Abstract
Thyroglossal duct cysts (TGDCs) are the most common congenital abnormality of the neck, accounting for approximately 70% of congenital neck lesions. Two-thirds of thyroglossal duct anomalies are diagnosed within the first three decades of life, with more than half being identified before 10 years of age. The age of presentation, clinical examination and imaging are essential for an accurate diagnosis. This review aims to summarize the imaging findings of TGDCs and their main differential diagnoses with emphasis on ultrasound assessment. A focus on site-specific key differentiating between them is also addressed.
Electronic supplementary material
The online version of this article (10.1007/s40477-020-00433-2) contains supplementary material, which is available to authorized users.
Keywords: Cystic neck lesions, Neck imaging, Neck ultrasound, Magnetic resonance imaging, Doppler techniques
Introduction
The thyroglossal duct (TGD) connects the thyroid to the tongue between the fourth and seventh weeks of development. This duct passes through the muscles of the tongue and then anterior to the hyoid bone and larynx and normally involutes by the tenth week of development (Fig. 1) [1, 2].
Fig. 1.
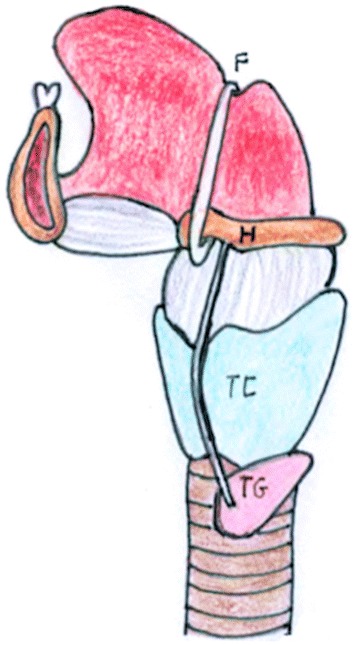
Schematic diagram illustrating the anatomic pathway of TGD. It originates from the foramen caecum (F) of the tongue, descends in the midline first anteriorly and then looping superiorly behind the hyoid bone (H). It descends further downwards anterior to the thyrohyoid membrane, thyroid cartilage (TC) and reaches its final position at the thyroid gland (TG)
The most common congenital anomaly related to the TGD is the thyroglossal duct cyst (TGDC), accounting for approximately 70% of the congenital lesions that occur in the neck [3].
Although they may occur at any age, the peak incidence is in the 1–10-year age group. However, recently it has been shown that TGDCs are more common in the adult population than previously believed. No gender predilection has been reported [4, 5].
It is thought that TGDC represents a segment of the duct that fails to regress and consequently differentiate into epithelial-lined cysts. TGDCs develop anywhere along the course of the remnant duct, from the base of the tongue to the suprasternal region [1–3]. As the thyroglossal duct is in close proximity to the hyoid bone during its development and descent, most TGDCs are located in the midline or just off-midline in the region of the hyoid bone [6]. About 20% to 25% are suprahyoid, 15% to 50% occurring at the level of the hyoid bone, where they may be anterior or posterior to the hyoid bone, and 25% to 65% occurring in the infrahyoid part of the neck [7]. In accordance with the literature, the majority of off-midline TGDCs are infrahyoid, characteristically located adjacent to the outer surface of the thyroid cartilage deep in the strap muscles: “the more inferior the cyst, the more likely it is to be off the midline” [8, 9].
Histologically, the cyst is composed of epithelial lining with or without ectopic thyroid gland tissue. Specifically, the epithelial lining varies with location: cysts located near the tongue are lined by stratified squamous epithelium, whereas cysts located in the neck near the thyroid gland are lined by cells similar to thyroidal acinar epithelium [10]. Functional thyroid tissue within the TGDC has been described and more than half contain normal thyroid tissue in their walls [11]. Cyst development or enlargment is thought to be a response to repeated local infection and inflammation [10, 11].
Clinically, TDC presents as painless median swelling or anterior neck mass that moves on protruding the tongue or swallowing, owing to attachment to the hyoid bone [1, 2].
However, although clinical history and examination may suggest the diagnosis, imaging is required to confirm the clinical diagnosis and assess the anatomical extent of the lesion before treatment [12, 13].
Aim of this review is to summarize the imaging findings of TGDCs and their main differential diagnoses with emphasis on ultrasound (US) assessment. A focus on site-specific key differentiating between them is also addressed.
Imaging techniques
US is an ideal initial imaging investigation for neck masses as it reveals the solid or cystic nature in most cases and localizes the lesion in relationship to the surrounding structures. US is readily available, relatively inexpensive, and does not involve ionizing radiation. Recently, modern ultrasound machines equipped with high-resolution transducers provide US images with excellent spatial and contrast resolution [14, 15]. Development of color—and power—Doppler (CD and PD) applications have further led to great improvements in diagnostic utility of US by providing a real-time evaluation of vascularity, which is an important clue in distinguishing benign from malignant lesions [16, 17]. US has also the unique advantage over other imaging techniques in providing reliable, real-time guidance for fine-needle aspiration cytology (FNAC) or core biopsy [14]. US imaging is not only to aid in the diagnosis, but also to identify a normal thyroid gland in orthotopic position [18]. Thus, if the US demonstrates a normal thyroid gland and the patient is clinically euthyroid, there is no need for preoperative thyroid function testing [19, 20]. Disadvantages of US may include its low specificity in the diagnosis of the lesions with complex appearance owing to the viscous nature of TGDC secretions; however, in Gupta series [20], there was a false-positive rate of only 5%.
Cross-sectional imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), serve a supplementary role in work-up of cystic neck lesions [13]. The multiplanar capability of both enables a precise pre-operative anatomical localization, particularly for more deep-seated and locally extensive lesions. The advantages of MRI over CT in imaging other parts of the body also apply to the neck, including better contrast resolution, lack of ionizing radiation and safer contrast agents. By comparison, CT examinations offer the advantages of superior assessment of osseous structures, shorter examination time, wider patient access and lower cost. However, CT scans are not performed as frequently on children as on adults because children require to limit radiation exposure. Similarly, MRI has some limitations related to its high costs and to the possible motion and breathing artifacts due to a poor patient collaboration, which likewise requires sedation in infants and young children [13, 14]. Therefore, CT and MRI of the neck, especially in children, should be performed selectively [20], being more useful for lesions that are present in unusual locations [21], in case of intralaryngeal involvement and for recurrent TGD remnants [22].
