Abstract
Purpose
To assess the role of intracavitary contrast-enhanced ultrasound (IC-CEUS) as a focused ultrasound (US) examination aimed at supporting a single physician in the management of interventional procedures for abdominal fluid collections/abscesses.
Methods
In 43 patients (27 M/16 F, median age 68 years, range 35–91), a single physician performed catheter drainage (42) or needle aspiration (3) for the following: 14 infected abdominal fluid collections, 11 non-infected abdominal fluid collections, 9 pyogenic liver abscesses, 8 gallbladder empyema, and 3 infected pancreatic fluid collections. IC-CEUS (0.1–0.2 mL of SonoVue in 20 mL of saline) was carried out during catheter/needle placement and during the follow-up for catheters left in place.
Results
Immediate IC-CEUS allowed to verify the (1) correct positioning of the needle/catheter inside the target in all cases and (2) communication with adjacent structures so as to choose a proper treatment in 21% of the cases. Follow-up IC-CEUS aided in the management of 40 catheters left in place. Appropriate treatment was implemented in 19.3% of the cases because of the presence of biliary fistulas and gallbladder perforation. IC-CEUS helped the physician with the appropriate timing of catheter removal by providing information on catheter malfunction (due to obstruction/dislodgement) and the size of residual undrained cavities. No side effects were registered following IC-CEUS.
Conclusion
Even if not strictly performed at bedside, IC-CEUS may represent an example of point-of-care ultrasound since it allows an interventional clinician to assess needle/catheter placement success, make treatment decisions, and choose the optimal timing for catheter removal with low costs and without side effects.
Keywords: CEUS, Intracavitary, Abscess, Liver, Abdomen, Drainage
Introduction
In the recent years, the value of contrast-enhanced ultrasound (CEUS) in US-guided hepatobiliary interventions has been demonstrated to have added value in procedure planning and guiding [1–6]. The benefits of CEUS in the evaluation of thermal ablation effectiveness are also well known [7–9].
On the contrary, the application of an ultrasound contrast agent (UCA) via drainage catheters [10] is far less known. Although endocavitary injection of UCA is currently an off-label use, several IC-CEUS applications are reported in the current non-liver CEUS guidelines of the EFSUMB [11]: voiding sonography for the study of vesicoureteral reflux in pediatric populations, contrast-enhanced hysterosalpingo-contrast sonography for imaging of the tubular patency, imaging of obstructed excretory systems, such as the urinary tract (through a nephrostomy tube) and biliary tree (via percutaneous or endoscopic drainage catheters), and treatment guidance for abdominal, pelvic, and thoracic fluid collections (infected and non-infected).
A common feature of all these applications is the added value in the management of patients, since immediate diagnostic and treatment decisions based on IC-CEUS can be made by physicians in charge of patients. In this sense, IC-CEUS seems to fulfill some requirements of point-of-care ultrasound (POCUS) [12–15], even if it is not strictly performed at the bedside.
The aim of this study was to describe the use of IC-CEUS by a single physician in the management of hepatic and abdominal fluid collections and abscesses, highlighting the findings that could allow this technique to be considered as an example of POCUS.
Materials and methods
An Institutional Review Board-approved patient database was collected over the last 3 years (2017–2019).
All of the patients had signed written informed consent forms at the time of the interventional procedures.
Forty-three consecutive patients (27 males/16 females, median age 68 years, range 35–91) were seen because of the following: infected abdominal fluid collection (IAFC) in 14 cases (median size 8 cm, range 7–15 cm), non-infected abdominal fluid collection (NIAFC) in 11 cases (median size 10 cm, range 8–14 cm), pyogenic liver abscess (PLA) in 9 cases (median size 8 cm, range 3–15 cm), gallbladder empyema (GE) in 8 cases, and infected pancreatic fluid collection (IPFC) in 3 cases (median size 12, range 10–20 cm).
