Abstract
Biomolecular motor systems are the smallest natural machines with an ability to convert chemical energy into mechanical work with remarkably high efficiency. Such attractive features enabled biomolecular motors to become classic tools in soft matter research over the past decade. For designing suitably engineered biomimetic systems, the biomolecular motors can potentially be used as molecular engines that can transform energy and ensure great advantages for the construction of bio-nanodevices and molecular robots. From the optimization of their prolonged lifetime to coordinate them into highly complex and ordered structures, enormous efforts have been devoted to make them useful in the synthetic environment. Synchronous operation of the biomolecular engines is one of the key criteria to coordinate them into certain different patterns, which depends on the local interaction of biomolecular motors. Utilizing chemical and physical stimuli, synchronization of biomolecular motor systems has become possible, which allows them to coordinate into different higher ordered patterns with different modes of functionality. Recently, programmed synchronous operation of the biomolecular engines has also been demonstrated, using a smart biomaterial to build up swarms reminiscent of nature. Here, we review the recent progress in the synchronized operation of biomolecular motors in engineered systems to explicitly program their interaction and further their applications. Such developments in the coordination of biomolecular motors have opened a broad way to explore the construction of future autonomous molecular machines and robots based on synchronization of biomolecular engines.
Keywords: Biomolecular motor system, Molecular engine, Synchronous behavior, Swarm, Molecular robot
Introduction
The development of molecular engines (Astumian 2017) has been highly beneficial in advancing towards fabrication artificial molecular machines. Fabrication of molecular machines requires synchronized operation of molecular engines that can lead to emergent functions, which has remained a major challenge for artificial molecular engines due to their restricted functions (Erbas-Cakmak et al. 2015). On the other hand, nature has her own set of molecular engines working in living organisms that are designed and optimized for performing specific functions. Biomolecular motor systems are the smallest natural machines that have several attractive features such as high efficiency of energy conversion that can drive many biological functions by forming highly complex structural organizations (Harold 2003; Howard 2001). For instance, the DNA polymerase can replicate DNA, and RNA polymerase transcribes genes by generating linear movement (Swan et al. 2009; Sainsbury et al. 2015). In biology, the F1F0-ATPase motor is used as an ATP generator, and vice versa, the hydrolysis of ATP can drive rotary motion (Kinosita et al. 1998; Yasuda et al. 1998). Through the hydrolysis of ATP, myosins exert forces on actin filaments and perform many cellular processes, including muscle contraction, cell division, cargo trafficking, and cell signaling (Frontera and Ochala 2015; Masters et al. 2016). On the other hand, kinesins and dyneins hydrolyze ATP to “walk” along microtubules (MT) and participate in intracellular cargo transport and cell division (Hirokawa et al. 2009; Riedel et al. 2015; Reck-Peterson et al. 2018). The coupling of mechanical and chemical events exerts the required force by a motor protein that can realize the specific tasks. These unique features distinguish them as powerful biological engines compared with man-made ones, and inspire to employ them for applications in various fields. In recent years, vast knowledge and understanding about these biological motors have been acquired, because of which, we now see a possibility of using natural machines or creating synthetic ones with criteria resembling nature’s components. The small size and force exerting capability give them opportunities for serving as a micro-actuator, as well as a sensor in designing and developing micro-machineries and molecular robots that can work autonomously in an artificial environment (Bachand et al. 2014). In this regard, among different biological motor protein systems, MT-kinesin systems have been attracting much attention nowadays (Hess and Bachand 2005; Hess 2011). Thanks to the latest biotechnological and chemical advancements, MTs and kinesins are nowadays used in the engineered systems, opening the opportunity for various bio- and nanotechnological applications. Alongside the utility of the “motor protein kinesin driven self-propelled MTs” as single actuators or sensors, self-assembly of them into hierarchically organized structures has also been extensively studied to understand the collective effect and their emergent behavior. In this regard, collective motion is one of the most spectacular phenomena in living systems, which is a typical display of moving or self-propelled objects with the formation of large-scale patterns (Vicsek and Zafeiris 2012). The idea is mainly inspired by swarming, where collective behavior of bees, flocks of birds, and schools of fish aggregate and move collectively in a certain direction (Bonabeau et al. 1999). An increasing focus has been seen over the past decade on the swarming of biomolecular motor-propelled protein filaments as self-propelled systems. Collective behavior of a large number of self-propelled biomolecular motors (Hess and Vogel 2001; Agarwal and Hess 2010) into different patterns was demonstrated which facilitated emergent functions through artificial swarm intelligence and swarm robots (Hess 2006; Lam et al. 2016). In this review, we will discuss the recent progress in the synchronized operation of the biomolecular engines, such as kinesin-driven cytoskeletal filaments MTs, leading to swarming. The programming of local interactions of MTs, dominated by their surrounding environment and external chemical and physical stimuli to reach the next level of operation of the biomolecular machines, is emphasized. Finally, the limitations and future perspectives of these fascinating phase transitions and their emergent functions to construct autonomous molecular robots are also highlighted.