More recently, US elastosonography [23–25] and US contrast agents [24] have been proposed as additional diagnostic tools for the evaluation of various organs including the structures of the neck.
Stiffness of the tissue structures may be accessed using ultrasound elastosonography (USE). By applying pressure to the inspection sites, USE acquires response information resulting from the pressure and determines the tissue stiffness [23]. The two most frequently used USE methods are strain elastosonography (SE) and shear wave elastography (SWE) [23, 24]. SE acquires the deformation information of the tissues under pressure, with greater deformations indicating lower tissue stiffness and less deformations representing greater tissue stiffness and presents the results in different colors or differing degrees of brightness. SWE obtains the shear wave information from the tissues under pressure, with faster propagation velocities of shear wave indicating greater tissue stiffness, and also presents the results in different colors or differing degrees of brightness. In addition, SWE also measures and quantifies the shear wave propagation velocities at the regions of interest and, therefore, provides more information compared with SE. Stiffness of a malignant tumor is typically higher compared with a benign tumor. Thus, USE has been widely accepted as an effective method for differentiating between benign and malignant lesions [23–25].
Contrast-enhanced ultrasound (CEUS) using second-generation contrast agents is a “new”, simple, immediate, and effective diagnostic implementation of conventional US [26–28]: microbubbles circulate freely inside the body and constitute an intravascular contrast agent; therefore, they permit analysis of both macro- [29, 30] and microvascular [31] blood flow giving more detailed and advanced results than the Doppler studies [32]. In the neck, CEUS could help both in the differentiation between a solid and a cystic neck lesion and also in determining whether enhancing septa or nodules are present within the cystic lesion by depicting more vessels and more intense perfusion than Doppler [32]. In recent years, CEUS has proved to be extremely useful in the evaluation of both liver lesions [33, 34] and complex renal cysts [35]. However, to date, the value of non-hepatic application of CEUS in the examination of superficial structures such as cystic neck lesions has not been studied in detail and the CEUS has not yet entered in standardized imaging protocols for clinical practice.
US findings
The typical US description of a TGDC is that of a well-circumscribed, round or oval anechoic lesion with thin walls and increased through-transmission; no internal flow with Doppler imaging [36]. However, this appearance is seen in 42% cases only [7]. Some studies have shown that most TGDCs are complicated with infection or hemorrhage presenting as homogeneous hypoechoic lesions with diffuse and fine internal echoes (Figs. 2, 3) or heterogeneous complex ones with solid-appearing internal septa and wall nodularity (Fig. 4) [3]. A pseudosolid pattern due to the diffuse proteinaceous fluid content of the cyst secreted by the epithelial lining has also been described [36]. In these cases, the uniform echogenicity may lead to an erroneous assumption that the lesion is solid. However, when pressure is applied on the cyst with the transducer, the entire contents shift, suggesting its cystic nature [7]. Intense posterior enhancement is a common but not specific finding of TGDC [3]. Ahuja et al. [7] reported that posterior enhancement was present in 88% of the cases and easily identified in lesions that were anechoic or had mixed echogenicity. Thus, radiologists must always look for it because posterior enhancement, even though subtle, is the key to identify the cystic rather than solid nature of the lesion, especially in lesions with a pseudosolid appearance [7]. Another US feature includes the depiction of thyroglossal tract [36]. Although US does not reveal a tract in all cases as MRI, this is not critical because, irrespective of the site or the size and appearance of a TGDC, a Sistrunk procedure is the recommended procedure of choice [7]. This entails resection of the cyst and any remaining tract and excision of the middle third of the hyoid bone. Incomplete resection invariably results in recurrence. With the use of the Sistrunk procedure, instead, recurrence rates have fallen from 50% to less than 4% [37].
Fig. 2.
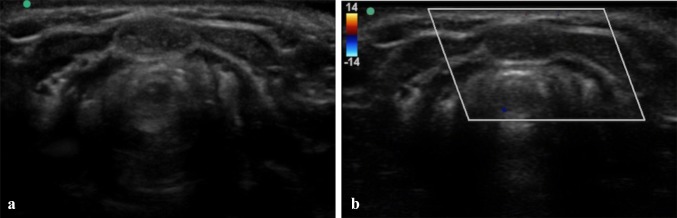
TGDC in a 44-year-old male with a mass in the midline of neck and erythema of the overlying skin. B-mode US (a) and PD (b) images show a homogeneous hypoechoic lesion with diffuse low-level internal echoes. Increased through transmission is present. No internal flow is observed with PD. Pathologic examination of surgical specimen showed a TGDC with infection-inflammation, lined by squamous epithelium
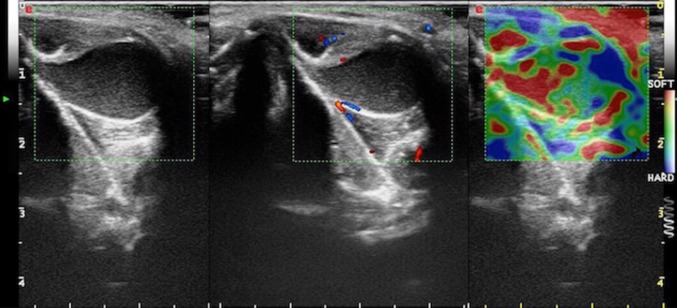
Fig. 3.
Complicated TGDC in a 21-year-old boy, slightly off-midline to the left, between the thyroid cartilage and strap muscles. Transverse B-mode US image (on the left) show a uniformly echogenic, pseudosolid appearance of a TGDC. Note, however, the posterior enhancement, suggesting its cystic nature. No internal flow is observed with CD (in the middle). SE (on the right) shows elasticity in the whole region of interest (homogeneously green with some red areas mixed in), an appearance suggestive of benign lesion. TGDC was confirmed by pathologic examination of surgical specimen, which showed no infection or hemorrhage. Pseudosolid pattern was due to proteinaceous content of cyst
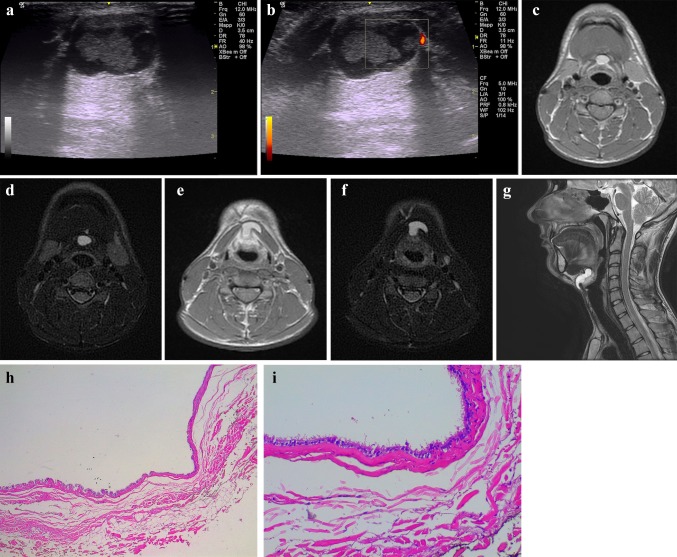
Fig. 4.