Convex or microconvex probes with or without a biopsy attachment were used to guide abscess drainage or needle aspiration and to perform intravenous CEUS and IC-CEUS.
A state-of-the-art US machine (Resona 7 system; Mindray Bio-Medical Electronics Co., Shenzhen, China) with dedicated software for CEUS was used throughout the study.
A single physician with more than 20 years of experience in interventional US-guided procedures performed all the interventional maneuvers and followed-up on the clinical and imaging evolution of all the patients.
For continuous drainage, 8–12 French pigtail catheters with thread locks were placed into hepatic and abdominal fluid collections using the Seldinger technique. Simple aspiration was carried out with 18–16 gauge needles in single or multiple sessions as needed.
Prior to the interventional procedures, all the patients underwent a thorough imaging work-up by means of US, CEUS, contrast-enhanced CT scan, and/or MRI.
For the purposes of IC-CEUS, 0.1–0.2 mL of SonoVue (sulfur hexafluoride with a phospholipid shell; Bracco SpA, Milan, Italy) in 20 mL physiological saline was used as a UCA and given in small doses (2–5 mL). The UCA was injected into catheters or needles immediately after catheter/needle introduction, after the completion of procedures and during the follow-up for catheters left in place. At the end of the IC-CEUS evaluation, the UCA was re-aspirated as much as possible. After drainage removal and needle aspiration, imaging techniques (US, CEUS, CT scan, and/or MRI) were used to check the patients’ clinical evolution on a case-by-case basis.
All the patients underwent antibiotic therapy based on the microbiological analysis of aspirates.
Results
Of the 43 patients, 40 (93%) received catheter drainage (in two patients, two catheters were simultaneously placed), and three patients with PLA were treated with needle aspirations in single or multiple (up to three) sessions.
In all cases, IC-CEUS was performed shortly after aspiration of 10–20 mL of intracavitary fluid for microbiological and chemical–physical examination purposes.
The injection of UCA in this phase allowed the following:
Demonstration of the correct position of the catheter/needle in 100% of the cases. This check proved especially relevant in cases of small targets, difficult sites (e.g., subdiaphragmatic) or when the US B-mode image was blurred because of the presence of either air or blood, the latter being caused by procedural trauma inside the abscess (Fig. 1).
Presence/lack of communication between multiple compartments of fluid collections with consequent treatment decisions. In 3 cases (1 PLA, 1 NIAFC, and 1 IPFC) with enhancement of all cavities, placement of a single catheter was the treatment of choice (Fig. 2). In contrast, in 2 cases (1 PLA and 1 NIAFC), lack of communication induced the operator to aspirate singular PLA loculations instead of perform catheter drainage (an additional session of multiple needle aspirations was thereafter carried out), while in the remaining patient, positioning of two catheters was necessary.
Demonstration of communication with adjacent structures. In one patient, UCA passed from an abdominal abscess into a nearby bowel loop, prompting immediate surgery (Fig. 3). In two PLAs, IC-CEUS showed communication with an apparently unobstructed biliary tree. In this case, not only was the etiology of PLA clarified, but endoscopic stenting was also thereafter programmed. In another patient with malignant obstruction of the common bile duct and two distinct PLAs, IC-CEUS enabled visualization of fistulous communications with the right pleural space and the biliary tree (Fig. 4). Thoracic drainage and replacement of the biliary endoprosthesis were carried out, along with deployment of two catheters to drain the PLAs.
Demonstration of the real size of the liquefied portion in a huge PLA. IC-CEUS only showed a small non-enhanced cavity; thus, inducing the operator to resort to simple needle aspiration, avoiding catheter deployment. This treatment option proved sufficient to heal the abscess, along with the appropriate antibiotic therapy.
