Abstract
This review briefly summarizes the effect of additives on the formation of liquid droplets and aggregates of proteins. Proteins have the property of forming liquid droplets and aggregates both in vivo and in vitro. The liquid droplets of proteins are mainly stabilized by electrostatic and cation–π interactions, whereas the amorphous aggregates are mainly stabilized by hydrophobic interactions. Crowders usually stabilize liquid droplets, whereas ions and hexandiols destabilize the droplets. Additives such as kosmotropes, sugars, osmolytes, and crowders promote the formation of amorphous aggregates, whereas additives such as arginine and chaotropes can prevent the formation of amorphous aggregates. Further, amyloid has a different mechanism for its formation from amorphous aggregates because it is primarily stabilized by a cross-β structure. These systematic analyses of additives will provide clues to controlling protein aggregations and will aid the true understanding of the transition of proteins from liquid droplets and aggregates.
Keywords: Liquid droplets, Dispersion, Aggregation, Amyloid, Additive
Introduction
Proteins comprise 20 naturally occurring amino acids, which have the property to form assemblies, typically liquid droplets and amorphous aggregates in vitro (Iwashita et al. 2018b). For example, when an antibody is mixed with a poly (amino acid) at a neutral pH, the protein solution becomes cloudy (Mimura et al. 2019). This cloudiness disappears with the addition of hundreds of mM of salt. The cloudiness is stabilized by the electrostatic interaction between the antibody and the poly (amino acid) and hence dissociated by the electrostatic shielding effect by increasing ionic strength (Matsuda et al. 2018). Similarly, hen egg-white lysozyme is mixed with ovalbumin (Iwashita et al. 2018a) or ovotransferrin (Iwashita et al. 2019), and cloudiness is observed in vitro. These data showed that proteins are prone to form liquid droplets and/or aggregates in vitro. In this review, we briefly summarize the stabilization factors of liquid droplets, amorphous aggregates, and amyloid fibrils. After that, we overview the solution additives that control these protein assemblies. This review will provide important information to understanding on liquid–liquid phase separation of proteins in living cells.
Stabilization of liquid droplets and aggregates
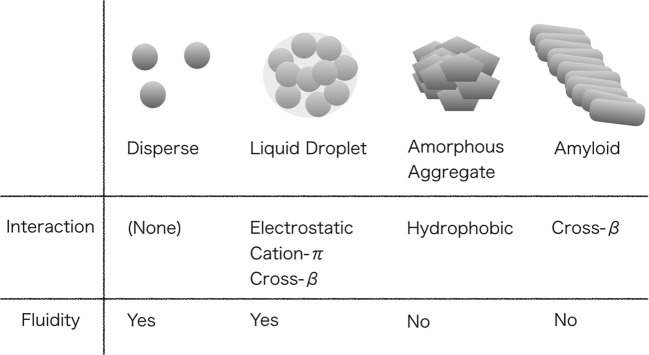
Protein solutions can be classified into four types according to their intermolecular interactions and fluidity (Fig. 1). The solution states are mainly influenced by the hydrophobicity that changes the fluidity of the protein assembly. (1) A dispersed solution of protein is an ideal state without intermolecular interactions. It should be noted that many researches on protein structure and function have been performed under the dispersed state. (2) The liquid droplet state of protein is a state stabilized by intermolecular interactions, although the molecules have fluidity inside the liquid droplets, which contain many water molecules. Hydrogels of proteins are more stable than droplets owing to mature intermolecular interactions (Kato et al. 2012). (3) Amorphous aggregates of proteins are solid precipitates without fluidity due to hydrophobic interactions between protein molecules. (4) The amyloid state is a typical aggregated state of proteins stabilized by cross-β structures.
Fig. 1.
