Abstract
Animals depend on light from their external environment to provide information for physiological functions such as vision, photoentrainment of circadian and circannual rhythms, photoperiodism, and background adaptation. Animals have a variety of photoreceptor cells that perform these functions, not only in the retina but also in other tissues, including brain tissue. In these cells, opsins function as universal photoreceptive proteins responsible for both visual and nonvisual photoreception. All opsins identified thus far bind either 11-cis or all-trans retinal as a chromophore and are classified into several groups based on their amino acid sequences. Opn5 forms an independent group that has diversified among vertebrate species. Most mammals only have one Opn5 gene, Opn5m, while nonmammalian vertebrates have two additional Opn5 subtypes, Opn5L1 and Opn5L2. Among these subtypes, Opn5m and Opn5L2 are UV-sensitive pigments in the dark. UV irradiation converts them into the visible light-sensitive active state, which converts back to the dark state by visible light irradiation. Opn5m and Opn5L2 therefore behave as bistable pigments. By contrast, Opn5L1 exclusively binds all-trans retinal to form the active state in the dark. Opn5L1 is converted to the resting state by light irradiation and subsequently reverts to the active state by a thermal process. Thus, Opn5L1 is categorized as a unique reverse photoreceptor whose activity is regulated by its photocyclic reaction. In this review, I introduce the diversity of molecular properties that have been described for vertebrate Opn5 subtypes and their physiological relevance.
Keywords: Photoreceptive protein, Retinal, Rhodopsin, Opn5
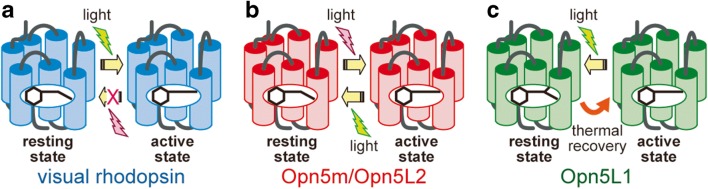
Opsins are universal photoreceptive molecules that form the molecular basis of visual and nonvisual photoreception in animals. Opsins have seven α-helical transmembrane domains and bind a chromophore retinal via a Schiff base linkage to a lysine residue. Vertebrate rhodopsin, the best-studied opsin, functions as a visual pigment in the retina. Rhodopsin binds 11-cis retinal in the dark, and photoisomerization of the retinal to all-trans retinal causes activation of rhodopsin and coupling with G protein (Fig. 1a). Activated rhodopsin spontaneously releases all-trans retinal, forming the apoprotein. The apoprotein can subsequently bind 11-cis retinal, thereby regenerating the resting state. The rhodopsin apoprotein cannot bind all-trans retinal directly to form the active state. Moreover, the resting state cannot be regenerated by irradiating the active state. Rhodopsin is characterized as a G protein-coupled receptor (GPCR) specialized for photoreception.
Fig. 1.
Comparison of the molecular properties of vertebrate visual rhodopsin (a) and Opn5 (b, c). a Vertebrate visual rhodopsin binds 11-cis retinal exclusively to form the resting state (dark state). Light irradiation converts retinal to the all-trans form, which, in turn, induces the formation of the active state. The resting state cannot be regenerated by irradiating the active state. b In general, Opn5m binds directly to either 11-cis or all-trans retinal to form the resting and active states, respectively. Interconversion between the resting and active state is triggered by light irradiation. Opn5L2 has the molecular property quite similar to Opn5m. c Opn5L1 binds all-trans retinal exclusively to form the active state. Light irradiation causes Opn5L1 to adopt the resting state, and subsequently the active state regenerates via a thermal process
The steady accumulation of genomic information has revealed that animals have various opsin genes in their genomes. These opsins are classified into several groups based on their amino acid sequences (Shichida and Matsuyama 2009). These groups show differences in their molecular properties, such as their photoreaction and G protein coupling selectivity. This review focuses on one independent opsin group, Opn5, on the phylogenetic tree.
