Abstract
Background
Diffusing capacity of the lung for carbon monoxide (Dlco) is inconsistently obtained in patients with COPD, and the added benefit of Dlco testing beyond that of more common tools is unknown.
Objective
The goal of this study was to determine whether lower Dlco is associated with increased COPD morbidity independent of emphysema assessed via spirometry and CT imaging.
Methods
Data for 1,806 participants with COPD from the Genetic Epidemiology of COPD (COPDGene) study 5-year visit were analyzed, including pulmonary function testing, quality of life, symptoms, exercise performance, and exacerbation rates. Dlco percent predicted was primarily analyzed as a continuous variable and additionally categorized into four groups: (1) Dlco and FEV1 > 50% (reference); (2) only Dlco ≤ 50%; (3) only FEV1 ≤ 50%; and (4) both ≤ 50% predicted. Outcomes were modeled by using multivariable linear and negative binomial regression, including emphysema and FEV1 percent predicted among other confounders.
Results
In multivariable analyses, every 10% predicted decrease in Dlco was associated with symptoms and quality of life (COPD Assessment Test, 0.53 [P < .001]; St. George’s Respiratory Questionnaire, 1.67 [P < .001]; Medical Outcomes Study Short Form 36 Physical Function, –0.89 [P < .001]), exercise performance (6-min walk distance, –45.35 feet; P < .001), and severe exacerbation rate (rate ratio, 1.14; P < .001). When categorized, severe impairment in Dlco alone, FEV1 alone, or both Dlco and FEV1 were associated with significantly worse morbidity compared with the reference group (P < .05 for all outcomes).
Conclusions
Impairment in Dlco was associated with increased COPD symptoms, reduced exercise performance, and severe exacerbation risk even after accounting for spirometry and CT evidence of emphysema. These findings suggest that Dlco should be considered for inclusion in future multidimensional tools assessing COPD.
Key Words: COPD, pulmonary diffusing capacity, pulmonary gas exchange
Abbreviations: 6MWD, 6-min walk distance; ATS, American Thoracic Society; CAT, COPD Assessment Test; Dlco, diffusing capacity of the lung for carbon monoxide; HRCT, high-resolution CT; KCO, efficiency of gas transfer; %LAA-950, percent low attenuation areas at or below –950 Hounsfield units consistent with emphysema; SF-36, Medical Outcomes Study Short Form 36; SGRQ, St. George’s Respiratory Questionnaire
COPD affects > 15.7 million adults1 and is the fourth leading cause of death in the United States.2 Spirometry has been the cornerstone of diagnosis, with the presence of airflow obstruction a key diagnostic criterion. More recently, to achieve a multidimensional evaluation, the approach to COPD assessment has broadened from primarily spirometry to include assessment of symptoms, impact on health status, quantitative assessment of emphysema by CT imaging, and risk of exacerbations.3, 4, 5, 6 One diagnostic tool that is noninvasive and widely available, but not currently integrated in commonly used COPD assessment models,5 is measurement of diffusing capacity of the lung for carbon monoxide (Dlco).
Dlco is a measure of gas transfer that reflects the complex interactions at the alveolar capillary interface. It has been strongly associated with decline in lung function and is one of the best correlates of emphysema in COPD.7, 8 Studies evaluating Dlco as a predictor of mortality are conflicting, with results dependent on the degree of airflow limitation and emphysema.9, 10, 11, 12, 13 A few small studies suggest an association between gas transfer defects and frequent exacerbations,14, 15 but they did not examine these associations independent of emphysema. Although Dlco provides insight regarding respiratory physiology beyond that provided with spirometry, including indirect measurement of pulmonary vascular abnormalities, little information is available on the clinical utility of Dlco in predicting outcomes independent of spirometry or emphysema.
As a large, well-characterized cohort, the Genetic Epidemiology of COPD (COPDGene) study provides an ideal opportunity to analyze the relation between Dlco and COPD morbidity. We hypothesized that Dlco, independent of FEV1 and quantitative CT measures of emphysema, is associated with worse COPD morbidity, including symptoms, quality of life, exercise performance, and exacerbations.