Microtubule-kinesin, an ATP-fueled biomolecular engine
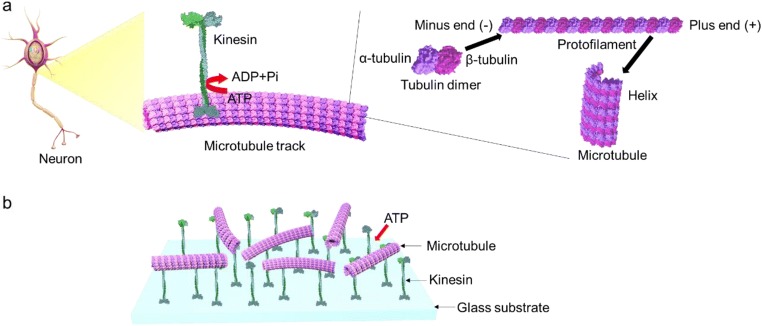
The biomolecular motor system is comprised of a class of proteins that performs various functions inside cells (Howard 2001). One of the proteins is a linear motor kinesin-1, along with cytoskeletal filament MT, which is found in living organisms and organs of animals. MT, the cytoskeletal track, is a hollow cylinder polymerized from α, β-tubulin (Tb) heterodimers (Nogales et al. 1998; Li et al. 2002). During polymerization, Tb dimers join head-to-tail to form a linear protofilament (PF). Generally, 13 PFs assemble into a cylindrical MT with an outer diameter of ~ 25 nm and a length of several micrometers (Chrétien et al. 1992; Drabik et al. 2007) (Fig. 1a). MTs serve as scaffolds for a wide variety of MT-associated molecular motors, e.g., kinesins and other families as well, in the cell. The conversion of chemical energy of ATP into mechanical work by kinesin enables a wide range of functions, such as intracellular transport of vesicles and organelles (Hirokawa and Takemura 2004) in neuron cells, cell division (Sharp et al. 2000), and the organization of cilia and flagella (Howard et al. 1989). Micromechanical recordings of single kinesin molecules indicate that one motor can exert a force as great as ~ 7 pN. The energy conversion efficiency of the biomolecular motor system exceeds 50% (Hunt et al. 1994; Visscher et al. 1999), a much higher efficiency compared with artificial motors. The small size and high efficiency of the MT-kinesin system makes it fascinating for application as molecular actuators for a wide range of purposes. The biomolecular motor system was successfully reconstructed using biotechnology and an in vitro motility assay system was further established (Schnapp et al. 1985; Gell et al. 2010; Malcos and Hancock 2011). In an in vitro motility assay, MTs are propelled by kinesins attached to a glass surface using the energy of ATP (Fig. 1b).
Fig. 1.
Structure of biomolecular motor system of the in vivo and in vitro motility assay. a Schematic representation of MT cylindrical-shaped structure formed from protofilaments of tubulin dimers and kinesin in neuron cells. b In vitro motility assay of MTs on a kinesin-coated glass surface in the presence of ATP
Biomolecular engines in engineered systems
The in vitro motility assay is the most promising engineered setup for nanotechnological applications of the biomolecular motor system. To investigate the property of biomolecular motor systems in vitro, motor proteins and their associated cytoskeletal filaments were reconstructed successfully by utilizing the bio-technological advancements (Sheetz and Spudich 1983). Vale and his colleagues discovered unidirectional gliding motion of MTs in the cell extract from squid axon on a substrate, which were driven by a biomolecular motor (kinesin and dynein) (Schnapp et al. 1985). They named this method “in vitro motility assay” as gliding of filaments was observed, which helps to investigate the property of biomolecular motor systems. Later, Spudich also developed a similar in vitro motility assay, where motility of F-actin on a glass surface coated with myosin was demonstrated (Kron and Spudich 1986). These works opened the door for understanding how biomolecular motors transport cargo molecules involved in numerous cellular processes, including cell polarity, cell division, cellular movement, and signal transduction. With the help of recent progress in nanotechnology, the in vitro gliding assay has been attracting interest in serving as a molecular shuttle (Hess and Vogel 2001). They provide transport of various nano- or micro-sized cargo molecules. This function is applicable for the development of various kinds of microdevices that sort, separate, concentrate, probe, analyze, and assemble materials (Böhm et al. 2001; Hess et al. 2002a, b, 2004; Bachand et al. 2004; Agarwal et al. 2009; Schmidt and Vogel 2010). Alongside many applicable features of self-propelled MTs, this molecular shuttle was also applied to demonstrate swarming in a discrete level controlled by external stimuli or guiding direction. For example, investigation on the control over molecular shuttles by sequestration of enzyme activities was studied by Hess et al. using gliding assay of MTs. Localized release and enzymatic sequestration of the substrate ATP creates a spatially and temporally well-defined concentration profile, which in turn leads to controlled activation of a small number of molecular shuttles, suggesting that these nanosystems are most efficiently addressed as a swarm rather than as individuals (Tucker et al. 2008). They also reported the mechanisms responsible for the dispersion of a swarm of “molecular shuttles,” consisting of functionalized MTs propelled by surface-adhered kinesin motor proteins. It was revealed that overall, the dispersion of such molecular shuttles is comparable with the dispersion of a sample plug transported by electroosmotic flow (Nitta and Hess 2005). However, these discrete level studies of the gathering of micro shuttles were still far from exhibiting synchronization like natural swarming. Therefore, an extensive study on swarming or collective behavior of biomolecular motor systems has been demonstrated that is based on guiding the moving direction of a large number of MTs in a distinctive pattern and energy dissipative active self-assembly process mediated by external stimuli.