Opn5 studies started with the initial cloning of human OPN5 and mouse Opn5. The human genome contains nine opsin genes, namely, rhodopsin (RHO), three cone opsins (OPN1SW, OPN1MW, and OPN1LW), OPN3, OPN4, OPN5, RGR, and RRH (Tarttelin et al. 2003). Among the nine opsins, OPN5 is the most recently identified. After the discovery of mammalian Opn5 genes, a search for opsin genes in vertebrate genomes showed that vertebrate Opn5 genes can be classified into at least three subgroups (Tomonari et al. 2008). Most mammals only have one Opn5 gene (Opn5m), while nonmammalian vertebrates have additional Opn5 gene subtypes (Opn5L1 and Opn5L2) (Fig. 2). This difference between mammals and nonmammals is probably due to the nocturnal period found in early mammalian evolution, during which mammals lost several nonvisual opsins and their cone opsin genes (Davies et al. 2015). Recently, we successfully analyzed the molecular properties of three Opn5 subtypes and uncovered an unexpected diversity within the Opn5 group.
Fig. 2.
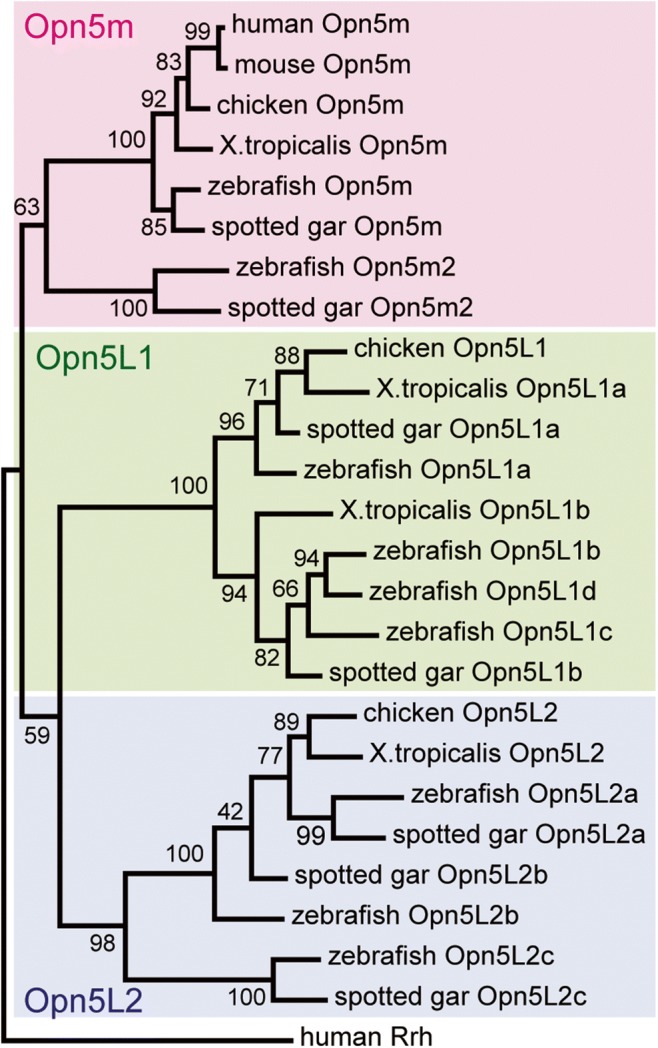
Molecular phylogenetic classification of vertebrate Opn5 subtypes. Mammals have only one Opn5 gene (Opn5m), whereas nonmammalian vertebrates have Opn5L1 and Opn5L2 genes in addition to Opn5m. The phylogenetic tree was inferred by the maximum-likelihood method after the alignment of amino acid sequences of opsins using ClustalW 2.1 (Larkin et al. 2007). The tree was constructed by MEGA X (Kumar et al. 2018) using JTT matrix-based model (Jones et al. 1992). The numbers at each node are bootstrap probabilities estimated by 1000 replications. Accession numbers of the sequence data in the tree are as follows: human (Homo sapiens) Opn5m, AY377391; mouse (Mus musculus) Opn5m, AY318865; chicken (Gallus gallus) Opn5m, AB368182; Xenopus tropicalis Opn5m, XM_002935990; zebrafish (Danio rerio) Opn5m, AY493740; zebrafish Opn5m2, XM_005157939; spotted gar (Lepisosteus oculatus) Opn5m, XM_015349763; spotted gar Opn5m2, XM_015337843; chicken Opn5L1, AB368181; X. tropicalis Opn5L1a, XM_031904599; X. tropicalis Opn5L1b, NM_001079378; zebrafish Opn5L1a, NM_001316948; zebrafish Opn5L1b, NM_001044992; zebrafish Opn5L1c, NM_001316949; zebrafish Opn5L1d, XM_001340566; spotted gar Opn5L1a, XM_015339535; spotted gar Opn5L1b, XM_015364771; chicken Opn5L2, AB368183; X. tropicalis Opn5L2, XM_004914948; zebrafish Opn5L2a, NM_001316947; zebrafish Opn5L2b, NM_001316945; zebrafish Opn5L2c, NM_001317764; spotted gar Opn5L2a, XM_015343997; spotted gar Opn5L2b, XM_015351606; spotted gar Opn5L2c, XM_015347092; and human Rrh, BC128401.
