Abstract
Nowadays, cranial ultrasonography (US) of the newborn represents the first imaging method in brain damage study and its possible outcomes. This exam is performed using the natural fontanelles, especially the anterior one. It is fast, non-invasive and does not produce any side effect. Ultrasonographic examination is usually performed in cases of prematurity, especially in children with birth weight less than 1500 g, because important informations about the possible presence of pathologies such as cerebral hemorrhage and hypoxic–ischemic encephalopathy are given. This approach can be useful also in the study of pre- and post-natal infections, for example, type II Herpes Simplex virus or Cytomegalovirus infections, or pointing out vascular malformations such as vein of Galen aneurysm. Although less important than methods such as computed tomography (CT) and magnetic resonance imaging (MRI) in the evaluation of trauma and tumors, ultrasound can provide useful informations or be used in first instance in the suspicion of a brain mass.
Keywords: Pediatrics, Ultrasonography, Brain, Hemorrhage, Periventricular leukomalacia
Sommario
Al giorno d’oggi l’ecografia encefalica del neonato rappresenta la prima metodica di imaging nello studio del danno cerebrale e dei suoi possibili esiti. Tale esame viene eseguito utilizzando le fontanelle naturali, in particolare quella anteriore. È veloce, non invasivo e privo di complicanze. L’esame ecografico è di solito eseguito in caso di prematurità, soprattutto nei bambini con un peso alla nascita inferiore a 1500 grammi, potendo fornire importanti informazioni riguardo la presenza di patologie come l’emorragia cerebrale e l’encefalopatia ipossico-ischemica. Questo approccio è utile anche per lo studio delle infezioni pre- e post-natali, quali ad esempio quelle da virus Herpes Simplex di tipo II o da Citomegalovirus, o per evidenziare malformazioni vascolari quali l’aneurisma della vena di Galeno. Sebbene meno importante rispetto a metodiche quali la tomografia computerizzata (TC) e risonanza magnetica (RM) nello studio dei traumi e dei tumori, l’ecografia può talvolta fornire informazioni utili o essere la metodica di prima istanza nel sospetto di una massa cerebrale.
Introduction and technical notes
During the last 30 years, sonographic examination of the brain has become a routine procedure in most neonatal intensive care structures and sonographic centers. Its accessibility, safety and low cost contributed to the diffusion of this technique [1]. Besides, ultrasound reports have proved to match anatomo-pathological examinations. As in any other organs, pediatric encephalic ultrasonography requires some both technical and methodological care: a strict collaboration with clinicians, a comfortable and properly heated environment. Furthermore, the room should not be crowded (only one parent allowed) and waiting time limited.
During both neonatal and early infancy, the skull is so thin and the ‘fontanelles’ are open allowing a clear view of the whole area with no need for anesthesia or sedation. The anterior fontanelle is used as cerebral acoustic window and maintains its patency until the age of 10/12 months [2]. Some pathological conditions (osteogenesis imperfecta, Down syndrome, cleidocranial dysostosis) can cause a delayed sealing of the fontanelles, allowing a successful scan even in older children.
Ultrasound probes need to be small enough (microconvex) as to allow neonatal brain examination, even when the anterior fontanelle is relatively narrowed. We usually employ 5–6.5 MHz frequencies or 5–10 MHz. In the last years, the diffusion of high-frequency linear probes (10–13 MHz) enabled a more detailed surface structures analysis.
The anterior fontanelle approach can sometimes be completed with transversal scanning involving the posterior and mastoid fontanelle, as well as cranial bone flaps [3].
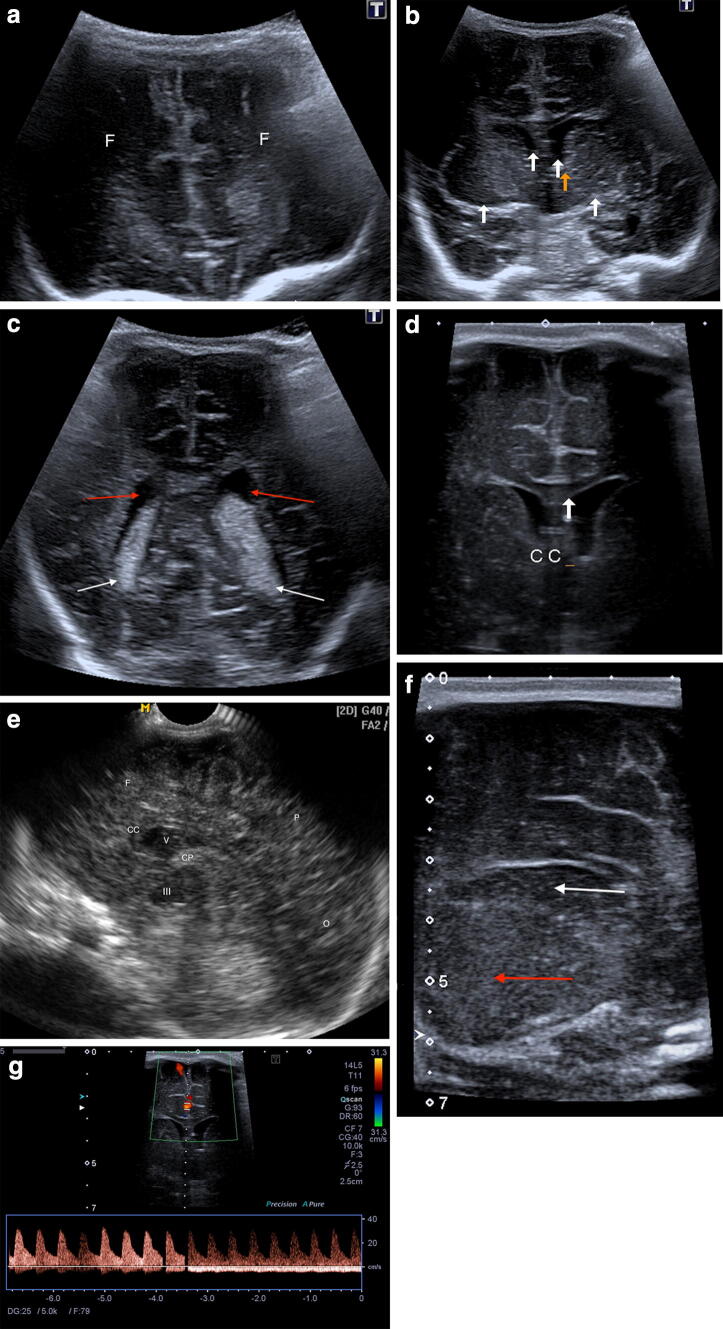
Standard brain ultrasonography needs both coronal and sagittal scans. Coronal scans allow a comparative of left and right hemispheres; usually executed in number of 5–7 from the median region of the bregmatic fontanelle through a slight inclination of the probe, evaluate the brain from the anterior to posterior region. Sagittal scans, usually 5 (median, parasagittal and parasagittal peripheral right and left), allow isolation of the ventricular and paraventricular structures [4, 5] (Fig. 1). With these series of scans, the ventricles (shape, size and content), the periventricular and subcortical white matter, basal ganglia, gray matter and pericerebral fluid spaces can be accurately evaluated. Anyhow, despite this standard approach, nowadays, real-time ultrasound allows to scan brain in any direction.
