Abstract
Background and aims
There is limited literature on endoscopic ultrasound-guided liver biopsy (EUS-LB), a new method of obtaining liver biopsy (LB).
Methods
We conducted a retrospective study of the efficacy and safety of EUS-LB compared to percutaneous liver biopsy (PC-LB) in patients with chronic liver disease at our center between January 2018 and August 2019.
Results
Thirty patients underwent EUS-LB and 60 patients underwent PC-LB were identified (median follow-up post-LB was 8 days; interquartile range (IQR), 3–5 days). The median number of portal tracts was significantly higher in the PC-LB group (13 vs. 5; P < 0.0001). A histologic diagnosis was established in 93% of the EUS-LB group, compared to 100% in the PC-LB group (P = 0.841). Patients in EUS-LB group had significantly shorter hospital stay (median time of hospital stay was 3 vs. 4.2 h in the EUS-LB vs. PC-LB group, respectively; P = 0.004) and reported less pain compared to PC-LB group (median pain score was 0 vs. 3.5; P = 0.0009). EUS-LB were performed using a 19-gauge (n = 27) or 22-gauge (n = 3); there was a tendency towards higher number of portal tracts in the 22- vs. the 19-gauge needle group (6 vs. 5; P = 0.501). No patient in either group had significant adverse events such as bleeding or death.
Conclusion
EUS-LB is safe and is associated with less pain, shorter hospital stay, and high diagnostic yield (93%) compared to PC-LB. Randomized trials are needed to standardize the utility of EUS-LB.
Keywords: Endoscopic ultrasound-guided liver biopsy, Percutaneous liver biopsy, Chronic liver disease
Introduction
Liver biopsy is crucial to the diagnosis, management, and prognosis of many patients with liver diseases. Many noninvasive methods such as Vibration-Controlled Transient Elastography (VCTE) are marvelous advancements in the assessment of fibrosis in patients with chronic liver disease. However, these methods are not as sensitive or specific as biopsy of hepatic tissue [1, 2]. Furthermore, noninvasive methods can be limited in determining the etiology of end-stage liver disease (ESLD) or be limited in certain populations such as those with increased body habitus. With increasing incidence of non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and other disorders that lead to chronic liver disease and ESLD, the need for liver histology is expected to rise in the future [3].
Traditionally, there are three ways to obtain hepatic tissue samples. In 1883, Paul Ehrlich performed the first documented liver biopsy using the percutaneous method, where he percussed the right lobe of the liver and pierced the skin with a large needle to obtain a core sample [4]. Today, percutaneous liver biopsy (PC-LB) is performed using ultrasound guidance for marking and guidance to avoid complications such as pneumothorax or puncturing other organs such as gallbladder or bowels in needle path [3, 5]. Transjugular liver biopsy was performed in the late 1900s where the biopsy needle is entered into the liver via hepatic vein and then the tissue is obtained. Transjugular liver biopsy is advantageous in patients with coagulopathy or significant ascites which makes the percutaneous approach impractical [5–7]. The newest method of acquisition of liver tissue is endoscopic ultrasound-guided liver biopsy (EUS-LB) which has only been established as an alternative method in the past few years. Endoscopic ultrasound-guided fine needle aspiration or biopsy (EUS-FNA/FNB) is performed using the curved linear-array echoendoscope using various needles. EUS imaging of the liver is currently limited to the left lobe, the proximal right lobe, the hilum, and part of the intrahepatic biliary tract. In EUS-LB, the biopsy needle is entered endoscopically via transgastric/transduodenal route and real-time endoscopic ultrasound guidance is employed during the procedure.[5, 6, 8–10]. EUS is also equipped with Ultrasound Doppler to interrogate for vascular flow signals in needle path.
There are advantages and disadvantages to each of these methods in obtaining liver tissue and additional data are emerging for the safety and efficacy of EUS-LB. For PC-LB, a significant advantage is the wide-spread availability of the procedure compared to the more complex EUS-LB. The procedure is generally performed through the transthoracic route by palpation/percussion-guided or imaging-guided. A larger suction or cutting needle can be employed in PC-LB which practically may allow for a large core tissue and increase diagnostic yield. The right lobe is more frequently accessed by this technique and the use of oral or intravenous anxiolytic therapy or conscious sedation is variable. Some disadvantages of PC-LB are pain and apprehension in patients, since the procedure requires minimal or no sedation [3, 5, 6, 11, 12]. Theoretically, there is a higher risk of puncturing a blood vessel with PC-LB, especially in obese patients. In contrast, EUS provides ability to view both lobes of the liver and avoid blood vessels as small as 1 mm in length [13]. Other known advantages of EUS-LB include less apprehension in patients due to use of sedation or monitored anesthesia care, and theoretically less pain due to avoidance of somatic nervous system [3, 6, 10, 14]. If patients need a concomitant diagnostic or therapeutic upper endoscopic procedure, then EUS-LB is deemed to be more cost-effective than other approaches [15]. A disadvantage of EUS-LB is the complexity of the procedure correlating to reduced availability and need of experienced endosonographer. To contribute to the current literature, we conducted a retrospective study to evaluate the safety and diagnostic yield of EUS-LB versus PC-LB at a single academic institution. Our institution represents a suitable setting to conduct this study, since we perform hundreds of liver biopsies annually at our institution, and the senior author (G.M.H.) maintains a prospective database of all patients who underwent EUS-LB. We hypothesize that EUS-LB is as efficacious as PC-LB in obtaining an adequate liver sample and may be better tolerated due to reduced pain and apprehension.