Molecular properties of Opn5m
First, to determine the molecular properties of Opn5m, we prepared recombinant chicken Opn5m, which formed a UV light-sensitive pigment after reconstitution with 11-cis retinal (Yamashita et al. 2010). UV light irradiation caused retinal isomerization into the all-trans form to generate the visible light-sensitive active state for G protein coupling. Subsequent visible light irradiation induced the conversion of retinal back to the 11-cis form to form the resting state of Opn5m. Thus, chicken Opn5m can be interconverted between resting and active states by light irradiation (Fig. 1b). In addition, chicken Opn5m can bind directly to both 11-cis and all-trans retinal. These molecular properties have been observed for visual rhodopsins in mollusks and arthropods and for several nonvisual opsins; collectively, opsins with such properties are called bistable opsins (Koyanagi and Terakita 2014).
Next, we studied the molecular properties of various vertebrate Opn5m, including those from human and mouse (Yamashita et al. 2014). We found that all tested vertebrate Opn5m are sensitive to UV light after reconstitution with 11-cis retinal. Among the nine human opsins, Opn5m is the only one predicted to be sensitive to UV light. Interestingly, human and mouse Opn5m lack the ability to bind directly to all-trans retinal. Mutational analysis revealed that a single mutation at amino acid position 168 in mammalian Opn5m (based on the bovine rhodopsin numbering system) can explain a loss of affinity for all-trans retinal. Close comparison of amino acid sequences revealed that Ala168, which is well conserved in Opn5m orthologues from nonmammalian vertebrates, monotremes, and marsupials, is replaced by a threonine residue in eutherian Opn5m. Thus, we speculate that the A168T mutation occurred after the divergence of eutherian from metatherian mammals. Direct binding to all-trans retinal can help to form the photopigments without a supply of 11-cis retinal. However, direct binding to all-trans retinal converts the receptor to the active state, which causes noise to increase under dark conditions. Thus, mammalian Opn5m is supposed to suppress the noise activity in order to become specialized for short wavelength reception. Histochemical analysis revealed that mouse and marmoset (primate) Opn5m is expressed in a subset of ganglion cells in the retina and in several areas within the hypothalamus such as the preoptic area of the brain (Kojima et al. 2011; Yamashita et al. 2014). Within the retina, the visual cycle functioning mainly in the retinal pigment epithelium supplies 11-cis retinal to rhodopsin and cone opsins in visual cells. Thus, Opn5m may be regenerated by the same 11-cis retinal supply system. We detected the expression of RPE65, which is responsible for the conversion of all-trans retinyl esters to 11-cis retinol in the visual cycle of the retina (Kiser and Palczewski 2016), in the brain near Opn5m-positive cells. Opn5m may function exclusively as a short wavelength sensor in the brain in the presence of an 11-cis retinal supply system.