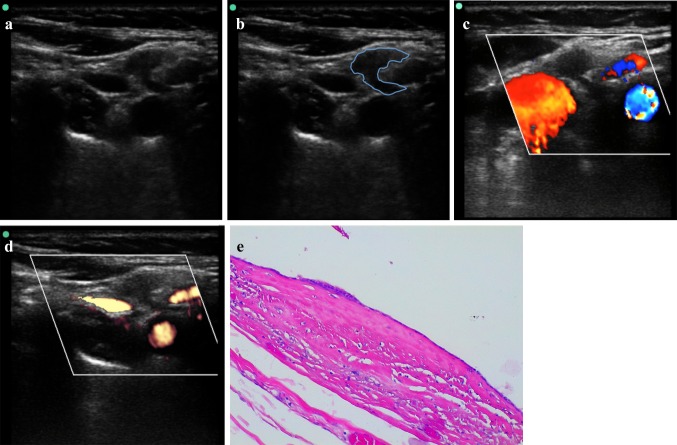
Young male (19-year-old) with a midline neck swelling, moving with deglutition. B-mode US (a) and PD (b) images show a well-circumscribed, heterogenous complex cystic lesion containing a solid-appearing echogenic nodularity due to a previous hemorrhage in the midline of neck, at the level of hyoid bone. Increased through transmission is present. No internal flow is observed with PD. A MRI of the neck was subsequently done, which demonstrated a large midline TGDC, attached to the body of the hyoid bone near the foramen cecum of the tongue base. Neck axial T1—(c) and T2-weighted fat-suppressed (d) images at the level of hyod bone and midline sagittal T2-weighted fat-suppressed (e) images show the high signal TGDC in a prevalent infrahyoid location with a superior component extending directly posterior to the hyoid bone. Axial T1—(f) and T2-weighted (g) images between the level of hyoid bone and thyroid cartilage allow to demonstrate the TGDC embedded in the left-sided strap muscles, a characteristic cross-sectional finding of TGDCs. h, i Photomicrographs—hematoxylin–eosin stain, magnification × 4 (h) and × 20 (i). TGDC characterized by pseudostratified ciliated epithelium and inflammatory stromal infiltrate; cystic content was found to be haemorragic
Cross-sectional imaging findings
On CT, a TGDC is seen as a low-density, usually unilocular thin-wall lesion along the course of embryologic thyroid migration, more commoly lying in the midline and closely related to the hyoid bone [36]. Mean internal density measurements are tipically less than 20 Hounsfield units (HU) [9]. Increased attenuation density within a cyst may be the results of a thicker, more viscous content, suggestive of infection. Septations and peripheral rim-enhancement following intravenous contrast somministration are sometimes observed [36].
Typical MRI features of uncomplicated TGDCs are homogeneous low signal on T1-weighted images and homogeneous high signal on T2-weighted images, reflecting the fluid content of the cyst. If there are previous episodes of infection or hemorrhage, signal intensity becomes more variable and hyperintensity on T1-weighted sequences is commonly encountered (Fig. 4) [36]. High signal on T1-weighted images can also be due to proteinaceous fluid content and the presence of thyroglobulin within the cyst fluid [36]. A tract ascending towards the tongue base is not infrequently identified on T2-weighted fat saturated sections [38]. Typically, there is no restricted diffusion within a TGDC [1]. Solid component as intracystic nodule is well demonstrated on pre- and post-contrast MRI studies. Calcifications shown on susceptibility weighted imaging (SWI) or gradient echo images should prompt suspicion of malignancy [2].
Malignancy of TGDCs
TGD carcinoma is an uncommon complication of TGDC, occurring in less than 1% of cases. It most frequently occurs in adults (median age of 40 years) [39]. All subtypes of thyroid carcinoma have been described in TGDCs with the exception of medullary carcinoma due to lack of parafollicular cells [36]. The vast majority of cases represent papillary carcinoma, similar to orthotopic thyroid malignancy. TGDC carcinoma usually presents as an asymptomatic mass, and diagnosis is only made postoperatively from histopathologic examination of a surgical specimen of the excised TGDC. Uncommonly dysphagia, change in voice or a draining cutaneous sinus may be the presenting symptom [1].
Preoperatively the presence of solid components (shown by any imaging technique including US), wall enhancing nodules (on CEUS or CT/MRI) or calcifications (on CT) within the thyroglossal duct cyst should raise the suspicion of carcinoma (Fig. 5) [1]. Doppler techniques, being able to produce an accurate map of lesional vessels, help to identify any solid intralesional element [40]. However, despite the modern US machines equipped with high-resolution transducers and low-flow scanner settings (high transmission frequency, low pulse repetition frequency, minimum wall filter, color gain increased up to the artifacts threshold), it is sometimes difficult or impossible to detect intralesional flow signals at CD and PD [41]. Calcifications are not appreciated on MR imaging, requiring a supplementary CT examination. According to some authors, calcifications are a more specific indicators of malignancy than solid components, as the latter can also be seen in inflammatory processes [1].
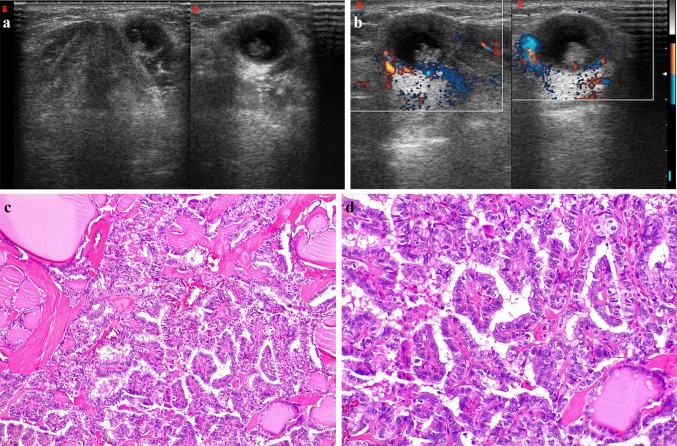
Fig. 5.