Representative solution states of proteins. Proteins has typically four states in aqueous solutions that stabilize different interactions
The main factors that stabilize liquid droplets are electrostatic and cation–π interactions. In addition, π–π interactions and multivalent interactions are effective in stabilizing the liquid droplet state of proteins. In contrast, the main factor that stabilizes aggregates is hydrophobic interaction. Cross-β interactions stabilize both liquid droplets and amyloid aggregates, and the former is mainly stabilized by short cross-β structures (Murray et al. 2017). These stabilizing factors are briefly summarized as follows.
Electrostatic interactions are generated by an electric attractive force or a repulsive force between charged groups of protein molecules. Electrostatic interaction is the most important factor in the stabilization of liquid droplets because it is the only long-ranged interaction among others. Additionally, electrostatic interactions are affected by post-translational modifications. For example, the C-terminal domain of RNA polymerase II has many sites that are phosphorylated, but phosphorylation allows it to form stable droplets with other histidine-rich proteins (Lu et al. 2018). The RNA-binding proteins Ddx4 (Nott et al. 2015) and hnRNPA2 (Ryan et al. 2018) become soluble at low temperatures when lysine and arginine are methylated. Similarly, two research groups have reported that the liquid droplet of fused-in sarcoma (FUS) protein is tightly controlled by arginine methylation in the disordered region (Qamar et al. 2018; Hofweber et al. 2018).
Cation–π interactions are interactions between the π electron and a cation. Compared with lysine, arginine contributes to a stronger cation–π interaction because arginine has a planar positive charge on the side chain that has the cation–π interaction alongside the aromatic ring. In fact, there are many RGG sequences in which arginine (R) and glycine (G) are aligned in the low complexity region that causes phase separation of RNA-binding proteins (Chong et al. 2018). It is thought that the small amino acid of glycine is present to facilitate the interaction between the large side chain of arginine and the target aromatic amino acid on proteins.
π–π interactions are those in which the side chains of aromatic amino acids are arranged. The π–π interaction stabilizes a dispersion force between the delocalized π electrons on the aromatic ring and is one of the stabilizing factors of liquid droplets; hence, tyrosine (Tyr) and phenylalanine (Phe) compose the low complexity region of proteins for phase separation (Boeynaems et al. 2018). Note that Trp, which is the most hydrophobic amino acid, is not found in the low complexity region. This may be because Trp is not favorable for the formation of liquid droplet.
Hydrophobic interactions are interactions between the nonpolar regions of macromolecules in aqueous solution. Generally, hydrophobic interactions are the most important factors that stabilize amorphous aggregates, as well as the tertiary structure of proteins (Chiti et al. 2002). To more clearly see the difference, the amorphous aggregates of proteins are mainly stabilized by hydrophobic interactions, whereas the liquid droplets of proteins are mainly stabilized by non-hydrophobic interactions (Iwashita et al. 2018b).
Cross-β structures are intermolecular interactions of hydrogen bonds between the main chains of proteins. The cross-β structure was originally known as a structure that stabilizes amyloid aggregates (Nelson et al. 2005). The amyloid fibrils associated with the pathogenesis of Alzheimer’s and Parkinson’s diseases exhibit a common structural feature known as the cross-β, known as “dry steric zipper structures” (Vitagliano et al. 2009). On the other hand, recent studies have suggested that many RNA-binding proteins such as FUS can form functional amyloid fibrils with distinct reversibility that cannot be interpreted by the dry steric zipper structure (Kato et al. 2012). Solid-state nuclear magnetic resonance and molecular dynamics simulations have revealed that liquid droplets of FUS, which is thought to cause amyotrophic lateral sclerosis, have a cross-β structure as short as several residues (Murray et al. 2017). In addition, the cross-β structure that stabilizes liquid droplets is basically characterized by a short bend (Luo et al. 2018); thus, it is naturally thought that amyloid fibrils grow by forming liquid droplets.