Fig. 1.
Normal anatomy. Coronal anterior (a), median (b superior white arrows lateral ventricles, inferior white arrows basal ganglia, orange arrow zone of caudothalamic notch) and posterior (c, white arrows choroid plexus, red arrow lateral ventricles) scan with magnified coronal scan (d) showing corpus callosum; g magnified coronal scan with normal color Doppler signal in cerebral anterior artery. Sagittal scan (e) and magnified parasagittal scan (f, thalamus red arrow and caudate nucleus white arrow). CC corpus callosum, III third ventricle, F frontal lobes, V lateral ventricle, O occipital lobe, P parietal lobe, CP choroid plexus
The integration with the color and power Doppler can give additional qualitative data, such as the visualization of aneurysms or the absence of flow in a venous vessel indicative of post-hemorrhagic venous infarct.
In this paper, we analyze the ultrasonographic approach to the study of the main cerebral pathologies: cerebral hemorrhage, hypoxic–ischemic encephalopathy (HIE), pre- and post-natal infections, trauma, vascular anomalies and tumors.
Cerebral hemorrhage
Cerebral hemorrhage can show different patterns depending on the gestational period. Prematures are at higher risk of germinal matrix hemorrhage and periventricular leukomalacia. Term babies can show hemorrhage at subdural, subarachnoid or choroid plexus levels [6, 7].
Germinative matrix is located between the caudate nucleus and the thalamus; its close proximity to the lateral ventricles explains their frequent secondary involvement.
Incidence varies between 20 and 25% in premature children aged < 32 weeks or less than 1500 g at birth and its onset occurs within the first 10 days of life, with a worsening in the first 2–3 days. It is usually bilateral; when unilateral, it tends to appear on the left side. Papile Classification (1978) is still employed today (Table 1) [8].
Table 1.
Papile classification [8]
| Grade I | Limited subependymal hemorrhage |
| Grade II | Intraventricular hemorrhage without hydrocephalus |
| Grade III | Intraventricular hemorrhage with hydrocephalus |
| Grade IV | Intraparenchymal and intraventricular hemorrhage |
Stage I
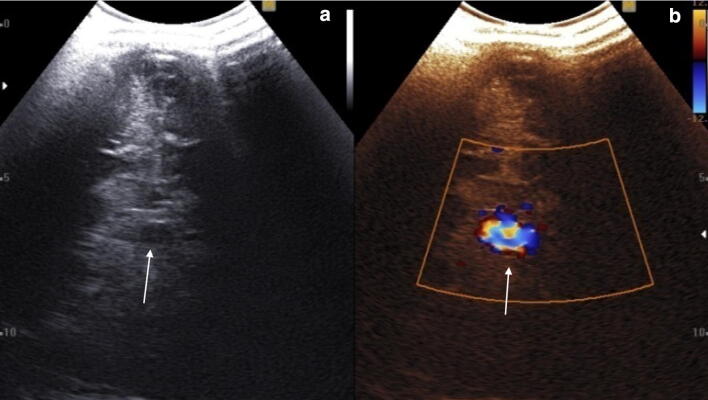
Subependymal hemorrhage can be single or bilateral, isolated or combined with a more extended hemorrhage. It appears as a homogeneously echoic area located in the caudothalamic groove. First-grade hemorrhage can resolve completely, or sometimes lead to the formation of a small cyst or to ventricular rupture (Fig. 2).
Fig. 2.
a Coronal scan. Hyperechoic subependymal hemorrhage (grade I, red arrow and calipers) without ventricular involvement (blue arrow thalamus; green arrow caudate nucleus), b coronal median scan with presence of a cystic area after 10 days from a grade I hemorrhage (white arrow)
Stage II
In the second stage, subependymal hemorrhage involves the adjacent ventricle (Fig. 3). Sonographically, it is characterized by hyperechoic images that partly or completely cover the ventricular cavities. The hemorrhage breaks the ependymal wall, floating into the lateral ventricle. The ventricle turns echoic, although it does not expand. Ventricular walls can be thickened (chemical ventriculitis). Sometimes, a ventricular declivous liquor-blood level can be present.
Fig. 3.
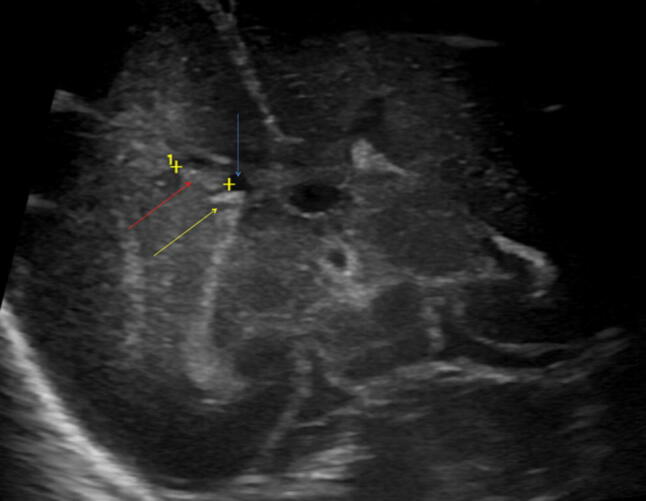
Coronal scan. Hyperechoic right intraventricular hemorrhage (grade II, red arrow; yellow arrow choroid plexus; blue arrow normal lateral ventricle)
Stage III
Hemorrhage floods into the lateral ventricle, distending it. It can affect one or both lateral ventricles (VVLL), sometimes involving III° and IV° ventricles.
It tends to resolve within 5–6 weeks. Secondary infections may occur with an inhomogeneous and corpusculated ventricular content; hyperechoic stripes or bands can result. Furthermore, it can cause post-hemorrhagic hydrocephalus.
The pathology regresses spontaneously 65/75% of the time, while 10% of the cases requires ventriculoperitoneal shunting (Fig. 4).