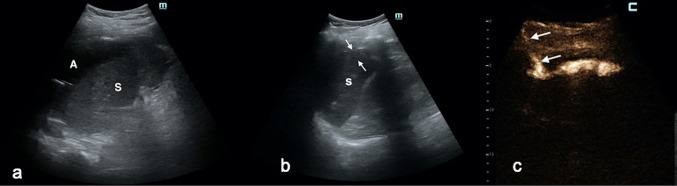
Fig. 1.
a A left subdiaphragmatic abscess (a) was displayed around the spleen (S) in a 40-year-old man after bariatric surgery. b After catheter positioning, the abscess cavity (between white arrows) was barely recognizable, whereas the catheter tip was not seen. B-mode scan was blurred with blood due to procedural trauma (S = Spleen). c IC-CEUS enabled the localization of the catheter inside the collection (white arrows) and the visualization of the real extension of the abscess; no extravasation into the adjacent splenic parenchyma was noted
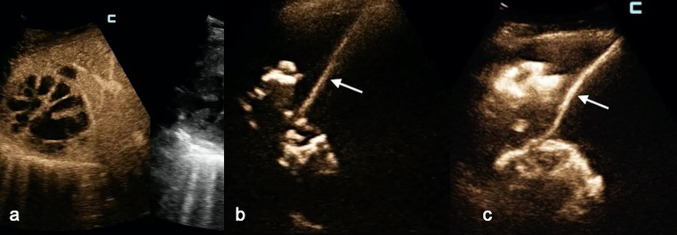
Fig. 2.
a CEUS evaluation (per venam) of a huge multiloculated liver abscess: this scanning modality could not establish whether or not the cavities were communicating. b Before catheter deployment, IC-CEUS via the needle (white arrow) placed in the greatest cavity demonstrated that all cavities were communicating. c A single catheter was positioned. IC-CEUS confirmed that all abscess pouches were communicating, and there was no need to place another drainage
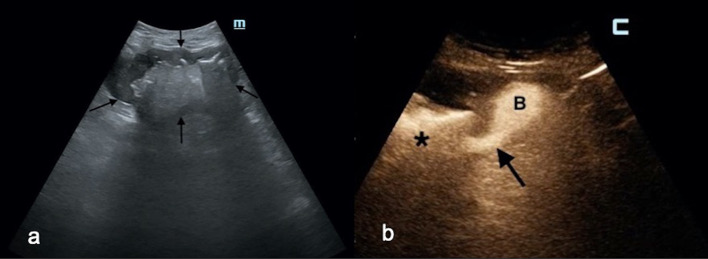
Fig. 3.
a A large abscess (between black arrows) was displayed in the middle abdomen in an 81-year-old man with high-grade fever. b After catheter placement, fecaloid material was aspirated; immediate IC-CEUS showed a fistulous communication (black arrow) between the abscess (black asterisk), and an adjacent bowel loop (b). This finding was confirmed at emergency surgery
Fig. 4.
a A large liver abscess (between white arrows) was displayed in a 70-year-old woman with malignant obstruction of the common bile duct already treated with endoscopic stenting. b IC-CEUS via the drainage catheter (white arrowheads) demonstrates communication (white arrows) between the abscess cavity (a) and the biliary tree (black star), suggesting obstruction of the biliary endoprosthesis. c In the same patient, another liver abscess (LA) at the dome was drained. The diluted contrast agent solution injected through a catheter (white arrowhead) was seen not only in the liver (black asterisk) but also in the thorax (white arrows), suggesting an extension of the inflammatory process through the diaphragm. d CT scan confirmed IC-CEUS findings and thoracic drainage was carried out
Overall, immediate IC-CEUS influenced the operator’s treatment options and management in 9 out of 43 patients (21%).
After catheter placement, IC-CEUS was carried out twice or thrice a week to check catheter status and possible complications. In this phase, IC-CEUS allowed demonstration of the following:
Catheter malfunction due to obstruction (four cases) or dislodgement (five cases) (Fig. 5) with immediate catheter withdrawal
Presence of communication between postoperative NIAFC and the intrahepatic biliary tree (six cases). Endoscopic stenting (two cases) and sphincterotomy (three cases) successfully contributed to the resolution of such complications (Fig. 6).