Notably, liquid droplets of proteins and RNAs are determined by the respective interactions described above and by the arrangement order and tertiary structure of the macromolecules. For example, proteins are prone to form liquid droplets when the distribution of positive and negative charges is biased (Chang et al. 2017). Additionally, RNA contained in RNA-granules is less likely to form liquid droplets when conformational change occurs by a mutation (Ditlev et al. 2018). These results indicate that the liquid droplet state of proteins and RNA is more difficult to understand than that of synthetic polymers of simple repetitive sequences.
In summary, proteins form liquid droplets, aggregates, as well as dispersed state (Fig. 1). This simple view may change the understanding of the molecular mechanisms occurring in living cells. For example, FUS protein is responsible for familial neurodegenerative diseases by point mutations (Vance et al. 2009). The mutations of FUS cause cytotoxicity because it tends to form aggregates after the formation of liquid droplets (Patel et al. 2015). Similar data were obtained from an RNA-binding protein hnRNPA1, which is thought to be involved in neurodegenerative diseases. The hnRNPA1 forms a highly fluid liquid droplet with short cross-β structures, which are highly reversible, although the formation of long cross-β structures results in insoluble amyloid (Gui et al. 2019). In another case of α-synuclein, first, the disordered region of the N-terminus forms a liquid droplet, followed by amyloid formation in the C-terminal region (Ray et al. 2019).
Effects of additives on the solution states of proteins
Here, we summarize the effects of small molecules on the stabilizing factors of liquid droplets, amorphous aggregates, and amyloids of proteins (Table 1). The additives that affect protein structure and solubility can be experimentally understood to some extent by considering the above interactions that stabilize the liquid droplets, amorphous aggregates, and amyloids of proteins.
Table 1.
Effect of typical additives on liquid droplets, amorphous aggregates, and amyloids of proteins
| Liquid droplet | Amorphous aggregate | Amyloid | |
|---|---|---|---|
| Stabilization |
TMAOa Crowderb Ethanolb |
Sugarg Crowderh Kosmotropei Polyelectrolytej |
Sugarg Crowderh Polyelectrolytem,n |
|
Destabilization Suppression |
Hexanediolc Sugarb Osmolyted Ione ATPf |
Argininek Denaturantl Chaotropei |
Catechino Polyphenolp Aromatic compoundq |
aChoi et al. 2018. bMimura et al. 2019. cLin et al. 2016. dChoi et al. 2018. eIwashita et al. 2018a. fPatel et al. 2017. gKatyal and Deep 2017. hKuznetsova et al. 2015. iZhang and Cremer 2010. jKurinomaru et al. 2012. kHamada et al. 2009. lHevehan and De Bernardez 1997. mKovachev et al. 2019. nZhang et al. 2015. oHe et al. 2009. pPorat et al. 2006. qLevy-Sakin et al. 2009
First, it should be noted that additives primarily affect the solubility of proteins, and their properties are divided into two types. Small-molecular-weight additives, such as amino acids and sugars, have the property of requiring high concentrations (typically 100 mM or more). This is because they change the properties of the solvent, rather than the binding to proteins. Conversely, polyamino acids and polyvalent metal ions have properties that are different from small molecules because they interact with proteins in multivalent interactions that change the solubility of the proteins. Accordingly, such polymers will work with just the same concentration of proteins (typically 1 mM or less).
Inorganic and organic ions weaken electrostatic interactions between solutes via the electrostatic shielding effect. For example, the addition of 50 mM sodium chloride completely dissociates the protein complex that is stabilized only by electrostatic interactions (Iwashita et al. 2018a). Notably, ions can be classified from kosmotropes to chaotropes depending on the strength of binding to solutes, so called Hofmeister series (Zhang and Cremer 2010). For example, sulfide and fluoride ions are typical kosmotropes, which tend to form amorphous aggregates of proteins. In contrast, iodide ions and thiocyanate ions are typical chaotropes, which tend to unfold the protein structure and suppress the formation of amorphous aggregates. Metal ions are prone to binding to protein, RNA, and DNA, which changes the ability of biomolecules to form liquid droplets, which is the different mechanism of monovalent ions. For example, magnesium and calcium ions accelerate the formation of nucleosome self-assembly liquid droplets by phase separation (Ohyama 2019).