Fig. 4.
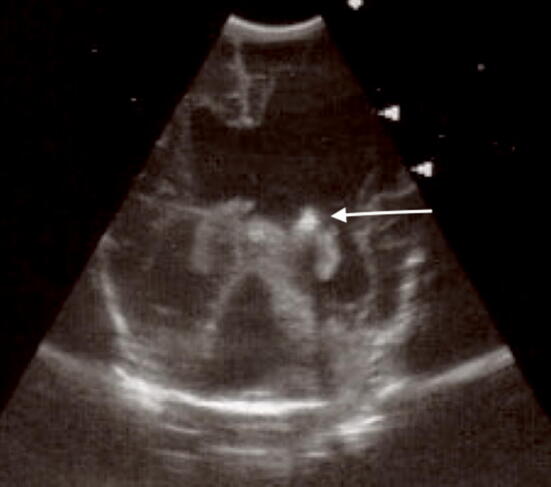
Coronal scan. Ventricular enlargement with the presence of derivation catheter (white arrow) after grade III hemorrhage
Stage IV
Subependymal hemorrhage, intraventricular hemorrhage with dilated ventricle and parenchymal hemorrhage (IPH) are sometimes found [7]. Sometimes, it can manifest through a periventricular hemorrhagic focus which can be uni- or bilateral. Most commonly, it is located in the frontal or parietal area, extending from the caudothalamic groove [9]. If the hemorrhage is unilateral, the median line can appear dislocated. Hemorrhagic stuffing can lead to a reduced venous draining, potentially degenerating into thrombosis with consequent parenchymal necrosis.
Hematoma appears as a hyperechoic intraparenchymal mass, with progressive reduction of its echogenicity over time, finally resulting in a porencephalic cavitation [10] (Figs. 5, 6).
Fig. 5.
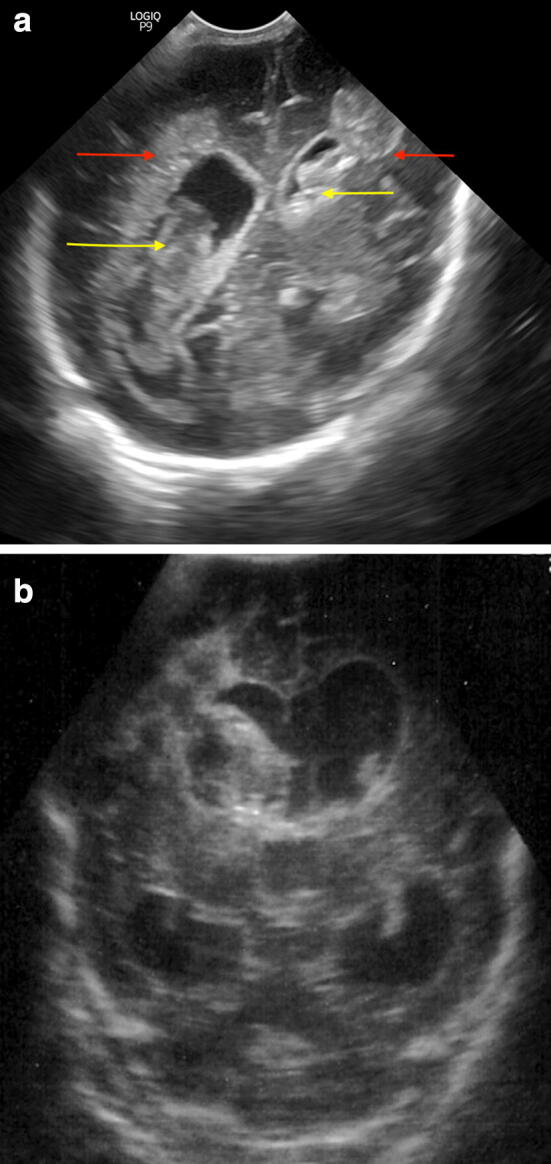
a Coronal scan. Intraparenchymal (red arrows) and intraventricular (yellow arrows) bilateral hemorrhage (grade IV); b Coronal scans showing right grade IV hemorrhage
Fig. 6.
Coronal scans. Right fronto-temporal subacute–chronic parenchymal hemorrhage with initial cystic malacic cavitation
Prognosis depends on the gravity of the disease, with more risk of hydrocephalus, neurological deficit or death for grades III and IV.
Hypoxic ischemic encephalopathy (HIE)
Hypoxic ischemic syndrome is characterized by a reduced hematic supply of oxygen to the brain (hypoxia) [11]. It is determined by respiratory deficit and reduced hematic flow to the brain which causes parenchymal damages (ischemia) [12].
Periventricular leukomalacia (PVL) is the main ischemic lesion of the preterm birth and, together with germinal matrix hemorrhage, the most important cause of permanent brain damage.
Asphyxia (prepartum, intrapartum and postpartum) appears to be the etiology [13]. Concurrent factors seem to be the absence of cerebral flow self-regulation and cardiovascular immaturity. HIE affects 25–40% of newborns with birth weight less than 1000 g. Different degrees of PVL are shown in Table 2 [14].
Table 2.
De Vries classification [14]
| Grade I | Periventricular hyperechogenicity, persisting for > 7 days |
| Grade II | Periventricular hyperechogenicity, evolving into small localized fronto-parietal cysts |
| Grade III | Periventricular hyperechogenicity, evolving into extensive periventricular cystic lesions |
| Grade IV | Hyperechogenicity extending into the deep white matter evolving into extensive cystic lesions |
HIE involves first the white matter located at the border regions of the arterial vascular supply, peripherally to the trigones of the lateral ventricles and in the frontal lobes [9].
In the early stages of disease, ultrasound can be normal or show a hyperechogenicity of the interested white matter, more than the adjacent choroid plexus [10, 15, 16]. A direct comparison between the two hemispheres on coronal view from the anterior and the posterior fontanelles should be used to point this out [17]. However, the alterations can be bilateral, symmetrical or asymmetrical. When pathology is limited, it can affect the periphery of the brain, the white matter next to the anterior horn of the lateral ventricles or trigonum cerebri. In its diffuse form, it involves the entirety of the periventricular white matter.
Consequent manifestations result as hypoechoic areas of cystic encephalomalacia (Fig. 7), that begin to appear within a month after the event at the site of the early hyperechogenicity. Such cysts may be single or multiple (ranging from 1 mm to 2–3 cm) and are located in the periventricular white matter varying according to the insult extent [9]. Subsequently (1–3 months), the cysts tend to disappear, leading to ex vacuo dilatation of the lateral ventricles. Necrosis of the affected zones leads to thinning of white matter and eventually of the corpus callosum [16–18].
