Abstract
Soil microbial biomass can reach its annual maximum pool size beneath the winter snowpack and is known to decline abruptly following snowmelt in seasonally snow-covered ecosystems. Observed differences in winter versus summer microbial taxonomic composition also suggests that phylogenetically conserved traits may permit winter- versus summer-adapted microorganisms to occupy distinct niches. In this study, we sought to identify archaea, bacteria, and fungi that are associated with the soil microbial bloom overwinter and the subsequent biomass collapse following snowmelt at a high-altitude watershed in central Colorado, United States. Archaea, bacteria, and fungi were categorized into three life strategies (Winter-Adapted, Snowmelt-Specialist, Spring-Adapted) based upon changes in abundance during winter, the snowmelt period, and after snowmelt in spring. We calculated indices of phylogenetic relatedness (archaea and bacteria) or assigned functional attributes (fungi) to organisms within life strategies to infer whether phylogenetically conserved traits differentiate Winter-Adapted, Snowmelt-Specialist, and Spring-Adapted groups. We observed that the soil microbial bloom was correlated in time with a pulse of snowmelt infiltration, which commenced 65 days prior to soils becoming snow-free. A pulse of nitrogen (N, as nitrate) occurred after snowmelt, along with a collapse in the microbial biomass pool size, and an increased abundance of nitrifying archaea and bacteria (e.g., Thaumarchaeota, Nitrospirae). Winter- and Spring-Adapted archaea and bacteria were phylogenetically clustered, suggesting that phylogenetically conserved traits allow Winter- and Spring-Adapted archaea and bacteria to occupy distinct niches. In contrast, Snowmelt-Specialist archaea and bacteria were phylogenetically overdispersed, suggesting that the key mechanism(s) of the microbial biomass crash are likely to be density-dependent (e.g., trophic interactions, competitive exclusion) and affect organisms across a broad phylogenetic spectrum. Saprotrophic fungi were the dominant functional group across fungal life strategies, however, ectomycorrhizal fungi experienced a large increase in abundance in spring. If well-coupled plant-mycorrhizal phenology currently buffers ecosystem N losses in spring, then changes in snowmelt timing may alter ecosystem N retention potential. Overall, we observed that snowmelt separates three distinct soil niches that are occupied by ecologically distinct groups of microorganisms. This ecological differentiation is of biogeochemical importance, particularly with respect to the mobilization of nitrogen during winter, before and after snowmelt.
Keywords: snowmelt, watershed, life history strategy, soil nitrogen, soil archaea and bacteria, soil fungi
Introduction
Snowmelt is a hydrologic event that exerts significant influence on annual water and nitrogen (N) export in seasonally snow-covered, mountainous catchments (Bales et al., 2006). Historically, the timing of snowmelt has coincided with a suite of environmental conditions that characterize the transition from winter to spring, including rising air temperature, longer days having higher photosynthetically active radiation, and greater soil moisture availability (Marks and Dozier, 1992; Harpold and Molotch, 2015; Musselman et al., 2017). These abiotic cues initiate phenological transitions between winter and spring metabolic states for both plants and soil microbial communities (Richardson et al., 2006; Inouye, 2008; Miller-Rushing et al., 2010; Contosta et al., 2016; Ladwig et al., 2016; Thackeray et al., 2016). As a consequence, snowmelt is found to be a critical period that influences nutrient mobilization, assimilation, and retention in terrestrial ecosystems (Brooks et al., 1998; Brooks and Williams, 1999; Grogan and Jonasson, 2003; Kielland et al., 2006; Campbell et al., 2007). Rising global air temperature has led to reductions in winter snowpack extent, earlier snowmelt dates, and altered growing season length in many mountainous catchments (Mote et al., 2005; Steltzer and Post, 2009; Harte et al., 2015; Sloat et al., 2015; Bormann et al., 2018; Hock et al., 2019; Prevéy et al., 2019). The ecological consequences of such environmental changes, however, are not well understood (Ernakovich et al., 2014).
Soil microbial biomass production beneath the winter snowpack can be significant, and in some ecosystems, microbial biomass may reach its annual maximum pool size beneath the winter snowpack (Brooks et al., 1998; Lipson et al., 1999; Grogan and Jonasson, 2003; Edwards et al., 2006; Larsen et al., 2007; Buckeridge and Grogan, 2010; Buckeridge et al., 2013). The microbial bloom in soil during winter immobilizes N within microbial biomass (Brooks et al., 1998). An abrupt collapse in the microbial biomass pool size after snowmelt results in the subsequent release of N from the microbial biomass pool (Lipson et al., 1999; Buckeridge et al., 2010; Isobe et al., 2018). Numerous mechanisms have been hypothesized to induce the microbial biomass crash, including substrate depletion and starvation of the winter community, cell lysis due to soil freeze-thaw events, sudden changes in the osmotic environment with snowmelt, grazing by protozoa and mesofauna, and mortality and replacement of winter-adapted psychrophiles by summer-adapted mesophiles (Jefferies et al., 2010). A better understanding of the factors that promote microbial biomass production under-snow and the crash after snowmelt is necessary because the flux of N released from microbial biomass after snowmelt can be the largest annual pulse of soil N in some ecosystems (Grogan and Jonasson, 2003; Schmidt et al., 2007).
In addition to differences in the size of the microbial biomass pool across seasons, bacterial and fungal community composition are also known to differ taxonomically in winter compared to summer (Schadt et al., 2003; Lipson and Schmidt, 2004; Aanderud et al., 2013; Isobe et al., 2018), although this has typically been reported at coarse (e.g., phylum) levels of taxonomic resolution. For example, Acidobacteria, Verrucomicrobia, and Bacteriodetes co-exist in alpine soils, but strong successional differences in relative abundance are observed among these phyla during winter and summer (Schmidt et al., 2007). Because two species competing for the same resources cannot persist together indefinitely, such co-occurrence and succession implies that these phyla do not occupy the same niche or partition resources in space or time (Hutchinson, 1957). Differences in growth rates and biomass yields among winter and summer bacterial isolates also indicate that phenotypic traits with physiological trade-offs may differentiate winter- and summer-adapted microorganisms (Lipson et al., 2009).
More broadly, an organism’s fitness across varying environmental gradients should be reflected by its abundance in the environment and constrained by a suite of underlying physiological traits, collectively referred to as an organism’s life strategy (Schimel et al., 2007; Placella et al., 2012; Evans et al., 2014; Ho et al., 2017). If such traits are adaptive and phylogenetically conserved (Webb et al., 2002), then quantifying the phylogenetic relatedness of organisms grouped into life strategies may provide insights into the community assembly processes (e.g., niche partitioning) that underlie the microbial bloom beneath the winter snowpack and subsequent biomass collapse after snowmelt.
Within this framework we addressed the following questions: (1) are closely related microorganisms responsible for the overwinter microbial biomass bloom, or is the phenomenon widespread and characteristic among distantly related taxa? (2) what is the mechanism responsible for the microbial biomass crash following snowmelt and how widespread is the snowmelt decline among distantly related bacterial, archaeal, and fungal taxa? (3) are there identifiable temporal abundance patterns that differentiate microorganisms into life strategies that can be used to infer an organism’s snowmelt niche?
To address these questions, we sampled soils along a 200-m upland hillslope-to-riparian floodplain transect in a mountainous catchment at the East River Watershed, Colorado, United States (Hubbard et al., 2018). Soil samples were collected over a time-course that began after plant senescence in autumn, through snow accumulation during winter, during snowmelt, and through plant green-up in spring and early summer. We monitored soil temperature and moisture and quantified the dynamics of the soil microbial biomass pool as well as extractable N in soil that was measured as extractable soil nitrate (). Bacterial, archaeal, and fungal species relative abundances were also measured over this seasonal time-course. Here, we show that soil temperature and soil moisture during winter are controlled by winter snowpack dynamics and that snowmelt induces rapid changes in the soil abiotic environment. In addition, snowmelt triggered marked increases in the size of the microbial biomass pool, which also reduced soil N availability. Finally, we show that specific bacterial, archaeal, and fungal taxa are associated with the rise and fall of microbial biomass overwinter and that the abundance responses can be used to differentiate taxa into three distinct life strategies separated in time by the snowmelt event.
Materials and Methods
The Upper East River Watershed is located in Gunnison County, CO, United States in the West Elk Mountains near the towns of Crested Butte and Gothic (38°57.5′ N, 106°59.3′ W) and is home to the Rocky Mountain Biological Laboratory. Elevation at the East River watershed spans from 2750 to 4000 m. The climate at East River is continental and characterized by periods of persistent snow cover in winter lasting 4 to 6 months (e.g., November through May) followed by an arid summer with intermittent, monsoonal rain events that occur from July through September. The lowest daily air temperatures typically occur in January (−14 ± 3°C), whereas the highest daily air temperatures typically occur in July (23.5 ± 3°C). The average summer air temperature has increased by 0.5 ± 0.1°C per decade since 1974 (CaraDonna and Inouye, 2015). The average annual precipitation is approximately 1200 cm, with the majority (>80%) falling as snowfall during winter (Harte and Shaw, 1995; Hubbard et al., 2018). The maximum annual snow depth between 1974 and 2017 was 465 cm and the date of snowmelt in spring has advanced by 3.5 ± 2 days per decade since 1974 (Iler et al., 2013).
We sampled a 200-m transect that transitioned from an upland hillslope (hereafter referred to as Hillslope) to a riparian floodplain (hereafter, Floodplain) adjacent to the main stem of the East River (elevation ∼2760 to 2775 m). We chose this transect because these ecosystem types are representative of dominant land cover adjacent to the East River. Vegetation in the Hillslope is a mix of perennial bunchgrasses (e.g., Festuca arizonica), forbs (e.g., Lupinus spp., Potentilla gracilis, Veratrum californicum), and shrubs (Artemisia tridentata), whereas plant communities in the Floodplain are dominated by dwarf birch (Betula grandulosa) and mountain willow (Salix monticola, Falco et al., 2019).
Soil temperature, soil volumetric water content, and water potential were measured continuously starting in October 2016 at three locations on the Hillslope and at three locations on the Floodplain. Soil temperature was measured continuously at 6 and 9 cm below the soil surface (sensor model RT-1, 5TE and MPS6, METER). Soil volumetric water content was measured at 9 cm using a time-domain reflectometry probe (model 5TE, METER Group). Soil water potential was measured at 17 cm using MPS6 from METER. We measured snow depth during winter with a marked snow pole or meter stick on the dates of soil sampling that had snow cover at the site.
Soil Sampling and Field Processing
Soil samples were collected on four dates to characterize the soil microbial biomass carbon (C) pool, extractable soil , and microbial community composition, starting first in autumn after plant senescence (September 12, 2016), at peak winter snow depth (March 7, 2017), during the snowmelt period (May 9, 2017), and following the complete loss of snow and the start of the plant growing season (June 9, 2017). During snow-free times of the year, soils were collected using a 4 cm diameter soil bulk density corer at 12 plots at the Hillslope and at 3 plots at the Floodplain. The six plots on the Hillslope were encompassed by a representative 80 m × 100 m area that was approximately 100 m upslope from the edge of the Floodplain. Each plot on the Hillslope was 20 m × 20 m and separated by at least 2.5 m. The Hillslope plots were arranged in three pairs that ran parallel to the river. The paired plots will serve as experimental blocks for a future field experiment. The three plots in the Floodplain were similarly 20 m × 20 m and arranged in a 100 m × 30 m transect that was perpendicular to the river and spanned the area from the toeslope-floodplain transition to near the edge of the river. Soil cores were subsampled and split into three discrete depth increments; 0 to 5 cm, 5 to 15 cm, and 15 cm + below the soil surface. These relatively shallow soils are best described as A-horizons at the Hillslope and Floodplain locations. Soil horizon development and differentiation is greatly reduced because of substantial bioturbation from burrowing mammals on the Hillslope and periodic sediment deposition during spring flooding events in the Floodplain.