Denaturants are substances that disrupt the tertiary structure of proteins. Denaturants, typically urea and guanidinium, have the function of weakening the hydrophobic interactions between solutes, resulting in the dispersion of hydrophobic substances into solution (Vanzi et al. 1998). Representative denaturants include guanidine hydrochloride and urea. In addition, protein aggregation is mainly driven by hydrophobic interactions, so the addition of denaturants can prevent protein aggregation (Hevehan and De Bernardez 1997). Similarly, it is naturally believed that liquid droplets are dissolved by the addition of denaturants if the liquid droplets are stabilized mainly by hydrophobic interactions.
Sugars stabilize the tertiary structure of proteins to increase the interfacial energy and promote the formation of amorphous aggregates. For example, glucose and trehalose dissolve the liquid droplets of immunoglobulin G and polyglutamic acid (Mimura et al. 2019). This may be because the addition of the sugars differs the hydration of poly (amino acids), rather than their tertiary structure, resulting in the dissolution of the liquid droplet. Trehalose accelerates the formation of amorphous aggregates, whereas it suppresses the formation of amyloid aggregates of prion domain (Katyal and Deep 2017) and amyloid-β 40 and 42 (Liu et al. 2005).
Osmolyte is a molecule found in the cells of marine organisms that are resistant to osmotic pressure (Yancey et al. 1982). For example, osmolytes such as betaine, taurine, and trimethylammonium N-oxide (TMAO) stabilize the three-dimensional structure of proteins. In cases where the structure of the natural denatured region plays an important role as a stabilizing factor for liquid droplets, the addition of osmolytes results in the dissolution of the liquid droplets in a similar way to sugars. TMAO stabilizes the liquid droplets of an intrinsically disordered protein of TDP-43, which prevents amyloid fibrilization (Choi et al. 2018). By contrast, TMAO promotes the amyloid formation of tau protein (Scaramozzino et al. 2006). Therefore, the effect of osmolytes remains unclear.
Arginine is a naturally occurring amino acid with a basic side chain. Arginine is the most versatile additive for various applications, such as suppressing aggregation due to heating, dissolving aromatic compounds, and improving refolding yield (Arakawa and Kita 2014). Because guanidinium in the side chain of arginine has a planar structure, arginine forms a stable cation–π interaction with the aromatic ring on the surface of the protein, resulting in an increase in the repulsion between protein molecules (Miyatake et al. 2016). Arginine dissolves the opalescence of high-concentration solutions of gamma globulin (Oki et al. 2018). Notably, alantoin and hydantoin are new aggregation suppressors for proteins that may complement arginine’s function (Nishinami et al. 2018).
Amine compounds, such as spermine, putrescine, and glycinamide, have the function of preventing aggregation and inactivation of proteins caused by heat treatment (Shiraki et al. 2015). A similar effect can be seen for ammonium ions (Hirano et al. 2007). The mechanism by which amine compounds prevent thermal stress mainly protects the amine on the side chain of asparagine (Tomita and Shiraki 2011). It is important to note that such chemical reactions affect the formation of liquid droplets and amorphous aggregates.
Alcohol solutions have a lower dielectric constant than water. Solutions with low dielectric constants enhance the electrostatic interactions and hydrogen bonds between the protein molecules; hence, alcohols tend to form α-helix-rich protein structures (Shiraki et al. 1995). For example, liquid droplets of immunoglobulin G and polyglutamic acid are stabilized in an ethanol solution (Mimura et al. 2019). In contrast, low concentration of 1,6-hexanediol dissolves liquid droplets because alcohol weakens the weak hydrophobic interactions between protein molecules (Lin et al. 2016). Accordingly, the molecular mechanism of alcohol on liquid droplets are controversial.