Fig. 7.
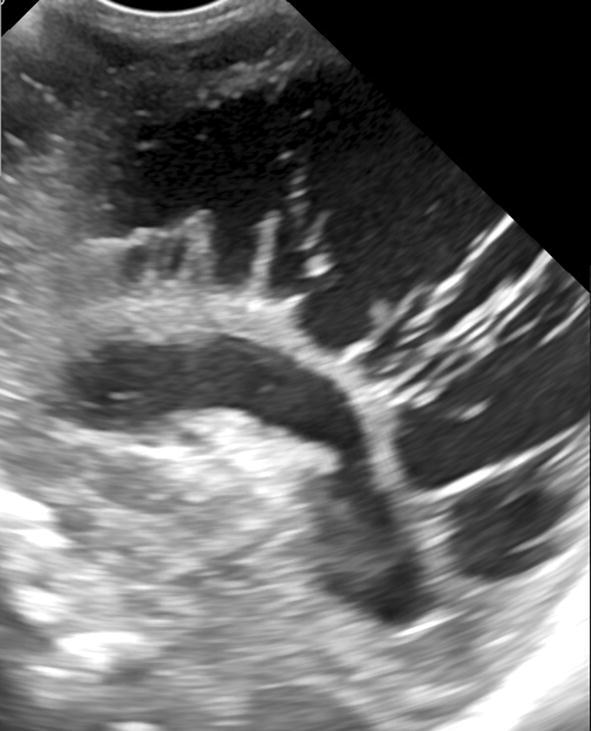
Sagittal scan showing large hypoechoic areas of cystic PVL
Ultrasound tends to underestimate the extent of the pathological process, which can be better assessed in its severity and long-term sequelae through the MRI. The MRI allows to highlight gliosis in the damaged areas, with thinning of the periventricular white matter and enlargement of the lateral ventricles [12, 19] and can better predict global neurodevelopmental outcome in affected patients [20].
It should be noted that a faint hyperechoic halo posterior to the ventricular trigones can be visible in preterm births when performing parasagittal scans. However, it is not considered as a pathological finding and tends to resolve in the first 7 days of life. The areas of PVL tend to be more marked and confluent and in any case persisting beyond the first week [18].
In term births, a frequent consequence of a hypoxic–ischemic event is the presence of a diffuse cerebral edema. A dimensional reduction of the ventricles can be observed in ultrasound, sometimes with a widespread increase in parenchymal echogenicity and a lesser recognition of sulci. Due to the severity of the event, long-term consequences may include a widespread atrophy, or localized encephalomalacia areas [9].
Infective pathology
Central nervous system infections of viral or bacterial origin can develop before or after birth. Pathogenic agents causing prenatal infections are classified under TORCH acronym (toxoplasmosis, rubella, cytomegalovirus, type II herpes simplex virus). These are responsible for necrotic and calcified meningoencephalitis. The alterations caused by such pathologies are seen as anechoic areas of malacia and ventriculomegaly ex vacuo, consequent to parenchymal destruction, and calcification. The latter appear as small hyperechoic images with or without posterior shadow cone depending on their size and the kind of frequency applied through the probe [21]. Among prenatal infections, cytomegalovirus is the most common, affecting between 0.2 and 2.2% of fetus.
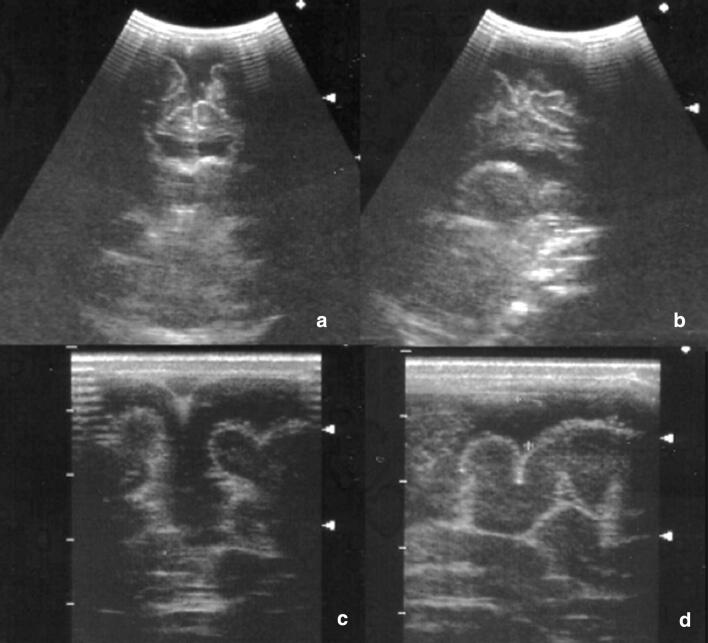
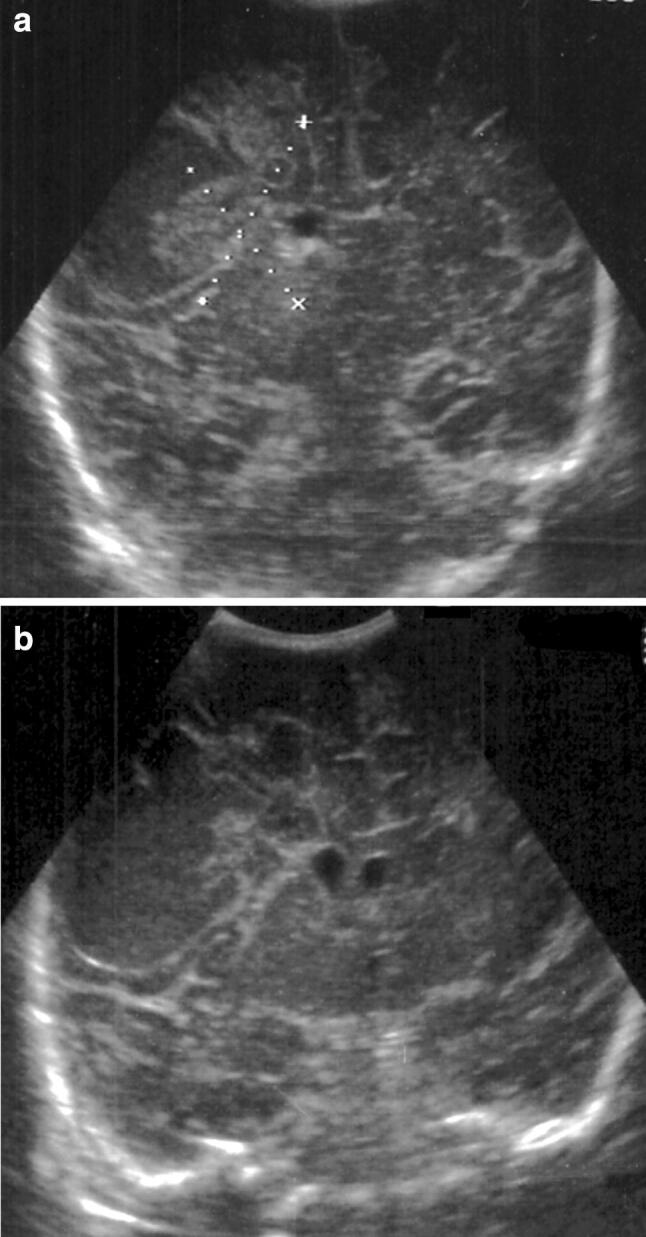
Typical ultrasonographic aspects are subependymal calcifications, punctiform or nodular, isolated or confluent [22] (Fig. 8).