During periods of winter snow cover (i.e., March and May 2017), snow-pits were dug down to the soil surface at three locations on the Hillslope and one location in the Floodplain in order to sample soils from beneath the snowpack. In each snow-pit, soils were sampled at two adjacent locations separated by more than 1 m using the soil coring method described above. New snow pits were dug in different locations on the Hillslope and Floodplain during snowmelt in May 2017 to avoid repeatedly sampling the same locations that were sampled at peak winter snow depth in March 2017. Thus, during the snow-free time of the year, we sampled and analyzed 180 total soil samples (2 ecosystem types × 15 Plots × 2 Time Points × 3 Depths) and 48 soils total during winter (4 Snow-pits × 2 Time Points × 3 Depths × 2 replicate cores). A ∼10 g subsample from each soil core at each depth was placed immediately on dry ice in the field, frozen, and archived for archaeal, bacterial and fungal community analysis. The remainder of the soil core was allocated to physical and chemical characterization (described below) and was stored at 4°C until further analysis.
Soil Physical and Chemical Properties
Gravimetric soil moisture content was measured for each sample on each sampling date by determining the mass of water lost during a 48-h incubation at 60°C. Soil pH was determined in a 1:1 w/v slurry in reagent water (18 MΩ resistance; GenPure UltraPure Water System; Thermo Scientific) using an Orion soil pH probe (Thermo Scientific). Total soil organic C and N concentrations and soil δ13C and δ15N were measured using a Costech ECS 4010 elemental analyzer coupled to a Thermo Delta V Plus isotope ratio mass spectrometer at the Center for Isotope Geochemistry at Lawrence Berkeley National Laboratory (Berkeley, CA, United States).
Extractable nitrate () as well as microbial biomass carbon (C) were quantified in 0.5M K2SO4 extracts. A 5 g subsample of each soil (field-moist) was extracted in 25 mL of 0.5M K2SO4 on an orbital shaker table for 60 min, then gravity filtered through pre-leached #42 Whatman filter paper and frozen until further analysis. concentration in the 0.5M K2SO4 extracts was measured colorimetrically using a Versamax microplate spectrophotometer (Molecular Devices) using a modified Greiss reaction protocol (Doane and Horwath, 2003). Microbial biomass carbon (C) was measured using the chloroform-fumigation extraction method (Brookes et al., 1985). A 5-g subsample was fumigated with ethanol-free chloroform for 7 days and then extracted as stated above. Microbial biomass C was estimated as organic C measured in the fumigated extract minus organic C measured in the non-fumigated sample (Brookes et al., 1985). We did not apply an extraction correction to account for incomplete microbial biomass lysis during the fumigation. The concentration of dissolved organic C in the fumigated and non-fumigated samples was quantified using the Mn(III) pyrophosphate oxidation method (Bartlett and Ross, 1988). We applied a universal correction factor to the measured organic C concentrations to account for differences in reaction efficiency across soil types (Giasson et al., 2014). All concentrations were corrected for the field-moist water content of the soil and values are reported based on the oven-dried soil mass used for the extractions.
Illumina Library Preparation and Bioinformatics
Total genomic DNA was extracted in triplicate from each soil sample collected on each date using the DNeasy PowerSoil DNA Extraction Kit (QIAGEN) with the protocol modified to include a 5-min incubation at 65°C prior to bead-beating to increase lysis efficiency. Replicate soil DNA extracts were combined prior to PCR amplification. PCR reaction conditions are summarized in the Supplementary Material (see Supplementary Datasheet S1). Purified PCR products were pooled in equimolar concentrations and sequenced on a single lane for 300-bp paired-end v3 Illumina MiSeq sequencing conducted at the Vincent J. Coates Genomics Sequencing Laboratory at the University of California, Berkeley.
Forward and reverse reads were aligned and paired using usearch [v8.1.1861 (Edgar, 2010)] fastq_mergepairs command (maximum diff = 3). The aligned reads were quality filtered (command fastq_filter with -fastq_trunclen = 230, -fastq_maxee = 0.1), concatenated into a single fasta file, and singletons were removed (command sortbysize with minsize = 2). These filtered sequences were used for operational taxonomic unit (OTU) clustering with the uparse pipeline (Edgar, 2013) setting the OTU cut-off threshold to 97%. Chimeric sequences were filtered with uchime (Edgar et al., 2011) with reference to the ChimeraSlayer database downloaded from http://drive5.com/uchime/gold.fa. OTU abundances across individual samples were calculated by mapping chimera-filtered OTUs against the quality-filtered reads (command usearch_global with -strand plus -id 0.97). Taxonomy was assigned to each OTU by a Naïve Bayes classifier using the assign_taxonomy. py command in QIIME (Wang et al., 2007) with reference to the SILVA database accessed from mothur (Schloss et al., 2009) release 119: https://mothur.org/wiki/Silva_reference_files#Release_119. For phylogenetic inference of bacterial and archaeal OTUs, representative sequences for each bacterial OTU were aligned to a SILVA SEED sliced alignment using the PyNAST algorithm (Caporaso et al., 2010) and archaeal and bacterial phylogeny was inferred using FastTree (Price et al., 2010).
This workflow resulted in 33 archaeal, 18,683 bacterial, and 4,194 fungal OTUs. OTUs were further filtered to include only OTUs that occurred in at least 25% of samples across sampling dates (25 archaeal, 10,555 bacterial, and 3,157 fungal OTUs after filtering). This prevalence filter was applied to decrease the effect of spatial variation during field sampling and to decrease the potential for sequencing artifacts that might affect our temporal analysis (see section “Statistical Analysis”). Fungal OTUs were assigned to functional guilds by referencing annotated databases using the open-source software FUNGuild (Nguyen et al., 2016). FUNGuild assigned functional attributes (e.g., trophic mode, guild) to 1,528 fungal OTUs. Fungal OTUs with ‘unknown’ FUNGuild functional annotations (1,629 OTUs) were also included in subsequent downstream analyses. Raw de-multiplexed sequences have been archived in the NCBI Bioproject database and are publicly available under accession no. PRJNA587134.
Statistical Analysis
All statistical analyses were completed using R v 3.5.2 (R Development Core Team, 2010). Differences in soil chemical properties between locations (Hillslope versus Floodplain) were tested using linear-mixed effect models using the R package ‘nlme’ (Pinheiro et al., 2016). Location was the fixed-effect and Plot was the random-effect in the model. The effect of sampling date on the size of the microbial biomass C pool, extractable soil , and gravimetric water content were also assessed using linear-mixed effect models with Time of Sampling as the fixed-effect and Plot as the random-effect in the model. Differences in bacterial and fungal community composition across sampling dates was determined by permutational multivariate analysis of variance (perMANOVA, permutations n = 999) using the R package ‘vegan’ (Oksanen et al., 2011). Dissimilarity between samples was calculated as Bray–Curtis distances for fungi or weighted-unifrac distances for bacteria and archaea (Bray and Curtis, 1957; Lozupone et al., 2007). Pairwise comparisons of community dissimilarity was tested using the R package ‘mctools’ (Leff, 2017).
We identified OTUs that had a statistically significant change in abundance between at least one of three paired time periods [September to March (i.e., ‘Winter’), March to May (i.e., ‘Snowmelt’), May to June (i.e., ‘Spring’)] by calculating log2foldchanges in relative abundance between time-points using the R package ‘gtools’ (Warnes et al., 2018). A 95% confidence interval for the log2foldchange was derived by applying a formula for error propagation for the product of a ratio,
| (1) |
where meant1,t2 was the mean relative abundance of each OTU across sampling locations on each date and 95% CI t1,t2 was the 95% confidence interval of each OTU on each date.
P-values were calculated from the confidence intervals (Altman and Bland, 2011) and were adjusted for multiple comparisons by the Benjimini and Hochberg false discovery rate (‘fdr’) method using the R package ‘stats.’ Only OTUs with at least one significant (e.g., FDR-adjusted P < 0.05) log2foldchange across time points were included in further analysis (8 archaeal, 4,583 bacterial, and 1,110 fungal OTUs).
Next, we applied agglomerative hierarchical clustering to group OTUs according to differences in relative abundance patterns across sampling time points (Placella et al., 2012; Evans et al., 2014). Distances between clusters were calculated by the average-linkage method. Dendrograms were visualized using ‘ggdendro’ (de Vries and Ripley, 2016) and cut at even height to categorize OTUs into one of three life strategies for archaea and bacteria. Here we define life strategy to be an assortment of physiological traits, selected for by abiotic and biotic factors, that determine an organism’s acquisition of resources, growth and reproduction, and responses to varying environmental gradients (Chagnon et al., 2013; Ho et al., 2017; Malik et al., 2018). In our study, we assume that changes in relative abundance underlie distinct life strategies related to winter snowpack dynamics and reflect each organism’s degree of adaptation and fitness during winter, snowmelt, and spring. For fungi, we observed a fourth life strategy that had high abundance in fall after plant senescence, but did not analyze this group further. There was substantial taxonomic overlap among strategies at all taxonomic ranks in both the Hillslope and Floodplain. Therefore, we interpreted the ecological significance of each life strategy based on the collective characteristics of a subset of OTUs that accounted for a significant portion of the temporal change in abundance or had large differential abundance across sampling dates.
Lastly, we calculated two measures of phylogenetic community relatedness within life strategies for bacteria and archaea, the net relatedness index (NRI) and the nearest taxon index (NTI), using the R package ‘picante’ with a null model based on random taxa shuffling and 1000 permutations (Webb et al., 2002; Kembel et al., 2010). Phylogenetic clustering (positive NRI/NTI) indicates that taxa in a group are more phylogenetically related than expected, compared to a random sampling from the regional species pool. Phylogenetic clustering suggests a group of taxa that are ecologically similar and share a common niche with traits that have been retained through speciation events (i.e., phylogenetic niche conservatism, Crisp and Cook, 2012). On the other hand, phylogenetic overdispersion (negative NRI/NTI) indicates a group of organisms less phylogenetically related than expected, as compared to the regional species pool. Phylogenetic overdispersion can arise from community assembly processes (e.g., trophic interactions, competitive exclusion) that result in a group of ecologically dissimilar taxa with non-overlapping niches (i.e., niche partitioning) or adaptive traits with a broad phylogenetic distribution.
Results
Soil Characteristics and Winter Snowpack Control Over Soil Temperature and Moisture
Average gravimetric soil moisture content and total soil C were greater on the Floodplain compared to the Hillslope (Table 1). Microbial biomass C in the top-soil (0 to 5 cm depth) was also approximately three times greater on the Floodplain compared to the Hillslope, but was comparable between the Hillslope and Floodplain in soils deeper than 5 cm (Table 1). Across all sampling depths, soils on the Hillslope had consistently greater δ13C and δ15N values than soils in the Floodplain (Table 1).
TABLE 1.