Recently, several papers have reported the physiological relevance of mammalian Opn5m by studying knockout mice. Opn5m-deficient mice maintain normal electroretinograms under dark- and light-adapted conditions but showed a loss of synchronization of local circadian oscillators in the retina, cornea, and skin to light/dark cycles (Buhr et al. 2019; Buhr et al. 2015). The knockout mice were also impaired in circadian photoentrainment of behavior under short wavelength conditions (Ota et al. 2018). Thus, the Opn5m-dependent reset of the circadian oscillator in the retina is thought to affect the behavioral rhythm in mammals. In addition, Opn5m mediates light-dependent vascular development in the mouse retina and vitreous body (Nguyen et al. 2019). Opn5m shows a relatively broad absorption spectrum with a smaller extinction coefficient and can cover an expanded wavelength range from the UV region to the violet and blue regions at the expense of photosensitivity (Yamashita et al. 2010; Yamashita et al. 2014). We recently reported that Drosophila melanogaster Rh7, which functions as a circadian photoreceptor in the brain (Ni et al. 2017), shows a similarly broad absorption spectrum (Sakai et al. 2017). This spectral characteristic may be advantageous for resetting circadian rhythm instead of visual color discrimination. In contrast to mounting evidence of important roles for Opn5m in the mammalian retina, there is less information concerning the physiological functions of Opn5m in the mammalian brain.
Most vertebrates have only one Opn5m gene in their genomes. However, several teleost fishes, such as zebrafish and pufferfish, possess an additional Opn5m gene, namely, Opn5m2 (Fig. 2) (Sato et al. 2016). The Opn5m2 gene can also be found in non-teleost fishes, such as spotted gar, sturgeon, and reedfish, in the class Actinopterygii. Thus, the gene duplication event that produced the Opn5m2 gene presumably occurred before the emergence of the Actinopterygii. Zebrafish Opn5m2 is also sensitive to UV light after reconstitution with 11-cis retinal and exhibits the characteristic behavior of a bistable pigment. However, Opn5m2 cannot bind all-trans retinal directly. Because Ala168 is conserved among Opn5m2 orthologues, the binding specificity of retinal isomers in Opn5m2 may be controlled by amino acid residue(s) at different position(s), and independent evolutionary events have enabled mammalian Opn5m and fish Opn5m2 to function as short wavelength sensors without an increase in noise triggered by the direct binding of all-trans retinal.
Molecular properties of Opn5L2
Most nonmammalian vertebrates only have one Opn5L2 gene in their genomes (Ohuchi et al. 2012). However, teleost fishes have paralogous Opn5L2 genes (Fig. 2). For example, zebrafish and pufferfish possess three Opn5L2 genes. We can also find multiple Opn5L2 genes in the genomes of non-teleost fishes, such as spotted gar, sturgeon, and reedfish, in the class Actinopterygii. Thus, as in the case of Opn5m2, the gene duplication event that produced multiple Opn5L2 paralogues presumably occurred before the Actinopterygii appeared.
To determine the molecular properties of Opn5L2, we prepared recombinant chicken Opn5L2, which formed a UV light-sensitive pigment after reconstitution with 11-cis retinal (Ohuchi et al. 2012). UV light irradiation induced the formation of the visible light-sensitive active state, which can be converted back to the resting state by isomerization of all-trans retinal to the 11-cis form. In addition, this active state was formed by the direct binding of all-trans retinal without light irradiation. Thus, Opn5L2 is also defined as a prototypical bistable opsin (Fig. 1b). Histochemical analysis of chicken tissues revealed that chicken Opn5L2 is expressed in a subset of amacrine and ganglion cells in the retina, several areas within the hypothalamus, and the adrenal gland. The retina and brain can receive light signals from the external environment and function as photoreceptive organs, whereas the adrenal grand is not thought to be photoreceptive. Opn5L2 may function as a retinal sensor due to its affinity for all-trans retinal in non-photoreceptive organs.
Molecular properties of Opn5L1
A genomic search for Opn5L1 genes revealed that most nonmammalian vertebrates have two Opn5L1 genes (Fig. 2) (Sato et al. 2018). However, teleost fishes have more Opn5L1 genes, e.g., three paralogues exist in pufferfish, and four are found in zebrafish. Because non-teleost fishes, such as spotted gar and sturgeon, in the class Actinopterygii have two Opn5L1 genes, the gene duplication event that produced multiple Opn5L1 genes in teleost fishes presumably occurred at the base of teleost fishes. By comparing amino acid sequences among vertebrate Opn5 proteins, we found that Opn5L1 proteins have characteristic mutations in the triad motif Asp-Arg-Tyr (DRY), which is well conserved among rhodopsin-like GPCRs and regulates GPCR conformational changes (Rovati et al. 2007), on the cytoplasmic side of the third transmembrane domain. Chicken is exceptional in that it only expresses one Opn5L1 protein, which contains Val-Cys-Cys (VCC) in place of the DRY motif. This suggested the possibility that Opn5L1 proteins are deficient in their G protein activation ability.