Fig. 8.
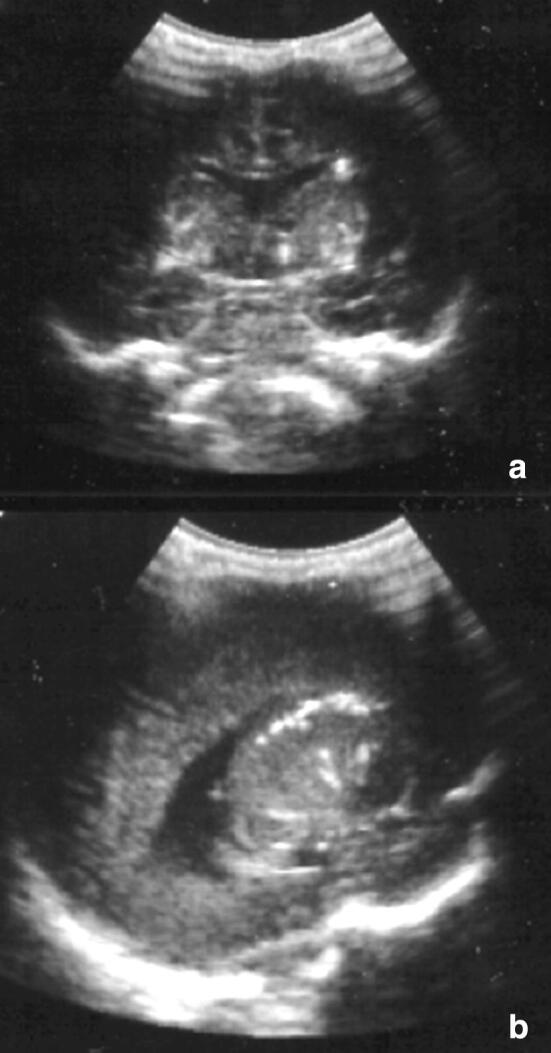
Coronal (a) and sagittal (b) scans. Multiple small periventricular hyperechoic spots in Cytomegalovirus infection
Other findings can coexist, namely ventriculomegaly, periventricular cysts and brain malformations. The presence of calcifications does not allow a differential diagnosis with other infectious diseases, as they are also found in toxoplasmosis and rubeola [23].
Another prenatal infection is type II herpes simplex virus, which has high necrotic potential and is typically diffuse, leading to serious cerebral atrophy.
Ultrasound examination can show multiple parenchymal cavitation areas, eventually with ventriculomegaly (Fig. 9).
Fig. 9.
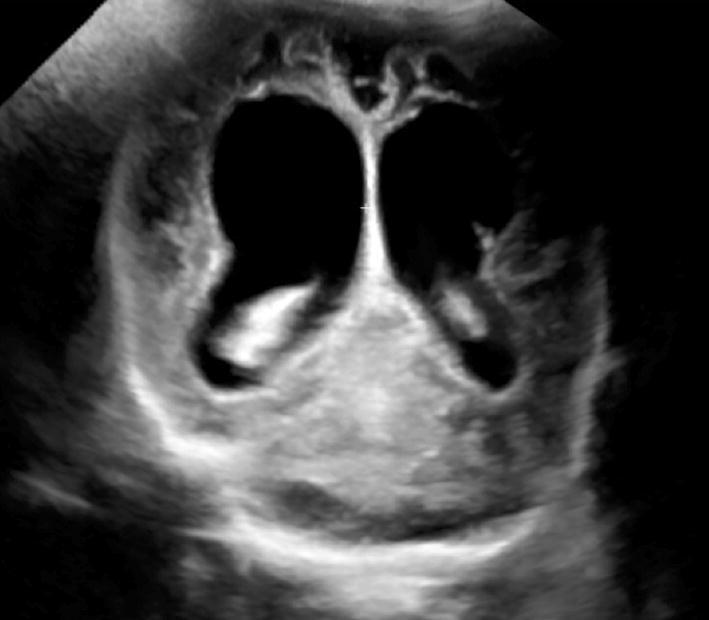
Cerebral atrophy and ventricular enlargement after herpes simplex virus encephalitis
Pathogenic agents most commonly involved in postnatal infections are haemolytic Streptococcus and Escherichia coli (affecting newborns), as well as Haemophilus influenzae.
Meningitis and Meningoencephalitis are direct consequences of those pathogenic agents. Meningoencephalitis frequently leads to complications such as ventriculitis, hydrocephalus, subdural collections and parenchymal damages such as atrophia, porencephaly, abscess and infarction.
Non-complicated bacterial acute meningitis shows an increased echogenicity of sulci, due to deposition of purulent material [24].
Ventriculitis is suspected when the ventricular walls thicken and increase their echogenicity showing inside echoic material, eventually associated with dimensional enlargement. Other possible complications are subdural collections that can appear as a hypo/anechoic sickle between the cranial table and the cerebral surface. Parenchymal infections can lead to porencephalic areas, whereas cerebral abscess is characterized by a homogenous or, more rarely, inhomogenous area of moderate echogenity, surrounded by an echoic ring of variable thickness [24–26] (Fig. 10).
Fig. 10.