Synchronization of biomolecular motor-driven filaments into swarming
Swarming or collective behavior is the most common spectacular manifestation of coordinated behavior in nature that can be described by some basic rules. For instance, from the observation of the fish school, ant colonies, bird flocks, etc., some basic rules were proposed that govern the swarming of the living beings. The major rules to synchronize the similar units are the following: (1) they should be moving with a nearly constant absolute velocity and can change their direction, (2) they interact within a specific range by changing their direction of motion in a way involving an effective alignment (Vicsek and Zafeiris 2012). Nevertheless, the rules are applicable not only for living beings, but also for the explanation of active matter of living and artificial interacting units (Narayan et al. 2007; Schaller et al. 2010). Swarming arising from the cytoskeletal filaments through the interaction of attractive and dispersive forces, which was extensively studied over the past decade, was developed based on these basic rules of collective motion. In response to engineered interactions, chemical interactions, induced forces, or confinement, the swarm of filaments can exhibit self-assembly, bundling, and collective movement into different patterns. Phase transitions from an isotropic phase to a nematic phase results from the steric interactions between filaments, which can be tuned by varying the filament length and density (Butt et al. 2010; Kim et al. 2018). The coexistence of nematic and polar states was demonstrated exclusively by the active motion of filaments. By creating a boundary, the organization of MTs filaments driven by kinesin can be tuned depending on the shape and size of the confinement (Islam et al. 2017). Such pattern formation in a confined space helps us to understand the self-organization of biomolecules like the orientation of cytoskeletal filaments during mitosis in cell division. Moreover, dynamic boundary conditions were created by applying mechanical stimuli, which helped to realize the insight into the rich dynamics in self-organization of MTs (Inoue et al. 2016, 2019). Alongside understanding the properties of biomolecular engine MT-kinesin as an active matter, realizing the energy dissipative discrete swarm pattern formation has also been getting a wide platform to study for the advancements of their nanotechnological applications. In order to achieve this, local interactions between a large number of active MT filaments were regulated, ranging from weak to strong, induced by cross-linking agents. Applying methylcellulose in the solution, weak interactions between the MT filaments can be induced from the resultant depletion forces, depending on the concentration of the depletant agent and the density of filaments (Inoue et al. 2015; Saito et al. 2017). A wide range of phase transitions from single filaments to different swarm patterns was obtained by strong interactions between filaments, induced by cross-linking of actin filaments by fascin (Takatsuki et al. 2014), whereas biotinylated MTs by streptavidin (Bachand et al. 2005; Hess et al. 2005) lead to the formation of long-lived patterns (Idan et al. 2011; Luria et al. 2011; Lam et al. 2014; Wada et al. 2014, 2015; Ito et al. 2016; VanDelinder et al. 2016; Martinez et al. 2019). By using this method, a wide variety of assembled structures was obtained, e.g., bundle, network, and ring-shaped structures, that differs in size or shape (Kawamura et al. 2008, 2009, 2010; Liu and Bachand 2013). It was found that short and stiffer MTs favored the production of linear bundles, where the size of the bundles depends on the initial density of MTs. On the other hand, MT spools assembled from longer and flexible MTs are an example of such non-equilibrium structures, capable of storing bending energies on the order of 105 kT (Lam et al. 2014). The phase diagram of ordered structures by changing the experimental conditions, such as the density of MTs and the ratio of streptavidin/biotin, was also thoroughly investigated (Tamura et al. 2011). Despite extensive studies of swarming of biomolecular motor systems that were effective for understanding their synchronized behavior, still, there are some major challenges in building them up into complex bio- and nano-devices with emergent functions. For instance, control over their collective behavior in a programmable way has been the earnest interest to open a considerable scope for synchronized swarming of biomolecular motor system with computing properties.
Swarming of biomolecular engines programmed by a biomolecular computer
Not only understanding the collective phenomena of cytoskeletal filaments, but also making the resultant swarm patterns sustainable in an engineered system with their emergent functions is very essential to construct molecular devices and micro-robots. However, to construct molecular swarm robots, utilizing the highly efficient biomolecular engines is a major challenge. The limitation lies in programming the synchronous operation among the self-propelled MTs to organize them into complex structures with the distinct division of labor, robustness, and flexibility reminiscent of nature. For instance, to address the challenge, recently, a system has been established where millions of self-propelled MTs on a kinesin-coated surface were used as similar interacting units that were modified by the biomolecular processor DNA (Keya et al. 2018b). The universal interface DNA can act as a crosslinker of motile MTs and program their interactions through their high specificity, selectivity, and reversibility features (Simmel et al. 2019). To control the interaction, at first, interacting units were prepared by attaching individual DNA strands to the MTs as receptors (r-DNAs) through a simple and non-invasive copper (Cu)-free click reaction (Früh et al. 2012). DNA crosslinker or linker DNA (l-DNA) that is complementary to the receptors was added in the in vitro motility assay system, which can pair up DNA-tethered MTs while moving on the kinesin-coated surface in the presence of ATP. Depending on the physical properties of MTs such as length and flexural rigidity, the swarms can exhibit translational and rotational modes of motion. Upon addition of another single-strand DNA, programmed to dissociate the swarms, the MT swarms disappeared quickly and reversed to the single state again (Fig. 2a, b). The quantity of swarm formation was estimated according to the following Eq. 1 (Keya et al. 2018b).
| 1 |
where R(t) is the association ratio at a given time; t was determined by counting the number of single MTs manually and dividing the number at time t associated by the number present initially (t = 0) with
- N (0)
initial number of single MTs,
- N (t)
number of single MTs after time t.
Fig. 2.
Swarming of molecular robots based on biomolecular motor system and DNA processor. a Schematic illustration of the association of r-DNA-conjugated red and green MTs by l-DNA and their dissociation by d-DNA via extraction of l-DNA. b Time-lapse fluorescence microscopy images showing the formation of a circular swarm from flexible red and green MTs (upper part) and dissociation of a swarm group with circular motion triggered by the d-DNA input signal (lower part). Change of the association ratio of MTs over time upon addition of l-DNA and d-DNA. c Orthogonal control of swarming of MTs. Schematic representation and fluorescence microscopy image of MTs with different rigidity. The flexible MTs (red) were conjugated with one set of r-DNAs, while the rigid MTs (green) were conjugated with another set of r-DNAs. Swarms with translational and circular motions were simultaneously formed in response to the introduction of one or both input l-DNA signals. d Visible light irradiation forming translational swarm and dissociation of them to single MTs upon UV light irradiation for rigid MTs (upper part) and flexible MTs (lower part). Changes in the association ratio upon repeated irradiation by visible and UV light. Scale bar, 20 μm. Reproduced with permission from Keya et al. (2018b)
The increase in association ratio of MTs with time in presence of l-DNA and then decrease of ratio in presence of dissociation DNA (d-DNA) with time indicates the correct response of DNA programming in the biomolecular motor system (Fig. 2b). Swarming of MTs by using DNA hybridization has allowed the design of different logic gate operations. Designing different simple logic gates, such as YES, AND, and OR gates, swarming of MTs was successfully programmed to a certain extent. Programming of swarming of MTs through bio-computation makes it more reliable in terms of speed and correct combination through specific DNA interaction rather than parallel computation using network of channels (Nakagaki et al. 2000; Nicolau et al. 2006, 2016). Such simple logic-gated programming of swarms allowed to exhibit complex orthogonal behavior with the translational and circular mode of swarming motion through the molecular recognition process (Fig. 2c). Further, a light switching azobenzene unit in DNA strands was incorporated as a sensor where MT swarming was repeatedly regulated by photoirradiation. Cis-trans isomerization of the azobenzene in response to irradiation of visible or ultraviolet light allows switch on/off behavior of swarming up to three consecutive cycles for both rigid and flexible MTs (Fig. 2d). Reversible regulation of MT swarming by inserting the photosensitive sensor enhanced the flexibility and robustness of the system to give the natural swarming behavior. It allowed repeated swarming and reversion back to the solitary state on signaling after performing a specified task, which can facilitate the future applications, such as spatiotemporally controlled nano-transportation.