Infrahyoid TDGC with malignancy in a 34-year-old man, slightly off-midline to the left, between the thyroid cartilage and strap muscles. B-Mode US (a) and color-Doppler (b) images reveal a well-circumscribed anechoic structure with a grossly irregular nodular component in its inferior portion, which shows vascular signals on Doppler imaging. Posterior acoustic enhancement is also evident. Resection revealed a papillary carcinoma(c, × 10 and d, × 20)
Preoperatively the diagnosis may be made by fine-needle aspiration (FNA). The presence of large, atypical squamous cells, or psammoma bodies, in the FNA material of a midline anterior cystic neck mass should suggest a papillary thyroglossal duct carcinoma [1]. The classic Sistrunk operation suffices for non-metastatic disease and medically high-risk surgical patients. Thyroidectomy and regional neck dissection are warranted if there is intrathyroidal involvement or local invasion which occurs in about one-third of cases. Postoperative radiotherapy and thyroid-stimulating hormone suppression are mandatory for effective cure in patients with extrathyroidal disease spread. With proper treatment, the prognosis is good. Squamous cell carcinoma arising in a thyroglossal duct remnant is more aggressive, requiring extensive surgery, radical neck dissection and postoperative radiotherapy [36].
Site-specific differential diagnoses of TGDCs
There are many mimics of TDGCs, and it is important to recognize these as each has different clinical implications [1]. Since the imaging findings of cystic neck lesions are not specific and the differential diagnosis is not easy, close attention to the age of presentation, lesion location, and association with surrounding structures can clue one into the correct diagnosis [14]. In this regard, an evidence-based diagnostic algorithm to guide clinicians and radiologists in the correct differential diagnosis has been proposed, which is summarized in Fig. 6 [42]. Briefly, if a neck lesion is detected, US can usually determine whether it is cystic or solid. Then, once established that the lesion is cystic, its location will often point to its nature [43].
Fig. 6.
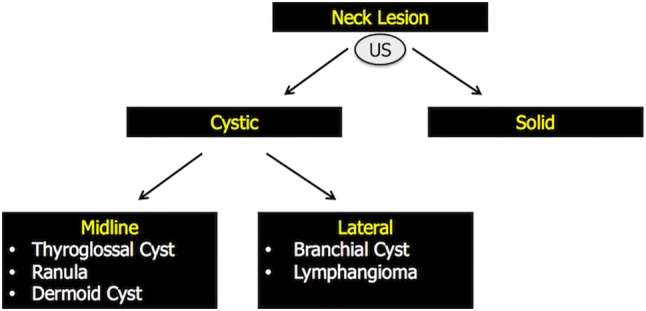
Evidence-based diagnostic algorithm proposed to guide clinicians and radiologists in the correct differential diagnosis of neck cystic lesions [40]. Briefly, if a neck lesion is detected, US can usually determine whether it is cystic or solid. Then, once established that the lesion is cystic, its location will often point to its nature. If located on the midline, the differential diagnosis narrows to TGDCs, ranulas or dermoid cysts. Off-midline lesions can be, instead, branchial cleft cysts or lymphangiomas
Midline lesions
Midline lesions are either TGDCs, ranulas or dermoid cysts (Fig. 7) [42].
A ranula is a mucous retention cyst resulting from obstruction of the sublingual gland or its duct, or rarely the minor salivatory glands in the sublingual space. Therefore, these are also called sublingual gland mucocele or mucous retention cyst [14]. No age is spared for ranulas. Kokong series [44] recorded an age range of 5 months-39 years which is at variance with a study which recorded 3–61 years [44]. There are two forms of ranula:
Simple ranula, the most common form, is a true cyst and invariably involves the sublingual gland. Anatomically, it is confined within the floor of the mouth to the level of mylohyoid muscle.
Diving ranula (pseudocyst), which forms from enlargement of a simple ranula with subsequent rupture that extends posteriorly around the free margin of mylohyoid muscle.
Fig. 7.
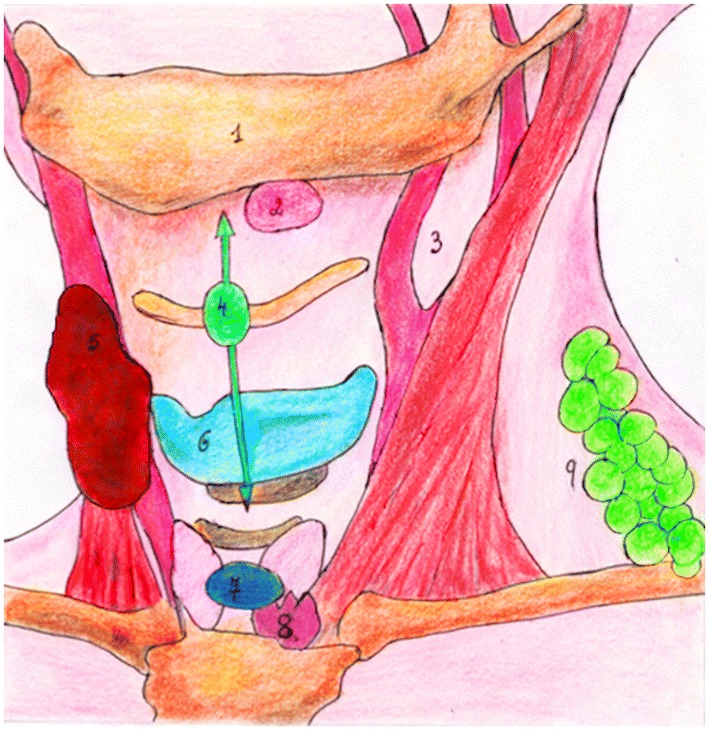
Midline lesions are either thyroglossal duct cysts, dermoid cysts or ranulas. Off-midline lesions can be branchial cleft cysts or lymphangiomas. (1) Mandible; (2) Ranula; (3) Sternocleidomastoid muscle—anterior border, lateral to the carotid artery bifurcation, space of lateral neck most frequently occupied by branchial cysts; (4)TGDC; (5)Fibromatosis colli; (6)Thyroid cartilage; (7) Dermoid cyst; (8) Thymus; (9) Lymphangioma
On US, a ranula appears as a unilocular, well-defined, cystic lesion in the submental region related to the sublingual gland [15]. On CT, a simple ranula usually appears as an ovoid-shaped cyst with an attenuation of 10–20 UH, which lies lateral to the genioglossal muscles and deep to the mylohyoid muscle. A diving ranula often infiltrates adjacent tissue planes, extending inferiorly and dorsally to the submandibular space [14, 15]. On MRI, a ranula usually shows low signal intensity on the T1 sequence and high signal on T2 weighted images. Occasionally, when there is a proteinaceous content, the lesion will appear hyperintense on the T1-weighted sequence [14].