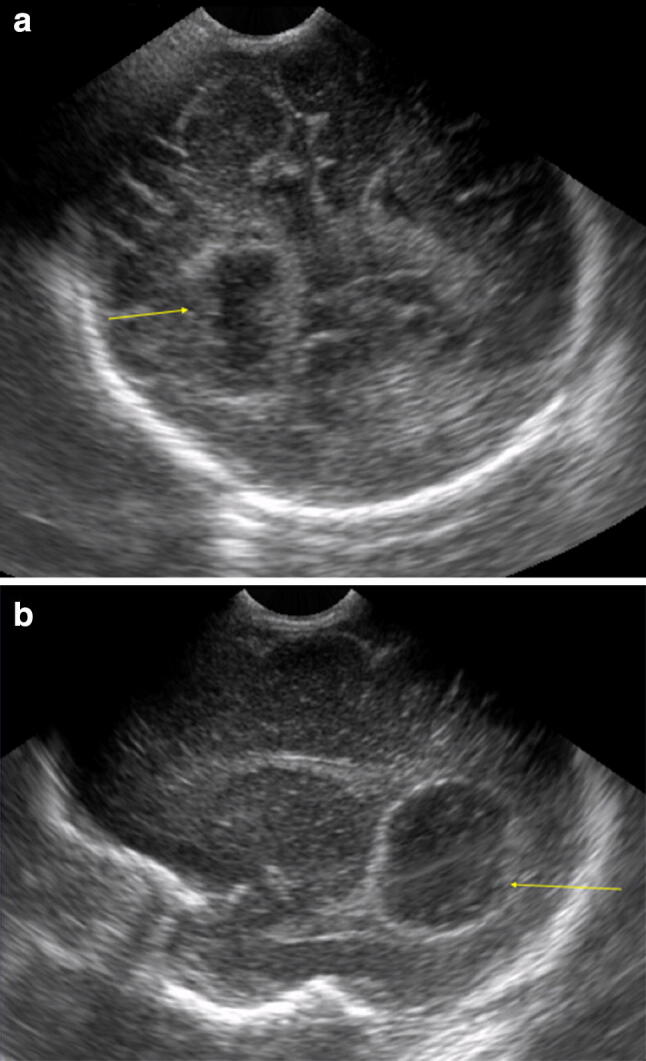
Coronal posterior (a) and sagittal (b) scans. Right abscessual round cavity (yellow arrow)
Vascular malformations
Vein of Galen aneurysm malformations are rare congenital vascular–cerebral pathologies. Nonetheless, they account for about 30% of all vascular malformations in pediatric age.
Vein of Galen aneurysm is a misnomer because it is not a true aneurysm. It is a venous ectasia secondary to an arteriovenous (a-v) shunt between deep choroidal vessels and the median prosencephalic vein of Markowski; vein of Galen fails to form because of the fistula [27].
Often, the pathology is identified through ultrasonographic examinations in fetal age [23, 28], though MRI can deliver a more precise characterization.
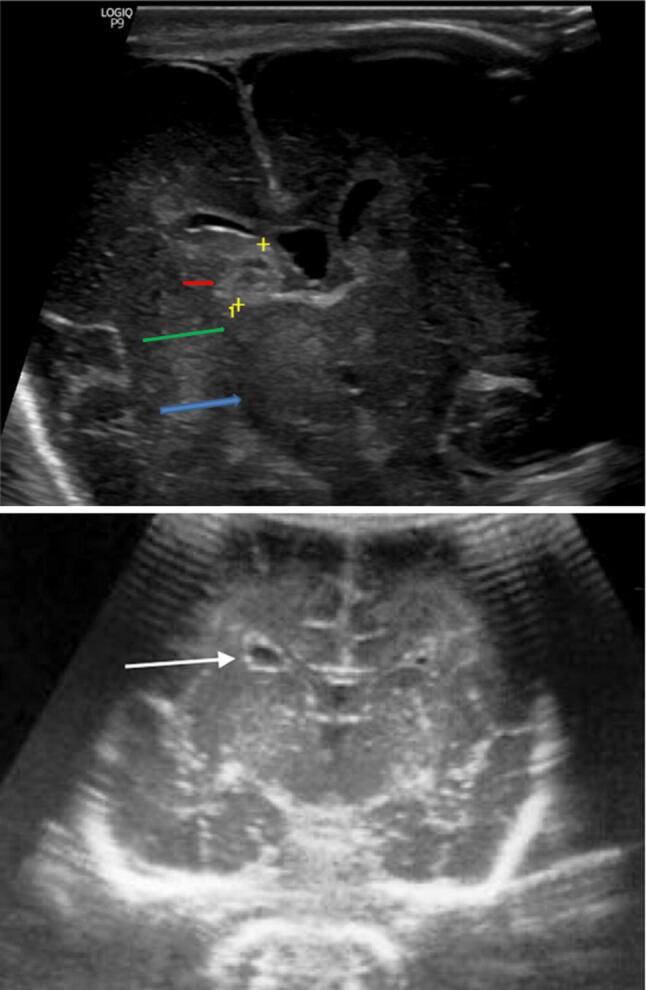
On a scan, it appears as a median anechoic formation with internal flow at Color Doppler, extended posteriorly to the III ventricle until the sinus junction. It shows a marked pulsatility of the satellite artery and vein (due to the a-v shunt) and hydrocephalus of variable degree [4, 29] (Fig. 11).
Fig. 11.
Coronal scans showing a posterior median pseudo-cystic lesion with internal vascular signal, Galen aneurysm malformation (white arrow)
Trauma
Cranial trauma is extremely common and one of the most frequent and important aspects of pediatric emergency room activity. Different lesions can be consequent to accidental or non-accidental cranial trauma:
Scalp wounds;
cranial fractures;
cerebral contusions;
obstructive hydrocephalus;
epidural hematoma (blood accumulation between bone inner layer and dura mater);
Subdural hematoma (blood accumulation between arachnoid and dura mater).
CT and MRI are the reference imaging techniques in head trauma; nevertheless, US could give useful information in diagnosis and follow-up. Ultrasonographic scan of traumatized newborns is a safe and repeatable examination, but patient sedation can be necessary, and medications and scalp wounds may obstacle the exploration. It allows identifying any presence of intraventricular, intra- or extra-parenchymal bleeding and its possible mass effect on the surrounding structures [30].
In fractures, a focused evaluation with the ultrasound probe can demonstrate an interruption of the continuity of the cranial table, evaluate its entity and also differentiate it from a normal suture. The latter typically shows serrated margins, which are more irregular and sharp in the event of a fracture [31, 32].
Epidural collections are more frequently associated with cranial fractures and adjacent to them in older children, but fractures are less frequent in neonates because of skull elasticity; typically, the epidural hematoma will appear as a biconvex-shaped, echogenic, mass adjacent to the cranial table [33].