Soil properties measured in the Hillslope and Floodplain watershed locations.
|
Hillslope |
Floodplain |
||||
| Depth | Mean | SE | Mean | SE | |
| 0 to 5 cm | Soil moisture | 0.42b | 0.10 | 1.16a | 0.3 |
| Soil pH | 6.7 | 0.1 | 6.8 | 0.2 | |
| Microbial biomass C | 203.7b | 21.6 | 629.4a | 117.5 | |
| Extractable organic C | 99.4 | 10.5 | 101.2 | 29.3 | |
| Total soil organic C | 4.2b | 0.3 | 7.4a | 2.1 | |
| Total soil organic N | 0.42 | 0.01 | 0.58 | 0.14 | |
| Soil δ13CVPDB | −24.3b | 0.1 | −26.6a | 0.5 | |
| Soil δ15NAIR | 4.0a | 0.1 | 2.6b | 0.9 | |
| 5 to 15 cm | Soil moisture | 0.34 | 0.1 | 0.72 | 0.1 |
| Soil pH | 6.7 | 0.1 | 6.9 | 0.1 | |
| Microbial biomass C | 217.2 | 24.8 | 276.8 | 35.7 | |
| Extractable organic C | 85.3 | 15.6 | 80.3 | 25.9 | |
| Total soil organic C | 3.3b | 0.2 | 7.0a | 0.4 | |
| Total soil organic N | 0.35b | 0.01 | 0.53a | 0.02 | |
| Soil δ13CVPDB | −24.3a | 0.1 | −26.4b | 0.2 | |
| Soil δ15NAIR | 4.0a | 0.2 | 2.0b | 1.2 | |
| 15 cm + | Soil moisture | 0.32 | 0.07 | 0.84 | 0.2 |
| Soil pH | 6.6 | 0.1 | 7.0 | 0.1 | |
| Microbial biomass C | 170.5 | 18.2 | 158.3 | 35.4 | |
| Extractable organic C | 61.7 | 7.0 | 127.2 | 35.0 | |
| Total soil organic C | 3.2 | 0.2 | 4.7 | 1.4 | |
| Total soil organic N | 0.34 | 0.01 | 0.57 | 0.08 | |
| Soil δ13CVPDB | −24.0a | 0.1 | −25.6b | 0.5 | |
| Soil δ15NAIR | 4.4a | 0.2 | 1.7b | 0.3 | |
Differences in means among Locations are denoted with different letters and represent statistically significant differences (Tukey post hoc P ≤ 0.05). (Units – Soil moisture (g H2O–1 g soil–1), microbial biomass C and extractable organic C (μg C g soil–1), total soil C & N (weight%), soil δ13C and δ15N are the isotopic composition of carbon and nitrogen in the conventional δ-notation (per mil, ‰) relative to Vienna Pee Dee Belemnite (VPDB) for carbon and relative to air for nitrogen.
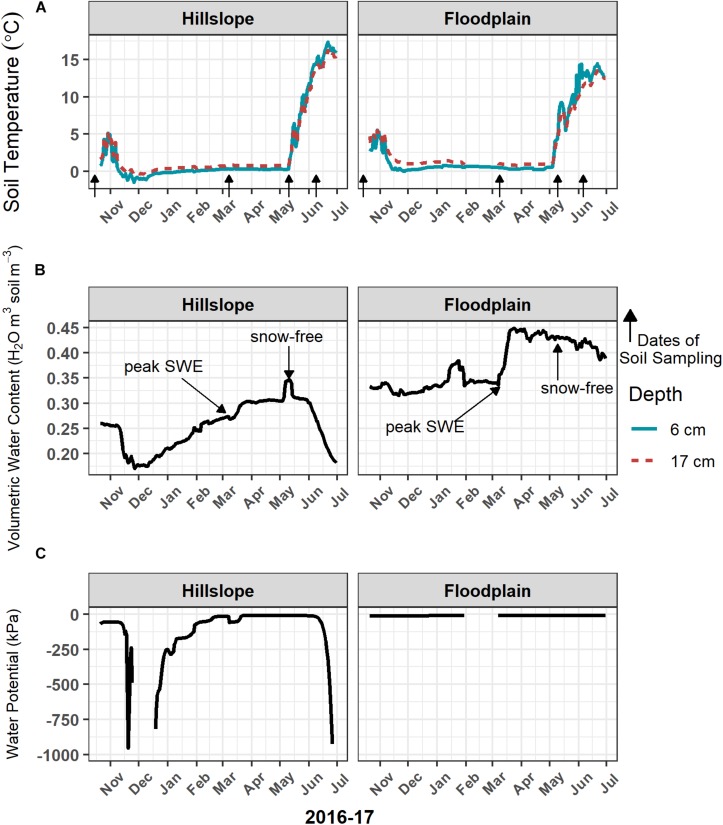
The onset of winter snowpack accumulation, persistence of snow cover, and complete loss of snow (approximately May 15, 2017) resulted in several different soil temperature and moisture regimes between November 2016 and July 2017 (Table 2 and Figure 1). Soil temperature at 6 cm depth was less than 0°C on the Hillslope in early December prior to the development of a persistent snowpack (Figure 1A). Snow accumulation and persistence coincided with a gradual increase in soil temperature to slightly above 0°C from January throughout the remainder of winter. Complete loss of snow led to higher soil temperatures at both the Hillslope and Floodplain (Figure 1A and Table 2) and trends in soil temperature in spring generally tracked trends in air temperature after snowmelt. We did not observe soil freeze-thaw cycles during or after snowmelt, but did observe soil freeze-thaw cycles in the winter time-period (Supplementary Table S1).
TABLE 2.
Snowpack effects on soil temperature and moisture.
|
Winter |
Snowmelt |
Spring |
|||||
| Depth | Mean | SE | Mean | SE | Mean | SE | |
| Hillslope | Snow depth | 144.1 | 4.1 | 15.4 | 3.1 | 0 | 0 |
| Soil T (6 cm) | 0.2c | 0.1 | 0.3b,c | 0.1 | 11.1a | 0.4 | |
| Soil T (17 cm) | 0.7c | 0.1 | 0.7b,c | 0.1 | 10.4a | 0.4 | |
| VWC | 0.22c | 0.01 | 0.30a | 0.01 | 0.27b | 0.01 | |
| Water potential | −721.0c | 85.6 | −19.0a,b | 1.2 | −197.0b | 40.2 | |
| Floodplain | Snow depth | 164.7 | 1.2 | 8.6 | 2.4 | 0 | 0 |
| Soil T (6 cm) | 1.0b | 0.1 | 0.5c | 0.1 | 10.8a | 0.3 | |
| Soil T (17 cm) | 1.9c | 0.1 | 1.0b | 0.1 | 9.6a | 0.3 | |
| VWC | 0.33c | 0.01 | 0.43a,b | 0.01 | 0.42a | 0.01 | |
| Water potential | −12.1c | 0.1 | −8.8a,b | 0.1 | −8.8a | 0.1 | |
Time periods were binned based on snow accumulation and loss as well as regime shifts in temperature and moisture. Winter was operationally defined as 10/21/2016 – 03/07/2017, Snowmelt was 03/08/2017-05/07/2017, and Spring was 05/08/2017-6/30/2017. Means and standard errors were calculated from daily means within a binned time period. Snow depth data were collected at multiple locations within a site (Hillslope and Floodplain) on the dates of soil sampling only. Significant differences in means among time-periods are denoted with different letters (P ≤ 0.05). [Units – snow depth (cm), temperature (°C), VWC-volumetric water content (m3 H2O soil m–3), water potential (kPa)].
FIGURE 1.
Soil temperature (A) at 6 cm and 17 cm below the soil surface remained above 0°C when soils were covered with snow during winter. Loss of snow cover in May 2017 resulted in a rapid increase in soil temperature. The onset of snowmelt in March triggered a large increase in soil volumetric water content (B), as well as soil water potential (C), which lasted through early June 2017. Volumetric water content was measured at 9-cm depth and water potential was measured at 17-cm depth below the soil surface. Arrows indicate the dates of soil sampling.
Soils in winter generally had lower volumetric water content and lower soil water potential compared to soils collected during snowmelt or spring (Table 2). Because over 90% of the winter snowpack was melted between early March and early May 2017, we refer to that time-period as the snowmelt period (Table 2). Snowmelt resulted in an increase in soil volumetric water content and soil water potential (Figures 1B,C and Table 2) with an extended period of soil saturation beginning more than 60 days prior to the complete loss of winter snow. At the Hillslope, soil moisture declined rapidly after snowmelt as plants broke winter dormancy in spring (Figures 1B,C). In contrast, soils on the Floodplain remained saturated long after snowmelt (Figure 1C and Table 2). In summary, the winter period was characterized by deep snow with generally cold and dry soils, the snowmelt period by the rapid loss of the winter snowpack with cold and wet soils, and the spring soil environment was characterized by rapid warming and soil drying (Table 2 and Figures 1A–C).
Microbial Biomass Bloom and Crash With Subsequent Pulse of Soil N
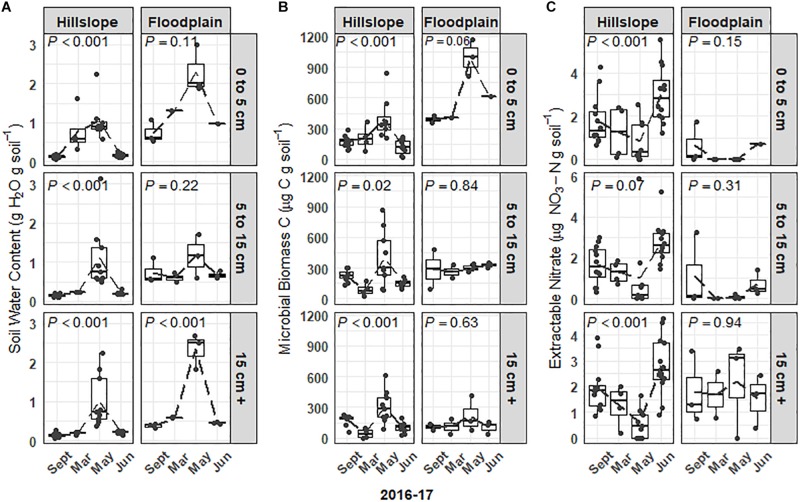
Soil microbial biomass and the extractable soil nitrate () pool responded strongly to variations in snowpack depth, soil temperature, and moisture (Figures 1, 2A–C). For example, soil microbial biomass C increased 2- to 5-fold at all three soil depths (0 to 5 cm, 5 to 15 cm, 15 cm +) during the snowmelt period on the Hillslope (e.g., March to May, Figure 2B) and trends in microbial biomass pool size were well-correlated with trends in soil water content (Figure 2A). Similar to observations for the Hillslope, microbial biomass C in the Floodplain also increased during snowmelt in the shallowest soils (e.g., 0 to 5 cm, Figure 2B), however, this was not observed in soils sampled more than 5 cm below the soil surface. Soil microbial biomass decreased more than twofold across all depths after snowmelt (between May and June) on the Hillslope (Figure 2B), which coincided with a 3- to 5-fold increase of extractable soil (Figure 2C). Although microbial biomass also collapsed on the Floodplain in topsoils after snowmelt (Figure 2B), in contrast to the Hillslope, we did not observe an increase in the soil pool size after snowmelt in the Floodplain (Figure 2C).
FIGURE 2.
Soil water content (A) and soil microbial biomass (B) in the Hillslope and Floodplain. A pulse of extractable soil nitrate (C) was observed in the Hillslope after snowmelt in June 2017. P-values are the outcome of mixed-models testing for the effect of time of sampling on soil water content, microbial biomass, and extractable nitrate.
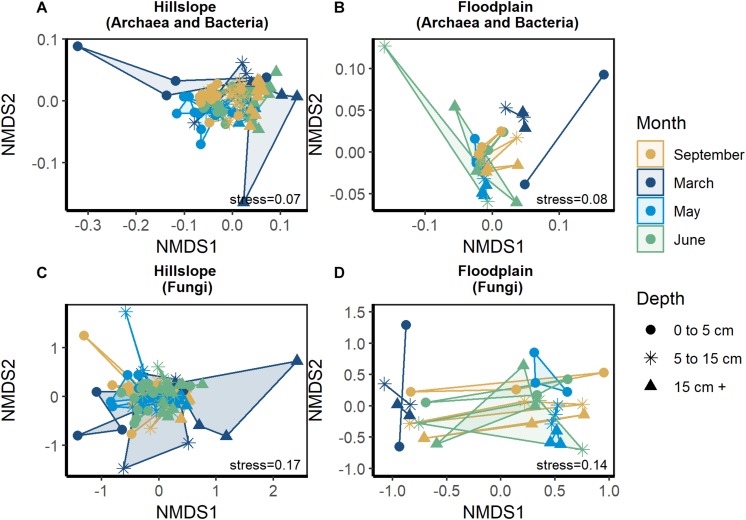
Similar to changes in the size of soil microbial biomass pool, archaeal and bacterial (Figures 3A,B) as well as fungal community structure (Figures 3C,D) varied significantly during winter, snowmelt, and following the loss of snow in spring. Date of sampling was a significant factor explaining archaeal and bacterial community structure in the Hillslope (perMANOVA pseudo-R2 = 0.11, P ≤ 0.001, see Supplementary Table S2 for pair-wise comparisons) and Floodplain (perMANOVA pseudo-R2 = 0.33, P ≤ 0.001) (Figures 3A,B). Fungal community structure was likewise significantly affected by the date of soil sampling in both the Hillslope (pseudo-R2 = 0.05, P ≤ 0.001) and Floodplain (perMANOVA pseudo-R2 = 0.22, P < 0.001, Figures 3C,D). Depth significantly affected archaeal and bacterial (perMANOVA pseudo-R2 = 0.33, P < 0.001) as well as fungal community structure (perMANOVA pseudo-R2 = 0.05, P < 0.001) in the Hillslope, but not in the Floodplain. We did not observe a significant Date × Depth of sampling interaction for either archaea and bacteria or for fungi in either the Hillslope or Floodplain (perMANOVA P ≥ 0.05 in all cases).