Gallbladder perforation in a patient with percutaneous cholecystostomy for GE. Emergency surgery ensued.
Size of the fluid collection/abscess residual cavity (24 cases). Along with improved clinical (apyretic status lasting at least 48 h) and laboratory (drop in C-reactive protein and white blood cell count values) findings and an improved amount (daily output less than 20 mL per day) and quality (absence of pus/bile/blood) of drain, significant shrinkage of the cavity shown on IC-CEUS helped the operator decide on the appropriate timing for catheter removal. The same was true for patients with percutaneous cholecystostomy. Other than the patient who underwent emergency surgery, the other seven cases had uneventful outcomes without relapse (at the time of writing, only two had undergone elective surgery for gallstone disease).
Fig. 5.
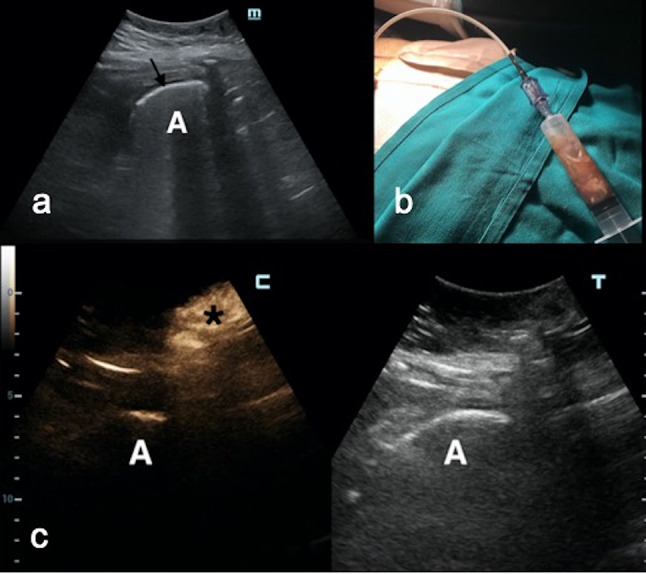
a A perigastric gas-filled (black arrow) abscess (a) in a 35-year-old woman after bariatric surgery was visualized at US B-mode. b: Percutaneous catheter placement allowed drainage of the abscess. c Three days after catheter deployment, drain output suddenly dropped. A subcutaneous depot of contrast medium (black asterisk) was depicted, but not a single bubble in the abscess (a) was seen
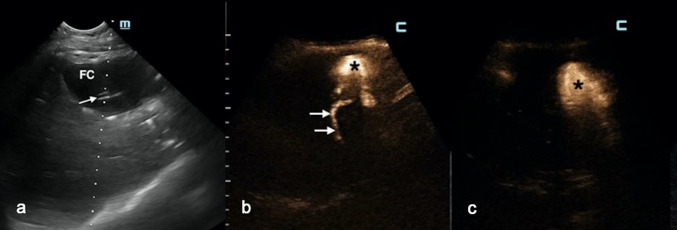
Fig. 6.
a Postoperative extrahepatic fluid collection (FC) with a catheter inside (white arrow). b After 2 days of continuous drainage, the fluid drained appeared stained with bile. IC-CEUS: a fistulous tract between the fluid collection (black asterisk) and the intrahepatic biliary tree (white arrows) was noted. c After endoscopic sphincterotomy, no bubble moved from the fluid collection (black asterisk) to the biliary tree (black liver)
After catheter removal, none of the patients relapsed and no additional catheter drainage or needle aspiration was needed.
IC-CEUS during follow-up helped the operator make quick decisions regarding treatment management in 6 out of 31 patients (19.3%). In general, catheter drainage and needle aspiration proved successful in 41 out of 43 cases (95.3%).
During the follow-up of those with catheters left in place, only two patients underwent CT scans for monitoring the evolution of their PLA.