Subdural blood collections are located superficially to the cerebral hemispheres or even in the interhemispheric falx region. They can have different echogenicity according to blood age (acute-hyperechoic, old-hypoechoic) [35]. In non-accidental traumas due to child abuse, the presence of inhomogeneous subdural hematomas with internal septa can reflect the coexistence of fresh and old blood [34, 35] (Fig. 12).
Fig. 12.
a, b Coronal and sagittal scans showing a hypoechoic subdural fluid collection; c, d magnified view of the same patient
Parenchymal lesions can be recognized primarily as contusion areas at the gray–white matter junction, particularly in the fronto-parietal region, which may appear as linear hypoechoic areas.
It should also be remembered that “secondary” encephalic damage is possible, such as the presence of cerebral edema characterized by an increase in echogenicity of the brain tissue, which is swollen, with a localized or diffused pattern depending on the extent of the damage [35]. Furthermore, recent literature is emerging about the role of transcranial Doppler ultrasound in traumatic brain injury as a useful tool to assess autoregulation, intracranial pressure, and vasospasm [36].
Neoplastic pathology
Cerebral tumors are quite rare in neonatal age through the first year of life. About 20% of tumors affecting pediatric patients originates in the central nervous system. They are divided into primitive or congenital/metastatic tumors such as neuroblastoma and leukemia lesions.
Primitive infratentorial tumors account for about 45–60% of all brain tumors in children (usually age between 4 and 10 years) and most commonly are cerebellar astrocytoma, medulloblastoma, ependymoma, cerebral trunk glioma. Supratentorial tumors are more common under 3 years (optical nerve glioma, germinoma, craniopharyngioma, pleomorphic xanthoastrocytoma, PNET/DNET, ganglioglioma); choroid plexus is the most common site involved in newborns [37, 38].
MRI and CT are the reference imaging methods in the suspicion of a brain tumor. Nevertheless, ultrasound can be the first-line imaging in those cases with aspecific clinical presentation or suspected hydrocephalus [9, 39].
In general, cerebral tumors can have various US appearances, more frequently hyperechoic, homogenous or inhomogenous depending on the presence of cystic, necrotic or hemorrhagic areas; depending on tumor localization, ventricular dilatation may occur. Sometimes, US can show a typical pattern, as in choroid plexus papilloma and callosal lipoma [31, 40].
Conclusions
MRI examination is the reference standard of cerebral pathology and related outcomes in neonatal age. However, cranial ultrasonography of the newborn is now widely diffused as a first-line imaging method in brain damage study, particularly in prematures at higher risk of cerebral hemorrhage and hypoxic–ischemic encephalopathy. It is fast, non-invasive and does not produce any side effect, so that it can be repeated in follow-up. This approach can be useful also in the study of pre- and post-natal infections or pointing out vascular malformations such as vein of Galen aneurysm.
Although less important than CT and MRI in the evaluation of trauma and tumors, ultrasound can provide some useful informations or be used in first instance in those cases with aspecific clinical presentation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and its later amendments. For this type of study, formal consent is not required.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent
Additional informed consent was obtained from all the patients for which identifying information is not included in this article.
References
- 1.Stanojevic M, Hafner T, Kurjak A. Three-dimensional (3D) ultrasound—a useful imaging technique in the assessment of neonatal brain. J Perinat Med. 2002;30(1):74–83. doi: 10.1515/JPM.2002.010. [DOI] [PubMed] [Google Scholar]
- 2.Slovis TL, Kuhns LR. Real-time sonography of the brain through the anterior fontanelle. Am J Roentgenol. 1981;136:277–286. doi: 10.2214/ajr.136.2.277. [DOI] [PubMed] [Google Scholar]
- 3.Di Salvo DN. A new view of the neonatal brain: clinical utility of supplemental neurologic US imaging windows. RadioGraphics. 2001;21:943–955. doi: 10.1148/radiographics.21.4.g01jl14943. [DOI] [PubMed] [Google Scholar]
- 4.Bhat V, Bhat V. Neonatal neurosonography: a pictorial essay. Indian J Radiol Imaging. 2014;24(4):389–400. doi: 10.4103/0971-3026.143901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe LH, Bailey Z. State-of-the-art cranial sonography: part 1, modern techniques and image interpretation. Am J Roentgenol. 2011;196:1028–1033. doi: 10.2214/AJR.10.6160. [DOI] [PubMed] [Google Scholar]
- 6.Zielonka-Lamparska E, Wieczorek AP. Usefulness of 3D sonography of the central nervous system in neonates and infants in the assessment of intracranial bleeding and its consequences when examined through the anterior fontanelle. J Ultrason. 2013;13(55):408–417. doi: 10.15557/JoU.2013.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rath C, Suryawanshi P. Point of care neonatal ultrasound—head, lung, gut and line localization. Indian Pediatr. 2016;53(10):889–899. doi: 10.1007/s13312-016-0954-5. [DOI] [PubMed] [Google Scholar]
- 8.Papile LS, Burstein J, Burstein R, Koffler H. Incidence and evolution of the subependymal intraventricular hemorrhage: a study of infants with weights less than 1500 grams. J Pediatr. 1978;92:529–534. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 9.Rumack CM, Drose JA, et al. Neonatal and infant brain imaging. In: Rumack CM, et al., editors. Diagnostic ultrasound. 4. Philadelphia: Elsevier; 2011. pp. 1558–1636. [Google Scholar]
- 10.Fox Traci B. Sonography of the neonatal brain. J Diagn Med Sonogr. 2009;25(6):331–348. doi: 10.1177/8756479309347801. [DOI] [Google Scholar]
- 11.Cassia GS, Faingold R, Bernard C, Sant’Anna GM. Neonatal hypoxic-ischemic injury: sonography and dynamic color Doppler sonography perfusion of the brain and abdomen with pathologic correlation. Am J Roentgenol. 2012;199:W743–W752. doi: 10.2214/AJR.11.8072. [DOI] [PubMed] [Google Scholar]