A dermoid cyst (DC) is the most common of the teratomatous lesions in the head and neck and approximately 7% occur of all dermoids occurring in this region [45]. Histologically it contains two germ cell layers and skin appendages (e.g., hair follicles and sebaceous glands). Dermoid cysts are frequently midline in location, typically arising either in the floor of mouth deep to the mylohyoid muscles or in the suprasternal notch. They may also occur in the orbit, nasal, and oral cavities [14, 15]. Like other congenital neck masses, DCs are usually noticed during the first few years of life, but if asymptomatic, may not be observed until much later [46].
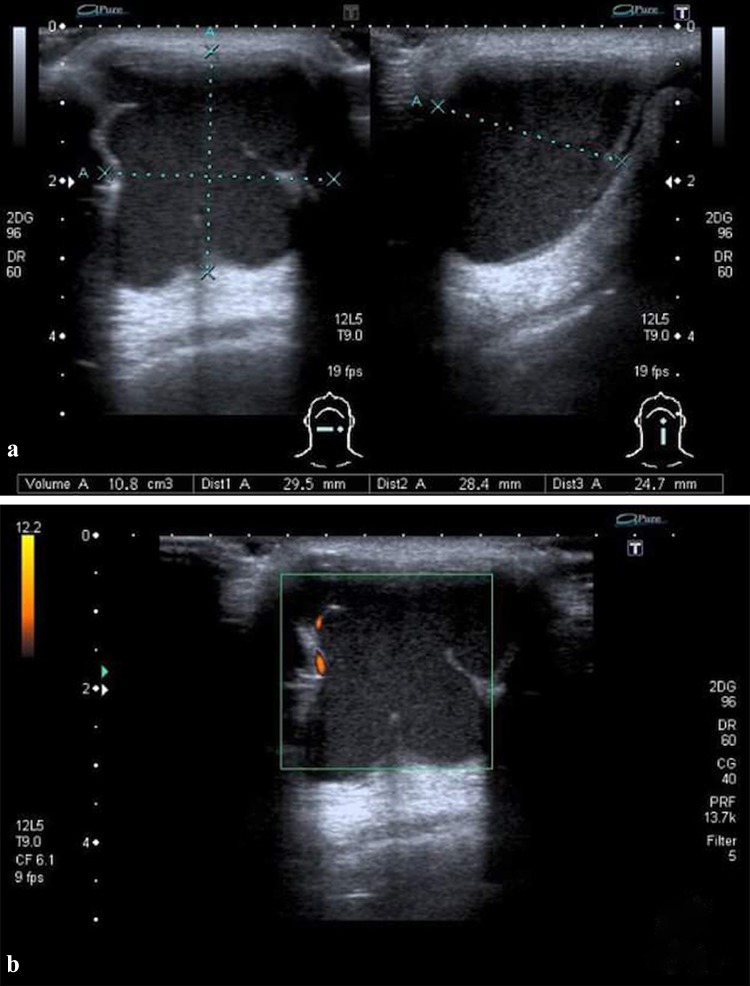
DCs usually present sonographically as well-defined, unilocular, homogeneously hypoechoic masses with internal echogenic dots because the lumen of DCs is filled with a mixture of keratin and sebaceous material, which may be reflective and cause echogenic dots on US. In other cases, they may exhibit a pseudosolid appearance on US due to the presence of dense cellular material within the cyst, (Fig. 8 and Video 1) [45]. Dermoid cysts may have also a complex internal echostructure because of its fat content and may show the presence of osseo-dental structures within, seen as echogenic foci with dense posterior acoustic shadowing. On CT or MRI, globules of fat floating within the lesion may produce a characteristic ‘‘sack of marbles’’ appearance, and fat and/or fluid levels may be present. Both CT and MRI clearly define their anatomical localization, internal appearance and extention [14, 15].
Fig. 8.
Dermoid cyst in a 38-year-old woman. a Transverse B-mode US image shows a well-defined, unilocular, homogeneously hypoechoic lesion with internal echogenic dots, midline in location and superficial to the strap muscles (thyrohyoid muscles) at the cross-section level of cricoid cartilage. b Corresponding CD images show no intenal flow
Off midline lesions
Off-midline lesions can be branchial cleft cysts or lymphatic malformations (Fig. 7) [42].
Branchial cleft anomalies arise from incomplete obliteration of any branchial tract, resulting in either a cyst (75%) or a sinus or fistulous tract (25%) [47]. Second branchial anomalies comprise 95% of all branchial cleft lesions, most commonly presenting as cystic masses rather than sinuses or fistulas [14, 15]. Branchial cleft cysts (BCCs) are the second most common pediatric head and neck masses, with patients most often presenting to medical attention between the ages of 11 and 30. However, the age of a patient should not exclude the diagnosis of a BCC as these lesions, although congenital, can present later in life with a literature reported case of a BCC in a 70-year-old patient [48].
A BCC occurs anywhere in the lateral aspect of neck, but it is classically seen as well-marginated anechoic masses with thin walls along the anterior border of the sternocleidomastoid muscle, lateral to the common carotid artery, and if more cranially, between the internal and external carotid artery (Fig. 9). It may show thick walls or internal echoes or septations. Sometimes a “beak sign” may be seen as a curved rim of the lesion pointing medially (Fig. 9) [14, 15]. On CT it is seen as a well-circumscribed, non-enhancing mass of homogenous low attenuation. Wall thickening and enhancement may occur due to associated inflammation. When a sinus or fistula is present, the external opening is typically along the anterior border of the sternocleidomastoid muscle at the junction of its middle and lower thirds, its internal opening being in the region of the palatine tonsillar fossa [14, 15].