Injection of the UCA was devoid of any collateral effect and/or complication. In particular, neither superinfection of sterile fluid collections nor allergic reactions occurred following UCA administration.
Discussion
There is limited existing literature on the efficacy and utility of IC-CEUS in the management of abdominal fluid collections or abscesses [2, 3, 16–20]. Among these papers, only two were case series. Girlich et al. [16] reported the clinical relevance of IC-CEUS in the percutaneous treatment of 15 abdominal abscesses (mostly located in the liver) since the exact position of the drainage, the real abscess size and the presence of septs or communication of different abscess regions could be demonstrated. In addition to these advantages, in a large cohort of 71 patients with abdominal abscesses, Ignee et al. [19] reported additional benefits of IC-CEUS: assessment of communication between the abscess cavity and adjacent anatomical structures (peritoneal cavity, pleural space, vessels, hollow viscera, biliary, or pancreatic duct) and in the follow-up for catheters left in place.
The present series confirms the added value of IC-CEUS in the management of abdominal abscesses during two phases: immediately after placement of a needle/catheter inside the pathological cavity and during the follow-up for catheters left in place. In the first case, several goals were achieved: (1) assessment of positioning of the needle/catheter inside the abscess cavities in 100%; this turned out to have relevant significance in difficult-to-reach sites, in small cavities and when the US B-mode vision was obscured because of blood or air; and (2) assessment of communication between a pathological cavity and other anatomic structures (i.e., hollow Viscera, biliary tree, pleural space or other abdominal collections). In this initial phase, IC-CEUS aided in treatment decisions in 21% of cases by inducing the operator to resort to emergency surgery or endoscopic stenting, and to modulate the interventional procedure by choosing both simple aspiration versus catheter deployment and the number of catheters to be placed.
The usefulness of IC-CEUS was confirmed during the follow-up of patients with a continuous drain [17–19]. In fact, treatment options (e.g., endoscopy stenting or sphincterotomy, emergency surgery) were made based on IC-CEUS results in approximately 19% of the cases. Overall, the operator in charge of all patients with abdominal fluid collections/abscesses relied on IC-CEUS results to decide on treatment options in 15 out of 43 cases (34.8%).
In addition, IC-CEUS helped the physician with the appropriate timing of catheter removal in 33 out of 40 cases (82.5%) by providing information on both catheter malfunction (due to obstruction/dislodgement) and the size of residual undrained cavities. Along with clinical and laboratory findings and daily drainage output, demonstration of the persistently very small size of the pathological cavities represents an important additional parameter to aid the operator in choosing the optimal timing for catheter withdrawal.
In the author’s opinion, the clinical value of IC-CEUS shown herein in the management of abdominal fluid collections/abscesses may qualify this technique as an example of POCUS [12–15].
Strictly speaking, IC-CEUS is not delivered at bedside, as is usually done with POCUS, even if the availability of compact, portable scanners offering contrast-enhanced technology could reasonably predict its bedside use, especially during the follow-up of patients with catheters left in place. However, if one considers the notable characteristics of POCUS, e.g., direct performance of US by the physician in charge of the patient and immediate assessment of new diagnostic hypotheses to make treatment/management decisions, the present series demonstrates that IC-CEUS fulfills all these requirements. In this sense, IC-CEUS stands as a problem-solving technique that provides invaluable information to immediately modify the management of a single case. It is therefore not a negligible matter that all decisions made based on IC-CEUS findings turned out to be useful for the clinical outcome of all the patients.
Of course, in the clinical scenario described herein, comprehensive knowledge of US, CEUS, and interventional maneuvers is required compared with the original definition of POCUS, i.e., ultrasonography performed at bedside and interpreted directly by the physician in charge of the patient, even with no advanced level of US training [12, 13, 21, 22]. In this sense, IC-CEUS could be merged into the definition for POCUS even if in a complex clinical scenario requiring high-level skills.