Neutral macromolecules, typically polyethylene glycol and ficoll, stabilize the tertiary structure of proteins via the crowding effect (Kuznetsova et al. 2015). The crowding effect prevents the formation of liquid droplets to make the protein structure more compact; however, it stabilizes the droplets once formed (Mimura et al. 2019). Crowders accelerate the formation of amyloids (Munishkina et al. 2004). The crowding effect plays an important role in understanding the inside of living cells.
Polyelectrolytes affect the formation of liquid droplets, amorphous aggregates, and amyloid. Polyelectrolytes change the solvent properties of proteins by complex formation. This is different from the above-described property of changing the solvent properties by the addition of small molecules in solution. For example, poly (lysine) and poly (glutamic acid) precipitate various types of proteins and peptides (Kurinomaru et al. 2014) because the complex of polyelectrolytes and proteins becomes electrostatically neutral; thus, it easily precipitates (Matsuda et al. 2018). On the other hand, a polyelectrolyte that binds strongly to proteins unfolds proteins to stabilize aggregates (Kurinomaru et al. 2012). One of the polyelectrolytes of RNA suppresses the formation of amyloids, such as mammalian prion (Kovachev et al. 2019) and poly Q (Zhang et al. 2015).
In conclusion, this review has summarized the fact that proteins easily assemble into liquid droplets and amorphous aggregates. The liquid droplets and aggregates of proteins have different stabilizing factors, typically hydrophobic, electrostatic, cation–π, and cross-β interactions. As a result, the effects of additives are systematically classified into liquid droplets and aggregates. This information will provide important clues for understanding how liquid–liquid phase separation occurs in living cells and will be useful in various biotechnological applications.
Funding information
This work was partly supported by JSPS KAKENHI (grant number 18H02383 and 19K22377).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arakawa T, Kita Y. Multi-faceted arginine: mechanism of the effects of arginine on protein. Curr Protein Pept Sci. 2014;15:608–620. doi: 10.2174/138920371506140818113015. [DOI] [PubMed] [Google Scholar]
- Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, Tompa P, Fuxreiter M. Protein phase separation: a new phase in cell biology. Trends Cell Biol. 2018;28:420–435. doi: 10.1016/j.tcb.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LW, Lytle TK, Radhakrishna M, Madinya JJ, Vélez J, Sing CE, Perry SL. Sequence and entropy-based control of complex coacervates. Nat Commun. 2017;8:1273. doi: 10.1038/s41467-017-01249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti F, Taddei N, Baroni F, Capanni C, Stefani M, Ramponi G, Dobson CM. Kinetic partitioning of protein folding and aggregation. Nat Struct Biol. 2002;9:137–143. doi: 10.1038/nsb752. [DOI] [PubMed] [Google Scholar]
- Choi KJ, Tsoi PS, Moosa MM, Paulucci-Holthauzen A, Liao SJ, Ferreon JC, Ferreon ACM. A chemical chaperone decouples TDP-43 disordered domain phase separation from fibrillation. Biochemistry. 2018;57:6822–6826. doi: 10.1021/acs.biochem.8b01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong PA, Vernon RM, Forman-Kay JD. RGG/RG motif regions in RNA binding and phase separation. J Mol Biol. 2018;430:4650–4665. doi: 10.1016/j.jmb.2018.06.014. [DOI] [PubMed] [Google Scholar]