Lymphatic malformations are congenital anomalies resulting from sequestration of embryonic lymphatic channels [49]. According to the International Society for the Study of Vascular Anomalies (ISSVA) [50], these malformations are divided into macrocystic, microcystic, and mixed type. The criterion of distinction between the macrocystic and microcystic form is not univocal. According to some, a 1–2 cm cut-off must be identified to distinguish between the two forms [51]. The term “lymphatic malformation” has now replaced the old name of “lymphangioma” (suffix ‘oma’ should be applied to lesions with cell proliferation) [49].
Fig. 9.
Second BCC in a 42-year-old girl, incidentally discovered during a screening US examination for thyroid pathologies. B-mode US (a) and corresponding US schematic (b) images of the lateral neck shows a small, well-marginated, anechoic lesion with thin walls and internal fine echoes along the anterior border of the sternocleidomastoid muscle, lateral to the carotid bifurcation. A “beak sign” is seen as a curved rim of the lesion pointing medially. c, d CD e PD landmarks (carotid bifurcation, giugular vein) are useful to better identify the BCC. e Photomicrograph–hematoxylin–eosin stain, magnification × 4. Second BCC lined by squamous epithelium
Macrocystic lymphatic malformations (MLMs) are the most common and usually present at birth as a painless swelling with most lesions (90%) appearing before the age of two. MLMs occur exceedingly rarely in adults. They are situated posterior to the sternocleidomastoid muscle in the posterior triangle. These lesions are characteristically infiltrative in nature and do not respect facial planes. Most are slow-growing; however, sudden enlargement can occur following infection or hemorrhage into the lesion [14, 15].
On US an anechoic mass with septae of variable thickness will be identified. Debris and fluid–fluid levels may be present when the lesions are complicated by hemorrhage (Fig. 10 and Video 2). The margins of the mass and the presence of mediastinal extension are better delineated with more panoramic CT or MRI. CT will show a loculated low-density mass with fluid attenuation. Hemorrhage or infection causes an increase in attenuation. MRI will demonstrate high signal on T2—and low signal on T1-weighted images. T1 hyper-intensity may be seen with hemorrhage. Fluid level may also be seen [14, 15].
Fig. 10.
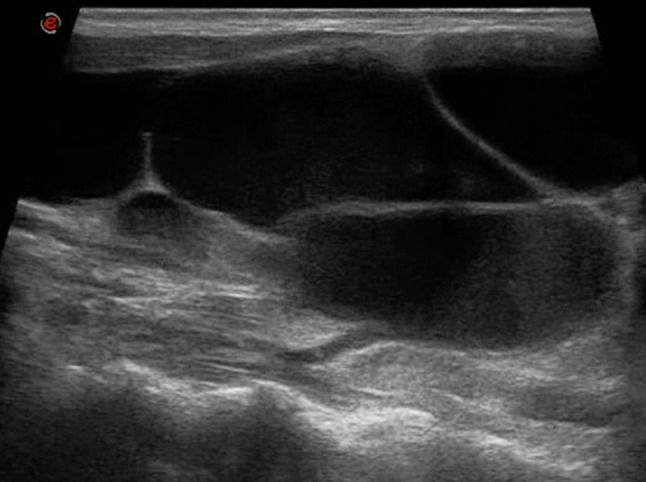
B-mode US image shows a large cystic lesion with internal septa and debris centered in the left neck base compatible with a MLM
Conclusions
During embryologic development, the thyroid primordium migrates from the foramen cecum to the pretracheal part of the neck. Accurate diagnosis of TGDCs requires knowledge of this anatomic course such as location and imaging features of these lesions. In addition, clinicians and radiologists must also be familiar with other cystic neck lesions that need to be excluded.
In this setting, US can in many cases provide all the informations that the surgeon requires, from diagnosis to preoperative assessment, thus avoiding in-depth with other time-consuming and more expensive radiologic procedures, such as CT and MR imaging.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Dermoid cyst in a 38-year-old woman. Transverse B-mode US sonograms show a well-defined, unilocular, homogeneously hypoechoic lesion with internal echogenic dots, midline in location and superficial to the strap muscles (thyrohyoid muscles).(MPG 3476 kb)
B-mode US image shows a multilocular large cystic lesion with internal septa and debris centered in the left neck base compatible with a MLM. Note the presence of grossly nodular clots within the cystic spaces, indicative of prior hemorrhage (not shown in Fig. 10). (MPG 25462 kb)
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest.
Informed consent
Written informed consent was obtained from all patients, and the study was approved by the ethics committee of the institution.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patel S, Bhatt AA. Thyroglossal duct pathology and mimics. Insights Imaging. 2019;10(1):12. doi: 10.1186/s13244-019-0694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zander DA, Smoker WR. Imaging of ectopic thyroid tissue and thyroglossal duct cysts. Radiographics. 2014;34(1):37–50. doi: 10.1148/rg.341135055. [DOI] [PubMed] [Google Scholar]
- 3.Wadsworth DT, Siegel MJ. Thyroglossal duct cysts: variability of sonographic findings. AJR Am J Roentgenol. 1994;163(6):1475–1477. doi: 10.2214/ajr.163.6.7992750. [DOI] [PubMed] [Google Scholar]
- 4.Dedivitis RA, Camargo DL, Peixoto GL, Weissman L, Guimarães AV. Thyroglossal duct: a review of 55 cases. J Am Coll Surg. 2002;194(3):274–277. doi: 10.1016/S1072-7515(01)01171-1. [DOI] [PubMed] [Google Scholar]
- 5.Brousseau VJ, Solares CA, Xu M, Krakovitz P, Koltai PJ. Thyroglossal duct cysts: presentation and management in children versus adults. Int J Pediatr Otorhinolaryngol. 2003;67(12):1285–1290. doi: 10.1016/j.ijporl.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Lee DH, Jung SH, Yoon TM, Lee JK, Joo YE, Lim SC. Computed tomographic evaluation of thyroglossal duct cysts in children under 11 years of age. Chonnam Med J. 2012;48(3):179–182. doi: 10.4068/cmj.2012.48.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahuja AT, King AD, King W, Metreweli C. Thyroglossal duct cysts: sonographic appearances in adults. AJNR Am J Neuroradiol. 1999;20(4):579–582. [PMC free article] [PubMed] [Google Scholar]
- 8.Pounds LA. Neck masses of congenital origin. Pediatr Clin North Am. 1981;28(4):841–844. doi: 10.1016/S0031-3955(16)34070-6. [DOI] [PubMed] [Google Scholar]