Materials and methods
Study design
This study was approved by the University of Missouri-Columbia Institutional Review Board (IRB # 2014436), and has been conducted in accordance with the institutional research ethics committee and the ethical standards as laid down in the Declaration of Helsinki. A database of all patients who underwent EUS-LB is prospectively maintained by the senior author (G.M.H). We conducted a retrospective analysis of the efficacy and safety of EUS-LB compared to PC-LB (ratio of 1:2) at the University of Missouri School of Medicine-Columbia Hospital between January 2018 and August 2019. Eligible patients were referred for EUS-LB or PC-LB by hepatologists and gastroenterologists from University of Missouri School of Medicine-Columbia outpatient clinics. Each patient was evaluated clinically and the need for liver biopsy was determined by the physician based on physical examination, labs, imaging, and the patient meeting appropriate criteria for liver biopsy. Patients were informed of the risks and benefits of both procedures and the choice between EUS-LB versus PC-LB was ultimately decided by the patient and the referring provider. Impaired hemostasis [being on potent antiplatelets and/or anticoagulants; and/or international normalized ratio (INR) > 1.5] are considered contraindications for liver biopsy at our institution, based on the experts’ opinion [3]. These minimal coagulation parameters are applied to both the EUS-LB and the PC-LB approaches at our institution.
Data collection
A comprehensive database of all liver biopsies, including both EUS-LB and PC-LB, is well maintained by the Pathology Department at the University of Missouri School of Medicine-Columbia. EUS-LB slides were re-examined by the gastrointestinal and liver pathologist (D.S.R) to confirm the diagnosis and determine adequacy of sample, fragmentation of the sample, the number of portal tracts, core biopsy length, and core biopsy number. Remaining information was collected by other co-authors of the study from patients’ charts including, patient demographics, complications and readmission rates, length of hospital stay, pain scores, and opiate use. For each readmission, the reason(s) for readmission to the hospital, investigations, outcomes, and vital status at the time of discharge were recorded. The length of hospital was defined as the time period (hours) between admission to the interventional radiology (if the procedure is to be performed by an interventional radiologist) or endoscopy suite (if the procedure is to be performed by an interventional gastroenterologist) and discharge from the facility.
EUS-LB technique
All the EUS-LB procedures at the University of Missouri School of Medicine-Columbia were performed by a single advanced therapeutic endoscopist (G.M.H), who performs hundreds of EUS procedures and other advanced therapeutic endoscopy procedures annually. After patient enters the endoscopy lab, a timeout is conducted prior to initiation of the procedure. Monitored anesthesia care is administered with propofol by an anesthesiologist or a certified registered nurse anesthetist. Vitals and hemodynamics are continuously monitored. The linear-array echoendoscope (GF-UCT180, Olympus America, Center Valley, PA, United States) was used for EUS-LB. All EUS-LB were performed using a 19- or 22-gauge Fork-tip SharkCore biopsy needle (Medtronic, Massachusetts, United States). The size of the needle used for the liver biopsy was recorded at each time in the procedure report. The 19-gauge needle is frequently used at our institution, because it has shown superiority over 22-gauge needle [16, 17]. The needle is prepped using ‘wet suction’ technique where needle stylet is removed and flushed with heparin prior to attaching a vacuum syringe and maximal suction was applied via a syringe. The echoendoscope is inserted under direct visualization and endoscopic ultrasound was performed to assess for any other pathology and evaluate for liver lesions. The site of the liver biopsy is determined using EUS guidance. Either right or left lobe of the liver was chosen for puncture. Color Doppler study was used to evaluate for any significant flow signals in needle path to avoid puncture of major blood vessels or adjacent biliary ducts. The right or left lobe of the liver is punctured either through transduodenal or transgastric approach, and suction syringe is applied (Fig. 1). Two to three deep actuations were performed per pass. A total of two passes were performed. The suction syringe is turned off and the needle is withdrawn from the liver. The stylet is inserted into the needle catheter to displace any liver tissue into the cell block container followed by flushing the needle with saline. The endoscopist separates tissue from any visualized clotted blood, and the biopsy sample is sent for histopathologic assessment (Fig. 2). At our institution, patients are monitored for 60 min post-procedure with intermittent vital signs’ checks. Patients are advised to avoid heavy lifting or strenuous activity for 72 h post-procedure. Patients were called on the following day to inquire about their overall wellbeing, and specifically about signs and symptoms of potential adverse events.
Fig. 1.
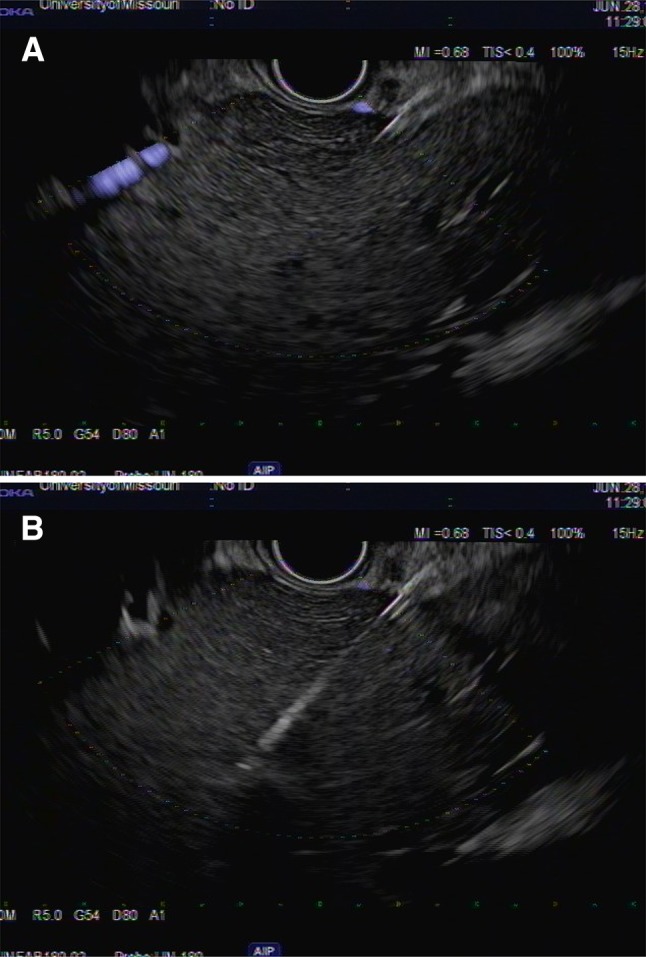
a Linear echoendoscope showing the right lobe of the liver. b Linear echoendoscope showing fine needle biopsy of the right lobe of the liver using 19-gauge fork-tip needle
Fig. 2.