Control of swarming of DNA-programmed biomolecular engines
Alongside programming with DNA interaction, different physicochemical parameters of biomolecular motors, DNA, and the surrounding environment can play important roles to control the swarming of MTs, which is required for making sustainable molecular swarm robot systems (Keya et al. 2018a). The DNA-based interaction is moderate in strength and specifically controls the interaction between MTs moving in the same direction, unlike the strong streptavidin-biotin crosslinker system, where MTs bind even moving in the opposite direction and results in sticking of MT swarms (Kawamura et al. 2011). The binding affinity of DNA-conjugated MTs promoting the swarm formation depends on the direction of motion and collision between MTs. Swarming was observed when the non-complementary r-DNA-modified MTs moved in the same direction, i.e., showed unidirectional motion in the presence of l-DNA, and collide with each other. MTs moving in the opposite direction passed each other without exhibiting swarming even in the presence of crosslinker DNA, which indicates the DNA interaction is moderate enough to form MT polarity selective swarm. Along with the binding interaction of DNA, the driving force of the surface-adhered kinesins is another factor that works behind the active swarming of MTs. The motility of the r-DNA-conjugated MTs is governed by the kinesins, which exert force to propel and self-assemble the MTs into swarms. The relationship between kinesin density on the surface and association ratio of swarming of MTs shows a bell-shaped curve which indicates that, at intermediate concentrations of kinesin, the motility and interaction between MTs are facilitated while lower and higher concentrations are not favorable for swarm formation.
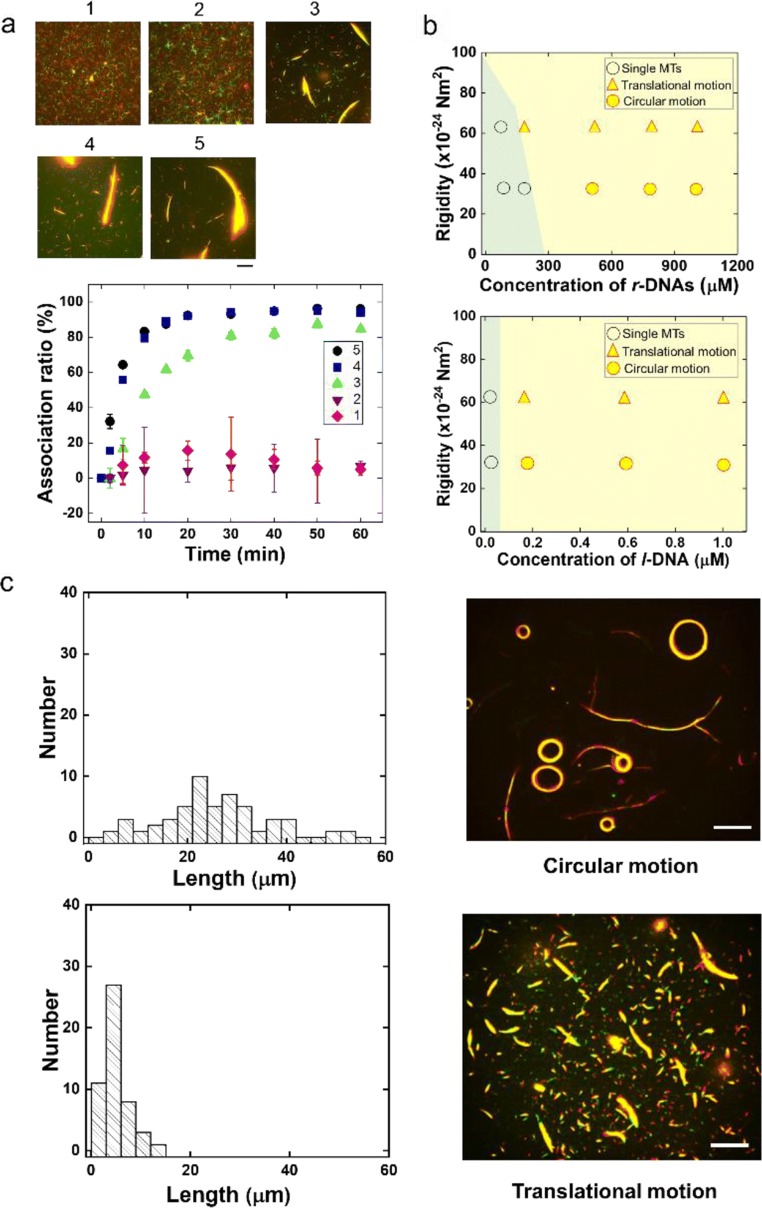
The kinetics of swarm formation also plays a significant role in the precise control of swarming MTs. By using different lengths of the l-DNA sequence to control the melting temperature of DNA hybridization (Tm), the swarming of MTs can be speeded up or slowed down. The kinetics of the swarming of the non-complementary r-DNA-conjugated MTs was investigated in the presence of designed l-DNAs with different lengths. In order to study the effect on the system, non-complementary r-DNA-conjugated MTs were allowed to move on a kinesin-coated surface. Upon addition of l-DNA with complementary base unit repeats shorter than 3, no swarming of MTs was observed. In the case of l-DNA where the number of base unit repeats was 3, the r-DNA-modified MTs started swarming with the l-DNA. For 4 and 5 base unit repeats, on the other hand, the preferential swarming was observed for a large number of MTs (Fig. 3a). Therefore, as the length of l-DNA, i.e., the length of complementary base sequences with r-DNA2, was increased, the swarming rate accordingly increased, which can be easily understood from the change in their association ratio (Fig. 3a).
Fig. 3.