- 9.Koeller KK, Alamo L, Adair CF, Smirniotopoulos JG. Congenital cystic masses of the neck: radiologicpathologic correlation. Radiographics. 1999;19(1):121–146. doi: 10.1148/radiographics.19.1.g99ja06121. [DOI] [PubMed] [Google Scholar]
- 10.Shahin A, Burroughs FH, Kirby JP, Ali SZ. Thyroglossal duct cyst: a cytopathologic study of 26 cases. Diagn Cytopathol. 2005;33(6):365–369. doi: 10.1002/dc.20346. [DOI] [PubMed] [Google Scholar]
- 11.Wei S, LiVolsi VA, Baloch ZW. Pathology of thyroglossal duct: an institutional experience. Endocr Pathol. 2015;26(1):75–79. doi: 10.1007/s12022-015-9354-y. [DOI] [PubMed] [Google Scholar]
- 12.Huoh KC, Durr ML, Meyer AK, Rosbe KW. Comparison of imaging modalities in pediatric thyroglossal duct cysts. Laryngoscope. 2012;122(6):1405–1408. doi: 10.1002/lary.23262. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Jung SH, Yoon TM, Lee JK, Joo YE, Lim SC. Preoperative computed tomography of suspected thyroglossal duct cysts in children under 10-years-of-age. Int J Pediatr Otorhinolaryngol. 2013;77(1):45–48. doi: 10.1016/j.ijporl.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Wong KT, Lee YY, King AD, Ahuja AT. Imaging of cystic or cyst-like neck masses. Clin Radiol. 2008;63(6):613–622. doi: 10.1016/j.crad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Mittal MK, Malik A, Sureka B, Thukral BB. Cystic masses of neck: a pictorial review. Indian J Radiol Imaging. 2012;22(4):334–343. doi: 10.4103/0971-3026.111488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corvino A, Sandomenico F, Corvino F, Campanino MR, Verde F, Giurazza F, Tafuri D, Catalano O. Utility of a gel stand-off pad in the detection of Doppler signal on focal nodular lesions of the skin. J Ultrasound. 2019 doi: 10.1007/s40477-019-00376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corvino A, Corvino F, Catalano O, Sandomenico F, Petrillo A. The Tail and the string sign: new sonographic features of subcutaneous melanoma metastasis. Ultrasound Med Biol. 2017;43(1):370–374. doi: 10.1016/j.ultrasmedbio.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Caprio MG, Di Serafino M, Pontillo G, Vezzali N, Rossi E, Esposito F, Zeccolini M, Vallone G. Paediatric neck ultrasonography: a pictorial essay. J Ultrasound. 2019;22(2):215–226. doi: 10.1007/s40477-018-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trovato P, Simonetti I, Verde F, Corvino A, Picchi SG, Lassandro G, Angelini V, Caprio MG. Sonographic findings of ectopic intrathyroidal thymus in childhood: our experience. Euromediterranean Biomed J. 2019;14(35):152–154. [Google Scholar]
- 20.Gupta P, Maddalozzo J. Preoperative sonography in presumed thyroglossal duct cysts. Arch Otolaryngol Head Neck Surg. 2001;127(2):200–202. doi: 10.1001/archotol.127.2.200. [DOI] [PubMed] [Google Scholar]
- 21.Ward RF, Selfe RW, St Louis L, Bowling D. Computed tomography and the thyroglossal duct cyst. Otolaryngol Head Neck Surg. 1986;95(1):93–98. doi: 10.1177/019459988609500118. [DOI] [PubMed] [Google Scholar]
- 22.Kacker A, Komisar A. Imaging quiz case 1. Thyroglossal duct cyst with intralaryngeal extension. Arch Otolaryngol Head Neck Surg. 1996;122(11):1266–1268. doi: 10.1001/archotol.1996.01890230110019. [DOI] [PubMed] [Google Scholar]
- 23.Bhatia KS, Lee YY, Yuen EH, Ahuja AT. Ultrasound elastography in the head and neck. Part I. Basic principles and practical aspects. Cancer Imaging. 2013;13(2):253. doi: 10.1102/1470-7330.2013.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia KS, Lee YY, Yuen EH, Ahuja AT. Ultrasound elastography in the head and neck. Part II. Accuracy for malignancy. Cancer Imaging. 2013;13(2):260–276. doi: 10.1102/1470-7330.2013.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McQueen AS, Bhatia KS. Head and neck ultrasound: technical advances, novel applications and the role of elastography. Clin Radiol. 2018;73(1):81–93. doi: 10.1016/j.crad.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Corvino A, Catalano O, Setola SV, Sandomenico F, Corvino F, Petrillo A. Contrast-enhanced ultrasound in the characterization of complex cystic focal liver lesions. Ultrasound Med Biol. 2015;41(5):1301–1310. doi: 10.1016/j.ultrasmedbio.2014.12.667. [DOI] [PubMed] [Google Scholar]
- 27.Corvino A, Catalano O, Corvino F, Petrillo A. Rectal melanoma presenting as a solitary complex cystic liver lesion: role of contrast-specific low-MI realtime ultrasound imaging. J Ultrasound. 2015;19(2):135–139. doi: 10.1007/s40477-015-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D’Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: update 2017 (Long Version) Ultraschall Med. 2018;39(2):e2–e44. doi: 10.1055/a-0586-1107. [DOI] [PubMed] [Google Scholar]
- 29.Corvino A, Sandomenico F, Setola SV, Corvino F, Pinto F, Catalano O. Added value of contrast-enhanced ultrasound (CEUS) with Sonovue® in the diagnosis of inferior epigastric artery pseudoaneurysm: report of a case and review of literature. J Ultrasound. 2019;22(4):485–489. doi: 10.1007/s40477-019-00398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corvino F, Giurazza F, Cangiano G, Cavaglià E, Amodio F, De Magistris G, Corvino A, Niola R. Safety and effectiveness of transcatheter embolization in the treatment of internal mammary artery injuries. Radiol Med. 2018;123(5):369–377. doi: 10.1007/s11547-017-0844-5. [DOI] [PubMed] [Google Scholar]