- Ditlev JA, Case LB, Rosen MK. Who's in and who's out-compositional control of biomolecular condensates. J Mol Biol. 2018;430:4666–4684. doi: 10.1016/j.jmb.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui X, Luo F, Li Y, Zhou H, Qin Z, Liu Z, Gu J, Xie M, Zhao K, Dai B, Shin WS, He J, He L, Jiang L, Zhao M, Sun B, Li X, Liu C, Li D. Structural basis for reversible amyloids of hnRNPA1 elucidates their role in stress granule assembly. Nat Commun. 2019;10:2006. doi: 10.1038/s41467-019-09902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Arakawa T, Shiraki K. Effect of additives on protein aggregation. Curr Pharm Biotechnol. 2009;10:400–407. doi: 10.2174/138920109788488941. [DOI] [PubMed] [Google Scholar]
- He J, Xing YF, Huang B, Zhang YZ, Zeng CM. Tea catechins induce the conversion of preformed lysozyme amyloid fibrils to amorphous aggregates. J Agric Food Chem. 2009;57:11391–11396. doi: 10.1021/jf902664f. [DOI] [PubMed] [Google Scholar]
- Hevehan DL, De Bernardez CE. Oxidative renaturation of lysozyme at high concentrations. Biotechnol Bioeng. 1997;54:221–230. doi: 10.1002/(SICI)1097-0290(19970505)54:3<221::AID-BIT3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Hirano A, Hamada H, Okubo T, Noguchi T, Higashibata H, Shiraki K. Correlation between thermal aggregation and stability of lysozyme with salts described by molar surface tension increment: an exceptional propensity of ammonium salts as aggregation suppressor. Protein J. 2007;26:423–433. doi: 10.1007/s10930-007-9082-3. [DOI] [PubMed] [Google Scholar]
- Hofweber M, Hutten S, Bourgeois B, Spreitzer E, Niedner-Boblenz A, Schifferer M, Ruepp MD, Simons M, Niessing D, Madl T, Dormann D. Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. Cell. 2018;173:706–719.e13. doi: 10.1016/j.cell.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Iwashita K, Handa A, Shiraki K. Coacervates and coaggregates: liquid-liquid and liquid-solid phase transitions by native and unfolded protein complexes. Int J Biol Macromol. 2018;120(Pt A):10–18. doi: 10.1016/j.ijbiomac.2018.08.063. [DOI] [PubMed] [Google Scholar]
- Iwashita K, Mimura M, Shiraki K. Control of aggregation, coaggregation, and liquid droplet of proteins using small additives. Curr Pharm Biotechnol. 2018;19:946–955. doi: 10.2174/1389201020666181204113054. [DOI] [PubMed] [Google Scholar]
- Iwashita K, Handa A, Shiraki K. Co-aggregation of ovotransferrin and lysozyme. Food Hydrocoll. 2019;89:416–424. doi: 10.1016/j.foodhyd.2018.11.022. [DOI] [Google Scholar]
- Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J, Grishin NV, Frantz DE, Schneider JW, Chen S, Li L, Sawaya MR, Eisenberg D, Tycko R, McKnight SL. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell. 2012;149:753–767. doi: 10.1016/j.cell.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyal N, Deep S. Inhibition of GNNQQNY prion peptide aggregation by trehalose: a mechanistic view. Phys Chem Chem Phys. 2017;19:19120–19138. doi: 10.1039/c7cp02912h. [DOI] [PubMed] [Google Scholar]
- Kovachev PS, Gomes MPB, Cordeiro Y, et al. RNA modulates aggregation of the recombinant mammalian prion protein by direct interaction. Sci Rep. 2019;9:12406. doi: 10.1038/s41598-019-48883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurinomaru T, Tomita S, Kudo S, Ganguli S, Nagasaki Y, Shiraki K. Improved complementary polymer pair system: switching for enzyme activity by PEGylated polymers. Langmuir. 2012;28:4334–4338. doi: 10.1021/la2043312. [DOI] [PubMed] [Google Scholar]
- Kurinomaru T, Maruyama T, Izaki S, Handa K, Kimoto T, Shiraki K. Protein-poly (amino acid) complex precipitation for high-concentration protein formulation. J Pharm Sci. 2014;103:2248–2254. doi: 10.1002/jps.24025. [DOI] [PubMed] [Google Scholar]
