This symposium session was intended to introduce to the society members the most recent achievements done by using SPring-8, the large-scale 3rd-generation synchrotron facility in Japan, and SACLA, the X-ray free-electron laser facility built next to SPring-8. The session had 5 talks. Of the 5 talks, 2 were about X-ray diffraction imaging, 2 were about conventional fiber diffraction, and the remaining one was about diffracted X-ray tracking (DXT), developed by Yuji Sasaki at the University of Tokyo.
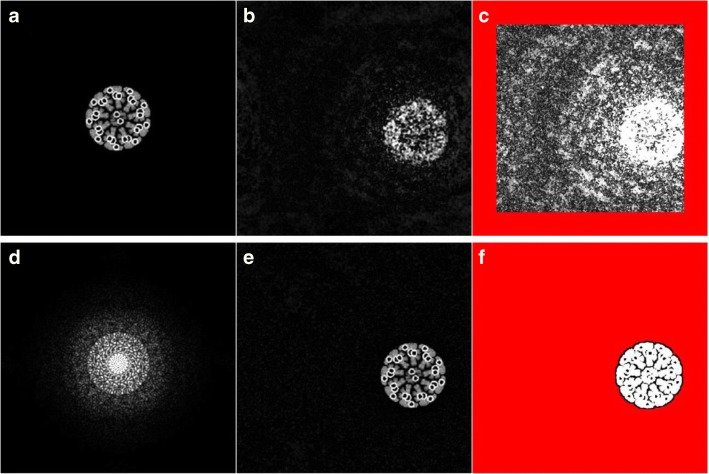
The first talk, given by Hiroyuki Iwamoto, SPring-8·JASRI, was titled “Recent progress in synchrotron radiation X-ray diffraction studies for non-crystalline biological specimens.” Compared with light- and electron-microscopic techniques, the development of high-resolution imaging techniques using X-rays is somewhat delayed, due to the much smaller population of researchers engaged in this field. Nevertheless, significant progress has been made recently (Iwamoto 2019), and 3 representative techniques were introduced in this talk: Fourier-transform holography, coherent diffractive imaging (CDI, Fig. 1), and the direct restoration of the 3D structure from fiber diffraction patterns developed by Iwamoto.
Fig. 1.
Example of coherent diffractive imaging (CDI, a computer experiment). a Image of the sample (cross section of a Drosophila sperm tail). d Its diffraction pattern. b, c Image restored from the diffraction pattern (d) after 10 iterative calculations. In c, the image is 10× intensified. The red area is outside the support area, in which the sample is expected to exist. d, e Restored image after 2000 iterative calculations. The support area fits the shape of the sample owing to the shrink-wrap algorithm
The second talk by Shinji Kamimura, Chuo University, was titled “Dynamic changes of tubulin dimer configurations on a scale of sub-second revealed by high flux X-ray fiber diffraction.” Kamimura and colleagues determined the axial repeat of tubulin monomers in microtubules very accurately by X-ray fiber diffraction and revealed that the axial repeat is affected differently by the addition of different kinds of microtubule-stabilizing agents (Estévez-Gallego et al. 2020). These agents are important candidates for anticancer drugs. The microtubules were aligned by using the shear-flow apparatus they developed.
The third speaker, Hiroshi Sekiguchi, SPring-8·JASRI, talked about the results of DXT applied to the group II chaperonin protein molecule (Sekiguchi et al. 2013: Yamamoto et al. 2016). DXT refers to the technique of labeling specific sites of protein molecules with gold nanocrystals and monitoring their local movements by tracking the diffraction spots from the nanocrystals. Previously, intense X-ray beams were required for these measurements, but recently Sekiguchi has developed a technique with much lower doses of X-ray, by observing the diffracted intensity fluctuation from the nanocrystals (Sekiguchi et al. 2018).