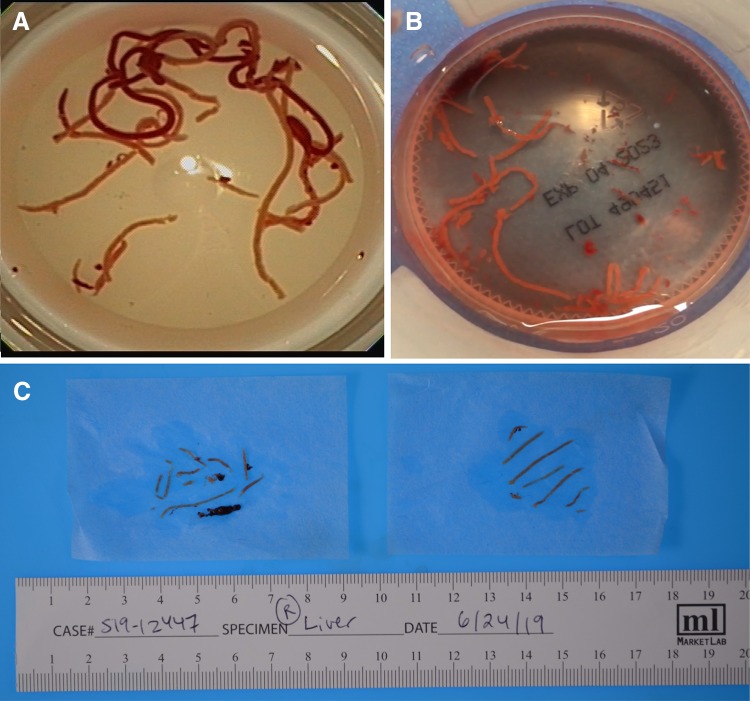
a and b Multiple cores of liver tissue obtained by EUS-FNB placed in a formalin jar. c Multiple cores of liver tissue obtained by EUS-FNB separated from blood clots
PC-LB technique
The PC-LB procedures were performed partly by the interventional radiology service and partly by the gastroenterology service. Patient is positioned in a supine manner with appropriate monitoring of vitals and hemodynamics in place. Preliminary ultrasound scan is performed to locate the site of hepatic parenchyma and avoid any blood vessels or bile ducts. A timeout is conducted prior to every procedure. The skin is appropriately prepped and draped to ensure asepsis followed by injection of a local anesthetic at the injection site. Patient is instructed to exhale when needle is inserted into liver parenchyma under ultrasound guidance to minimize the risk of perforation to the pleural cavity. For the procedure, an 18-gauge CorVocet needle (Meritmedical, Sought Jordan, Utah, United States) is typically used by intervention radiology or 15-gauge Jamshidi needle (CareFusion, Vernon Hills, Illinois, United States) if performed by the interventional gastroenterologist. A post-procedure scan is performed to ensure that there is no internal bleeding after the liver biopsy. At our institution, patients are typically monitored for 4 h post-procedure with regular vital checks. Patients are advised to avoid heavy lifting or strenuous activity for 72 h post-procedure. All patients were contacted the next day of procedure to assess if any adverse events encountered.
Pathological assessment
Liver biopsies were placed in 10% buffered formalin. For each liver biopsy, hematoxylin and eosin; Masson’s trichrome; reticulin; iron; periodic acid-Schiff (PAS); and PAS with diastase stains were performed when applicable. For NASH, biopsies were graded and staged using the Clinical Research Network (CRN), non-alcoholic fatty liver disease (NAFLD), activity score (NAS), and fibrosis staging system [18]. All liver biopsies were read by a gastrointestinal and liver pathologist (D.S.R.).
Quality of the liver biopsy
For the purposes of this study, we used the core biopsy numbers, core biopsy length, and number of portal tracts, as objective measures of the quality of the liver biopsy samples. We also used the direct subjective assessment of the interpreting pathologist whether the biopsy samples are “adequate” or “fragmented” and if pathologist was able to reach a diagnosis.
Pain assessment
All patients were monitored and assessed for pain, using the pain numeric rating scale (NRS), on which patients rate their pain from 0 “no pain” to 10 “worse possible pain” [19].
Statistical analysis
Continuous data were expressed as median (Q2) and interquartile range (IQR; [Q1–Q3]), and categorical data were expressed as frequency and percentage. Chi-square test, Fisher’s exact test, and the Wilcoxon rank-sum test were used to compare between the EUS-LB and the PC-LB groups. Linear regression was used to examine the relationship between the age, core biopsy numbers, core length, and the number of portal tracts. A P value < 0.05 was considered statistically significant. Statistical analyses were conducted using STATA v12.1 (StataCorp LP, College Station, TX, USA), and graphs were constructed using the Prism v7 GraphPad Software (La Jolla, California, USA).
Results
Characteristics of the entire study population
A total of 90 patients (EUS-LB, n = 30; and PC-LB, n = 60) were included in the study. Their median age was 53 years (IQR, 45–59 years) at the time of LB, and 63% (57/90) were female. The indications for liver biopsy were staging of viral hepatitis, NAFLD, autoimmune, and metabolic liver diseases (n = 48), elevated liver transaminases (n = 27), and evaluation of suspected NASH on imaging studies (n = 15).
Characteristics of endoscopic ultrasound-guided liver biopsy group
During the study period, a total of 30 patients had undergone EUS-LB (Table 1). Their median age was 54 years (IQR, 46–63 years) at the time of the liver biopsy, and 63% (19/30) were female. Indications for liver biopsy were elevated liver transaminases (n = 17), evaluation of suspected NASH (n = 8), and staging of fibrosis (n = 5).
Table 1.
