We have reported our present work about the phase separation behavior and molecular diffusion inside cell mimicking droplets in the session entitled “Crossover of physics theories and experiments” at the 2019 BSJ Meeting. Cells contain highly concentrated polymer solution in the micrometer-sized cell membrane. Molecular Crowding is one of the central issues to tackle biophysical basis of cell system (Zhou et al. 2008). Under crowding conditions, nano-sized space has been reported to affect biopolymer stabilities (Eggers and Valentine 2001), or phase transition (Klein and Kumacheva 1995; Luengo et al. 1997). Recently, from experiments using model cells, not only the nano-scale but also micrometric confinement (i.e., the cell mimetic membrane confinement) is reported to provide a characteristic environment which induces different phenomenon than bulk solution (Watanabe and Yanagisawa 2018) (Biswas et al. 2012) (Yanagisawa et al. 2014). However, the cause is yet to be elucidated. In this work, we quantitatively study molecular diffusion using fluorescence correlation spectroscopy (FCS) (Ochiishi et al. 2016) in cell-mimicking droplets, i.e., water-in-oil droplets of an aqueous polymer solution covered by a lipid monolayer (Fig. 1) (Watanabe and Yanagisawa 2018). In these studies, polymers such as globular proteins (bovine serum albumin—BSA) and linear chain polymers (polyethylene glycol—PEG) are used.
Fig. 1.
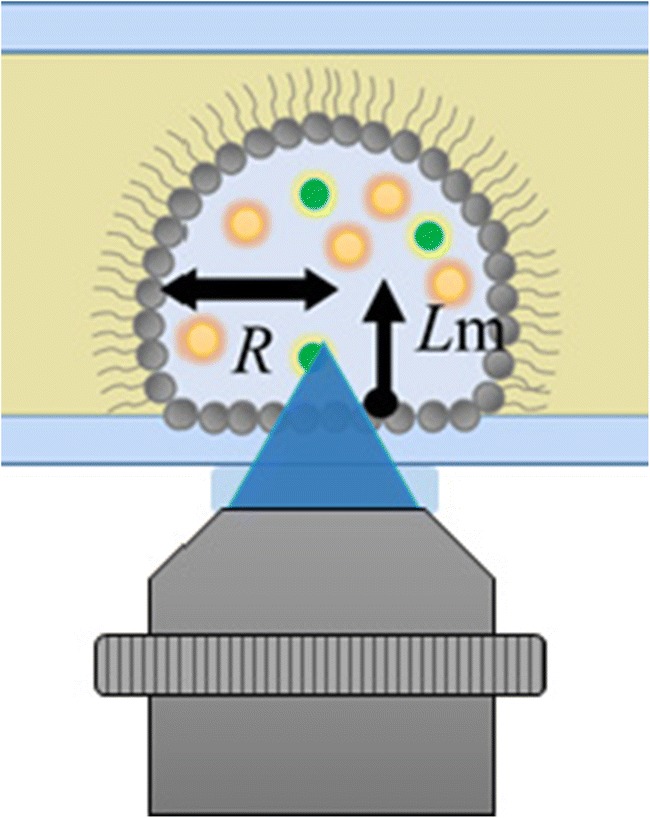
FCS measurement inside adhering polymer droplet on a glass plate
Our result shows that the molecular diffusion slows down at high concentration of BSA and PEG solution in the spherical droplet for radii R below 20–30 μm. In addition, the slow diffusion is independent of the distance from the membrane surface Lm. Further, the slow diffusion is observed in disk-shaped droplets of which the gap between the membrane interface is less than 20 μm, even when the volume of the droplets exceeds the volume of spherical droplets with a radius of 20–30 μm. These results show that the slow diffusion is due to the confinement between the membranes but not due to the small volume effect. We hypothesize that the membrane micrometric confinement induces increased polymer-polymer interaction that possibly leads to transient polymer cluster formation causing slow diffusion.
We also found that liquid-liquid phase separation (LLPS) of BSA-PEG system is induced by membrane confinement at a size corresponding to where the slow diffusion occurs (Watanabe et al. 2020). These results strongly suggest correlation between cell-sized membrane confinement and phase behaviors of biomolecules. Our quest for understanding the “cell-size confinement effect” keeps going.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Biswas N, Ichikawa M, Datta A, Sato YT, Yanagisawa M, Yoshikawa K. Phase separation in crowded micro-spheroids: DNA-PEG system. Chem Phys Lett. 2012;539:157–162. doi: 10.1016/j.cplett.2012.05.033. [DOI] [Google Scholar]
- Eggers DK, Valentine JS. Molecular confinement influences protein structure and enhances thermal protein stability. Protein Sci. 2001;10:250–261. doi: 10.1110/ps.36201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Kumacheva E. Confinement-Induced Phase Transitions in Simple Liquids. Science. 1995;269(5225):816–819. doi: 10.1126/science.269.5225.816. [DOI] [PubMed] [Google Scholar]
- Luengo G, Schmitt FJ, Hill R, Israelachvili J. Thin film rheology and tribology of confined polymer melts: contrasts with bulk properties. Macromolecules. 1997;30:2482–2494. doi: 10.1021/ma9519122. [DOI] [Google Scholar]
- Ochiishi T, Doi M, Yamasaki K, Hirose K, Kitamura A, Urabe T, Hattori N, Kinjo M, Ebihara T, Shimura H (2016) Development of new fusion proteins for visualizing amyloid-beta oligomers in vivo Sci Rep 6:22712 doi:10.1038/srep22712 [DOI] [PMC free article] [PubMed]
- Watanabe C, Yanagisawa M. Cell-size confinement effect on protein diffusion in crowded poly(ethylene)glycol solution. Phys Chem Chem Phys. 2018;20:8842–8847. doi: 10.1039/c7cp08199e. [DOI] [PubMed] [Google Scholar]
- Watanabe Chiho, Kobori Yuta, Yamamoto Johtaro, Kinjo Masataka, Yanagisawa Miho. Quantitative Analysis of Membrane Surface and Small Confinement Effects on Molecular Diffusion. The Journal of Physical Chemistry B. 2020;124(6):1090–1098. doi: 10.1021/acs.jpcb.9b10558. [DOI] [PubMed] [Google Scholar]
- Yanagisawa Miho, Sakaue Takahiro, Yoshikawa Kenichi. International Review of Cell and Molecular Biology. 2014. Characteristic Behavior of Crowding Macromolecules Confined in Cell-Sized Droplets; pp. 175–204. [DOI] [PubMed] [Google Scholar]
- Zhou HX, Rivas G, Minton AP. Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys. 2008;37:375–397. doi: 10.1146/annurev.biophys.37.032807.125817. [DOI] [PMC free article] [PubMed] [Google Scholar]