The safety of the procedure should be emphasized; it confirms the results of all previous studies dealing with endocavitary use of SonoVue [10, 11, 16–20]. Indeed, side effects or complications were never observed in any of the patients included in the present study. In addition, substantial sparing of X-ray exposure was achieved since additional CT scans were needed in only two cases during the follow-up of patients with continuous drainage.
Finally, IC-CEUS is a very inexpensive method since only a few drops are needed to perform a thorough examination of a single patient without substantial impairment of the UCA vial devoted to intravenous use.
To still consider all endocavitary applications of UCA off-label uses seems to be unjustified in light of the substantial benefit of these diagnostic modalities and the lack of risk reported by all the studies available in the existing literature.
In conclusion, the present experience of IC-CEUS by a single clinician in charge of patients with abdominal fluid collections/abscesses confirms the pivotal role of this safe and inexpensive technique in the management of such pathological conditions and could suggest the possibility of including this technique in a wider definition of POCUS.
Author contributions
The author (GF) was the sole contributor to the study conception and design, material preparation, data collection and analysis, interpretation, literature research, and manuscript drafting and editing.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflicts of interest
The author declare that they have no conflict of interest.
Ethical approval
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the IRB of Pineta Grande Hospital who determined that our study did not need ethical approval. At the time of interventional procedures all patients signed an informed consent.
Informed consent and consent for publication
Not applicable (information is anonymized and the submission does not include images that may identify the person). Not applicable (information is anonymized and the submission does not include images that may identify the person).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Claudon M, Dietrich CF, Choi BI, et al. World Federation for Ultrasound in Medicine European Federation of Societies for Ultrasound: Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver—update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Nolsøe CP, Nolsøe AB, Klubien J, Pommergaard HC, Rosenberg J, Meloni MF, Lorentzen T. Use of ultrasound contrast agents in relation to percutaneous interventional procedures: a systematic review and pictorial essay. J Ultrasound Med. 2018;37:1305–1324. doi: 10.1002/jum.14498. [DOI] [PubMed] [Google Scholar]
- 3.Lorentzen T, Nolsøe CP, Ewertsen C, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part I general aspects (long version) Ultraschall Med. 2015;36:E1–E14. doi: 10.1055/s-0035-1553593. [DOI] [PubMed] [Google Scholar]
- 4.Francica G, Meloni MF, de Sio I, et al. Biopsy of liver target lesions under contrast-enhanced ultrasound guidance—a multi-center study. Ultraschall Med. 2018;39:448–453. doi: 10.1055/s-0043-122496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparchez Z, Mocan T, Hagiu C, Kacso G, Zaharie T, Rusu I, Al Hajjar N, Leucuta DC, Sparchez M. Real-time contrast-enhanced-guided biopsy compared with conventional ultrasound-guided biopsy in the diagnosis of hepatic tumors on a background of advanced chronic liver disease: a prospective, randomized, clinical trial. Ultrasound Med Biol. 2019;45:2915–2924. doi: 10.1016/j.ultrasmedbio.2019.07.678. [DOI] [PubMed] [Google Scholar]