Control of swarming of molecular robots based on biomolecular motor system and DNA processor. a Fluorescence microscopy images showing the extent of swarming of rigid MTs upon varying the length of l-DNA (1–5). The images were captured 60 min after ATP addition. Change in the association ratio of the MTs with time in the presence of l-DNA with different lengths. Scale bar, 20 μm. b A phase diagram showing the effect of the rigidity of MTs and the concentration of r-DNAs on the swarming mode of the MTs (upper part) and the concentration of l-DNA on the swarming mode of the MTs (lower part). Rigidity values used for flexible MTs and rigid MTs were 34 × 10−24 Nm2 and 62 × 10−24 Nm2, respectively (Mickey and Howard 1995). c Histograms of the length of r-DNA-conjugated MTs just after their preparation. The fluorescence microscopy image shows the swarms of MTs that exhibited circular motion. The average lengths of the flexible MTs were 22.2 ± 12.5 μm (n = 50). Histograms of the length of the r-DNA-conjugated MTs after shear treatment (ten times). The fluorescence microscopy image shows the swarms of MTs that exhibited translational motion. The length of the flexible MTs was 4.7 ± 2.5 μm. Scale bar, 50 μm. Reproduced with permission from Keya et al. (2018a)
The phase change and corresponding phase diagram were depicted depending on the concentration of r-DNAs and l-DNA. Both for translational and rotational swarm formation, the phases were increased by increasing the concentration of receptor and crosslinker DNAs (Fig. 3b). However, alongside the aforementioned parameters, the density of MTs is also an important factor that can precisely control the phase of MT swarm formation. The higher binding affinity of dissociation DNA (d-DNA) with l-DNA resulting from high Tm facilitates the dissociation of swarming which also can be tuned by changing the concentration of d-DNA. Alongside different parameters of DNA, the physical properties of MTs are also important in regulating the phase transition of swarming. By changing the length of MTs from longer to shorter ones, the swarm can be transformed from circular to translational mode of motion (Fig. 3c) (Jeune-Smith and Hess 2010; Wada et al. 2015). Thus, by tuning the parameters, a new concept can be introduced, not only to synchronize biomolecular motors into swarms, but also to control the synchronization of swarms of millions of bio-engines to develop micro-scale devices and swarm robots.
Conclusion and future perspectives
The recent studies on the synchronization of the biomolecular motor system will enable the precise designing of the highly computed swarming of self-propelled biomolecular motor systems (Kassem et al. 2017; Sato et al. 2017; Matsuda et al. 2019). Moreover, the observations point towards a way of greatly improving the selectivity of many nanotechnological devices and robotic systems, such as active biosensors, sequential signaling, adaptive actuators, or analyte concentrators by optimization of these systems (Kumar et al. 2012; Jia et al. 2015; Chaudhuri et al. 2017). However, the lifetime of the system is limited by degradation of MTs from the kinesin-coated surface due to mechanical activity, reduced density of kinesin, or photodegradation (Dumont et al. 2015; Keya et al. 2017). Also, detachment of MTs with time due to thermal denaturation of kinesins shortens the lifetime of the system (Kawaguchi et al. 2008; Munmun et al. 2020). To make the system sustainable, efforts are underway to prevent the degradation or loss of MTs in the system by using reactive oxygen species (ROS)-free environment, and osmolytes (Kabir et al. 2011; Islam et al. 2016; Bachand et al. 2018; Munmun et al. 2020). In the future, this knowledge would expand potential applications of biomolecular motors with precisely controlled synchronization, which may ultimately benefit molecular robotics (Hagiya et al. 2014; Saper and Hess 2019).
Funding information
This study was financially supported by the Grant-in-Aid for Scientific Research on Innovative Areas “Molecular Engine” (No. 18H05423) and a Grant-in-Aid for Scientific Research (A) (Grant No. 18H03673) from JSPS.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
In case of using tubulin protein from pig brain, all applicable institutional guidelines for the care and use of animals were followed.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agarwal A, Hess H. Biomolecular motors at the intersection of nanotechnology and polymer science. Prog Polym Sci. 2010;35:252–277. [Google Scholar]
- Agarwal A, Katira P, Hess H. Millisecond curing time of a molecular adhesive causes velocity-dependent cargo-loading of molecular shuttles. Nano Lett. 2009;9:1170–1175. doi: 10.1021/nl803831y. [DOI] [PubMed] [Google Scholar]
- Astumian RD. How molecular motors work–insights from the molecular machinist’s toolbox: the Nobel prize in Chemistry 2016. Chem Sci. 2017;8:840–845. doi: 10.1039/c6sc04806d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachand GD, Rivera SB, Boal AK, et al. Assembly and transport of nanocrystal CdSe quantum dot nanocomposites using microtubules and kinesin motor proteins. Nano Lett. 2004;4:817–821. [Google Scholar]
- Bachand M, Trent AM, Bunker BC, Bachand GD. Physical factors affecting kinesin-based transport of synthetic nanoparticle cargo. J Nanosci Nanotechnol. 2005;5:718–722. doi: 10.1166/jnn.2005.112. [DOI] [PubMed] [Google Scholar]
- Bachand GD, Bouxsein NF, VanDelinder V, Bachand M. Biomolecular motors in nanoscale materials, devices, and systems. Wiley Interdiscip Rev Nanomedicine Nanobiotechnology. 2014;6:163–177. doi: 10.1002/wnan.1252. [DOI] [PubMed] [Google Scholar]