- Kuznetsova IM, Zaslavsky BY, Breydo L, Turoverov KK, Uversky VN. Beyond the excluded volume effects: mechanistic complexity of the crowded milieu. Molecules. 2015;20:1377–1409. doi: 10.3390/molecules20011377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Sakin M, Shreberk M, Daniel Y, Gazit E. Targeting insulin amyloid assembly by small aromatic molecules: toward rational design of aggregation inhibitors. Islets. 2009;1:210–215. doi: 10.4161/isl.1.3.9609. [DOI] [PubMed] [Google Scholar]
- Lin Y, Mori E, Kato M, Xiang S, Wu L, Kwon I, McKnight SL. Toxic PR poly-dipeptides encoded by the C9orf72 repeat expansion target LC domain polymers. Cell. 2016;167:789–802.e12. doi: 10.1016/j.cell.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Barkhordarian H, Emadi S, Park CB, Sierks MR. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol Dis. 2005;20:74–81. doi: 10.1016/j.nbd.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Lu H, Yu D, Hansen AS, Ganguly S, Liu R, Heckert A, Darzacq X, Zhou Q. Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II. Nature. 2018;558:318–323. doi: 10.1038/s41586-018-0174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Gui X, Zhou H, Gu J, Li Y, Liu X, Zhao M, Li D, Li X, Liu C. Atomic structures of FUS LC domain segments reveal bases for reversible amyloid fibril formation. Nat Struct Mol Biol. 2018;25:341–346. doi: 10.1038/s41594-018-0050-8. [DOI] [PubMed] [Google Scholar]
- Matsuda A, Mimura M, Maruyama T, Kurinomaru T, Shiuhei M, Shiraki K. Liquid droplet of protein-polyelectrolyte complex for high-concentration formulations. J Pharm Sci. 2018;107:2713–2719. doi: 10.1016/j.xphs.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Mimura M, Tsumura K, Matsuda A, Akatsuka N, Shiraki K. Effect of additives on liquid droplet of protein-polyelectrolyte complex for high-concentration formulations. J Chem Phys. 2019;150:064903. doi: 10.1063/1.5063378. [DOI] [PubMed] [Google Scholar]
- Miyatake T, Yoshizawa S, Arakawa T, Shiraki K. Charge state of arginine as an additive on heat-induced protein aggregation. Int J Biol Macromol. 2016;87:563–569. doi: 10.1016/j.ijbiomac.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Munishkina LA, Cooper EM, Uversky VN, Fink AL. The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J Mol Recognit. 2004;17:456–464. doi: 10.1002/jmr.699. [DOI] [PubMed] [Google Scholar]
- Murray DT, Kato M, Lin Y, Thurber KR, Hung I, McKnight SL, Tycko R. Structure of FUS protein fibrils and its relevance to self-assembly and phase separation of low-complexity domains. Cell. 2017;171:615–627.e16. doi: 10.1016/j.cell.2017.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Sawaya MR, Balbirnie M, Madsen AØ, Riekel C, Grothe R, Eisenberg D. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435:773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishinami S, Yoshizawa S, Arakawa T, Shiraki K. Allantoin and hydantoin as new protein aggregation suppressors. Int J Biol Macromol. 2018;114:497–503. doi: 10.1016/j.ijbiomac.2018.03.011. [DOI] [PubMed] [Google Scholar]
- Nott TJ, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones DP, Pawson T, Forman-Kay JD, Baldwin AJ. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol Cell. 2015;57:936–947. doi: 10.1016/j.molcel.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T. New aspects of magnesium function: a key regulator in nucleosome self-assembly, chromatin folding and phase separation. Int J Mol Sci. 2019;20:4232. doi: 10.3390/ijms20174232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki S, Iwashita K, Kimura M, Kano H, Shiraki K. Mechanism of co-aggregation in a protein mixture with small additives. Int J Biol Macromol. 2018;107:1428–1437. doi: 10.1016/j.ijbiomac.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, Pozniakovski A, Poser I, Maghelli N, Royer LA, Weigert M, Myers EW, Grill S, Drechsel D, Hyman AA, Alberti S. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–1077. doi: 10.1016/j.cell.2015.07.047. [DOI] [PubMed] [Google Scholar]