Liver biopsy data on patients who underwent EUS-LB from January 2018 to August 2019 (n = 30)
| Case # | Age | Needle gauge | Core length (mm) | n portal tracts | Fibrosis stage | Histological diagnosis |
|---|---|---|---|---|---|---|
| 1 | 63 | 19 | 30 | 5 | 4 | CHC |
| 2 | 56 | 19 | 43 | 1 | 3 | NASH |
| 3 | 39 | 22 | 80 | 6 | 1 | NASH |
| 4 | 37 | 19 | 25 | 9 | 1 | FLD |
| 5 | 33 | 19 | 68 | 9 | 0 | GH |
| 6 | 64 | 19 | 15 | 6 | 0 | FLD |
| 7 | 46 | 19 | 37 | 5 | 0 | FLD |
| 8 | 53 | 19 | 16 | 8 | 0 | FLD |
| 9 | 51 | 19 | 27 | 5 | 2 | PBC |
| 10 | 53 | 19 | 20 | 6 | 2 | PFIC |
| 11 | 48 | 19 | 19 | 5 | 1 | FLD |
| 12 | 54 | 22 | 33 | 5 | 0 | NASH |
| 13 | 52 | 19 | 25 | 5 | 1 | NASH |
| 14 | 46 | 19 | 26 | 6 | 0 | CHC |
| 15 | 39 | 22 | 45 | 8 | 2 | NASH |
| 16 | 54 | 19 | 23 | 4 | 2 | CHC |
| 17 | 32 | 19 | 25 | 9 | 1 | NASH |
| 18 | 72 | 19 | 24 | 4 | 1 | NASH |
| 19 | 62 | 19 | 25 | 1 | 0 | EH |
| 20 | 65 | 19 | 25 | 5 | 2 | NASH |
| 21 | 55 | 19 | 20 | 6 | 3 | NASH |
| 22 | 48 | 19 | 15 | 5 | 3 | NASH |
| 23 | 63 | 19 | 25 | 4 | 4 | NASH |
| 24 | 55 | 19 | 21 | 8 | 2 | CHC |
| 25 | 48 | 19 | 28 | 6 | 1 | NASH |
| 26 | 70 | 19 | 55 | 42 | 4 | NASH |
| 27 | 59 | 19 | 21 | 5 | 2 | NASH |
| 28 | 54 | 19 | 24 | 10 | 4 | NASH |
| 29 | 64 | 19 | 20 | 6 | 2 | Hemochromatosis |
| 30 | 33 | 19 | 22 | 5 | 3 | NASH |
CHC chronic hepatitis C, GH granulomatous hepatitis, EH eosinophilic hepatitis, FLD fatty liver disease, NASH non-alcoholic steatohepatitis, PBC primary biliary cholangitis, PFIC progressive familial intrahepatic cholestasis
The 19-gauge SharkCore needle was used in 90% (27/30) of cases. The number of portal tracts tended to be higher in the 22-gauge needle group compared to the 19-gauge needle group (median, 6 vs. 5; P = 0.501).
Comparison between the endoscopic ultrasound-guided liver biopsy versus the percutaneous liver biopsy group
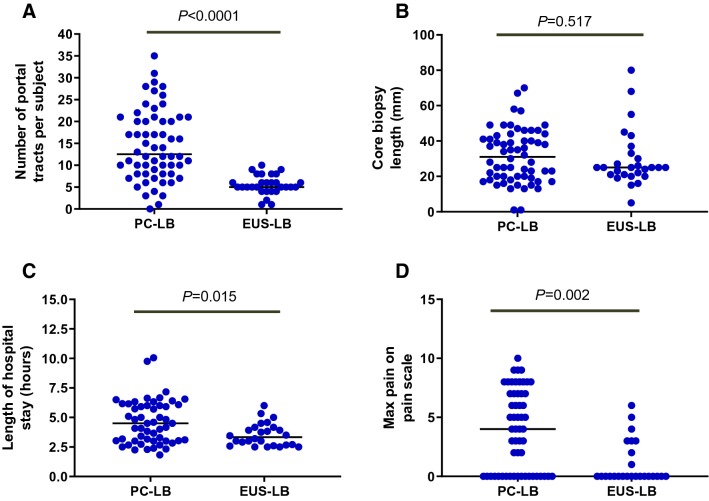
We compared the safety and efficacy of EUS-LB to PC-LB in an age- and gender-matched group (1:2 ratio study design). Table 2 and Fig. 3 outline the comparison between the two groups. The median age of the two groups was comparable. There were significantly more patients who undergone EUS-LB for evaluation of elevated liver transaminases (57% vs. 17%, respectively; P < 0.0001) and suspected NASH (27% vs. 12%, respectively; P < 0.0001) compared to PC-LB. In contrast, there were significantly more patients in the PC-LB group who underwent LB for staging of fibrosis (72% vs. 17%, respectively; P < 0.0001). The median LB core length tended to be longer in the PC-LB group compared to the EUS-LB group (31 vs. 25 mm; P = 0.517). The median number portal tract per LB sample was significantly higher in the PC-LB group compared to the EUS-LB group (13 vs. 5 portal tracts; P < 0.0001). There were significantly less fragmented liver biopsy samples in the PC-LB group compared to the EUS-LB group (10% (6/60) vs. 40% (12/30); P = 0.005).
Table 2.