FIGURE 3.
Archaeal and bacterial community structure represented by non-metric dimensional scaling (NMDS) in the Hillslope (A) and Floodplain (B) as well as fungal community structure in the Hillslope (C) and Floodplain (D).
Snowmelt Selected for Phylogenetically Clustered Bacterial Life Strategies
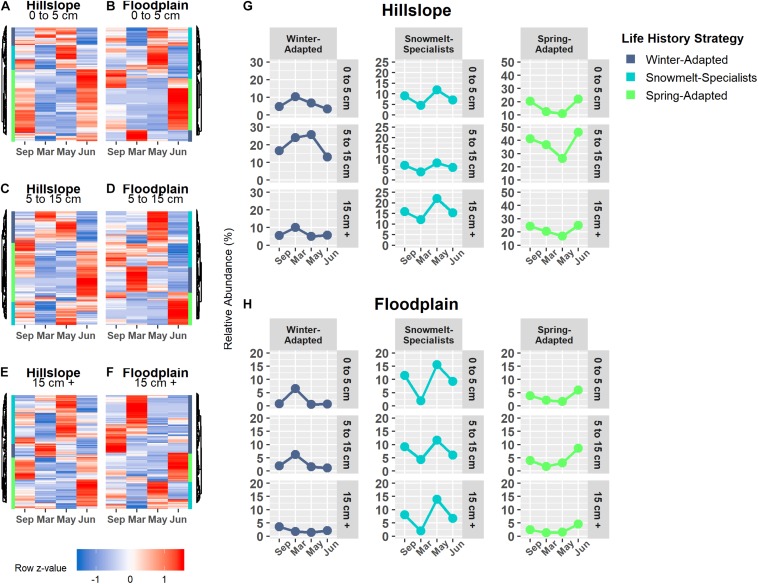
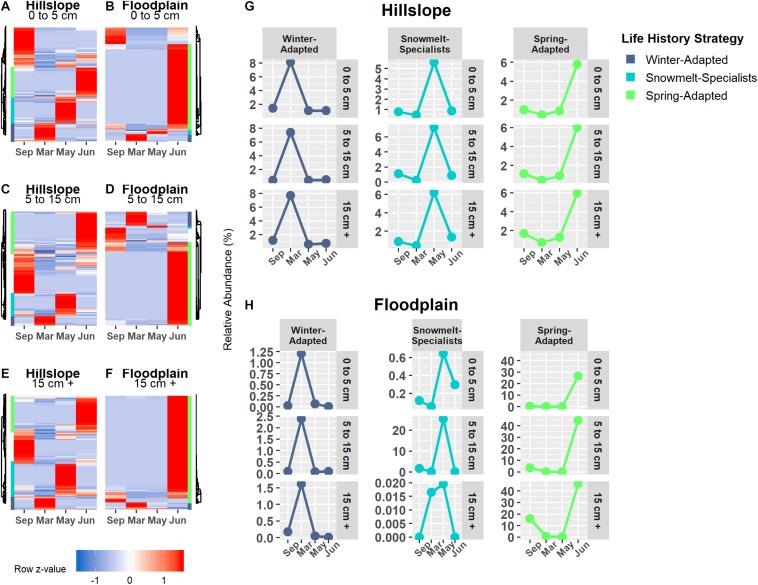
Bacterial and archaeal species were grouped together into one of three life strategies based on changes in relative abundance in response to snow accumulation, snowmelt, and the onset of spring (Figures 4A–H). As a group, Winter-Adapted archaea and bacteria had the highest group relative abundance (i.e., all Winter-Adapted OTUs in group summed together, separated by depth) in March (Figures 4G,H), a time when winter snow depth was greatest and soils were relatively cool and dry (Table 2). The relative abundance of Snowmelt-Specialist archaea and bacteria increased 1.8 to 2.4-fold on the Hillslope (Figure 4G) and 2- to 6-fold in the Floodplain (Figure 4H) during the snowmelt period (i.e., March–May). The majority of bacterial and archaeal taxa across life strategies were Spring-Adapted (Table 3). Spring-Adapted archaea and bacteria group relative abundance increased after snowmelt and ranged from 20 to 45% of the total community in the Hillslope or 5 to 8% of the total community in the Floodplain (Figures 4G,H).
FIGURE 4.
Archaeal and bacterial OTUs that had a significant change in abundance between any two sampling time points (September, March, May, June) were grouped by hierarchical clustering in the Hillslope (A,C,E) and Floodplain (B,D,F). Three life-history strategies related to winter snow cover, snowmelt, and loss of snow cover were identified and the group relative abundance (i.e., sum of all OTUs in a group at each depth) patterns of each strategy is shown (G,H).
TABLE 3.
Phylogenetic relatedness of bacteria and archaea grouped by life-history strategy.
| Location | Depth | Life history strategy | #OTUs | NRI | NTI |
| Hillslope | 0 to 5 cm | Winter-adapted | 55 | −0.48 | 2.18 |
| Snowmelt-specialists | 335 | −2.15 | 1.13 | ||
| Spring-adapted | 1093 | 5.28 | 2.75 | ||
| 5–15 cm | Winter-adapted | 138 | −1.67 | 5.16 | |
| Snowmelt-specialists | 433 | −0.89 | 0.33 | ||
| Spring-adapted | 1120 | 9.53 | 5.21 | ||
| 15 cm + | Winter-adapted | 132 | 1.69 | 3.37 | |
| Snowmelt-specialists | 461 | −0.16 | 0.31 | ||
| Spring-adapted | 771 | 1.77 | 0.47 | ||
| Floodplain | 0 to 5 cm | Winter-adapted | 83 | 2.05 | 3.49 |
| Snowmelt-specialists | 269 | 3.13 | 2.25 | ||
| Spring-adapted | 631 | 0.05 | 0.19 | ||
| 5–15 cm | Winter-adapted | 123 | −0.24 | 3.32 | |
| Snowmelt-specialists | 153 | 3.48 | 3.88 | ||
| Spring-adapted | 387 | 3.63 | −0.01 | ||
| 15 cm + | Winter-adapted | 119 | 1.60 | −1.27 | |
| Snowmelt-specialists | 200 | 3.49 | 3.07 | ||
| Spring-adapted | 276 | 1.32 | 1.00 |
Values in bold indicate a significant pattern of phylogenetic relatedness. Positive NTI/NRI values indicate that groups are phylogenetically clustered, with a low probability that the observed phylogenetic distance within groups is greater compared to the null model (P < 0.025). Negative NTI/NRI values indicate phylogenetic overdispersion, with a high probability that the observed phylogenetic distance within groups is greater than the null model (P > 0.975).
Generally speaking, the direction of the phylogenetic relatedness patterns within archaeal and bacterial life strategies were most often toward phylogenetic clustering (positive NRI/NTI values), however, the responses were not always observed at every sampling depth in the Hillslope or Floodplain (Table 3). For example, Spring-Adapted archaea and bacteria were phylogenetically clustered across all soil depths in the Hillslope based on the Net Relatedness Index (NRI, Table 3). In the Floodplain, Snowmelt-Specialist archaea and bacteria were similarly phylogenetically clustered across all sampling depths. In contrast to observations for the Floodplain, Snowmelt-Specialist archaea and bacteria sampled 0 to 5 cm below the soil surface were phylogenetically overdispersed on the Hillslope (NRI = −2.15, Table 3).
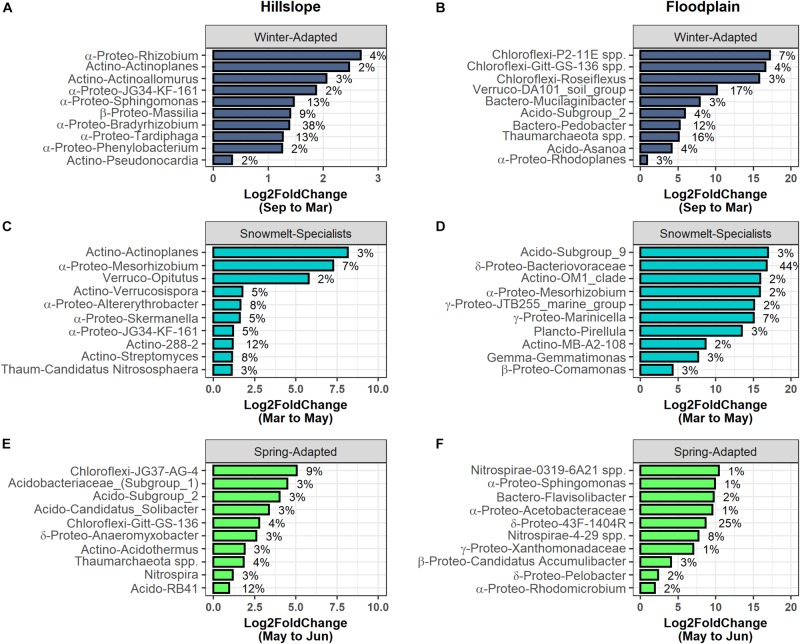
Bradyrhizobium (α-Proteobacteria), Tardiphaga (α-Proteobacteria), Sphingomonas (α-Proteobacteria), and Massilia (β-Proteobacteria) collectively accounted for 73% of the total increase in group relative abundance of Winter-Adapted bacteria at the Hillslope during winter (i.e., September 2016 to March 2017; Figure 5A). In the Floodplain, the DA101 soil group (Verrucomicrobia), Thaumarchaeota spp., and Pedobacter (Bacteroidetes) likewise contributed substantially to the increase in relative abundance of Winter-Adapted bacteria (Figure 5B). Streptomyces (Actinobacteria) and Candidatus Nitrososphaera (Thaumarchaeota) together accounted for 11% of the total increase in relative abundance of Snowmelt-Specialist archaea and bacteria from March to May on the Hillslope (Figure 5C), whereas Bacteriovoraceae (δ-Proteobacteria; 44% contribution alone) dominated the response of Snowmelt-Specialists in the Floodplain (Figure 5D). Various phylotypes of Acidobacteria [e.g., Acidobacteriaceae (Subgroup 1) spp., Subgroup 2 spp., Candidatus Solibacter, RB41 spp.] composed the largest fraction of Spring-Adapted bacteria in the Hillslope (Figure 5E). Nitrifying taxa (e.g., Nitrospira spp. and Thaumarchaeota spp.) also contributed 7% to the increase of Spring-Adapted archaea and bacteria from May to June on the Hillslope (Figure 5E). Similar to the Hillslope, other potentially nitrifying organisms belonging to the phylum Nitrospirae (e.g., 4-29 spp.) were major contributors to the increase of Spring-Adapted bacteria after snowmelt and during plant-green up in the Floodplain (Figure 5F). All archaeal and bacterial OTUs with life strategies assigned are listed in Supplementary Table S3.
