–119. doi: 10.1016/j.cocis.2016.09.010. [DOI] [Google Scholar]
- Estévez-Torres A, Baigl D. DNA compaction: fundamentals and applications. Soft Matter. 2011;7:6746–6756. doi: 10.1039/C1SM05373F. [DOI] [Google Scholar]
- Feric M, Vaidya N, Harmon TS, Mitrea DM, Zhu L, Richardson TM, Kriwacki RW, Pappu RV, Brangwynne CP. Coexisting liquid phases underlie nucleolar subcompartments. Cell. 2016;165:1686–1697. doi: 10.1016/j.cell.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara I, Takahashi S, Tadakuma H, Funatsu T, Si I. Microscopic analysis of polymerization dynamics with individual actin filaments. Nat Cell Biol. 2002;4:666–673. doi: 10.1038/ncb841. [DOI] [PubMed] [Google Scholar]
- Hamada T, Fujimoto R, Shimobayashi SF, Ichikawa M, Takagi M. Molecular behavior of DNA in a cell-sized compartment coated by lipids. Phys Rev E. 2015;91:062717. doi: 10.1103/PhysRevE.91.062717. [DOI] [PubMed] [Google Scholar]
- Han C, Takayama S, Park J. Formation and manipulation of cell spheroids using a density adjusted PEG/DEX aqueous two phase system. Sci Rep. 2015;5:11891. doi: 10.1038/srep11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. Evidence from electron microscope studies on actin paracrystals concerning the origin of the cross-striation in the thin filaments of vertebrate skeletal muscle. Proc R Soc Lond B Biol Sci. 1973;183:39–58. doi: 10.1098/rspb.1973.0003. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Yoshida A, Ohta T, Taniguchi H, Sadakane K, Yoshikawa K. Formation of stable cell–cell contact without a solid/gel scaffold: non-invasive manipulation by laser under depletion interaction with a polymer. Chem Phys Lett. 2016;655-656:11–16. doi: 10.1016/j.cplett.2016.05.019. [DOI] [Google Scholar]
- Hyman AA, Weber CA, Jülicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30:39–58. doi: 10.1146/annurev-cellbio-100913-013325. [DOI] [PubMed] [Google Scholar]
- Iwaki T, Shew CY, Gumbs G. Integral equation theory for hard spheres confined on a cylindrical surface: anisotropic packing entropically driven. J Chem Phys. 2005;123:124712. doi: 10.1063/1.2038727. [DOI] [PubMed] [Google Scholar]
- Jacobs WM, Frenkel D. Phase transitions in biological systems with many components. Biophys J. 2017;112:683–691. doi: 10.1016/j.bpj.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemura A, Yoshikawa Y, Fukuda W, Tsumoto K, Kenmotsu T, Yoshikawa K. Opposite effect of polyamines on in vitro gene expression: enhancement at low concentrations but inhibition at high concentrations. PLoS One. 2018;13:e0193595. doi: 10.1371/journal.pone.0193595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai M, Asakura S, Oosawa F. The G-F equilibrium in actin solutions under various conditions. Biochim Biophys Acta. 1962;57:13–21. doi: 10.1016/0006-3002(62)91072-7. [DOI] [PubMed] [Google Scholar]
- Klosin A, Oltsch F, Harmon T, Honigmann A, Jülicher F, Hyman AA, Zechner C. Phase separation provides a mechanism to reduce noise in cells. Science. 2020;367:464–468. doi: 10.1126/science.aav6691. [DOI] [PubMed] [Google Scholar]
- Larson AG, Elnatan D, Keenen MM, et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature. 2017;547:236–240. doi: 10.1038/nature22822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman-Aiden E, van Berkum NL, Williams L, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Hansen GP, Venancio-Marques A, Baigl D. Cell-free preparation of functional and triggerable giant proteoliposomes. ChemBioChem. 2013;14:2243–2247. doi: 10.1002/cbic.201300501. [DOI] [PubMed] [Google Scholar]
- Luckel F, Kubo K, Tsumoto K, Yoshikawa K. Enhancement and inhibition of DNA transcriptional activity by spermine: a marked difference between linear and circular templates. FEBS Lett. 2005;579:5119–5122. doi: 10.1016/j.febslet.2005.07.095. [DOI] [PubMed] [Google Scholar]
- Mayama M, Iwataki T, Yoshikawa K. Thermodynamics in the folding phase-transition of single T4DNA molecules in poly (ethylene glycol) solution. Chem Phys Lett. 2000;318:113–117. doi: 10.1016/S0009-2614(00)00004-X. [DOI] [Google Scholar]
- Minagawa K, Matsuzawa Y, Yoshikawa K, Khokhlov AR, Doi M. Direct observation of the coil-globule transition in DNA molecules. Biopolymers. 1994;34:555–558. doi: 10.1002/bip.360340410. [DOI] [Google Scholar]
- Mitrea DM, Chandra B, Ferrolino MC, Gibbs EB, Tolbert M, White MR, Kriwacki RW. Methods for physical characterization of phase-separated bodies and membrane-less organelles. J Mol Biol. 2018;430:4773–4805. doi: 10.1016/j.jmb.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama H, Yoshikawa K. Thermodynamics of the collapsing phase transition in a single duplex DNA molecule. J Phys Chem B. 1999;103:10517–10523. doi: 10.1021/jp990721o. [DOI] [Google Scholar]