- 12.StarkJE Seibert JJ. Cerebral artery Doppler ultrasonography for prediction of outcome after perinatal asphyxia. J Ultrasound Med. 2012;13:595–600. doi: 10.7863/jum.1994.13.8.595. [DOI] [PubMed] [Google Scholar]
- 13.Huang BY, Castillo M. Hypoxic-ischemic brain injury: imaging findings from birth to adulthood. RadioGraphics. 2008;28:417–439. doi: 10.1148/rg.282075066. [DOI] [PubMed] [Google Scholar]
- 14.de Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49(1):1–6. doi: 10.1016/S0166-4328(05)80189-5. [DOI] [PubMed] [Google Scholar]
- 15.Epelman M, Daneman A, Kellenberger CJ, Aziz A, Konen O, Moineddin R, Whyte H, Blaser S. Neonatal encephalopathy: a prospective comparison of head US and MRI. Pediatr Radiol. 2010;40:1640–1650. doi: 10.1007/s00247-010-1634-6. [DOI] [PubMed] [Google Scholar]
- 16.Salas J, Tekes A, Hwang M, Northington FJ, Huisman TAGM. Head ultrasound in neonatal hypoxic-ischemic injury and its mimickers for clinicians: a review of the patterns of injury and the evolution of findings over time. Neonatology. 2018;114(3):185–197. doi: 10.1159/000487913. [DOI] [PubMed] [Google Scholar]
- 17.Benson JE, Bishop MR, Cohen HL. Intracranial neonatal neurosonography: an update. Ultrasound Q. 2002;18:89–114. doi: 10.1097/00013644-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Magnaguagno F, Tomà P. Encefalo, midollo, faccia e collo del neonato e del lattante. In: Busilacchi P, Rapaccini GL, editors. Ecografia clinica. Napoli: Idelson-Gnocchi; 2006. pp. 1569–1594. [Google Scholar]
- 19.Shroff MM, Soares-Fernandes JP, Whyte H, Raybaud C. MR imaging for diagnostic evaluation of encephalopathy in the newborn. Radiographics. 2010;30(3):763–780. doi: 10.1148/rg.303095126. [DOI] [PubMed] [Google Scholar]
- 20.Sewell EK, Andescavage NN. Neuroimaging for neurodevelopmental prognostication in high-risk neonates. Clin Perinatol. 2018;45(3):421–437. doi: 10.1016/j.clp.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 21.de Vries LS, Verboon-Maciolek MA, Cowan FM, Groenendaal F. The role of cranial ultrasound and magnetic resonance imaging in the diagnosis of infections of the central nervous system. Early Hum Dev. 2006;82(12):819–825. doi: 10.1016/j.earlhumdev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Siegel MJ. Brain. In: Siegel MJ, editor. Pediatric sonography. Philadelphia: Wolters Kluwer, Lippincott Williams & Wilkins; 2011. pp. 43–117. [Google Scholar]
- 23.Yoon HK, Cho SW. Neonatal head ultrasound: systematic approach to congenital central nervous system anomalies. A pictorial essay. Med Ultrason. 2016;18(3):386–393. doi: 10.11152/mu.2013.2066.183.hye. [DOI] [PubMed] [Google Scholar]
- 24.Gupta N, Grover H, Bansal I, Hooda K, Sapire JM, Anand R, Kumar Y. Neonatal cranial sonography: ultrasound findings in neonatal meningitis—a pictorial review. Quant Imaging Med Surg. 2017;7(1):123–131. doi: 10.21037/qims.2017.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernanz-Schulman M, Wendy Cohen W, Genieser NB. Sonography of cerebral infarction in infancy. AJNR. 1988;9:131–136. doi: 10.2214/ajr.150.4.897. [DOI] [PubMed] [Google Scholar]
- 26.Raju VS, Rao MN, Rao VS. Cranial sonography in pyogenic meningitis in neonates and infants. J Trop Pediatr. 1995;41:68–73. doi: 10.1093/tropej/41.2.68. [DOI] [PubMed] [Google Scholar]
- 27.Epelman M, et al. Differential diagnosis of intracranial cystic lesions at head US: correlation with CT and MR imaging. RadioGraphics. 2006;26:173–196. doi: 10.1148/rg.261055033. [DOI] [PubMed] [Google Scholar]
- 28.Abbitt PL, Hurst RW, Ferguson RD, McIlhenny J, Alford BA. The role of ultrasound in the management of vein of Galen aneurysm in infancy. Neuroradiology. 1990;32:86–89. doi: 10.1007/BF00588554. [DOI] [PubMed] [Google Scholar]
- 29.Cohen HL, Haller JO. Advances in perinatal neurosonography. Am J Roentgenol. 1994;163:801–810. doi: 10.2214/ajr.163.4.7916529. [DOI] [PubMed] [Google Scholar]
- 30.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents-second edition. Pediatr Crit Care Med. 2012;13(supplement 1):S1–S82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 31.Riccarbona M. Pediatric ultrasound, requisites and applications. Berlin: Springer; 2014. [Google Scholar]
- 32.Parri N, Crosby BJ, Mills L, et al. Point-of-care ultrasound for the diagnosis of skull fractures in children younger than two years of age. J Pediatr. 2018;196:230–236. doi: 10.1016/j.jpeds.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 33.Lam A, Cruz GB, Johnson I. Extradural hematoma in neonates. J Ultrasound Med. 1991;10(4):205–209. doi: 10.7863/jum.1991.10.4.205. [DOI] [PubMed] [Google Scholar]
- 34.Lam AH, Cruz GB. Ultrasound evaluation of subdural haematoma. Australas Radiol. 1991;35(4):330–332. doi: 10.1111/j.1440-1673.1991.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 35.De Bruyn R. Pediatric ultrasound, how, why and when. London: Churchil Livingstone (Elsevier limited); 2005. [Google Scholar]
- 36.LaRovere KL, O’Brien NF, Tasker RC. Current opinion and use of transcranial Doppler ultrasonography in traumatic brain injury in the pediatric intensive care unit. J Neurotrauma. 2016;33(23):2105–2114. doi: 10.1089/neu.2015.4344. [DOI] [PubMed] [Google Scholar]
- 37.Mata-Mbemba D, Donnellan J, Krishnan P, Shroff M, Muthusami P. Imaging features of common pediatric intracranial tumours: a primer for the radiology trainee. Can Assoc Radiol J. 2018;69(1):105–117. doi: 10.1016/j.carj.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Osborn AG, et al. Diagnostic imaging brain. Salt Lake City: Amirsys Inc; 2004. [Google Scholar]
- 39.Woodward PJ, Sohaey R, Kennedy A, Koeller KK. From the archives of the AFIP: a comprehensive review of fetal tumors with pathologic correlation. Radiographics. 2005;25(1):215–242. doi: 10.1148/rg.251045156. [DOI] [PubMed] [Google Scholar]
- 40.Lysyy O, Puzhevsky A, Strauss S. Choroid plexus papilloma in an infant: ultrasound diagnosis. Eur J Pediatr. 2012;171(11):1717–1718. doi: 10.1007/s00431-012-1842-1. [DOI] [PubMed] [Google Scholar]