FIGURE 5.
The top 10 archaeal and bacterial phylotypes within each life-history strategy in the Hillslope (A,C,E) and Floodplain (B,D,F). Phylotypes were ranked by their percent change in abundance between the specified sampling time-points (x-axis). The bars represent log2fold changes and the percent contributions to the change in group relative abundance are given at the side of the bar. Abbreviations for taxonomy (phylum or subphylum level) are Acido – Acidobacteria; Actino – Actinobacteria; Bactero – Bacteriodetes; Gemmo – Gemmatimonadetes; Plancto – Planctomycetes; α-Proteo – Alphaproteobacteria; β-Proteo – Betaproteobacteria; δ-Proteo – Deltaproteobacteria; γ-Proteo – Gammproteobacteria; Verruco – Verrucomicrobia.
Fungal Taxa and Functional Groups Were Also Differentiated by Snowmelt Niche
Winter-Adapted, Snowmelt-Specialist, and Spring-Adapted fungi were also observed across all three soil depths in both the Hillslope and Floodplain (Figures 6A–F). Similar to the archaeal and bacterial communities, Winter-Adapted fungi had the highest group abundance (∼8% of total fungal community) at peak winter snow depth (Figure 6G). Snowmelt-Specialist fungi increased in abundance during the snowmelt period (i.e., March to May), and Spring-Adapted fungi had the highest group abundance during plant green-up in June (Figures 6G,H). The majority of fungal taxa in the Floodplain were Spring-Adapted, with far fewer fungal species categorized as either Winter-Adapted or Snowmelt-Specialists (Figures 6B,D,F and Table 4). For example, Spring-Adapted fungi accounted for 27 to 43% of the total fungal community in the Floodplain in June (Figure 6H), whereas Winter-Adapted and Snowmelt-Specialist fungi were rarely more than 2% of the total fungal community at any time.
FIGURE 6.
Fungal OTUs that had a significant change in abundance between any two sampling time points were grouped by hierarchical clustering in the Hillslope (A,C,E) and Floodplain (B,D,F). Similar to bacterial OTUs, we observed Winter-Adapted, Snowmelt-Specialists, and Spring-Adapted fungi across all depths and in both the Hillslope (G) and Floodplain (H). A fourth group with high abundance in autumn was also observed, but this group was not included in further analyses.
TABLE 4.
Composition of functional guilds for fungal life-history strategies related to snowmelt.
| Location | Depth | Strategy | #OTUs | AMF | EMF | Endophyte | Saprotroph |
| Hillslope | 0 to 5 cm | Winter-adapted | 97 | 0% | 5% | 7% | 88% |
| Snowmelt-specialists | 186 | 3% | 5% | 4% | 87% | ||
| Spring-adapted | 181 | 6% | 12% | 4% | 78% | ||
| 5 to 15 cm | Winter-adapted | 65 | 2% | 0% | 5% | 94% | |
| Snowmelt-specialists | 184 | 2% | 4% | 3% | 91% | ||
| Spring-adapted | 263 | 2% | 15% | 3% | 80% | ||
| 15 cm + | Winter-adapted | 88 | 5% | 13% | 3% | 80% | |
| Snowmelt-specialists | 261 | 5% | 5% | 4% | 85% | ||
| Spring-adapted | 200 | 4% | 11% | 1% | 85% | ||
| Floodplain | 0 to 5 cm | Winter-adapted | 8 | 0% | 0% | 13% | 88% |
| Snowmelt-specialists | 2 | 0% | 0% | 0% | 100% | ||
| Spring-adapted | 107 | 0% | 19% | 5% | 76% | ||
| 5 to 15 cm | Winter-adapted | 12 | 0% | 0% | 0% | 100% | |
| Snowmelt-specialists | 2 | 0% | 100% | 0% | 0% | ||
| Spring-adapted | 77 | 0% | 36% | 6% | 58% | ||
| 15 cm + | Winter-adapted | 5 | 0% | 0% | 20% | 80% | |
| Snowmelt-specialists | 4 | 0% | 0% | 0% | 100% | ||
| Spring-adapted | 141 | 0% | 18% | 9% | 72% |
The percent within a functional group was calculated by dividing the total number of OTUs within the functional group by the total numbers of OTUs within a life-history strategy at each depth. [AMF, arbuscular mycorrhizae; EMF, ectomycorrhizae; Saprotrophs also included OTUs assigned to multiple trophic modes (i.e., mixotrophic) and various types of pathogenesis (e.g., plant pathogen)].
Saprotrophy was the dominant fungal trophic mode at both the Hillslope and Floodplain (Table 4). For example, across all soil depths, 80 to 100% of Winter-Adapted fungal taxa were classified as saprotrophic. Symbiotic fungi that associate with plant roots (e.g., arbuscular mycorrhizae, ectomycorrhizae, and root-endophytes) had higher prevalence within the Spring-Adapted life strategy compared to Winter-Adapted or Snowmelt-Specialist fungi (Table 4). Across soil depths, root-associated fungi constituted 16 to 22% of all Spring-Adapted fungi on the Hillslope and 24 to 38% of Spring-Adapted fungi in the Floodplain. Ectomycorrhizal fungi were the predominant root-associated functional group, especially in the Floodplain, where arbuscular mycorrhizal fungi were not observed among any of the life strategies (Table 4).
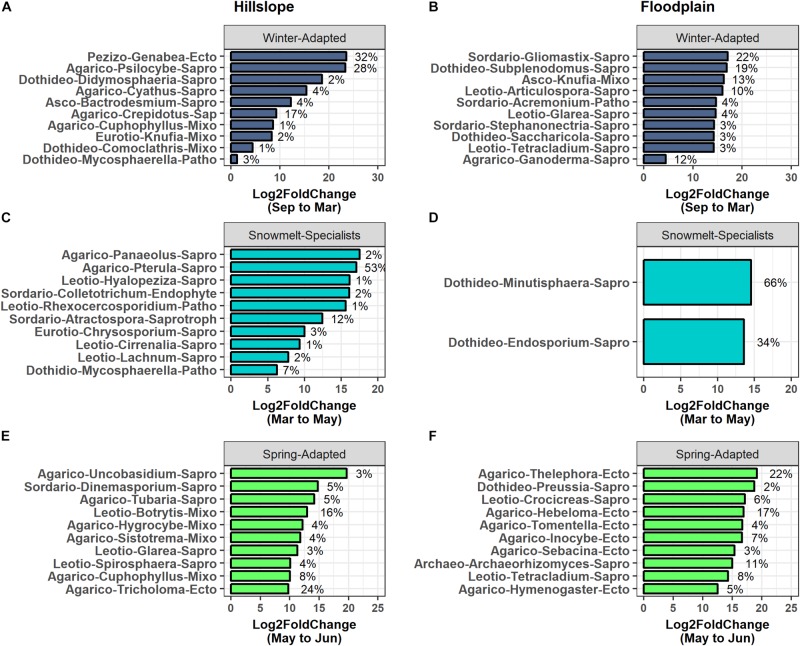
Genabea (Pezizomycetes), Psilocybe (Agaricomycetes), and Crepidotus (Agaricomycetes) collectively accounted for 77% of the increase in group relative abundance observed for Winter-Adapted fungi occurring from September to March on the Hillslope (Figure 7A). A few select fungal genera also contributed disproportionately to changes in group abundance for Snowmelt-Specialists and Spring-Adapted fungi during and after snowmelt (Figures 7C–F). For example, Pterula (Agaricomycetes) alone accounted for 53% of the increase in relative abundance for Snowmelt-Specialist fungi during the snowmelt period (i.e., March to May) and Tricholoma (Agaricomyetes), Botrytis (Leotiomycetes), and Cuphophyllus (Agaricomycetes) together accounted for 48% of the increase in group abundance for Spring-Adapted fungi from May to June on the Hillslope (Figures 7C,E). Gliomastix (Sordariomycetes), Subplenodomus (Dothideomycetes), Knufia (Ascomycetes familia incertae), Articulospora (Leotiomycetes), and Ganoderma (Agaricomycetes) accounted for 78% of the increase in group relative abundance of Winter-Adapted fungi from September to March in the Floodplain (Figure 7B). Thelephora (Agaricomycetes), Hebeloma (Agaricomycetes), Archaeorhizomyces (Archaeorhizomycetes), and Tetracladium (Leotiomycetes) were Spring-Adapted fungi with substantial increases in relative abundance in the Floodplain after snowmelt (Figure 7F). Very few fungal OTUs employed the Snowmelt-Specialist life strategy in the Floodplain (Table 4), which was composed of only two fungal genera [i.e., Minutisphaera (Dothideomycetes) and Endosporium (Dothideomycetes); Figure 7D]. All fungal OTUs with life strategies assigned are listed in Supplementary Table S4.
FIGURE 7.
The top 10 fungal phylotypes within each life-history strategy in the Hillslope (A,C,E) and Floodplain (B,D,F). Phylotypes were ranked by their percent change in abundance between the specified sampling time-points. The bars represent log2fold changes and the percent contributions to the change in group relative abundance are given at the side of the bar. Only two fungal genera contributed to the significant change in fungal Snowmelt-Specialists from March to May in the Floodplain location (D). Abbreviations for taxonomy (class-level) are Agarico – Agaricomycetes; Archaeo – Archaeorhizomycetes; Asco – Ascomycetes; Dothideo – Dothideomycetes; Eurotio – Eurotiomycetes; Leotio – Leotiomycetes; Pezizo – Pezizomycetes; Sordario – Sordariomycetes. Abbreviations for functional groups are Ecto – ectomycorrhizae; Endophyte – root endophyte; Mixo – mixotrophic; Patho – pathotrophic; Sapro – saprotrophic.
Discussion
Soil microbial biomass is commonly observed to bloom beneath the winter snowpack and subsequently decline following snowmelt, often resulting in a significant pulse of soil N in spring (Grogan and Jonasson, 2003; Edwards et al., 2006; Schmidt et al., 2007). In this study, we primarily sought to identify bacteria, archaea, and fungi that were associated with the microbial biomass bloom during winter and biomass crash following snowmelt. We also sought to infer whether the traits that govern microbial community assembly during and after snowmelt were phylogenetically conserved. This was accomplished by measuring the phylogenetic relatedness of bacteria and archaea, or by comparing guilds of soil fungi, that were grouped into life strategies based upon relative abundance patterns before, during, and after snowmelt.
Overall, we found that (1) increases in soil microbial biomass production beneath the winter snowpack were observed at both locations in our study (Hillslope and Floodplain) and appeared to be induced by a pulse of snowmelt infiltration (Figure 2), (2) the microbial biomass collapse was associated with a significant pulse of N measured as extractable soil (Figure 2), (3) three microbial life strategies (Winter-Adapted, Snowmelt-Specialist, and Spring-Adapted) were identified at each soil depth at both locations (Figures 4, 6), (4) life strategies were most often phylogenetically clustered (bacteria and archaea) or shared similar trophic modes (e.g., saprotrophic fungi; Tables 3, 4), and finally that (5) a few select taxa within each life strategy contributed disproportionately to the abundance responses (Figures 5, 7). Thus, we have shown that bacteria, archaea, and fungi with similar responses to snow accumulation and snowmelt are likely to share adaptive traits and we conclude that this framework may be useful in understanding an organism’s snowmelt niche and response to changing winter snowpack conditions.