To determine the detailed molecular properties of Opn5L1, we prepared recombinant chicken Opn5L1 after incubation with 11-cis or all-trans retinal (Sato et al. 2018). After purifying these proteins, we found that they have similar absorption spectra (λmax = 510 nm). We also determined that these proteins were predominantly bound to all-trans retinal, even though it was originally incubated with 11-cis retinal. This shows that Opn5L1 does not bind 11-cis but does bind all-trans retinal, which could be formed through isomerization of 11-cis retinal before the incorporation into the protein. We also observed a significant increase in G protein activation ability after direct binding of all-trans retinal despite mutations in the conserved triad “DRY” motif on the cytoplasmic side of the third transmembrane domain. Thus, all-trans retinal can function as an Opn5L1 agonist; however, other all-trans retinoids, such as retinol and retinoic acid, cannot function as agonists. Yellow light irradiation on all-trans retinal bound Opn5L1 suppresses G protein activation ability, which indicates that Opn5L1 can be deactivated by light. Moreover, light irradiation causes the complete elimination of absorbance in the visible and near-UV regions and a small increase in absorbance at 270 nm.
Formation of the 270-nm product is unique to retinal proteins and has been thought to originate from an unknown mechanism other than retinal isomerization. Time-resolved spectral analysis of Opn5L1 revealed that a 500-nm product forms before the 270-nm product following photoreception. Analysis of the retinal configuration revealed that we could extract 11-cis retinal from the 500-nm product by breaking the Schiff base linkage between lysine residue and the retinal but could not extract retinal isomers from the 270-nm product using the same method. This shows that the primary event of photoreaction in Opn5L1 is trans/cis isomerization of retinal and that the 500-nm product contains 11-cis retinal. We also observed a subsequent spectral change following light irradiation. We detected the recovery of absorbance at 510 nm and G protein activation ability during incubation in the dark. We could extract all-trans retinal from our samples following dark incubation. Thus, we concluded that the 270-nm product can undergo thermal reversion to the dark state (Fig. 1c).
Next, we searched for the amino acid residue(s) responsible for this unique photoreaction by Opn5L1. We successfully identified a key cysteine residue at position 188, which is well conserved in the Opn5L1 subtype and not in either the Opn5m or Opn5L2 subtype. After photoreception, the Opn5L1 C188T mutant formed a 500-nm product, which could not convert to the 270-nm product. This shows that formation of the 270-nm product depends on some molecular mechanism involving Cys188. Because the λmax of the 270-nm product is similar to that of protonated 11,12-dihydro-retinylidene Schiff base in methanol (Gawinowicz et al. 1977), we hypothesized that the retinal-conjugated double bond system is disrupted by the formation of a covalent adduct between retinal and the cysteine residue. The liquid chromatography-mass spectrometry analysis confirmed that a short peptide fragment containing Cys188, which is formed by proteolysis of Opn5L1, binds to the retinal in the 270-nm product. This result supports the hypothesis that an adduct is formed between Cys188 and retinal in the 270-nm product. Based on these experimental data, the predicted molecular mechanism underlying the photoreaction of Opn5L1 is as follows (Fig. 3):
Fig. 3.
Conformational change of Opn5L1-bound retinal to trigger the photocyclic reaction. The dark state binds all-trans retinal to activate G protein. Light irradiation causes retinal isomerization to the 11-cis form to suppress G protein activation. In this state, Cys188 and C11 of retinal form an adduct, thereby converting the double bond of C11=C12 to a single bond. Thermal rotation of the C11–C12 single bond is facile and permits conversion to the all-trans form. In this state, Cys188 and C11 of retinal dissociate to regenerate the original dark state containing all-trans retinal.
1. The dark state binds exclusively to all-trans retinal, which photoisomerizes to the 11-cis form.
2. Cys188 approaches C11 of retinal to form a covalent adduct, which induces the conversion of the C11=C12 double bond in retinal to a single bond.