- 6.Francica G, Meloni MF, Riccardi L, et al. Ablation treatment of primary and secondary liver tumors under contrast-enhanced ultrasound guidance in field practice of interventional ultrasound centers. A multicenter study. Eur J Radiol. 2018;105:96–101. doi: 10.1016/j.ejrad.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Mauri G, Porazzi E, Cova L, et al. Intraprocedural contrast-enhanced ultrasound (CEUS) in liver percutaneous radiofrequency ablation: clinical impact and health technology assessment. Insights Imaging. 2014;5:209–216. doi: 10.1007/s13244-014-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meloni MF, Smolock A, Cantisani V, et al. Contrast enhanced ultrasound in the evaluation and percutaneous treatment of hepatic and renal tumors. Eur J Radiol. 2015;84:1666–1674. doi: 10.1016/j.ejrad.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Meloni MF, Francica F, Eisenbrey J. CEUS in treatment response evaluation: RFA, microwave. In: Lyshchik A, editor. Specialty imaging: fundamentals of CEUS. 1. Amsterdam: Elsevier; 2019. pp. 322–327. [Google Scholar]
- 10.Ignee A, Schuessler G, Cui XW, Dietrich CF. Intracavitary contrast medium ultrasound—different applications, a review of the literature ad future prospects. Ultraschall Med. 2013;34:504–525. doi: 10.1055/s-0033-1354852. [DOI] [PubMed] [Google Scholar]
- 11.Sidhu PS, Cantisani V, Dietrich CF, et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (short version) Ultraschall Med. 2018;39:154–180. doi: 10.1055/s-0044-101254. [DOI] [PubMed] [Google Scholar]
- 12.Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364:749–757. doi: 10.1056/NEJMra0909487. [DOI] [PubMed] [Google Scholar]
- 13.Piscaglia F, Dietrich CF, Nolsoe C, Gilja OH, Gaitini D. Birth of ‘‘echoscopy’’: the EFSUMB point of view. Ultraschall Med. 2013;34:92. doi: 10.1055/s-0032-1319207. [DOI] [Google Scholar]
- 14.Solomon SD, Saldana F. Point-of-care ultrasound in medical education-stop listening and look. N Engl J Med. 2014;370:1083–1085. doi: 10.1056/NEJMp1311944. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich CF, Goudie A, Chiorean L, Cui XW, Gilja OH, Dong Y, et al. Point of care ultrasound: a WFUMB position paper. Ultrasound Med Biol. 2017;43(1):49–58. doi: 10.1016/j.ultrasmedbio.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 16.Girlich C, Buttner R, Schacherer D, Klebl F. Contrast-enhanced sonographic drainage control: a feasibility study [in German] Z Gastroenterol. 2011;49:1470–1474. doi: 10.1055/s-0031-1281579. [DOI] [PubMed] [Google Scholar]
- 17.Heinzmann A, Muller T, Leitlein J, Braun B, Kubicka S, Blank W. Endocavitary contrast enhanced ultrasound (CEUS)—work in progress. Ultraschall Med. 2012;33:76–84. doi: 10.1055/s-0031-1299056. [DOI] [PubMed] [Google Scholar]
- 18.Muller T, Blank W, Leitlein J, Kubicka S, Heinzamann A. Endocavitary contrast-enhanced ultrasound: a technique whose time has come? J Clin Ultrasound. 2015;43:71–80. doi: 10.1002/jcu.22250. [DOI] [PubMed] [Google Scholar]
- 19.Ignee A, Jenssen C, Cui XW, Schuessler G, Dietrich CF. Intracavitary contrast-enhanced ultrasound in abscess drainage: feasibility and clinical value. Scand J Gastroenterol. 2016;51:41–47. doi: 10.3109/00365521.2015.1066423. [DOI] [PubMed] [Google Scholar]
- 20.Kessner R, Nakamoto DA, Kondray V, Partovi S, Ahmed Y, Azar N. Contrast-enhanced ultrasound guidance for interventional procedures. J Ultrasound Med. 2019;38:2541–2557. doi: 10.1002/jum.14955. [DOI] [PubMed] [Google Scholar]
- 21.Arienti V, Camaggi V. Clinical applications of bedside ultrasonography in internal and emergency medicine. Intern Emer Med. 2011;6:195–201. doi: 10.1007/s11739-010-0424-3. [DOI] [PubMed] [Google Scholar]
- 22.Sabatino V, Caramia MR, Curatola A, Deidda A, Cinicola B, Iodice F, et al. Point-of-care ultrasound (POCUS) in a remote area of Sierra Leone: impact on patient management and training program for community health officers. J Ultrasound. 2020 doi: 10.1007/s40477-019-00426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.