- Bachand GD, Jain R, Ko R, et al. Inhibition of microtubule depolymerization by osmolytes. Biomacromolecules. 2018;19:2401–2408. doi: 10.1021/acs.biomac.7b01799. [DOI] [PubMed] [Google Scholar]
- Böhm KJ, Stracke R, Mühlig P, Unger E. Motor protein-driven unidirectional transport of micrometer-sized cargoes across isopolar microtubule arrays. Nanotechnology. 2001;12:238. [Google Scholar]
- Bonabeau E, Dorigo M, Marco D de RDF et al (1999) Swarm intelligence: from natural to artificial systems. Oxford University Press, Oxford
- Butt T, Mufti T, Humayun A, et al. Myosin motors drive long range alignment of actin filaments. J Biol Chem. 2010;285:4964–4974. doi: 10.1074/jbc.M109.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S, Korten T, Korten S, et al. Label-free detection of microvesicles and proteins by the bundling of gliding microtubules. Nano Lett. 2017;18:117–123. doi: 10.1021/acs.nanolett.7b03619. [DOI] [PubMed] [Google Scholar]
- Chrétien D, Metoz F, Verde F, et al. Lattice defects in microtubules: protofilament numbers vary within individual microtubules. J Cell Biol. 1992;117:1031–1040. doi: 10.1083/jcb.117.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabik P, Gusarov S, Kovalenko A. Microtubule stability studied by three-dimensional molecular theory of solvation. Biophys J. 2007;92:394–403. doi: 10.1529/biophysj.106.089987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont ELP, Do C, Hess H. Molecular wear of microtubules propelled by surface-adhered kinesins. Nat Nanotechnol. 2015;10:166. doi: 10.1038/nnano.2014.334. [DOI] [PubMed] [Google Scholar]
- Erbas-Cakmak S, Leigh DA, McTernan CT, Nussbaumer AL. Artificial molecular machines. Chem Rev. 2015;115:10081–10206. doi: 10.1021/acs.chemrev.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- Früh SM, Steuerwald D, Simon U, Vogel V. Covalent cargo loading to molecular shuttles via copper-free “click chemistry”. Biomacromolecules. 2012;13:3908–3911. doi: 10.1021/bm301437c. [DOI] [PubMed] [Google Scholar]
- Gell C, Bormuth V, Brouhard GJ, et al (2010) Microtubule dynamics reconstituted in vitro and imaged by single-molecule fluorescence microscopy. In: Methods in cell biology. Elsevier 95:221–245 [DOI] [PubMed]
- Hagiya M, Konagaya A, Kobayashi S, et al. Molecular robots with sensors and intelligence. Acc Chem Res. 2014;47:1681–1690. doi: 10.1021/ar400318d. [DOI] [PubMed] [Google Scholar]
- Harold FM (2003) Way of the cell: molecules, organisms, and the order of life. Oxford University Press, Oxford
- Hess H. Self-assembly driven by molecular motors. Soft Matter. 2006;2:669–677. doi: 10.1039/b518281f. [DOI] [PubMed] [Google Scholar]
- Hess H. Engineering applications of biomolecular motors. Annu Rev Biomed Eng. 2011;13:429–450. doi: 10.1146/annurev-bioeng-071910-124644. [DOI] [PubMed] [Google Scholar]
- Hess H, Bachand GD. Biomolecular motors. Mater Today. 2005;8:22–29. [Google Scholar]
- Hess H, Vogel V. Molecular shuttles based on motor proteins: active transport in synthetic environments. Rev Mol Biotechnol. 2001;82:67–85. doi: 10.1016/s1389-0352(01)00029-0. [DOI] [PubMed] [Google Scholar]
- Hess H, Clemmens J, Howard J, Vogel V. Surface imaging by self-propelled nanoscale probes. Nano Lett. 2002;2:113–116. [Google Scholar]
- Hess H, Howard J, Vogel V. A piconewton forcemeter assembled from microtubules and kinesins. Nano Lett. 2002;2:1113–1115. [Google Scholar]
- Hess H, Bachand GD, Vogel V. Powering nanodevices with biomolecular motors. Chem Eur J. 2004;10:2110–2116. doi: 10.1002/chem.200305712. [DOI] [PubMed] [Google Scholar]
- Hess H, Clemmens J, Brunner C, et al. Molecular self-assembly of “nanowires” and “nanospools” using active transport. Nano Lett. 2005;5:629–633. doi: 10.1021/nl0478427. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Takemura R. Kinesin superfamily proteins and their various functions and dynamics. Exp Cell Res. 2004;301:50–59. doi: 10.1016/j.yexcr.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- Howard J. Mechanics of motor proteins and the cytoskeleton. Sunderland: Sinauer Associates; 2001. [Google Scholar]
- Howard J, Hudspeth AJ, Vale RD. Movement of microtubules by single kinesin molecules. Nature. 1989;342:154. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- Hunt AJ, Gittes F, Howard J. The force exerted by a single kinesin molecule against a viscous load. Biophys J. 1994;67:766–781. doi: 10.1016/S0006-3495(94)80537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idan O, Lam A, Kamcev J, et al. Nanoscale transport enables active self-assembly of millimeter-scale wires. Nano Lett. 2011;12:240–245. doi: 10.1021/nl203450h. [DOI] [PubMed] [Google Scholar]
- Inoue D, Mahmot B, Kabir AMR, et al. Depletion force induced collective motion of microtubules driven by kinesin. Nanoscale. 2015;7:18054–18061. doi: 10.1039/c5nr02213d. [DOI] [PubMed] [Google Scholar]
- Inoue D, Nitta T, Kabir AMR, et al. Sensing surface mechanical deformation using active probes driven by motor proteins. Nat Commun. 2016;7:12557. doi: 10.1038/ncomms12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue D, Gutmann G, Nitta T, et al. Adaptation of patterns of motile filaments under dynamic boundary conditions. ACS Nano. 2019;13:12452–12460. doi: 10.1021/acsnano.9b01450. [DOI] [PubMed] [Google Scholar]
- Islam MS, Kabir AMR, Inoue D, et al. Enhanced dynamic instability of microtubules in a ROS free inert environment. Biophys Chem. 2016;211:1–8. doi: 10.1016/j.bpc.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Islam MS, Kuribayashi-Shigetomi K, Kabir AMR, et al. Role of confinement in the active self-organization of kinesin-driven microtubules. Sensors Actuators B Chem. 2017;247:53–60. [Google Scholar]