3. The C11–C12 single bond in retinal undergoes thermal rotation.
4. The Cys188-retinal adduct dissociates in a trans conformation to induce the regeneration of the original dark state.
Opn5L1 has unique characteristics compared with vertebrate rhodopsin and prototypical bistable opsins (Fig. 2). In particular, Opn5L1 is not activated by photoreception but is deactivated instead. Comparing the molecular properties of opsins led to the proposal that the ancestral opsin is bistable like Opn5m and Opn5L2 (Shichida and Matsuyama 2009). Vertebrate rhodopsin lost the ability to be deactivated by light and evolved to become activated exclusively by light, i.e., a forward photoreceptor. By contrast, Opn5L1 is thought to have evolved to become exclusively deactivated by light. Opn5L1 is therefore considered a reverse photoreceptor. In addition, after photoreception, Opn5L1 can thermally self-regenerate to the dark state by thermal isomerization of the retinal. The combination of photoisomerization and thermal isomerization of retinal regulates the G protein activation ability by Opn5L1, making this the first animal opsin whose ability is controlled by its photocyclic reaction. The physiological relevance of Opn5L1, however, remains unknown. Opn5L1 rapidly loses activity following photoreception and gradually recovers its activity in the dark. Thus, Opn5L1 may be used as a timer to measure time elapsed since the last illumination, such as after sunset.
Concluding remarks
Our analysis of vertebrate Opn5 subtypes revealed that the molecular properties of this group are unexpectedly diverse (Fig. 1). It surprised us that Opn5m proteins from a wide range of vertebrate species, including human, form a UV light-sensitive pigment. Moreover, Opn5L1 is the first opsin known to behave as a reverse photoreceptor. However, little is known about the molecular mechanisms that underlie such diverse molecular properties, e.g., key amino acid residues responsible for UV light reception by Opn5m and Opn5L2, and the low affinity of Opn5L1 for 11-cis retinal. The recent increase in genomic information revealed that Opn5 genes have been identified in many deuterostome and protostome species, but not in cnidarian species (Ramirez et al. 2016). Thus, the Opn5 gene has been proposed to be acquired by an ancestor of the bilateria. The analysis of Opn5 proteins from a wide range of animal species will reveal the evolutionary route that led to the diverse molecular properties of vertebrate Opn5.
Moreover, Opn5L1 exclusively binds all-trans retinal in the dark state and self-regenerate to the dark state after photoreception, which is quite similar to the molecular property of microbial rhodopsins (Ernst et al. 2014). Among microbial rhodopsins, channelrhodopsin is known to function as a light-gated cation channel (Nagel et al. 2002) and control the electrical excitability of mammalian neurons using light in a less-invasive way (Boyden et al. 2005; Ishizuka et al. 2006). This is because channelrhodopsin ectopically expressed in mammalian neurons can uptake endogenous all-trans retinal to form photopigments and repeatedly regulate its channel activity by using the photocyclic reaction. GPCRs are known to function in most mammalian tissues and control a wide variety of cellular responses. Thus, by the ectopic expression of Opn5L1 in various mammalian tissues, the opsin has the potential to repeatedly control the activity of intracellular G protein signaling using light in place of endogenous GPCRs. Optimizing the molecular properties of Opn5L1 will help provide a novel optogenetic tool.
Acknowledgments
I thank Profs. Y. Shichida (Ritsumeikan University) and H. Ohuchi (Okayama University) and Dr. K. Sato (Okayama University) for their long-term collaboration on the Opn5 analysis.