Baseline features at the time of liver biopsy in 90 patients who underwent liver biopsy between January 2018 and August 2019
| Variable | EUS-guided liver biopsy (n = 30) | PC-guided liver biopsy (n = 60) | P value |
|---|---|---|---|
| Demographics | |||
| Agea | 54 (46–63) | 53 (45–59) | 0.694 |
| Gender, male:female | 11:19 | 22:38 | 1 |
| Indications for LB | |||
| Fibrosis staging, n (%) | 5 (17%) | 43 (72%) | < 0.0001 |
| Elevated liver enzymes, n (%) | 17 (57%) | 10 (17%) | |
| Evaluation of suspected NASH, n (%) | 8 (27%) | 7 (12%) | |
| Liver biopsy properties | |||
| Core length (mm)a | 25 (21–33) | 31 (20–42) | 0.517 |
| Core numbersa | 5 (5–6) | 3 (3–4) | 0.001 |
| Portal tract numbersa | 5 (5–8) | 13 (8–21) | < 0.0001 |
| Fragmented LB sample, yes, n (%) | 12/30 (40%) | 6/60 (10%) | 0.005 |
| Histological diagnosis established, yes, n (%) | 28 (93%) | 60 (100%) | 0.84 |
| Others | |||
| Hospital stay, hoursa | 3 (2.6–3.9) | 4.2 (3–5.9) | 0.004 |
| Pain severitya,b | 0 (0–3) | 3.5 (2–7) | 0.0009 |
| Opiate use, yes, n (%) | 2 (7%) | 29 (48%) | < 0.0001 |
| Readmission, yes, n (%) | 1 (3%) | 0 | 0.303 |
aData are expressed as median (IQR) [Q2: Q1 − Q3]
bPain severity scale ranges from 0 to 10, where 0 is no pain and 10 is intolerable pain
Fig. 3.
Comparison between the number of portal tracts (a), core biopsy length (b), length of hospital stay (c), and maximum pain in the PC-LB (n = 60) vs. the EUS-LB (n = 30) groups. Descriptive statistics are expressed as median (interquartile range)
Patients in the EUS-LB group had significantly shorter hospital stay post-procedure, compared to those in the PC-LB group (median, 3 vs. 4.2 h; P = 0.004). Patients in the EUS-LB group had significantly less pain on the pain scale (median, 0 vs. 3.5; P = 0.0009) and significantly less frequency of use of opiates post-procedure (7% vs. 48%; P < 0.0001). Furthermore, histological diagnosis was established in 93% of the EUS-LB group, compared to 100% of the PC-LB group (P = 0.84).
Linear regression analysis
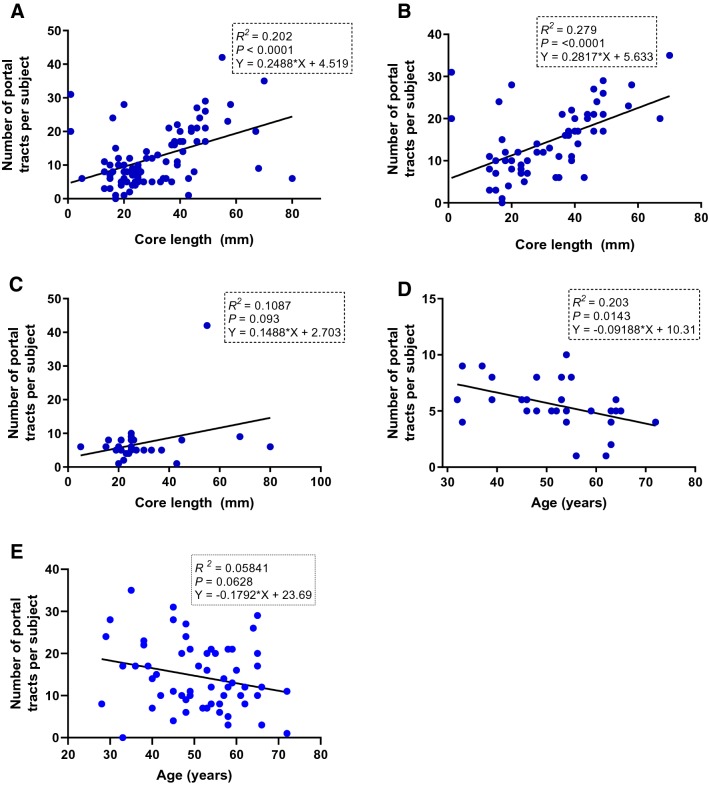
We carried out simple and multiple regression analyses to investigate the relationship between age at the time of the liver biopsy and the core biopsy length, core biopsy numbers, and the number of portal tracts. The scatter slope showed a strong positive linear correlation between the core LB length and the number of portal tracts in the entire sample (Fig. 4a) and in the PC-LB group (Fig. 4b); there was a positive, but weak, correlation between the core LB length and the number of portal tracts in the EUS-LB group (Fig. 4c).
Fig. 4.
a Linear regression showing the relationship between the number of portal tracts per subject and the core biopsy length in the entire sample; b number of portal tracts per subject and the core biopsy length in the PC-LB group; c number of portal tracts per subject and core biopsy length in the EUS-LB group; d relationship between age and number of portal tracts per subject in the EUS-LB group; and e relationship between age and number of portal tracts per subject in the PC-LB group
A multiple regression analysis was run to predict the number of portal tracts from the core biopsy number, core biopsy length, and the type of liver biopsy (PC vs. EUS). All three variables added statistically significantly to the prediction of number of portal tracts [core biopsy number, P = 0.037; core biopsy length, P < 0.0001; and type of liver biopsy, P < 0.0001; F (3, 72) = 18.44; R2 = 0.44].
Additionally, we sought to determine if there was a relationship between the following variables in the EUS-LB group: age at the time of liver biopsy; core LB length; and the number of portal tracts. A linear regression showed a statistically significant negative relationship between age at the time of EUS-LB and the number of portal tracts (F (1, 23) = 7.4; P = 0.012; R2 = 0.245; predicted number of portal tracts = – 0.1382 * age + 12.26; Fig. 4d). Because of this unexpected nature of relationship between age and the number of portal tracts in the EUS-LB group, we sought to examine the relationship between age and the number of portal tracts in the PC-LB group. Interestingly, there was a negative linear relationship between the two variables; however, it was not statistically significant (Fig. 4e).
Adverse events
We continued follow-up of the patients after LB for a median of 8 days (IQR, 3–5 days). Only one patient in the EUS-LB group was readmitted 2 days following the procedure for abdominal pain. Thorough investigations including cross-sectional imaging revealed no evidence of intraabdominal hemorrhage or iatrogenic trauma of the intrathoracic/intraabdominal organs. The patient was discharged home on the following day; he was called 24 h and 48 h after discharge and reported resolution of pain. No patient in the PC-LB was readmitted. No patient in either group experienced any significant adverse events such as bleeding, puncture of other organ, or death post-procedure or when called on the following day.
