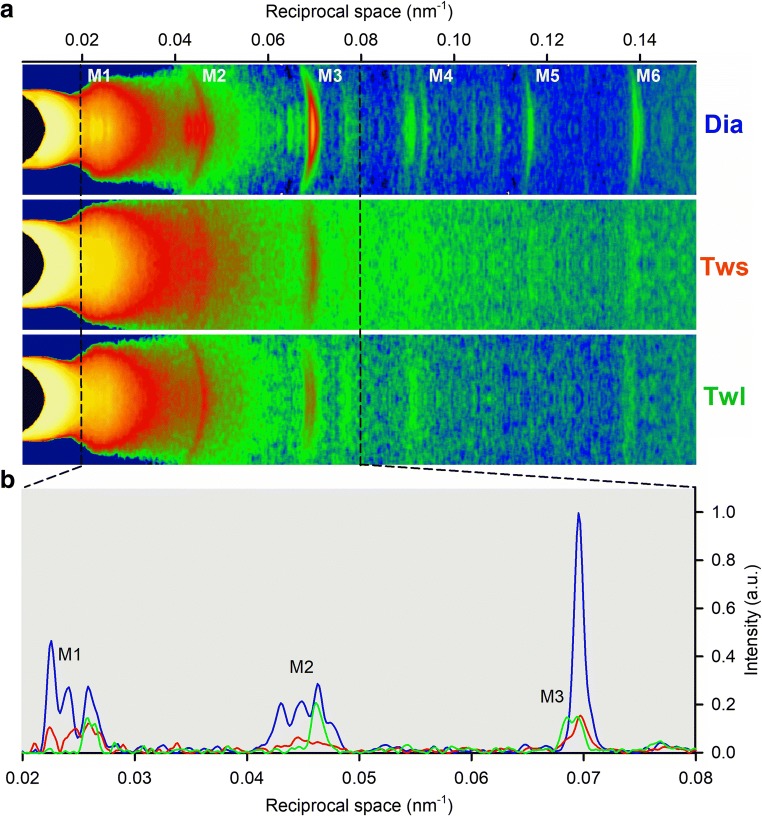
This is a Session Commentary describing the “Frontiers in multi-scale mechanobiology of muscle and vascular systems - Session 1SGA” of the Annual Meeting of the Biophysical Society of Japan (Miyazaki 2019). The field of mechanobiology has grown dramatically in the past decade (Iskratsch et al. 2014) and importance of physical stimuli and the response in muscle and vascular system is well known at the phenomenological level; however, the molecular mechanism and multi-scale relationships between molecules, cells, tissues, and organ is still elusive. This session provided an overview of the latest findings in the field, revealing the relationship between physical stimuli and activities of muscle and vascular system at each hierarchy. Prof. Vincenzo Lombardi (University of Florence) discussed his finding on mechanosensing mechanism of myosin-containing thick filament in cardiac myocytes by X-ray diffraction experiments (Linari et al. 2015; Reconditi et al. 2017), which accounts for heart length-dependent activation (Fig. 1).
Fig. 1.
a Meridional reflection slices of 2D X-ray patterns collected from an electrically paced cardiac trabecula during diastole (Dia) and at the peak of the short length twitch (Tws) and long length twitch (Twl). b Superimposed intensity profiles from the region indicated by the dashed lines. Same color code as in a. Image credit: Prof. Vincenzo Lombardi
Dr. Lorenzo Marcucci (Padova University) is a theorist and presented a heart simulator (Marcucci et al. 2019), a multi-scale model bridging from single myosin to whole heart mechanics. He incorporated geometrical constraints of myosins in sarcomere and Prof. Lombardi’s mechanosensing mechanism into the model, then argued the linkage between mechanosensitive molecular mechanism and the whole heart function.
Dr. Seine Shintani (Chubu University) reported a thermally stimulated contraction of cardiac cells. This is called as Hyperthermic Sarcomeric Oscillations (HSOs), having a physiological significance when heart functions in our body. He analyzed the HSOs by a fluorescence microscopy and found rhythmic homeostasis at varying calcium concentration.
Several talks suggested roles of the plasma membrane and the extracellular matrix as mechanosensors. Dr. Motoi Kanagawa (Kobe University) provided the evidence showing that glycans on membrane proteins play a role in maintaining the membrane integrity of skeletal myofibers. Based on his findings, ribitol phosphate, a specific type of glycans that were only found in gram-positive bacteria, is critical for a membrane glycoprotein dystroglycan to associate with extracellular matrix proteins such as laminin (Kanagawa et al. 2016). Moreover, he showed that mutations in FKTN (whose gene product is responsible to transfer ribitol phosphates to dystroglycan sugar chains) altered mechanosensation systems in skeletal myofibers. Dr. Kimiko Yamamoto (University of Tokyo) has focused on the signal transduction in vascular endothelial cells, and reveals that mechanical stimulation change the physical properties (e.g., membrane fluidity, lipid order, and lipid content) of the plasma membrane; thus, the plasma membrane serves as the mechanosensor to activate a series of signal transduction pathways in vascular endothelial cells in response to shear stress (Yamamoto and Ando 2018).
Finally, Dr. Satoshi Arai (Kanazawa University) developed novel compounds that regulate a variety of cellular functions by nano-heating. He showed that gold nanoshells can induce contraction of C2C12 myotubes based upon mild heating after near-infrared excitation. This approach could be useful for future application such as engineering of cellular and tissue functions.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mitsuhiro Iwaki, Email: iwaki@riken.jp.
Yuji Hara, Email: hara@sbchem.kyoto-u.ac.jp.
References
- Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Biol. 2014;15(12):825–833. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa M, Kobayashi K, Tajiri M, Manya H, Kuga A, Yamaguchi Y, Akasaka-Manya K, Furukawa JI, Mizuno M, Kawakami H, Shinohara Y, Wada Y, Endo T, Toda T. Identification of a post-translational modification with ribitol-phosphate and its defect in muscular dystrophy. Cell Rep. 2016;14:2209–2223. doi: 10.1016/j.celrep.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Linari M, Brunello E, Reconditi M, Fusi L, Caremani M, Narayanan T, Piazzesi G, Lombardi V, Irving M. Force generation by skeletal muscle is controlled by mechanosensing in myosin filaments. Nature. 2015;528(7581):276–279. doi: 10.1038/nature15727. [DOI] [PubMed] [Google Scholar]
- Marcucci L, Washio T, Yanagida T. Proposed mechanism for the length dependence of the force developed in maximally activated muscles. Sci Rep. 2019;9(1):1317. doi: 10.1038/s41598-018-36706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reconditi M, Caremani M, Pinzauti F, Powers JD, Narayanan T, Stienen GJ, Linari M, Lombardi V, Piazzesi G. Myosin filament activation in the heart is tuned to the mechanical task. Proc Natl Acad Sci U S A. 2017;114(12):3240–3245. doi: 10.1073/pnas.1619484114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Ando J. Emerging role of plasma membranes in vascular endothelial mechanosensing. Circ J. 2018;82:2691–2698. doi: 10.1253/circj.CJ-18-0052. [DOI] [PubMed] [Google Scholar]