The fourth talk, given by James T. Pearson, National Cerebral and Cardiovascular Center, was titled “Dynamic changes in cardiac myosin head regulation during hyperglycemic events in insulin resistant rats.” This study was to expose the beating heart of a rat in anesthesia, and monitor the movement of contractile proteins by monitoring the changes in the intensities of X-ray reflections and the lattice constant of protein filaments (Pearson et al. 2004). He presented the results of the effects of hyperglycemia on cardiac functions by using hyperglycemia model rats. In normal rats, the contractile proteins in the heart are to some extent in an activated state even in diastole, but it was revealed that in hyperglycemia, the proteins approach a completely relaxed state. Administration of insulin was found to remedy this situation.
The last talk, given by Masayoshi Nakasako, Keio University, was titled “Distribution of nucleic acids in yeast nucleus of G1 phase visualized by X-ray diffraction imaging using X-ray free electron laser.” This work was done in SACLA, and he used CDI to study the structure of chromatin in the nucleus of budding yeast. He used a cryochamber, specially designed for the use in SACLA, to cryofix yeast cells on the silicon nitride membrane (Kobayashi et al. 2016). By moving the membrane in synchronization with the X-ray pulses, he was able to record many diffraction patterns from the nuclei. He showed a model of chromatin folding in the nucleus by using images restored from the diffraction patterns.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Estévez-Gallego J, Josa-Prado F, Ku S, Buey RM, Balaguer FA, Prota AE, Lucena-Agell D, Kamma-Lorger C, Yagi T, Iwamoto H, Duschesne L, Barasoain I, Steinmetz MO, Chrétien D, Kamimura S, Díaz JF, Oliva MA (2020) Structural model for differential cap maturation at growing microtubule ends. eLife in press [DOI] [PMC free article] [PubMed]
- Iwamoto H. Synchrotron radiation X-ray diffraction studies on muscle: past, present and future. Biophys Rev. 2019;11:547–558. doi: 10.1007/s12551-019-00554-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Yuki Sekiguchi T, Tomotaka Oroguchi T, Okajima K, Fukuda A, Oide M, Yamamoto M, Nakasako M. Specimen preparation for cryogenic coherent X-ray diffraction imaging of biological cells and cellular organelles by using the X-ray free-electron laser at SACLA. J Synchrotron Radiat. 2016;23:975–989. doi: 10.1107/S1600577516007736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JT, Shirai M, Ito H, Tokunaga N, Tsuchimochi H, Nishiura N, Schwenke DO, Ishibashi-Ueda H, Akiyama R, Mori H, Kangawa K, Suga H, Yagi N. In situ measurements of crossbridge dynamics and lattice spacing in rat hearts by X-ray diffraction. Sensitivity to regional ischemia. Circulation. 2004;109:2976–2979. doi: 10.1161/01.CIR.0000133322.19340.EF. [DOI] [PubMed] [Google Scholar]
- Sekiguchi H, Nakagawa A, Moriya K, Makabe K, Ichiyanagi K, Nozawa S, Sato T, Adachi S, Kuwajima K, Yohda M, Sasaki YC. ATP dependent rotational motion of group II chaperonin observed by X-ray single molecule tracking. PLoS One. 2013;8:e64176. doi: 10.1371/journal.pone.0064176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi H, Kuramochi M, Ikezaki K, Okamura Y, Yoshimura K, Matsubara K, Chang J-W, Ohta N, Kubo T, Mio K, Suzuki Y, Chavas LMG, Sasaki YC. Diffracted X-ray blinking tracks single protein motions. Sci Rep. 2018;8:17090. doi: 10.1038/s41598-018-35468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto YY, Tsuchida K, Noguchi K, Ogawa N, Sekiguchi H, Sasaki YC, Yohda M. Characterization of group II chaperonins from an acidothermophilic archaeon Picrophilus torridus. FEBS Open Bio. 2016;6:751–764. doi: 10.1002/2211-5463.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]