Discussion
In this retrospective comparative single-center study, we found that the safety of EUS-LB is comparable to the safety of PC-LB. Moreover, EUS-LB was associated with shorter hospital stay, less frequency of reported pain, and, as a result, less use of opiates. Although PC-LB was associated with higher core biopsy numbers, longer core biopsy specimens, and less frequency of fragmented liver tissue samples, EUS-LB had a diagnostic yield with respect to ascertainment of the histological diagnosis of 93%, which is comparable to that of the PC-LB group.
Over the last decade, EUS-LB has been increasingly used as a tool for obtaining liver tissue samples for histological assessment. Since January 2018, we maintained a prospective database of patients who underwent EUS-LB. To better understand the safety and efficacy of EUS-LB, we compared the EUS-LB group with an age- and gender-matched group of patients who underwent PC-LB (1:2 study design ratio). With respect to efficacy, we chose core biopsy numbers, core biopsy length, number of portal tracts, and the subjective pathologists’ assessment whether liver biopsy was adequate and/or fragmented as measurements of the quality of liver biopsies, since these parameters are frequently taken into account by pathologists who are specialized in liver biopsy interpretation [20].
There are no randomized clinical trials comparing the safety and efficacy of EUS-LB to that of PC-LB [6]. The safety and diagnostic yield of EUS-LB has been reported by several groups, with a diagnostic yield of up to 100% [8]. We observed a diagnostic yield of 93% in the EUS-LB group in our study. Analysis of our data using linear regression revealed a statistically significant positive relationship between the core biopsy numbers, core biopsy length, and the number of portal tracts obtained per liver biopsy sample; the positive linear relationship between the core liver biopsy length and number of portal tracts has been previously reported [21]. These data suggest that these parameters might be of clinical and diagnostic significance when using the EUS as a tool for assessment of the liver parenchyma.
Our data show that the number of portal tracts in liver biopsies obtained by EUS is inversely related to the patients’ age. We initially thought this relationship pertains only to liver biopsies obtained by EUS. This led us to examine the nature of the relationship between age of patients and number of portal tracts in the PC-LB group. Interestingly, although not statistically significant, we found a negative linear relationship between the age of patients and the number of portal tracts in the PC-LB group. It is unclear why older patients in our study had fewer portal tracts compared to younger patients. This could be due to technical difficulties in obtaining the liver tissue sample in the older population; age-related increased fibrosis which could theoretically result in less intact portal tracts; or age-related loss of portal tracts. These potential reasons are speculative at this time; however, we believe that these factors should be carefully considered when designing future randomized clinical trials assessing the utility of EUS-LB and when performing EUS-LB in clinical practice.
Although not powered enough due to the small-sample size of our study, an interesting and unexpected finding is the tendency towards a higher number of portal tracts in the EUS-LB group of patients who underwent the procedure using a 22-gauge compared to the 19-gauge EUS needle. The reported data on diagnostic yield of liver tissue samples using different needle sizes/calibers are conflicting. One would assume that sampling the liver tissue using larger caliber needles would yield higher numbers of portal tracts, as previously demonstrated by Mok et al. [9]. However, at least one study published recently by Bazerbachi et al. using the 22-gauge needle reported a higher number of portal tracts in comparison with earlier studies using the 19-gauge needle [22]. There are at a few plausible theories that could be proposed, none of which can be confirmed at this time. One plausible explanation is the nature of the liver disease being sampled. We noted all three patients in the EUS-LB group in our study who underwent the procedure using the 22-gauge needle had NASH, which is the same patient population studied by Bazerbachi et al. [22]. The EUS allows visualization of the areas that are “fatty” in the liver, thus guiding to the representative areas in the liver. Another plausible explanation is the length of the specimen. In our study, further sub-analysis of the EUS-LB group revealed that the median length of specimen was higher in the 22-gauge vs. the 19-gauge (45 mm vs. 25 mm, respectively). Thus, it is reasonable to hypothesize that length of specimen is directly proportional to the number of portal tracts. This hypothesis deserves further testing in future clinical studies.
The retrospective nature and the small-sample size are important limitations to this study. Patients included in this study were not consecutively enrolled due to the retrospective nature of the study. The purpose of consecutive screening combined with enrollment of patients is to assure that representative patients who fulfill all inclusion criteria are enrolled and to avoid active selection to gain a reliable assessment of the different diagnostic/therapeutic methods and apply to clinical practice [23]. Furthermore, in the EUS-LB group, all patients chose to undergo the endoscopic approach, likely because they preferred a procedure that is tolerable and associated with less pain and anxiety. However, we acknowledge that patients in the EUS-LB group may have been influenced by the referring physician’s preference of the endoscopic over the percutaneous approach, which could have led to introducing selection bias. Standardization and optimization of the liver tissue sampling using EUS-guided approach are lacking. An important limitation to our study is that needles with varying calibers have been used in the PC-LB approach (18-gauge and 15-gauge), depending on the specialization of the performer; however, it has been consistently reported that the choice of the type and caliber of the LB needle depends largely on the local preference [20, 24–30]. Investigators should carefully consider the type and caliber of the LB needle when designing future clinical trials comparing the two approaches, accounting for patient factors such as body habitus, weight, suspected nature of liver disease, etc.