- Nakai T, Hizume K, Yoshimura SH, Takeyasu K, Yoshikawa K. Phase transition in reconstituted chromatin. Europhys Lett. 2005;69:1024–1030. doi: 10.1209/epl/i2004-10444-6. [DOI] [Google Scholar]
- Nakatani N, Sakuta H, Hayashi M, Tanaka S, Takiguchi K, Tsumoto K, Yoshikawa K. Specific spatial localization of actin and DNA in a water/water microdroplet: self-emergence of a cell-like structure. ChemBioChem. 2018;19:1370–1374. doi: 10.1002/cbic.201800066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio T, Yoshikawa Y, Fukuda W, Umezawa N, Higuchi T, Fujiwara S, Imanaka T, Yoshikawa K. Branched-chain polyamine found in hyperthermophiles induces unique temperature-dependent structural changes in genome-size DNA. ChemPhysChem. 2018;19:2299–2304. doi: 10.1002/cphc.201800396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio T, Yoshikawa Y, Shew CY, Umezawa N, Higuchi T, Yoshikawa K. Specific effects of antitumor active norspermidine on the structure and function of DNA. Sci Rep. 2019;9:14971. doi: 10.1038/s41598-019-50943-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott TJ, Craggs TD, Baldwin AJ. Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nat Chem. 2016;8:569–575. doi: 10.1038/nchem.2519. [DOI] [PubMed] [Google Scholar]
- Ozer G, Luque A, Schlick T. The chromatin fiber: multiscale problems and approaches. Curr Opin Struct Biol. 2015;31:124–139. doi: 10.1016/j.sbi.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LJ, Rajfur Z, Maddox AS, Freel CD, Chen Y, Edlund M, Otey C, Burridge K. Simultaneous stretching and contraction of stress fibers in vivo. Mol Biol Cell. 2004;15:3497–3508. doi: 10.1091/mbc.e03-09-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudyal RR, Pir Cakmak F, Keating CD, Bevilacqua PC. Physical principles and extant biology reveal roles for RNA-containing membraneless compartments in origins of life chemistry. Biochemistry. 2018;57:2509–2519. doi: 10.1021/acs.biochem.8b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev. 2018;47:8572–8610. doi: 10.1039/c8cs00162f. [DOI] [PubMed] [Google Scholar]
- Rivas G, Minton AP. Macromolecular crowding in vitro, in vivo, and in between. Trends Biochem Sci. 2016;41:970–981. doi: 10.1016/j.tibs.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuta H, Fujimoto T, Yamana Y, Hoda Y, Tsumoto K, Yoshikawa K. Aqueous/aqueous micro phase separation: construction of an artificial model of cellular assembly. Front Chem. 2019;7:44. doi: 10.3389/fchem.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiessel H. The physics of chromatin. J Phys Condens Matter. 2003;15:R699–R774. doi: 10.1088/0953-8984/15/19/203. [DOI] [PubMed] [Google Scholar]
- Schuster BS, Reed EH, Parthasarathy R, Jahnke CN, Caldwell RM, Bermudez JG, Ramage H, Good MC, Hammer DA. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat Commun. 2018;9:2985. doi: 10.1038/s41467-018-05403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya A, Park S, Rana N, King JT. Liquid-liquid phase separation of histone proteins in cells: role in chromatin organization. Biophys J. 2020;118:753–764. doi: 10.1016/j.bpj.2019.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew CY. Conformational behavior of a single polymer chain confined by a two-dimensional harmonic potential in good solvents. J Chem Phys. 2003;119:10428. doi: 10.1063/1.1616512. [DOI] [Google Scholar]
- Solovei I, Thanisch K, Feodorova Y. How to rule the nucleus: divide et impera. Curr Opin Cell Biol. 2016;40:47–59. doi: 10.1016/j.ceb.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Song Y, Michaels TCT, Ma Q, Liu Z, Yuan H, Takayama S, Knowles TPJ, Shum HC. Budding-like division of all-aqueous emulsion droplets modulated by networks of protein nanofibrils. Nat Commun. 2018;9:2110. doi: 10.1038/s41467-018-04510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom AR, Brangwynne CP. The liquid nucleome - phase transitions in the nucleus at a glance. J Cell Sci. 2019;132:jcs235093. doi: 10.1242/jcs.235093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X, Karpen GH. Phase separation drives heterochromatin domain formation. Nature. 2017;547:241–245. doi: 10.1038/nature22989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Yamazaki M, Ito T. Osmoelastic coupling in biological structures: formation of parallel bundles of actin filaments in a crystalline-like structure caused by osmotic stress. Biochemistry. 1989;28:6513–6518. doi: 10.1021/bi00441a052. [DOI] [PubMed] [Google Scholar]