- Patel A, Malinovska L, Saha S, Wang J, Alberti S, Krishnan Y, Hyman AA. ATP as a biological hydrotrope. Science. 2017;356:753–756. doi: 10.1126/science.aaf6846. [DOI] [PubMed] [Google Scholar]
- Porat Y, Abramowitz A, Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- Qamar S, Wang G, Randle SJ, Ruggeri FS, Varela JA, Lin JQ, Phillips EC, Miyashita A, Williams D, Ströhl F, Meadows W, Ferry R, Dardov VJ, Tartaglia GG, Farrer LA, Kaminski Schierle GS, Kaminski CF, Holt CE, Fraser PE, Schmitt-Ulms G, Klenerman D, Knowles T, Vendruscolo M, St George-Hyslop P. FUS phase separation is modulated by a molecular chaperone and methylation of arginine cation-π interactions. Cell. 2018;173:720–734.e15. doi: 10.1016/j.cell.2018.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Singh N, Pandey S, Kumar R, Gadhe L, Datta D, Patel K, Mahato J, Navalkar A, Panigrahi R, Chatterjee D (2019) Liquid-liquid phase separation and liquid-to-solid transition mediate α-synuclein amyloid fibril containing hydrogel formation. bioRxiv:619858. 10.1101/619858
- Ryan VH, Dignon GL, Zerze GH, Chabata CV, Silva R, Conicella AE, Amaya J, Burke KA, Mittal J, Fawzi NL. Mechanistic view of hnRNPA2 low-complexity domain structure, interactions, and phase separation altered by mutation and arginine methylation. Mol Cell. 2018;69:465–479.e7. doi: 10.1016/j.molcel.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaramozzino F, Peterson DW, Farmer P, Gerig JT, Graves DJ, Lew J. TMAO promotes fibrillization and microtubule assembly activity in the C-terminal repeat region of tau. Biochemistry. 2006;4:3684–3691. doi: 10.1021/bi052167g. [DOI] [PubMed] [Google Scholar]
- Shiraki K, Nishikawa K, Goto Y. Trifluoroethanol-induced stabilization of the alpha-helical structure of beta-lactoglobulin: implication for non-hierarchical protein folding. J Mol Biol. 1995;245:180–194. doi: 10.1006/jmbi.1994.0015. [DOI] [PubMed] [Google Scholar]
- Shiraki K, Tomita S, Inoue N. Small amine molecules: solvent design toward facile improvement of protein stability against aggregation and inactivation. Curr Pharm Biotechnol. 2015;17:116–125. doi: 10.2174/1389201017666151029110229. [DOI] [PubMed] [Google Scholar]
- Tomita S, Shiraki K. Why do solution additives suppress the heat-induced inactivation of proteins? Inhibition of chemical modifications. Biotechnol Prog. 2011;27:855–862. doi: 10.1002/btpr.597. [DOI] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzi F, Madan B, Sharp K. Effect of the protein denaturants urea and guanidinium on water structure: a structural and thermodynamic study. J Am Chem Soc. 1998;120:10748–10753. doi: 10.1021/ja981529n. [DOI] [Google Scholar]
- Vitagliano L, Stanzione F, De Simone A, Esposito L. Dynamics and stability of amyloid-like steric zipper assemblies with hydrophobic dry interfaces. Biopolymers. 2009;91:1161–1171. doi: 10.1002/bip.21182. [DOI] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cremer PS. Chemistry of Hofmeister anions and osmolytes. Annu Rev Phys Chem. 2010;61:63–83. doi: 10.1146/annurev.physchem.59.032607.093635. [DOI] [PubMed] [Google Scholar]
- Zhang H, Elbaum-Garfinkle S, Langdon EM, et al. RNA controls PolyQ protein phase transitions. Mol Cell. 2015;60:220–230. doi: 10.1016/j.molcel.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]