We used the number of portal tracts per sample as one of the parameters for assessment of the quality of liver specimens in our study. The median number of portal tracts was significantly lower in the EUS-LB compared to the PC-LB group (5 vs. 13); and this has the potential drawback of inadequate assessment of the liver parenchyma, and thus potentially missing important histological findings. While the median number of portal tracts in our study is lower than the number of portal tracts proposed by the American Association for the Study of Liver Diseases position paper on liver biopsy (11 portal tracts) [3], it is worth noting that this recommendation is based on weak evidence (level C). Although it is generally agreed on that the higher the number of portal tracts, the higher the diagnostic yield of a liver tissue sample, in several occasions, even small biopsy specimens may be large enough to establish a diagnosis. A recently published meta-analysis of nine EUS-LB studies by Mohan et al. [31] reported an overall diagnostic yield of 93.9%; the median number of portal tracts in the studies included ranged from 2 to 32. We relied on the pathologist’s ability to interpret and reach a final histopathologic diagnosis in this study, aided by the parameters and requirements for adequate liver specimens, given that there are no data available to define the adequacy of liver tissue obtained by EUS-guided biopsy needles. Fragmentation of liver tissue occurs in high rates, up to 72% of the EUS-obtained liver tissue samples, which could have a negative impact on the overall quality of EUS-LB [8]. Moreover, we acknowledge that the staging of fibrosis may have been underestimated in EUS-LB group due to the shorter core LB length, less number of portal tracts, or both, compared to the PC-LB; a 2003 prospective study showed that core LB length can influence the staging of chronic viral hepatitis [32]. Nevertheless, our findings are concordant with the current literature, and add to the impression that EUS-LB is safe with a high diagnostic yield. Our finding that a tendency towards higher numbers of portal tracts in the 22-gauge vs. the 19-gauge needle group should be interpreted with caution, because the sample size of those who underwent EUS-LB using the 22-gauge needle was too small to allow for an adequately powered subgroup analyses.
Although our study found less number of portal tracts in the EUS-LB group in comparison to the PC-LB group, we believe that this can be modified and improved by increasing the number of passes and/or actuations. Furthermore, EUS-LB allows to easily access and biopsy both hepatic lobes, and thus decreasing the possibility of underestimating diseases characterized by patchy involvement of the liver.
Even though the primary aim of this study was to assess the safety and efficacy of EUS-LB, we acknowledge that a cost-effectiveness analysis comparing the two approaches is crucial. The use of EUS-LB may seem to be associated with increased cost in comparison to PC-LB, because not only it requires an interventional endosonographer, but also deep sedation and anesthesiological assistance, which are not needed for PC-LB. However, per our hospital protocol, patients who undergo PC-LB are generally admitted for 4 h of observation post-procedure with frequent monitoring of vital signs in comparison to 1 h of observation in patients who undergo EUS-LB which could add more costs to PC-LB.
Our findings may not apply to different populations, since nearly two-third of the study subjects were females, and more than 90% were Caucasians. Furthermore, the excellent diagnostic yield of EUS-LB in our study may be due to selection and/or referral bias. Moreover, EUS-LB requires the availability of endosonographers experienced in the performance of EUS-LB. All of our EUS-LB were performed by a single experienced therapeutic endoscopist (G.M.H), and thus, the finding may not be generalized to less-experienced or multiple endosonographers.
In summary, our study results show that compared to PC-LB, EUS-LB can be safely used in evaluating patients with abnormal liver chemistries and/or for staging chronic liver disease, and is as effective as PC-LB in terms of establishing a histological diagnosis. The main advantage of EUS-LB over PC-LB appears to be the reduction of post-procedural pain and anxiety for the patient. Until large-scale randomized comparative clinical trials are needed, EUS-LB should not replace PC-LB, but should be considered as an alternative in the evaluation of patients with chronic liver disease. Furthermore, although EUS-LB was associated with shorter hospital stay, cost-effectiveness analysis studies are needed to estimate the overall healthcare costs of both procedures to help the patients and their healthcare providers decide on optimum methods of evaluation of liver disease.
Abbreviations
- CHC
Chronic hepatitis C
- CRN
Clinical Research Network
- EH
Eosinophilic hepatitis
- ESLD
End-stage liver disease
- EUS-LB
Endoscopic ultrasound-guided liver biopsy
- EUS-FNA/FNB
Endoscopic ultrasound-guided fine needle aspiration or biopsy
- FLD
Fatty liver disease
- IRB
Institutional Review Board
- LB
Liver biopsy
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NAS
NAFLD activity score
- PBC
Primary biliary cholangitis
- PC-LB
Percutaneous-guided liver biopsy
- PFIC
Progressive familial intrahepatic cholestasis
- VCTE
Vibration-controlled transient elastography
Author contribution
GMH, AHA, and SP contributed to the concept, design, literature search, clinical studies, data acquisition, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. DSR and YG re-examined the liver biopsies. AA, SS, and JAI reviewed and edited the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This is a retrospective study that involved only chart review. No animals were involved in any aspect of this study.
Informed consent
Obtaining informed consent has been waived by the institution’s IRB due to nature of the study (retrospective and chart review study).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ahmad Hassan Ali, Email: ali.ahmad@mayo.edu.
Sarjukumar Panchal, Email: Sarjukumar.Panchal@Pennmedicine.upenn.edu.
Deepthi S. Rao, Email: raods@health.missouri.edu
Yujun Gan, Email: ganyu@health.missouri.edu.
Alhareth Al-Juboori, Email: aljubooria@health.missouri.edu.
Sami Samiullah, Email: samiullah@health.missouri.edu.