Funding information
This work was supported in part by grants-in-aid for Scientific Research of MEXT, a grant from the Takeda Science Foundation, a grant from Daiichi Sankyo Foundation of Life Science, and CREST, JST JPMJCR1753.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Buhr ED, Vemaraju S, Diaz N, Lang RA, Van Gelder RN. Neuropsin (OPN5) mediates local light-dependent induction of circadian clock genes and circadian photoentrainment in exposed murine skin. Curr Biol. 2019;29(3478–3487):e3474. doi: 10.1016/j.cub.2019.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, et al. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc Natl Acad Sci U S A. 2015;112:13093–13098. doi: 10.1073/pnas.1516259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies WI, et al. An extended family of novel vertebrate photopigments is widely expressed and displays a diversity of function. Genome Res. 2015;25:1666–1679. doi: 10.1101/gr.189886.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst OP, Lodowski DT, Elstner M, Hegemann P, Brown LS, Kandori H. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem Rev. 2014;114:126–163. doi: 10.1021/cr4003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawinowicz MA, Balogh-Nair V, Sabol JS, Nakanishi K. A nonbleachable rhodopsin analogue formed from 11, 12-dihydroretinal. J Am Chem Soc. 1977;99:7720–7721. doi: 10.1021/ja00465a059. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Kakuda M, Araki R, Yawo H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci Res. 2006;54:85–94. doi: 10.1016/j.neures.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Kiser PD, Palczewski K. Retinoids and retinal diseases. Annu Rev Vis Sci. 2016;2:197–234. doi: 10.1146/annurev-vision-111815-114407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima D, Mori S, Torii M, Wada A, Morishita R, Fukada Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS One. 2011;6:e26388. doi: 10.1371/journal.pone.0026388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Terakita A. Diversity of animal opsin-based pigments and their optogenetic potential. Biochim Biophys Acta. 2014;1837:710–716. doi: 10.1016/j.bbabio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbey/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, et al. An opsin 5-dopamine pathway mediates light-dependent vascular development in the eye. Nat Cell Biol. 2019;21:420–429. doi: 10.1038/s41556-019-0301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JD, Baik LS, Holmes TC, Montell C. A rhodopsin in the brain functions in circadian photoentrainment in Drosophila. Nature. 2017;545:340–344. doi: 10.1038/nature22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H, Yamashita T, Tomonari S, Fujita-Yanagibayashi S, Sakai K, Noji S, Shichida Y. A non-mammalian type opsin 5 functions dually in the photoreceptive and non-photoreceptive organs of birds. PLoS One. 2012;7:e31534. doi: 10.1371/journal.pone.0031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota W, Nakane Y, Hattar S, Yoshimura T. Impaired circadian photoentrainment in Opn5-null mice. iScience. 2018;6:299–305. doi: 10.1016/j.isci.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez MD, Pairett AN, Pankey MS, Serb JM, Speiser DI, Swafford AJ, Oakley TH. The last common ancestor of most bilaterian animals possessed at least nine opsins. Genome Biol Evol. 2016;8:3640–3652. doi: 10.1093/gbe/evw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovati GE, Capra V, Neubig RR. The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol Pharmacol. 2007;71:959–964. doi: 10.1124/mol.106.029470. [DOI] [PubMed] [Google Scholar]
- Sakai K, Tsutsui K, Yamashita T, Iwabe N, Takahashi K, Wada A, Shichida Y. Drosophila melanogaster rhodopsin Rh7 is a UV-to-visible light sensor with an extraordinarily broad absorption spectrum. Sci Rep. 2017;7:7349. doi: 10.1038/s41598-017-07461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yamashita T, Haruki Y, Ohuchi H, Kinoshita M, Shichida Y. Two UV-sensitive photoreceptor proteins, Opn5m and Opn5m2 in ray-finned fish with distinct molecular properties and broad distribution in the retina and brain. PLoS One. 2016;11:e0155339. doi: 10.1371/journal.pone.0155339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, et al. Opn5L1 is a retinal receptor that behaves as a reverse and self-regenerating photoreceptor. Nat Commun. 2018;9:1255. doi: 10.1038/s41467-018-03603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichida Y, Matsuyama T (2009) Evolution of opsins and phototransduction. Philos Trans R Soc Lond Ser B Biol Sci 364:2881–2895 doi: 364/1531/2881. 10.1098/rstb.2009.0051 [DOI] [PMC free article] [PubMed]
- Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett. 2003;554:410–416. doi: 10.1016/S0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- Tomonari S, Migita K, Takagi A, Noji S, Ohuchi H. Expression patterns of the opsin 5-related genes in the developing chicken retina. Dev Dyn. 2008;237:1910–1922. doi: 10.1002/dvdy.21611. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ohuchi H, Tomonari S, Ikeda K, Sakai K, Shichida Y. Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc Natl Acad Sci U S A. 2010;107:22084–22089. doi: 10.1073/pnas.1012498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, et al. Evolution of mammalian Opn5 as a specialized UV-absorbing pigment by a single amino acid mutation. J Biol Chem. 2014;289:3991–4000. doi: 10.1074/jbc.M113.514075. [DOI] [PMC free article] [PubMed] [Google Scholar]