- Ito M, Kabir AMR, Islam MS, et al. Mechanical oscillation of dynamic microtubule rings. RSC Adv. 2016;6:69149–69155. [Google Scholar]
- Jeune-Smith Y, Hess H. Engineering the length distribution of microtubules polymerized in vitro. Soft Matter. 2010;6:1778–1784. [Google Scholar]
- Jia Y, Dong W, Feng X, et al. A self-powered kinesin-microtubule system for smart cargo delivery. Nanoscale. 2015;7:82–85. doi: 10.1039/c4nr04454a. [DOI] [PubMed] [Google Scholar]
- Kabir AMR, Inoue D, Kakugo A, et al. Prolongation of the active lifetime of a biomolecular motor for in vitro motility assay by using an inert atmosphere. Langmuir. 2011;27:13659–13668. doi: 10.1021/la202467f. [DOI] [PubMed] [Google Scholar]
- Kassem S, Lee ATL, Leigh DA, et al. Stereodivergent synthesis with a programmable molecular machine. Nature. 2017;549:374. doi: 10.1038/nature23677. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Ishiwata S, Yamashita T. Temperature dependence of the flexural rigidity of single microtubules. Biochem Biophys Res Commun. 2008;366:637–642. doi: 10.1016/j.bbrc.2007.11.162. [DOI] [PubMed] [Google Scholar]
- Kawamura R, Kakugo A, Shikinaka K, et al. Ring-shaped assembly of microtubules shows preferential counterclockwise motion. Biomacromolecules. 2008;9:2277–2282. doi: 10.1021/bm800639w. [DOI] [PubMed] [Google Scholar]
- Kawamura R, Kakugo A, Osada Y, Gong JP. Selective formation of a linear-shaped bundle of microtubules. Langmuir. 2009;26:533–537. doi: 10.1021/la902197f. [DOI] [PubMed] [Google Scholar]
- Kawamura R, Kakugo A, Osada Y, Gong JP. Microtubule bundle formation driven by ATP: the effect of concentrations of kinesin, streptavidin and microtubules. Nanotechnology. 2010;21:145603. doi: 10.1088/0957-4484/21/14/145603. [DOI] [PubMed] [Google Scholar]
- Kawamura R, Kakugo A, Shikinaka K, et al. Formation of motile assembly of microtubules driven by kinesins. Smart Mater Struct. 2011;20:124007. [Google Scholar]
- Keya JJ, Inoue D, Suzuki Y et al (2017) High-resolution imaging of a single gliding protofilament of tubulins by HS-AFM. Sci Rep. 10.1038/s41598-017-06249-1 [DOI] [PMC free article] [PubMed]
- Keya JJ, Kabir AMR, Inoue D, et al (2018a) Control of swarming of molecular robots. Sci Rep. 10.1038/s41598-018-30187-1 [DOI] [PMC free article] [PubMed]
- Keya JJ, Suzuki R, Kabir AMR et al (2018b) DNA-assisted swarm control in a biomolecular motor system. Nat Commun. 10.1038/s41467-017-02778-5 [DOI] [PMC free article] [PubMed]
- Kim K, Yoshinaga N, Bhattacharyya S, et al. Large-scale chirality in an active layer of microtubules and kinesin motor proteins. Soft Matter. 2018;14:3221–3231. doi: 10.1039/c7sm02298k. [DOI] [PubMed] [Google Scholar]
- Kinosita K, Yasuda R, Noji H, et al. F1-ATPase: a rotary motor made of a single molecule. Cell. 1998;93:21–24. doi: 10.1016/s0092-8674(00)81142-3. [DOI] [PubMed] [Google Scholar]
- Kron SJ, Spudich JA. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci. 1986;83:6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, ten Siethoff L, Persson M, et al. Antibodies covalently immobilized on actin filaments for fast myosin driven analyte transport. PLoS One. 2012;7:e46298. doi: 10.1371/journal.pone.0046298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AT, Curschellas C, Krovvidi D, Hess H. Controlling self-assembly of microtubule spools via kinesin motor density. Soft Matter. 2014;10:8731–8736. doi: 10.1039/c4sm01518e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam AT, VanDelinder V, Kabir AMR, et al. Cytoskeletal motor-driven active self-assembly in in vitro systems. Soft Matter. 2016;12:988–997. doi: 10.1039/c5sm02042e. [DOI] [PubMed] [Google Scholar]
- Li H, DeRosier DJ, Nicholson WV, et al. Microtubule structure at 8 Å resolution. Structure. 2002;10:1317–1328. doi: 10.1016/s0969-2126(02)00827-4. [DOI] [PubMed] [Google Scholar]
- Liu H, Bachand GD. Effects of confinement on molecular motor-driven self-assembly of ring structures. Cell Mol Bioeng. 2013;6:98–108. [Google Scholar]
- Luria I, Crenshaw J, Downs M, et al. Microtubule nanospool formation by active self-assembly is not initiated by thermal activation. Soft Matter. 2011;7:3108–3115. [Google Scholar]
- Malcos JL, Hancock WO. Engineering tubulin: microtubule functionalization approaches for nanoscale device applications. Appl Microbiol Biotechnol. 2011;90:1–10. doi: 10.1007/s00253-011-3140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez H, VanDelinder V, Imam ZI, et al. How non-bonding domains affect the active assembly of microtubule spools. Nanoscale. 2019;24:11562–11568. doi: 10.1039/c9nr02059d. [DOI] [PubMed] [Google Scholar]
- Masters Thomas A., Kendrick-Jones John, Buss Folma. The Actin Cytoskeleton. Cham: Springer International Publishing; 2016. Myosins: Domain Organisation, Motor Properties, Physiological Roles and Cellular Functions; pp. 77–122. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Kabir AMR, Akamatsu N, et al. Artificial smooth muscle model composed of hierarchically ordered microtubule asters mediated by DNA origami nanostructures. Nano Lett. 2019;19:3933–3938. doi: 10.1021/acs.nanolett.9b01201. [DOI] [PubMed] [Google Scholar]
- Mickey B, Howard J. Rigidity of microtubules is increased by stabilizing agents. J Cell Biol. 1995;130:909–917. doi: 10.1083/jcb.130.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munmun T, Kabir AMR, Katsumoto Y, et al. Controlling the kinetics of interaction between microtubules and kinesins over a wide temperature range using the deep-sea osmolyte trimethylamine N-oxide. Chem Commun. 2020;56:1187–1190. doi: 10.1039/c9cc09324a. [DOI] [PubMed] [Google Scholar]