Role of Moisture and Substrate Utilization in Structuring the Winter-Adapted Niche
Phylogenetic clustering of Winter-Adapted archaea and bacteria may partly be explained by poor connectivity of soil pore spaces due to dry soil conditions that also lead to specific substrate utilization patterns during the winter. Because plant detritus is thought to be the primary substrate utilized by bacteria and fungi during winter (Taylor and Jones, 1990; Uchida et al., 2005; Hooker and Stark, 2012; Isobe et al., 2018), low substrate diffusion rates, combined with high spatial heterogeneity and low resource availability, probably selects for bacteria and fungi with specialized abilities to degrade complex soil organic matter and plant root or leaf litter. A predominance of α-Proteobacteria (e.g., Tardiphaga, Massilia, Phenylobacteria, Rhodoplanes) known to be early- to mid-stage colonizers of decomposing root and leaf-litter (Purahong et al., 2016; Bonanomi et al., 2019) supports this hypothesis (Figure 5). Similar to the bacterial community, we observed that Winter-Adapted fungi were predominantly saprotrophic, with a few select taxa that are known wood or detritus decomposers (e.g., Gliomastix, Psilocybe, Ganoderma) contributing significantly to the increase in Winter-Adapted fungi (Figure 7). Additional fungal taxa commonly found during early- to mid-stages of decomposition of leaf or root litter (e.g., Acremonium, Mycosphaerella, Saccharicola, Tetracladium; Kuhnert et al., 2012) also had significant positive responses to winter snow accumulation at our study site.
Lipson et al. (2009) previously found that bacteria and fungi isolated from beneath the snowpack in winter had a propensity for high growth rates, high mass-specific respiration, and low growth yields. If those observations are generalizable to the winter-adapted taxa observed here, we hypothesize that such physiological traits would select for a winter-adapted phenotype characterized by enhanced investment in extracellular enzyme production to optimize nutrient acquisition via depolymerization of soil organic matter at the expense of traits that optimize stress tolerance and growth yield (Schimel et al., 2007; Malik et al., 2018; Zhalnina et al., 2018).
We also observed two potentially N-fixing genera (e.g., Rhizobium and Bradyrhizobium) that contributed significantly to the overwinter increase in Winter-Adapted archaea and bacteria on the Hillslope, but these genera were not observed in the Floodplain (Figure 5). Recent studies have shown high abundance of N-fixing Bradyrhizobium and Rhizobium in mid- to late-stages of litter decomposition and their presence is thought to alleviate N-limitation that occurs during late stages of decomposition, which thereby facilitates the activity of decomposers that degrade more complex biopolymers (Purahong et al., 2016; Lladó et al., 2017; Bonanomi et al., 2019). In seasonally snow-covered ecosystems, like the East River study site, a similar effect might occur as winter progresses due to the prolonged absence of fresh C inputs from plants aboveground and the potential for progressive N-limitation as the decomposer community propagates. In contrast to the Hillslope, we observed greater total soil N availability in the Floodplain as well as autotrophic, nitrifying archaea (e.g., Thaumarchaeota) during winter. These observations, along with a lack of a significant contribution from N-fixing genera (e.g., Rhizobium and Bradyrhizobium), might indicate that the N-fixing niche is not present beneath the snowpack in the Floodplain.
Snowmelt Infiltration Triggers Microbial Biomass Bloom
The microbial biomass bloom occurred during a period of extended soil saturation that lasted for approximately 65 days and that coincided with net snowpack loss and snowmelt infiltration (Figures 1, 2). Snowmelt-triggered biomass production stands in contrast to previous hypotheses predicting that a sudden decrease in osmolarity due to snowmelt infiltration should cause lysis of microbial cells (Schimel et al., 2007; Jefferies et al., 2010). Our results are similar to those of other studies that have observed an increase in soil microbial biomass production and peak microbial biomass during snowmelt, when soil temperatures were near or above 0°C, followed by a collapse in the microbial biomass pool size when soils became snow-free and were relatively drier in spring (Brooks et al., 1998; Lipson et al., 1999; Edwards et al., 2006; Buckeridge and Grogan, 2010; Isobe et al., 2018). However, we acknowledge that the time intervals of our sampling do not allow us to fully resolve the temporal trends of the microbial biomass bloom and crash or to identify the exact date of peak microbial biomass.
We hypothesize that alleviation from moisture and substrate limitation may have triggered the microbial biomass bloom that we observed under the winter snowpack. While microbial metabolic activity has been shown to occur in soils with temperatures well below 0°C (McMahon et al., 2009), our findings suggest that soil and air temperatures need to be warm enough to induce net snowmelt infiltration and for soil water to be in the liquid phase in order to induce the soil microbial bloom. In addition, the microbial bloom did not occur in Floodplain soils deeper than 5 cm, where higher soil moisture availability existed throughout the winter.
We also observed phylogenetic overdispersion of Snowmelt-Specialist bacteria and archaea on the Hillslope in our study (Table 3), whereas Snowmelt-Specialists were phylogenetically clustered in the Floodplain. For the Hillslope, these results imply that ecologically dissimilar organisms with a broad phylogenetic distribution contribute to the biomass bloom during snowmelt and biomass collapse thereafter. Thus, antagonistic biotic interactions, such as competitive exclusion and density-dependent regulation of population size (e.g., trophic interactions), are likely to be the community assembly mechanisms underlying the microbial biomass bloom, biomass crash, and taxonomic succession that we observed before and after snowmelt. For example, antagonism resulting from toxin production may promote biomass production by reducing competition during a time of high resource availability (Moons et al., 2009; Hibbing et al., 2010). After snowmelt, the population decline on the Hillslope also indicates far greater mortality compared to reproduction rates, which may result from resource depletion or predation by viruses, microfauna, and mesofauna (Moons et al., 2009; Buckeridge et al., 2013; Phaiboun et al., 2015; Georgiou et al., 2017).
Alternatively, competitive exclusion of Winter-Adapted organisms may occur during snowmelt if Snowmelt-Specialist archaea and bacteria have higher growth efficiencies compared to Winter-Adapted taxa (Georgiou et al., 2017). Dissolved organic substrate quality and quantity is known to increase during snowmelt (Campbell et al., 2014; Patel et al., 2018), thus organisms that are characteristic of the biomass bloom (i.e., Snowmelt-Specialists) should be able to re-allocate energy resources away from extracellular enzyme production and nutrient uptake toward protein/fatty acid/DNA synthesis to maximize growth efficiency (Schimel et al., 2007; Malik et al., 2018). Furthermore, it is unlikely that organisms with high growth efficiencies or whole communities with high rates of biomass production would be closely related phylogenetically, but rather could possess genomic features and adaptive traits that are shared due to convergent evolutionary trajectories (Roller et al., 2016; Muscarella and Lennon, 2018).
Spring Nitrogen Dynamics and Spring-Adapted Bacteria and Fungi
We mainly attribute the pulse of extractable soil observed in spring to elevated N mineralization and nitrification rates resulting from a flush of labile C and N that was released from microbial biomass after snowmelt. Lipson et al. (1999) similarly showed a pulse of soil protein that was attributed to the lysis of soil microbial biomass after snowmelt. Furthermore, four ammonia- or nitrite-oxidizing groups [e.g., 0319-6A21 (Nitrospirae), unassigned Nitrospirae spp., and unassigned Thaumarchaeota spp.] contributed substantially to the increase of Spring-Adapted archaea and bacteria on the Hillslope (Figure 5), which also coincided with the pulse of soil in spring (Figure 2) and suggests substantial autotrophic nitrification. It should be noted that nitrogen in the snowpack also likely contributed to the pulse of soil that we observed after snowmelt, however, previous studies have shown that snow is usually a minor contributor compared to nitrogen that has passed through, and been released from, the soil microbial pool (Williams et al., 1996; Williard et al., 2001; Burns and Kendall, 2002; Grogan and Jonasson, 2003; Campbell et al., 2007). In contrast to the Hillslope, we did not observe accumulation in the Floodplain, despite a twofold decline in microbial biomass in the upper soil profile and increases in the abundance of some nitrifying organisms. Lower nitrate accumulation could arise from any combination of the following: lower mineralization and nitrification rates, higher N uptake rates (plant and microbial) with higher N immobilization, or higher rates of assimilation and denitrification at the Floodplain compared to the Hillslope.
In addition to the prevalence of ammonia-oxidizing genera, we also found that four groups of Acidobacteria (i.e., Subgroup 1, Subgroup 2, Candidatus Solibacter, and RB41) made large contributions to the increase in Spring-Adapted bacteria on the Hillslope (Figure 5E). Acidobacteria are traditionally considered to be oligotrophs with abundances negatively correlated to soil organic C (Fierer et al., 2007). However, an increasing number of studies have shown that Acidobacteria responses to gradients in organic C availability can be either positive or negative, challenging their status as strictly oligotrophic (Kielak et al., 2016; Lladó et al., 2017). In addition, some Acidobacteria can be isolated from the environment under anoxic conditions using microbial necromass (i.e., gellan gum) as a growth substrate and Ca. Solibacter has 4- to 6-fold higher nutrient-transporting genes in its genome compared to most other bacteria (Kielak et al., 2016). Therefore, some Acidobacteria phylotypes may occupy the Spring-Adapted niche by capitalizing on the necromass from the biomass crash along with high investment in nutrient transporters after snowmelt.
Mycorrhizal fungi made significant contributions to the increase in Spring-Adapted fungi in both the Hillslope and Floodplain (Figure 7); this pattern was driven primarily by an increase in ectomycorrhizal fungi. For example, in the Floodplain, 6 of the top 10 Spring-Adapted fungi were ectomycorrhizae. If ecosystem N retention in spring is enhanced by well-coupled plant-mycorrhizae phenology, then the implications of our results are noteworthy with respect to changing winter conditions. For example, although earlier snowmelt could result in an earlier pulse of N released from the crash in microbial biomass, floodplain ecosystems may buffer ecosystem N losses via well-coupled plant-mycorrhizal N uptake in spring. It may also be possible to predict the capacity of other watershed locations to retain N in response to earlier snowmelt based upon plant distributions and plant-mycorrhizae associations, given that plant species assemblages and plant-mycorrhizal associations can be mapped and inferred at high spatial resolution using remote-sensing methods (Fisher et al., 2016; Falco et al., 2019).
Conclusion
Because snowmelt rates have been declining in mountainous regions in recent decades (Musselman et al., 2017; Harpold and Brooks, 2018), the length of time during which soils are saturated beneath a melting snowpack may increase in the future and thereby promote a larger snowmelt microbial biomass bloom and crash. Alternatively, a future with warmer air temperatures and a shallower winter snowpack would be expected to result in an increase in the frequency of sustained soil freezing or more frequent soil freeze-thaw events during winter (Hardy et al., 2001; Brown and DeGaetano, 2011; Kreyling and Henry, 2011), which would most likely reduce the magnitude of the microbial biomass bloom, biomass collapse, and pulse of N after snowmelt (Brooks et al., 1998; Buckeridge et al., 2010; Ueda et al., 2013). Whether future environmental conditions will sustain an overwinter microbial bloom warrants further study, because the nutrient flush from microbial biomass following snowmelt can be one of the largest annual soil N pulses in some high-latitude or high-altitude ecosystems (Grogan and Jonasson, 2003; Schmidt et al., 2007).
Here, we have provided novel evidence that the snowmelt period is an environmental filter that differentiates soil bacteria, archaea, and fungi into three distinct life strategies. The phylogenetic relatedness of bacteria and archaea and enrichment of fungal functional groups within life strategies suggests that selective, deterministic processes allow Winter-Adapted, Snowmelt-Specialist, and Spring-Adapted organisms to occupy distinct niches that are related to the winter snowpack and snowmelt. Although our main goal was not to compare and contrast two distinct watershed locations (Hillslope and Floodplain), we did observe differences in the magnitude of the microbial biomass bloom and collapse among locations and soil depths, patterns of accumulation in spring, and differences in contributions from various taxa to each life strategy. These results indicate that local factors, in part, shape the response of these watershed locations to snowmelt. We contend, however, that common microbial traits allow for ecologically similar organisms to occupy the same life strategy, in spite of watershed location. This hypothesis can be tested using high-resolution, culture-independent methods (e.g., genome-resolved metagenomics and metatranscriptomics) and by quantifying traits in controlled laboratory experiments and with isolates to further explore the microbial snowmelt niche.
