In this symposium, six speakers introduced the cutting-edge technologies and researches in optogenetics (Fig. 1). Optogenetics markedly revolutionized life science. This technique allows fast and precise control of a defined biological event, such as neuronal excitation, cell locomotion, gene expression, and so on, even in a complex system such as freely moving animals. Optogenetics has been realized through understanding the molecular properties of photoreceptors, developing new optical techniques, genetics in model systems, and modern brain science.
Fig. 1.
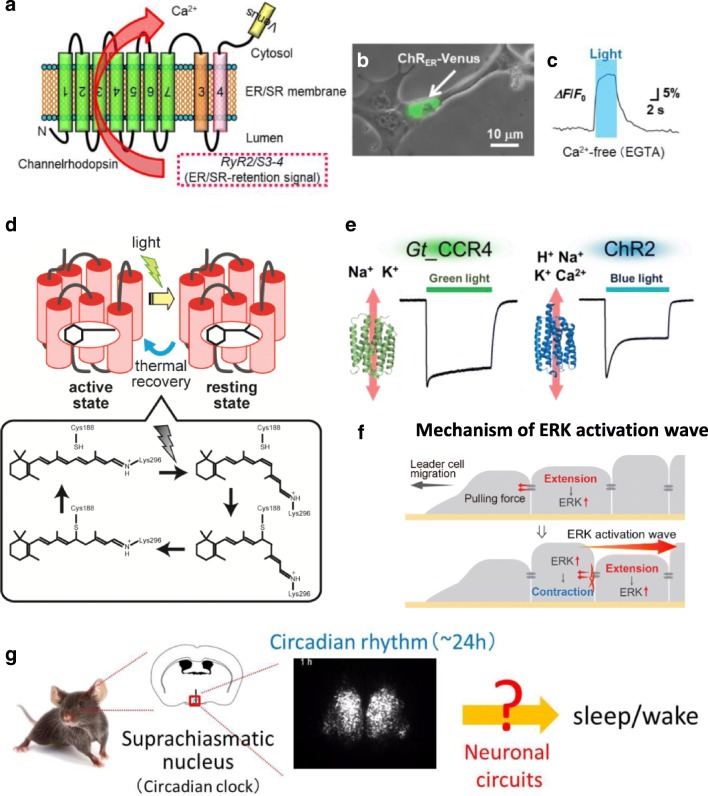
Schematic drawing of several presentations at the symposium. a–c Optical manipulation of intracellular Ca2+ dynamics by a newly engineered channelrhodopsin targeted on ER/SR membrane. d Reaction scheme of vertebrate non-visual opsin Opn5L1. e Cation channel properties of a novel cation channelrhodopsin Gt_CCR4 from cryptophyte flagellates, Guillardia theta, and a well-known ChR2 from chlorophyte algae, Chlamydomonas reinhardtii.f Mechanism of ERK activation wave during collective cell migration. g Mammalian central circadian clock regulates wakefulness via corticotropin-releasing factor (CRF) neurons of the hypothalamus
Dr. Hiromu Yawo presented his research entitled “Organelle optogenetics: Optical manipulation of intracellular Ca2+ dynamics” (Fig. 1a-c). He introduced a novel method to manipulate the Ca2+ release from ER/SR by using a channelrhodopsin and a ryanodine receptor (RYR) in myoblast (Asano et al. Front Neurosci. 2018). The organelle optogenetics would reveal the physiological significance of intracellular Ca2+ dynamics under spatiotemporal precision.
Dr. Takahiro Yamashita presented his research entitled “Engineering of photocyclic animal opsin as a potential optogenetic tool” (Fig. 1d). Optogenetic tools which can control G protein signaling are useful for the analysis of the intercellular signaling. He introduced novel photocyclic opsins, Opn5, and proposed their potential as an optogenetic tool (Sato et al. Nat. Commun. 2018).
Dr. Shoko Hososhima introduced a novel cation channelrhodopsin Gt_CCR4 by her talk entitled “Novel optogenetics tool: A light-gated cation channel with high-reactivity to weak light” (Fig. 1e). She reported electrophysiological properties and optogenetical application of Gt_CCR4 and a well-known channelrhodopsin, ChR2. Gt_CCR4 exhibited powerful channel activity with a high light sensitivity compared with ChR2 (Shigemura et al. Applied Sciences, 2019).
Dr. Takeaki Ozawa presented his research entitled “Optical switches of membrane receptor activities using CRY2.” He focused on an optogenetic method using an optical dimerizer, cryptochrome2 (CRY2) and its partner protein CIB, to control the trafficking of GPCRs. The method allows to manipulate the interaction between β-arrestin and β2-adrenergic receptor (ADRB2) by external light (Takenouchi et al. Sci Rep. 2018).
Dr. Daisuke Ono presented his research entitled “Mammalian central circadian clock regulates wakefulness via corticotropin-releasing factor (CRF) neurons of the hypothalamus” (Fig. 1g). He showed that γ-amino-butyric acid (GABA) in the hypothalamic suprachiasmatic nucleus (SCN) is necessary for the refinement of the circadian firing rhythm (Ono et al. J Physiol Sci. 2018).
Dr. Naoya Hino presented his study entitled “ERK activation waves mediated by intercellular mechanical signals during collective cell migration” (Fig. 1f). He introduced a novel optogenetic approach for manipulating activity of ERK (extracellular signal-regulated kinase).