- 31.Corvino A, Sandomenico F, Setola SV, Corvino F, Tafuri D, Catalano O. Morphological and dynamic evaluation of complex cystic focal liver lesions by contrast-enhanced ultrasound: current state of the art. J Ultrasound. 2019;22(3):251–259. doi: 10.1007/s40477-019-00385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catalano O, Varelli C, Sbordone C, Corvino A, De Rosa D, Vallone G, Wortsman X. A bump: what to do next? Ultrasound imaging of superficial soft tissue palpable lesions. J Ultrasound. 2019 doi: 10.1007/s40477-019-00415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corvino A, Catalano O, Corvino F, Sandomenico F, Petrillo A. Diagnostic performance and confidence of contrast-enhanced ultrasound in the differential diagnosis of cystic and cysticlike liver lesions. AJR Am J Roentgenol. 2017;209(3):W119–W127. doi: 10.2214/AJR.16.17062. [DOI] [PubMed] [Google Scholar]
- 34.Guarino B, Catalano O, Corvino A, Corvino F, Amore A, Petrillo A. Hepatic inflammatory pseudotumor: educational value of an incorrect diagnosis at contrast-enhanced ultrasound. J Med Ultrason. 2015;42(4):547–552. doi: 10.1007/s10396-015-0624-6. [DOI] [PubMed] [Google Scholar]
- 35.Ascenti G, Mazziotti S, Zimbaro G, Settineri N, Magno C, Melloni D, Caruso R, Scribano E. Complex cystic renal masses: characterization with contrast-enhanced US. Radiology. 2007;243(1):158–165. doi: 10.1148/radiol.2431051924. [DOI] [PubMed] [Google Scholar]
- 36.Ahuja AT, Wong KT, King AD, Yuen EH. Imaging for thyroglossal duct cyst: the bare essentials. Clin Radiol. 2005;60(2):141–148. doi: 10.1016/j.crad.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Righini CA, Hitter A, Reyt E, Atallah I. Thyroglossal duct surgery Sistrunk procedure. Eur Ann Otorhinolaryngol Head Neck Dis. 2016;133(2):133–136. doi: 10.1016/j.anorl.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 38.King AD, Ahuja AT, Mok CO, Metreweli C. MR imaging of thyroglossal duct cysts in adults. Clin Radiol. 1999;54(5):304–308. doi: 10.1016/S0009-9260(99)90559-7. [DOI] [PubMed] [Google Scholar]
- 39.Jang DW, Sikora AG, Leytin A. Thyroglossal duct cyst carcinoma: case report and review of the literature. Ear Nose Throat J. 2013;92(9):E12–E14. [PubMed] [Google Scholar]
- 40.Corvino F, Giurazza F, Cangiano G, Silvestre M, Cavaglià E, de Magistris G, Amodio F, Corvino A, Niola R. Endovascular treatment of peripheral vascular blowout syndrome in end-stage malignancies. Ann Vasc Surg. 2019;58:382.e1–382.e5. doi: 10.1016/j.avsg.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 41.Catalano O, Roldán FA, Varelli C, Bard R, Corvino A, Wortsman X. Skin cancer: findings and role of high-resolution ultrasound. J Ultrasound. 2019;22(4):423–431. doi: 10.1007/s40477-019-00379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Littooij A, Ravesloot C, Beek E (2016) Neck masses in children. Radiology Assistant. https://radiologyassistant.nl/head-neck/neck-masses-in-children(Accessed 2016-11-01)
- 43.Corvino A, Catalano O, Corvino F, Sandomenico F, Setola SV, Petrillo A. Superficial temporal artery pseudoaneurysm what is the role of ultrasound. J Ultrasound. 2016;19(3):197–201. doi: 10.1007/s40477-016-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kokong D, Iduh A, Chukwu I, Mugu J, Nuhu S, Augustine S. Ranula: current concept of pathophysiologic basis and surgical management options. World J Surg. 2017;41(6):1476–1481. doi: 10.1007/s00268-017-3901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi HI, Choi YH, Cheon JE, Kim WS, Kim IO. Ultrasonographic features differentiating thyroglossal duct cysts from dermoid cysts. Ultrasonography. 2018;37(1):71–77. doi: 10.14366/usg.17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Görür K, Talas DU, Ozcan C. An unusual presentation of neck dermoid cyst. Eur Arch Otorhinolaryngol. 2005;262(4):353–355. doi: 10.1007/s00405-004-0813-1. [DOI] [PubMed] [Google Scholar]
- 47.Valentino M, Quiligotti C, Carone L. Branchial cleft cyst. J. Ultrasound. 2013;16(1):17–20. doi: 10.1007/s40477-013-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vemula R, Greco G. An unusual presentation of presentation of a branchial cleft cyst. J Craniofac Surg. 2012;23(3):e270–e272. doi: 10.1097/SCS.0b013e31824e6d64. [DOI] [PubMed] [Google Scholar]
- 49.Esposito F, Ferrara D, Di Serafino M, Diplomatico M, Vezzali N, Giugliano AM, Colafati GS, Zeccolini M, Tomà P. Classification and ultrasound findings of vascular anomalies in pediatric age: the essential. J Ultrasound. 2019;22(1):13–25. doi: 10.1007/s40477-018-0342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahlawat S, Fayad LM, Durand DJ, Puttgen K, Tekes A. International society for the study of vascular anomalies classification of soft tissue vascular anomalies: survey-based assessment of musculoskeletal radiologists’ use in clinical practice. Curr Probl Diagn Radiol. 2019;48(1):10–16. doi: 10.1067/j.cpradiol.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Wassef M, Blei F, Adams D, Alomari A, Baselga E, Berenstein A, Burrows P, Frieden IJ, Garzon MC, Lopez-Gutierrez JC, Lord DJ, Mitchel S, Powell J, Prendiville J, Vikkula M, ISSVA Board and Scientific Committee Vascular anomalies classification: recommendations from the international society for the study of vascular anomalies. Pediatrics. 2015;136(1):e203–e214. doi: 10.1542/peds.2014-3673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dermoid cyst in a 38-year-old woman. Transverse B-mode US sonograms show a well-defined, unilocular, homogeneously hypoechoic lesion with internal echogenic dots, midline in location and superficial to the strap muscles (thyrohyoid muscles).(MPG 3476 kb)
B-mode US image shows a multilocular large cystic lesion with internal septa and debris centered in the left neck base compatible with a MLM. Note the presence of grossly nodular clots within the cystic spaces, indicative of prior hemorrhage (not shown in Fig. 10). (MPG 25462 kb)