- Takagi S, Tsumoto K, Yoshikawa K. Intra-molecular phase segregation in a single polyelectrolyte chain. J Chem Phys. 2001;114:6942–6949. doi: 10.1063/1.1342810. [DOI] [Google Scholar]
- Takahashi M, Yoshikawa K, Vasilevskaya VV, Khokhlov AR. Discrete coil−globule transition of single duplex DNAs induced by polyamines. J Phys Chem B. 1997;101:9396–9401. doi: 10.1021/jp9716391. [DOI] [Google Scholar]
- Tongu C, Kenmotsu T, Yoshikawa Y, Zinchenko A, Chen N, Yoshikawa K. Divalent cation shrinks DNA but inhibits its compaction with trivalent cation. J Chem Phys. 2016;144:205101. doi: 10.1063/1.4950749. [DOI] [PubMed] [Google Scholar]
- Toyama H, Yoshikawa K, Kitahata H. Homogenization of a phase-separated droplet in a polymer mixture caused by the dielectric effect of a laser. Phys Rev E. 2008;78:060801R. doi: 10.1103/PhysRevE.78.060801. [DOI] [PubMed] [Google Scholar]
- Tsumoto K, Luckel F, Yoshikawa K. Giant DNA molecules exhibit on/off switching of transcriptional activity through conformational transition. Biophys Chem. 2003;106:23–29. doi: 10.1016/S0301-4622(03)00138-8. [DOI] [PubMed] [Google Scholar]
- Tsumoto K, Arai M, Nakatani N, Watanabe SN, Yoshikawa K. Does DNA exert an active role in generating cell-sized spheres in an aqueous solution with a crowding binary polymer? Life (Basel) 2015;5:459–466. doi: 10.3390/life5010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Yoshikawa K. Phase transition and phase segregation in a single double-stranded DNA molecule. Phys Rev Lett. 1996;77:2133–2136. doi: 10.1103/PhysRevLett.77.2133. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Intrinsically disordered proteins in overcrowded milieu: membrane-less organelles, phase separation, and intrinsic disorder. Curr Opin Struct Biol. 2017;44:18–30. doi: 10.1016/j.sbi.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Vasilevskaya VV, Khokhlov AR, Matsuzawa Y, Yoshikawa K. Collapse of single DNA molecule in poly (ethylene glycol) solutions. J Chem Phys. 1995;102:6595–6602. doi: 10.1063/1.469375. [DOI] [Google Scholar]
- Venancio-Marques A, Bergen A, Rossi-Gendron C, Rudiuk S, Baigl D. Photosensitive polyamines for high-performance photocontrol of DNA higher-order structure. ACS Nano. 2014;8:3654–3663. doi: 10.1021/nn500266b. [DOI] [PubMed] [Google Scholar]
- Woodcock CL. A milestone in the odyssey of higher-order chromatin structure. Nat Struct Mol Biol. 2005;12:639–640. doi: 10.1038/nsmb0805-639. [DOI] [PubMed] [Google Scholar]
- Yamada A, Kubo K, Nakai T, Tsumoto K, Yoshikawa K. All-or-none switching of transcriptional activity on single DNA molecules caused by a discrete conformational transition. Appl Phys Lett. 2005;86:223901. doi: 10.1063/1.1937990. [DOI] [Google Scholar]
- Yamazaki T, Kishimoto T, Leszczyński P, et al. Construction of 3D cellular composites with stem cells derived from adipose tissue and endothelial cells by use of optical tweezers in a natural polymer solution. Materials (Basel) 2019;12:1759. doi: 10.3390/ma12111759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Tsuji S, Taniguchi H, Kenmotsu T, Sadakane K, Yoshikawa K. Manipulating living cells to construct a 3D single-cell assembly without an artificial scaffold. Polymers (Basel) 2017;9:319. doi: 10.3390/polym9080319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K. Controlling the higher-order structure of giant DNA molecules. Adv Drug Deliv Rev. 2001;52:235–244. doi: 10.1016/S0169-409X(01)00210-1. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Takahashi M, Vasilevskaya VV, Khokhlov AR. Large discrete transition in a single DNA molecule appears continuous in the ensemble. Phys Rev Lett. 1996;76:3029–3031. doi: 10.1103/PhysRevLett.76.3029. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Velichko YS, Ichiba Y, Yoshikawa K. Self-assembled pearling structure of long duplex DNA with histone H1. Eur J Biochem. 2001;268:2593–2599. doi: 10.1046/j.1432-1327.2001.02144.x. [DOI] [PubMed] [Google Scholar]
- Zinchenko AA, Pyshkina OA, Lezov AV, Sergeyev VG, Yoshikawa K. Single DNA molecules: compaction and decompaction. In: Lindman B, Dias R, editors. DNA interactions with polymers and surfactants. Hoboken: Wiley; 2008. [Google Scholar]
- Zinchenko A, Hiramatsu H, Yamaguchi H, et al. Amino acid sequence of oligopeptide causes marked difference in DNA compaction and transcription. Biophys J. 2019;116:1836–1844. doi: 10.1016/j.bpj.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]