Data Availability Statement
Sequencing reads can be found in the NCBI Bioproject database accession no. PRJNA587134. Data for this manuscript were downloaded from the Watershed Function SFA data portal (http://wfsfa-data.lbl.gov) and are archived at the U.S. Department of Energy’s Environmental System Science Data Infrastructure for a Virtual Ecosystem (ESS-DIVE, Sorensen et al., 2019).
Author Contributions
PS, HB, and EB designed the study, which is a contribution to the Watershed Function Scientific Focus Area lead by SH and KW. Data collection and analysis was performed by PS (primarily) and SW, AP, MB, UK, NB, HB, and EB. PS, HB, and EB wrote the manuscript. All authors contributed to revising the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Erica Dorr, Biz Whitney, Hans Wu Singh, and Chelsea Wilmer for their assistance in the lab and in the field. Mark Conrad, Rosemary Carroll, and Wendy Brown were extremely helpful digging snow pits during winter. We also wish to thank Jenny Reithel and Shannon Sprott at Rocky Mountain Biological Laboratory (RMBL) for their help at the field site. Lastly, we thank Zarine Kakalia and Joan Damerow for their help to archive data on ESS-Dive.
Footnotes
Funding. RMBL lab spaces and equipment are funded in part by the RMBL Equipment (Understanding Genetic Mechanisms) Grant, DBI-1315705. This material is based upon work supported as part of the Watershed Function Scientific Focus Area funded by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number DE-AC02-05CH11231 to Lawrence Berkeley National Laboratory.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00871/full#supplementary-material
Daily soil freeze-thaw cycles measured in the Hillslope and Floodplain during winter and before and after snowmelt in 2016/17. We considered a freeze-thaw cycle to have occurred if the daily maximum soil temperature was > 0°C and the daily minimum soil temperature was < 0°C.
Pair-wise comparisons of permANOVAs for bacteria and fungi.
Life strategies assigned to archaeal and bacterial OTUs.
Life strategies assigned to fungal OTUs.
References
- Aanderud Z. T., Jones S. E., Schoolmaster D. R., Jr., Fierer N., Lennon J. T. (2013). Sensitivity of soil respiration and microbial communities to altered snowfall. Soil Biol. Biochem. 57 217–227. [Google Scholar]
- Altman D. G., Bland J. M. (2011). How to obtain the P value from a confidence interval. BMJ 343:d2304 10.1590/S1806-37562018000000096 [DOI] [PubMed] [Google Scholar]
- Bales R. C., Molotch N. P., Painter T. H., Dettinger M. D., Rice R., Dozier J. (2006). Mountain hydrology of the western United States. Water Resour. Res. 42:13. [Google Scholar]
- Bartlett R. J., Ross D. S. (1988). Colorimetric determination of oxidizable carbon in acid soil solutions. Soil Sci. Soc. Am. J. 52 1191–1192. [Google Scholar]
- Bonanomi G., De Filippis F., Cesarano G., La Storia A., Zotti M., Mazzoleni S., et al. (2019). Linking bacterial and eukaryotic microbiota to litter chemistry: combining next generation sequencing with 13C CPMAS NMR spectroscopy. Soil Biol. Biochem. 129 110–121. [Google Scholar]
- Bormann K. J., Brown R. D., Derksen C., Painter T. H. (2018). Estimating snow-cover trends from space. Nat. Clim. Change 8 924–928. [Google Scholar]
- Bray J. R., Curtis J. T. (1957). An ordination of the upland forest communities of Southern Wisconsin. Ecol. Monogr. 27 325–349. [Google Scholar]
- Brookes P. C., Landman A., Pruden G., Jenkinson D. S. (1985). Chloroform fumigation and the release of soil-nitrogen - a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 17 837–842. [Google Scholar]
- Brooks P. D., Williams M. W. (1999). Snowpack controls on nitrogen cycling and export in seasonally snow-covered catchments. Hydrol. Process. 13 2177–2190. [Google Scholar]
- Brooks P. D., Williams M. W., Schmidt S. K. (1998). Inorganic nitrogen and microbial biomass dynamics before and during spring snowmelt. Biogeochemistry 43 1–15. [Google Scholar]
- Brown P. J., DeGaetano A. T. (2011). A paradox of cooling winter soil surface temperatures in a warming northeastern United States. Agric. For. Meteorol. 151 947–956. [Google Scholar]
- Buckeridge K. M., Banerjee S., Siciliano S. D., Grogan P. (2013). The seasonal pattern of soil microbial community structure in mesic low arctic tundra. Soil Biol. Biochem. 65 338–347. [Google Scholar]
- Buckeridge K. M., Cen P. Y., Layzell D. B., Grogan P. (2010). Soil biogeochemistry during the early spring in low arctic mesic tundra and the impacts of deepened snow and enhanced nitrogen availability. Biogeochemistry 99 127–141. [Google Scholar]
- Buckeridge K. M., Grogan P. (2010). Deepened snow increases late thaw biogeochemical pulses in mesic low arctic tundra. Biogeochemistry 101 105–121. 10.1111/gcb.14084 [DOI] [Google Scholar]
- Burns D. A., Kendall C. (2002). Analysis of δ15N and δ18O to differentiate NO sources in runoff at two watersheds in the Catskill Mountains of New York. Water Resour. Res. 38 9–1. [Google Scholar]
- Campbell J., Reinmann A., Templer P. (2014). Soil freezing effects on sources of nitrogen and carbon leached during snowmelt. Soil Sci. Soc. Am. J. 78 297–308. [Google Scholar]
- Campbell J. L., Mitchell M. J., Mayer B., Groffman P. M., Christenson L. M. (2007). Mobility of nitrogen-15-labeled nitrate and sulfur-34 labeled sulfate during snowmelt. Soil Sci. Soc. Am. J. 71 1934–1944. [Google Scholar]
- Caporaso J. G., Bittinger K., Bushman F. D., DeSantis T. Z., Andersen G. L., Knight R. (2010). PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26 266–267. 10.1093/bioinformatics/btp636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CaraDonna P. J., Inouye D. W. (2015). Phenological responses to climate change do not exhibit phylogenetic signal in a subalpine plant community. Ecology 96 355–361. 10.1890/14-1536.1 [DOI] [PubMed] [Google Scholar]
- Chagnon P.-L., Bradley R. L., Maherali H., Klironomos J. N. (2013). A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 18 484–491. 10.1016/j.tplants.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Contosta A. R., Adolph A., Burchsted D., Burakowski E., Green M., Guerra D., et al. (2016). A longer vernal window: the role of winter coldness and snowpack in driving spring transitions and lags. Glob. Change Biol. 23 1610–1625. 10.1111/gcb.13517 [DOI] [PubMed] [Google Scholar]
- Crisp M. D., Cook L. G. (2012). Phylogenetic niche conservatism: what are the underlying evolutionary and ecological causes? New Phytol. 196 681–694. 10.1111/j.1469-8137.2012.04298.x [DOI] [PubMed] [Google Scholar]
- de Vries A., Ripley B. D. (2016). ggdendro:Create Dendrograms and Tree Diagrams Using ggplot2 Version 0.1. [Google Scholar]
- Doane T. A., Horwath W. R. (2003). Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 36 2713–2722. [Google Scholar]
- Edgar R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10:996 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- Edgar R. C., Haas B. J., Clemente J. C., Quince C., Knight R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K. A., McCulloch J., Peter Kershaw G., Jefferies R. L. (2006). Soil microbial and nutrient dynamics in a wet Arctic sedge meadow in late winter and early spring. Soil Biol. Biochem. 38 2843–2851. [Google Scholar]
- Ernakovich J. G., Hopping K. A., Berdanier A. B., Simpson R. T., Kachergis E. J., Steltzer H., et al. (2014). Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob. Chang. Biol. 20 3256–3269. 10.1111/gcb.12568 [DOI] [PubMed] [Google Scholar]
- Evans S. E., Wallenstein M. D., Burke I. C. (2014). Is bacterial moisture niche a good predictor of shifts in community composition under long-term drought? Ecology 95 110–122. 10.1890/13-0500.1 [DOI] [PubMed] [Google Scholar]
- Falco N., Wainwright H., Dafflon B., Léger E., Peterson J., Steltzer H., et al. (2019). Investigating microtopographic and soil controls on a mountainous meadow plant community using high-resolution remote sensing and surface geophysical data. J. Geophys. Res. Biogeosciences 124 1618–1636. [Google Scholar]
- Fierer N., Bradford M. A., Jackson R. B. (2007). Toward an ecological classification of soil bacteria. Ecology 88 1354–1364. 10.1890/05-1839 [DOI] [PubMed] [Google Scholar]
- Fisher J. B., Sweeney S., Brzostek E. R., Evans T. P., Johnson D. J., Myers J. A., et al. (2016). Tree-mycorrhizal associations detected remotely from canopy spectral properties. Glob. Chang. Biol. 22 2596–2607. 10.1111/gcb.13264 [DOI] [PubMed] [Google Scholar]
- Georgiou K., Abramoff R. Z., Harte J., Riley W. J., Torn M. S. (2017). Microbial community-level regulation explains soil carbon responses to long-term litter manipulations. Nat. Commun. 8:1223 10.1038/s41467-017-02134-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson M. A., Averill C., Finzi A. C. (2014). Correction factors for dissolved organic carbon extracted from soil, measured using the Mn(III)-pyrophosphate colorimetric method adapted for a microplate reader. Soil Biol. Biochem. 78 284–287. [Google Scholar]
- Grogan P., Jonasson S. (2003). Controls on annual nitrogen cycling in the understory of a subarctic birch forest. Ecology 84 202–218. [Google Scholar]
- Hardy J. P., Groffman P. M., Fitzhugh R. D., Henry K. S., Welman A. T., Demers J. D., et al. (2001). Snow depth manipulation and its influence on soil frost and water dynamics in a northern hardwood forest. Biogeochemistry 56 151–174. [Google Scholar]
- Harpold A. A., Brooks P. D. (2018). Humidity determines snowpack ablation under a warming climate. Proc. Natl. Acad. Sci. U.S.A. 115:1215 10.1073/pnas.1716789115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold A. A., Molotch N. P. (2015). Sensitivity of soil water availability to changing snowmelt timing in the western US. Geophys. Res. Lett. 42 8011–8020. [Google Scholar]
- Harte J., Saleska S. R., Levy C. (2015). Convergent ecosystem responses to 23-year ambient and manipulated warming link advancing snowmelt and shrub encroachment to transient and long-term climate-soil carbon feedback. Glob. Chang. Biol. 21 2349–2356. 10.1111/gcb.12831 [DOI] [PubMed] [Google Scholar]
- Harte J., Shaw R. (1995). Shifting dominance within a montane vegetation community: results of a climate-warming experiment. Science 267:876 10.1126/science.267.5199.876 [DOI] [PubMed] [Google Scholar]
- Hibbing M. E., Fuqua C., Parsek M. R., Peterson S. B. (2010). Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8 15–25. 10.1038/nrmicro2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho A., Di Lonardo D. P., Bodelier P. L. E. (2017). Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 93:14 10.1093/femsec/fix006 [DOI] [PubMed] [Google Scholar]
- Hock R., Rasul G., Adler A., Caceres B., Gruber S., Hirabayashi Y., et al. (2019). High Mountain Areas. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Geneva: IPCC. [Google Scholar]
- Hooker T. D., Stark J. M. (2012). Carbon flow from plant detritus and soil organic matter to microbes—linking carbon and nitrogen cycling in semiarid soils. Soil Sci. Soc. Am. J. 76 903–914. [Google Scholar]
- Hubbard S. S., Williams K. H., Agarwal D., Banfield J., Beller H., Bouskill N., et al. (2018). The East River, Colorado, Watershed: a mountainous community testbed for improving predictive understanding of multiscale hydrological–biogeochemical dynamics. Vadose Zone J. 17 1–25. [Google Scholar]