- Nakagaki T, Yamada H, Tóth Á. Intelligence: maze-solving by an amoeboid organism. Nature. 2000;407:470. doi: 10.1038/35035159. [DOI] [PubMed] [Google Scholar]
- Narayan V, Ramaswamy S, Menon N. Long-lived giant number fluctuations in a swarming granular nematic. Science. 2007;317:105–108. doi: 10.1126/science.1140414. [DOI] [PubMed] [Google Scholar]
- Nicolau DV, Nicolau DV, Jr, Solana G, et al. Molecular motors-based micro-and nano-biocomputation devices. Microelectron Eng. 2006;83:1582–1588. [Google Scholar]
- Nicolau DV, Lard M, Korten T, et al. Parallel computation with molecular-motor-propelled agents in nanofabricated networks. Proc Natl Acad Sci. 2016;113:2591–2596. doi: 10.1073/pnas.1510825113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hess H. Dispersion in active transport by kinesin-powered molecular shuttles. Nano Lett. 2005;5:1337–1342. doi: 10.1021/nl050586t. [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, Downing KH. Correction: structure of the αβ tubulin dimer by electron crystallography. Nature. 1998;393:191. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- Reck-Peterson SL, Redwine WB, Vale RD, Carter AP. The cytoplasmic dynein transport machinery and its many cargoes. Nat Rev Mol Cell Biol. 2018;19:382. doi: 10.1038/s41580-018-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel C, Gabizon R, Wilson CAM, et al. The heat released during catalytic turnover enhances the diffusion of an enzyme. Nature. 2015;517:227. doi: 10.1038/nature14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury S, Bernecky C, Cramer P. Structural basis of transcription initiation by RNA polymerase II. Nat Rev Mol Cell Biol. 2015;16:129. doi: 10.1038/nrm3952. [DOI] [PubMed] [Google Scholar]
- Saito A, Farhana TI, Kabir AMR, et al. Understanding the emergence of collective motion of microtubules driven by kinesins: role of concentration of microtubules and depletion force. RSC Adv. 2017;7:13191–13197. [Google Scholar]
- Saper G, Hess H. Synthetic systems powered by biological molecular motors. Chem Rev. 2019;120:288–309. doi: 10.1021/acs.chemrev.9b00249. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hiratsuka Y, Kawamata I, et al. Micrometer-sized molecular robot changes its shape in response to signal molecules. Sci Robot. 2017;2:1–10. doi: 10.1126/scirobotics.aal3735. [DOI] [PubMed] [Google Scholar]
- Schaller V, Weber C, Semmrich C, et al. Polar patterns of driven filaments. Nature. 2010;467:73. doi: 10.1038/nature09312. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Vogel V. Molecular shuttles powered by motor proteins: loading and unloading stations for nanocargo integrated into one device. Lab Chip. 2010;10:2195–2198. doi: 10.1039/c005241h. [DOI] [PubMed] [Google Scholar]
- Schnapp BJ, Vale RD, Sheetz MP, Reese TS. Single microtubules from squid axoplasm support bidirectional movement of organelles. Cell. 1985;40:455–462. doi: 10.1016/0092-8674(85)90160-6. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000;407:41. doi: 10.1038/35024000. [DOI] [PubMed] [Google Scholar]
- Sheetz MP, Spudich JA. Movement of myosin-coated structures on actin cables. Cell Motil. 1983;3:485–489. doi: 10.1002/cm.970030515. [DOI] [PubMed] [Google Scholar]
- Simmel FC, Yurke B, Singh HR. Principles and applications of nucleic acid strand displacement reactions. Chem Rev. 2019;119:6326–6369. doi: 10.1021/acs.chemrev.8b00580. [DOI] [PubMed] [Google Scholar]
- Swan MK, Johnson RE, Prakash L, et al. Structural basis of high-fidelity DNA synthesis by yeast DNA polymerase δ. Nat Struct Mol Biol. 2009;16:979. doi: 10.1038/nsmb.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki H, Bengtsson E, Månsson A. Persistence length of fascin-cross-linked actin filament bundles in solution and the in vitro motility assay. Biochim Biophys Acta (BBA)-General Subj. 2014;1840:1933–1942. doi: 10.1016/j.bbagen.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Kawamura R, Shikinaka K, et al. Dynamic self-organization and polymorphism of microtubule assembly through active interactions with kinesin. Soft Matter. 2011;7:5654–5659. [Google Scholar]
- Tucker R, Katira P, Hess H. Herding nanotransporters: localized activation via release and sequestration of control molecules. Nano Lett. 2008;8:221–226. doi: 10.1021/nl072516n. [DOI] [PubMed] [Google Scholar]
- VanDelinder V, Brener S, Bachand GD. Mechanisms underlying the active self-assembly of microtubule rings and spools. Biomacromolecules. 2016;17:1048–1056. doi: 10.1021/acs.biomac.5b01684. [DOI] [PubMed] [Google Scholar]
- Vicsek T, Zafeiris A. Collective motion. Phys Rep. 2012;517:71–140. [Google Scholar]
- Visscher K, Schnitzer MJ, Block SM. Single kinesin molecules studied with a molecular force clamp. Nature. 1999;400:184. doi: 10.1038/22146. [DOI] [PubMed] [Google Scholar]
- Wada Shoki, Kabir Arif Md. Rashedul, Kawamura Ryuzo, Ito Masaki, Inoue Daisuke, Sada Kazuki, Kakugo Akira. Controlling the Bias of Rotational Motion of Ring-Shaped Microtubule Assembly. Biomacromolecules. 2014;16(1):374–378. doi: 10.1021/bm501573v. [DOI] [PubMed] [Google Scholar]
- Wada S, Kabir AMR, Ito M, et al. Effect of length and rigidity of microtubules on the size of ring-shaped assemblies obtained through active self-organization. Soft Matter. 2015;11:1151–1157. doi: 10.1039/c4sm02292k. [DOI] [PubMed] [Google Scholar]
- Yasuda R, Noji H, Kinosita K, Jr, Yoshida M. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120 steps. Cell. 1998;93:1117–1124. doi: 10.1016/s0092-8674(00)81456-7. [DOI] [PubMed] [Google Scholar]