Jamal A. Ibdah, Email: ibdahj@health.missouri.edu
Ghassan M. Hammoud, Email: hammoudg@health.missouri.edu
References
- 1.Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5(10):1214–1220. doi: 10.1016/j.cgh.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. American Association for the Study of Liver D. Liver biopsy. Hepatology. 2009;49(3):1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 4.Strassburg CP, Manns MP. Approaches to liver biopsy techniques–revisited. Sem Liver Dis. 2006;26(4):318–327. doi: 10.1055/s-2006-951599. [DOI] [PubMed] [Google Scholar]
- 5.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344(7):495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 6.Diehl DL, Johal AS, Khara HS, Stavropoulos SN, Al-Haddad M, Ramesh J, et al. Endoscopic ultrasound-guided liver biopsy: a multicenter experience. Endosc Int Open. 2015;3(3):E210–E215. doi: 10.1055/s-0034-1391412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yavuz K, Geyik S, Barton RE, Petersen B, Lakin P, Keller FS, et al. Transjugular liver biopsy via the left internal jugular vein. J Vasc Interv Radiol. 2007;18(2):237–241. doi: 10.1016/j.jvir.2006.12.730. [DOI] [PubMed] [Google Scholar]
- 8.Mok SRS, Diehl DL. The role of EUS in liver biopsy. Curr Gastroenterol Rep. 2019;21(2):6. doi: 10.1007/s11894-019-0675-8. [DOI] [PubMed] [Google Scholar]
- 9.Mok SRS, Diehl DL, Johal AS, Khara HS, Confer BD, Mudireddy PR, et al. Endoscopic ultrasound-guided biopsy in chronic liver disease: a randomized comparison of 19-G FNA and 22-G FNB needles. Endosc Int Open. 2019;7(1):E62–E71. doi: 10.1055/a-0655-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pineda JJ, Diehl DL, Miao CL, Johal AS, Khara HS, Bhanushali A, et al. EUS-guided liver biopsy provides diagnostic samples comparable with those via the percutaneous or transjugular route. Gastrointest Endosc. 2016;83(2):360–365. doi: 10.1016/j.gie.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991;178(1):253–258. doi: 10.1148/radiology.178.1.1984314. [DOI] [PubMed] [Google Scholar]
- 12.Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28(5):705–712. doi: 10.1111/j.1478-3231.2008.01691.x. [DOI] [PubMed] [Google Scholar]
- 13.Johal AS, Khara HS, Maksimak MG, Diehl DL. Endoscopic ultrasound-guided liver biopsy in pediatric patients. Endosc Ultrasound. 2014;3(3):191–194. doi: 10.4103/2303-9027.138794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parekh PJ, Majithia R, Diehl DL, Baron TH. Endoscopic ultrasound-guided liver biopsy. Endosc Ultrasound. 2015;4(2):85–91. doi: 10.4103/2303-9027.156711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shruti MSI, Vyas N, Sofi A, Das A (2018) Eus guided liver biopsy is more cost-effective than percutaneous liver biopsy in patients with non-alcoholic fatty liver disease (NAFLD). Gastrointest Endosc AB326–AB7
- 16.Sey MS, Al-Haddad M, Imperiale TF, McGreevy K, Lin J, DeWitt JM. EUS-guided liver biopsy for parenchymal disease: a comparison of diagnostic yield between two core biopsy needles. Gastrointest Endosc. 2016;83(2):347–352. doi: 10.1016/j.gie.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Nieto J, Khaleel H, Challita Y, Jimenez M, Baron TH, Walters L, et al. EUS-guided fine-needle core liver biopsy sampling using a novel 19-gauge needle with modified 1-pass, 1 actuation wet suction technique. Gastrointest Endosc. 2018;87(2):469–475. doi: 10.1016/j.gie.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 19.Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22(10):1453–1458. doi: 10.1007/s11606-007-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demetris AJ, Ruppert K. Pathologist's perspective on liver needle biopsy size? J Hepatol. 2003;39(2):275–277. doi: 10.1016/s0168-8278(03)00282-4. [DOI] [PubMed] [Google Scholar]
- 21.Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: a quantitative reference standard. Hepatology. 1998;28(2):323–331. doi: 10.1002/hep.510280206. [DOI] [PubMed] [Google Scholar]
- 22.Bazerbachi F, Vargas EJ, Matar R, Storm AC, Mounajjed TM, Topazian MD, et al. EUS-guided core liver biopsy sampling using a 22-gauge fork-tip needle: a prospective blinded trial for histologic and lipidomic evaluation in nonalcoholic fatty liver disease. Gastrointest Endosc. 2019;90(6):926–932. doi: 10.1016/j.gie.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Bjørn M, Brendstrup C, Karlsen S, Carlsen JE. Consecutive screening and enrollment in clinical trials: the way to representative patient samples? J Cardiac Fail. 1998;4(3):225–230. doi: 10.1016/s1071-9164(98)80009-2. [DOI] [PubMed] [Google Scholar]
- 24.Firpi RJ, Soldevila-Pico C, Abdelmalek MF, Morelli G, Judah J, Nelson DR. Short recovery time after percutaneous liver biopsy: should we change our current practices? Clin Gastroenterol Hepatol. 2005;3(9):926–929. doi: 10.1016/s1542-3565(05)00294-6. [DOI] [PubMed] [Google Scholar]
- 25.Friedman LS. Controversies in liver biopsy: who, where, when, how, why? Curr Gastroenterol Rep. 2004;6(1):30–36. doi: 10.1007/s11894-004-0023-4. [DOI] [PubMed] [Google Scholar]
- 26.Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. Gut. 1999;45(Supplement 4):iv1–iv11. doi: 10.1136/gut.45.2008.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindor KD, Bru C, Jorgensen RA, Rakela J, Bordas JM, Gross JB, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology. 1996;23(5):1079–1083. doi: 10.1002/hep.510230522. [DOI] [PubMed] [Google Scholar]
- 28.Takyar V, Etzion O, Heller T, Kleiner DE, Rotman Y, Ghany MG, et al. Complications of percutaneous liver biopsy with Klatskin needles: a 36-year single-centre experience. Aliment Pharmacol Therap. 2017;45(5):744–753. doi: 10.1111/apt.13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tublin ME, Blair R, Martin J, Malik S, Ruppert K, Demetris A. Prospective study of the impact of liver biopsy core size on specimen adequacy and procedural complications. Am J Roentgenol. 2017;210(1):183–188. doi: 10.2214/AJR.17.17792. [DOI] [PubMed] [Google Scholar]
- 30.Vijayaraghavan GR, David S, Bermudez-Allende M, Sarwat H. Imaging-guided parenchymal liver biopsy: how we do it. J Clin Imaging Sci. 2011;1:30. doi: 10.4103/2156-7514.82082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan BP, Shakhatreh M, Garg R, Ponnada S, Adler DG. Efficacy and safety of EUS-guided liver biopsy: a systematic review and meta-analysis. Gastrointest Endosc. 2019;89(2):238–46.e3. doi: 10.1016/j.gie.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39(2):239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]