- Hutchinson G. E. (1957). Concluding remarks. J. Cold Spring Harb. Symp. Quant. Biol. 22 415–427. [Google Scholar]
- Iler A. M., Høye T. T., Inouye D. W., Schmidt N. M. (2013). Nonlinear flowering responses to climate: are species approaching their limits of phenological change? Phil. Trans. R Soc. B 368:20120489 10.1098/rstb.2012.0489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye D. W. (2008). Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89 353–362. 10.1890/06-2128.1 [DOI] [PubMed] [Google Scholar]
- Isobe K., Oka H., Watanabe T., Tateno R., Urakawa R., Liang C., et al. (2018). High soil microbial activity in the winter season enhances nitrogen cycling in a cool-temperate deciduous forest. Soil Biol. Biochem. 124 90–100. [Google Scholar]
- Jefferies R. L., Walker N. A., Edwards K. A., Dainty J. (2010). Is the decline of soil microbial biomass in late winter coupled to changes in the physical state of cold soils? Soil Biol. Biochem. 42 129–135. [Google Scholar]
- Kembel S. W., Cowan P. D., Helmus W. K., Cornwell W. K., Morlon H., Ackerly D. D. (2010). Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26 1463–1464. 10.1093/bioinformatics/btq166 [DOI] [PubMed] [Google Scholar]
- Kielak A. M., Barreto C. C., Kowalchuk G. A., van Veen J. A., Kuramae E. E. (2016). The ecology of acidobacteria: moving beyond genes and genomes. Front. Microbiol. 7:744 10.3389/fmicb.2016.00744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielland K., Olson K., Ruess R. W., Boone R. D. J. B. (2006). Contribution of winter processes to soil nitrogen flux in taiga forest ecosystems. Biogeochemistry 81 349–360. [Google Scholar]
- Kreyling J., Henry H. A. L. (2011). Vanishing winters in Germany: soil frost dynamics and snow cover trends, and ecological implications. Clim. Res. 46 269–276. [Google Scholar]
- Kuhnert R., Oberkofler I., Peintner U. (2012). Fungal growth and biomass development is boosted by plants in snow-covered soil. Microb. Ecol. 64 79–90. 10.1007/s00248-011-0001-y [DOI] [PubMed] [Google Scholar]
- Ladwig L. M., Ratajczak Z. R., Ocheltree T. W., Hafich K. A., Churchill A. C., Frey S. J. K., et al. (2016). Beyond arctic and alpine: the influence of winter climate on temperate ecosystems. Ecology 97 372–382. 10.1890/15-0153.1 [DOI] [PubMed] [Google Scholar]
- Larsen K. S., Grogan P., Jonasson S., Michelsen A. (2007). Respiration and microbial dynamics in two subarctic ecosystems during winter and spring thaw: effects of increased snow depth. Arctic Antarctic Alpine Res. 39 268–276. [Google Scholar]
- Leff J. W. (2017). mctoolsr: Microbial Community Data Analysis Tools. R package version 0.1.1.2. [Google Scholar]
- Lipson D. A., Monson R. K., Schmidt S. K., Weintraub M. N. (2009). The trade-off between growth rate and yield in microbial communities and the consequences for under-snow soil respiration in a high elevation coniferous forest. Biogeochemistry 95 23–35. [Google Scholar]
- Lipson D. A., Schmidt S. K. (2004). Seasonal changes in an alpine soil bacterial community in the colorado rocky mountains. Appl. Environ. Microbiol. 70 2867–2879. 10.1128/aem.70.5.2867-2879.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson D. A., Schmidt S. K., Monson R. K. (1999). Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology 80 1623–1631. [Google Scholar]
- Lladó S., López-Mondéjar R., Baldrian P. (2017). Forest soil bacteria: diversity, involvement in ecosystem processes, and response to global change. Microbiol. Mol. Biol. Rev. 81:e00063-16 10.1128/MMBR.00063-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C. A., Hamady M., Kelley S. T., Knight R. (2007). Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 73 1576–1585. 10.1128/AEM.01996-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A. A., Martiny J. B. H., Brodie E. L., Martiny A. C., Treseder K. K., Allison S. D. (2018). Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. bioRxiv [preprint]. 10.1101/445866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D., Dozier J. (1992). Climate and energy exchange at the snow surface in the alpine region of the sierra-nevada. 2. Snow cover energy-balance. Water Resour. Res. 28 3043–3054. [Google Scholar]
- McMahon S. K., Wallenstein M. D., Schimel J. P. (2009). Microbial growth in Arctic tundra soil at-2 degrees C. Environ. Microbiol. Rep. 1 162–166. 10.1111/j.1758-2229.2009.00025.x [DOI] [PubMed] [Google Scholar]
- Miller-Rushing A. J., Hoye T. T., Inouye D. W., Post E. (2010). The effects of phenological mismatches on demography. Philos. Trans. R. Soc. B Biol. Sci. 365 3177–3186. 10.1098/rstb.2010.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons P., Michiels C. W., Aertsen A. (2009). Bacterial interactions in biofilms. Crit. Rev. Microbiol. 35 157–168. [DOI] [PubMed] [Google Scholar]
- Mote P. W., Hamlet A. F., Clark M. P., Lettenmaier D. P. (2005). Declining mountain snowpack in western north america. Bull. Am. Meteorol. Soc. 86 39–50. [Google Scholar]
- Muscarella M. E., Lennon J. T. (2018). Trait-based approach to bacterial growth efficiency. bioRxiv [preprint]. 10.1101/427161 [DOI] [PubMed] [Google Scholar]
- Musselman K. N., Clark M. P., Liu C., Ikeda K., Rasmussen R. (2017). Slower snowmelt in a warmer world. Nat. Clim. Chang. 7:214. [Google Scholar]
- Nguyen N. H., Song Z., Bates S. T., Branco S., Tedersoo L., Menke J., et al. (2016). FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fung. Ecol. 20 241–248. [Google Scholar]
- Oksanen J., Blanchet F. G., Kindt R., Legendre P., O’Hara R. B., Simpson G. L., et al. (2011). Vegan: Community Ecology Package. R Package Version 2.2-0. [Google Scholar]
- Patel K. F., Tatariw C., MacRae J. D., Ohno T., Nelson S. J., Fernandez I. J. (2018). Soil carbon and nitrogen responses to snow removal and concrete frost in a northern coniferous forest. Can. J. Soil Sci. 98 436–447. [Google Scholar]
- Phaiboun A., Zhang Y., Park B., Kim M. (2015). Survival kinetics of starving bacteria is biphasic and density-dependent. PLoS Comput. Biol. 11:e1004198 10.1371/journal.pcbi.1004198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J., Bates D., Debroy S., Sarkar S. (2016). nlme: Linear and non-linear mixed-effects models. R package version 3.1–126. [Google Scholar]
- Placella S. A., Brodie E. L., Firestone M. K. (2012). Rainfall-induced carbon dioxide pulses result from sequential resuscitation of phylogenetically clustered microbial groups. Proc. Natl. Acad. Sci. U.S.A. 109 10931–10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevéy J. S., Rixen C., Rüger N., Høye T. T., Bjorkman A. D., Myers-Smith I. H., et al. (2019). Warming shortens flowering seasons of tundra plant communities. Nat. Ecol. Evol. 3 45–52. [DOI] [PubMed] [Google Scholar]
- Price M. N., Dehal P. S., Arkin A. P. (2010). FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purahong W., Wubet T., Lentendu G., Schloter M., Pecyna M. J., Kapturska D., et al. (2016). Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 25 4059–4074. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2010). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Richardson A. D., Bailey A. S., Denny E. G., Martin C. W., O’Keefe J. (2006). Phenology of a northern hardwood forest canopy. Glob. Chang. Biol. 12 1174–1188. [Google Scholar]
- Roller B. R. K., Stoddard S. F., Schmidt T. M. (2016). Exploiting rRNA operon copy number to investigate bacterial reproductive strategies. Nat. Microbiol. 1:16160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadt C. W., Martin A. P., Lipson D. A., Schmidt S. K. (2003). Seasonal dynamics of previously unknown fungal lineages in tundra soils. Science 301 1359–1361. [DOI] [PubMed] [Google Scholar]
- Schimel J., Balser T. C., Wallenstein M. (2007). Microbial stress-response physiology and its implications for ecosystem function. Ecology 88 1386–1394. [DOI] [PubMed] [Google Scholar]
- Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. K., Costello E. K., Nemergut D. R., Cleveland C. C., Reed S. C., Weintraub M. N., et al. (2007). Biogeochemical consequences of rapid microbial turnover and seasonal succession in soil. Ecology 88 1379–1385. [DOI] [PubMed] [Google Scholar]
- Sloat L. L., Henderson A. N., Lamanna C., Enquist B. J. (2015). The effect of the foresummer drought on carbon exchange in subalpine meadows. Ecosystems 18 533–545. [Google Scholar]
- Sorensen P., Brodie E., Beller H., Wang S., Bill M., Bouskill N. (2019). Sample collection metadata for soil cores from the east river watershed, colorado collected in 2017. Watershed Function SFA. 10.21952/WTR/1573029 [DOI] [Google Scholar]
- Steltzer H., Post E. (2009). Seasons and life cycles. Science 324:886. [DOI] [PubMed] [Google Scholar]
- Taylor B. R., Jones H. G. (1990). Litter decomposition under snow cover in a balsam fir forest. Can. J. Bot. 68 112–120. [Google Scholar]
- Thackeray S. J., Henrys P. A., Hemming D., Bell J. R., Botham M. S., Burthe S., et al. (2016). Phenological sensitivity to climate across taxa and trophic levels. Nature 535:241. [DOI] [PubMed] [Google Scholar]
- Uchida M., Mo W., Nakatsubo T., Tsuchiya Y., Horikoshi T., Koizumi H. (2005). Microbial activity and litter decomposition under snow cover in a cool-temperate broad-leaved deciduous forest. Agric. For. Meteorol. 134 102–109. [Google Scholar]
- Ueda M. U., Muller O., Nakamura M., Nakaji T., Hiura T. (2013). Soil warming decreases inorganic and dissolved organic nitrogen pools by preventing the soil from freezing in a cool temperate forest. Soil Biol. Biochem. 61 105–108. [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M., Cole J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnes G. R., Bolker B., Lumley T. (2018). gtools:Various R Programming Tools R package version 3. [Google Scholar]
- Webb C. O., Ackerly D. D., McPeek M. A., Donoghue M. J. (2002). Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33 475–505. [Google Scholar]
- Williams M. W., Brooks P. D., Mosier A., Tonnessen K. A. (1996). Mineral nitrogen transformations in and under seasonal snow in a high-elevation catchment in the Rocky Mountains, United States. Water Resour. Res. 32 3161–3171. [Google Scholar]
- Williard K. W. J., DeWalle D. R., Edwards P. J., Sharpe W. E. (2001). 18O isotopic separation of stream nitrate sources in mid-Appalachian forested watersheds. J. Hydrol. 252 174–188. [Google Scholar]
- Zhalnina K., Louie K. B., Hao Z., Mansoori N., da Rocha U. N., Shi S., et al. (2018). Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 3 470–480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Daily soil freeze-thaw cycles measured in the Hillslope and Floodplain during winter and before and after snowmelt in 2016/17. We considered a freeze-thaw cycle to have occurred if the daily maximum soil temperature was > 0°C and the daily minimum soil temperature was < 0°C.
Pair-wise comparisons of permANOVAs for bacteria and fungi.
Life strategies assigned to archaeal and bacterial OTUs.
Life strategies assigned to fungal OTUs.
Data Availability Statement
Sequencing reads can be found in the NCBI Bioproject database accession no. PRJNA587134. Data for this manuscript were downloaded from the Watershed Function SFA data portal (http://wfsfa-data.lbl.gov) and are archived at the U.S. Department of Energy’s Environmental System Science Data Infrastructure for a Virtual Ecosystem (ESS-DIVE, Sorensen et al., 2019).