Patients and Methods
Study Population
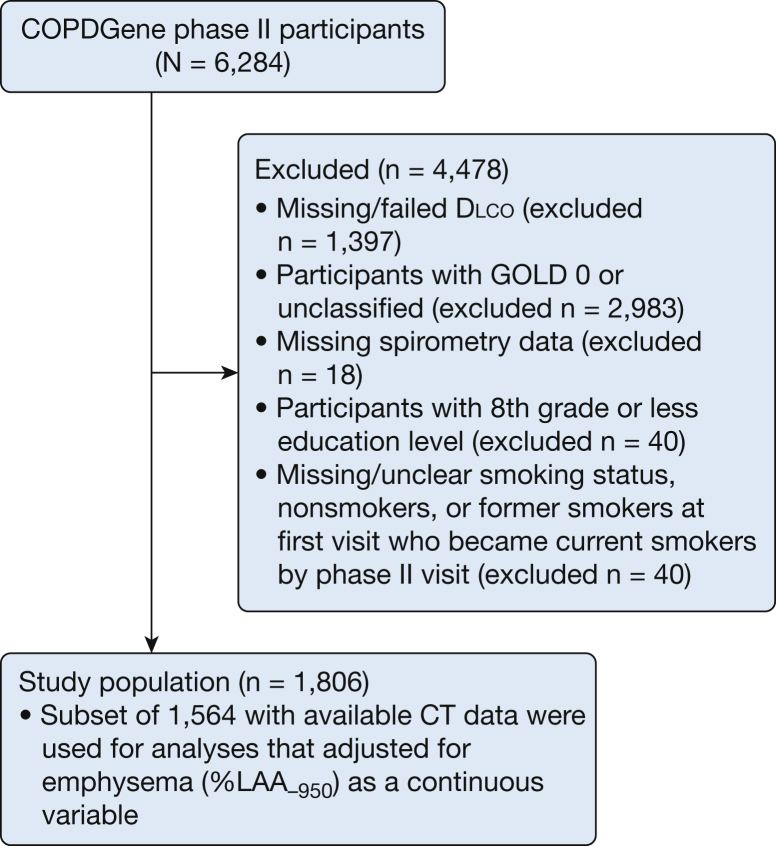
COPDGene was approved by institutional review boards at all participating centers. Each participant provided written informed consent. COPDGene study methods have been previously reported.16 Smokers with a history of ≥ 10 pack-years and age 45 to 80 years from 21 clinical centers were enrolled in the years 2007 to 2012. Participants with postbronchodilator FEV1/FVC < 70% were defined as having COPD, and they were further categorized according to severity based on postbronchodilator percent predicted FEV1 using Global Obstructive Lung Disease spirometry stage I to IV criteria.5 Participants with COPD who completed the COPDGene visit conducted 5 years following the original enrollment (2013-2017) and who had Dlco measurements and complete data for all relevant covariates were included. Of these 1,806 participants, 1,564 had CT data available (Fig 1).
Figure 1.
Participant selection. Models with and without emphysema (%LAA-950) are presented for all outcomes in Tables 2, 3, and e-Table 2. COPDGene = Genetic Epidemiology of COPD; Dlco = diffusing capacity of the lung for carbon monoxide; GOLD = Global Obstructive Lung Disease; %LAA-950 = percent low attenuation areas at or below –950 Hounsfield units consistent with emphysema.
Physiologic Testing
Spirometry and Dlco measurements were conducted by using the EasyOne Pro (ndd Medical Technologies) in accordance with American Thoracic Society (ATS)/European Respiratory Society guidelines, with standardization of protocols and quality control procedures across clinical sites.17 Only subjects with tests judged acceptable and reproducible were included. FEV1 and Dlco percent predicted values were calculated by using Global Lung Initiative reference equations,18, 19 with Dlco values adjusted for hemoglobin and altitude.18 Six-minute walk distance (6MWD) tests were conducted according to ATS guidelines.20
CT Scans
CT scans were acquired by using individual site CT scanners, with previously published protocols for each scanner type.21 Total percent emphysema, defined as the percentage of voxels with attenuation at or below –950 Hounsfield units (%LAA-950), was calculated by using parametric response mapping software.
Patient-reported Outcomes
COPD Assessment Test (CAT), St. George’s Respiratory Questionnaire (SGRQ), and Medical Outcomes Study Short Form 36 (SF-36) were administered and scored to assess quality of life and impact of symptoms.
Exacerbations
Exacerbation rate was determined from self-reported episodes of increased COPD symptoms requiring antibiotics or steroids in the 12 months preceding this study. Severe exacerbations were defined as the subset of exacerbations requiring an ED visit or hospitalization, whereas moderate exacerbations were those that did not.
Statistical Analysis
All analyses were performed by using SAS version 9.4 (SAS Institute, Inc.), and P values < .05 were considered significant. Participant baseline characteristics were described by using medians with interquartile ranges. Multivariable linear regressions were constructed wherein Dlco and FEV1 were modeled as continuous variables with results presented for every 10% predicted value decrease. Models included age, sex, ethnicity, BMI categories (≤ 21, 21-30, and > 30 kg/m2), education, smoking exposure in pack-years, smoking status (current, former, or current-to-former smoker), anemia, diabetes, congestive heart failure, sleep apnea, %LAA-950, percent predicted Dlco, and percent predicted FEV1. Generalized estimating equations were used to account for correlation between participants at each center. Identical analyses were conducted evaluating percent predicted KCO, a measure of the efficiency of gas transfer accounting for alveolar volume.
Participants were also categorized into four groups based on the presence or absence of severe impairment in FEV1 and Dlco, defined by a value ≤ 50% predicted. The groups are described as follows: (1) reference (FEV1 and Dlco both > 50% predicted); (2) Dlco impaired (FEV1 > 50% and Dlco ≤ 50%); (3) FEV1 impaired (FEV1 ≤ 50% and Dlco > 50%); and (4) both impaired (FEV1 and Dlco ≤ 50%). Multivariable regression models were adjusted for confounders as noted earlier, comparing the latter three groups vs the reference group.
Exacerbation rate was modeled by using multivariable negative binomial regressions with the confounders described earlier. Given the low number of events in the study population, emphysema was categorized into those with missing high-resolution CT (HRCT) data, ≤ 5% LAA-950, and > 5% LAA-950. Categorization in this manner allowed for inclusion of participants with missing HRCT data (e-Table 1).
Results
Participant Characteristics
A total of 1,806 participants with COPD Global Obstructive Lung Disease spirometry stages I to IV in whom Dlco measurements were available from the year 5 visit were identified; of these, 1,564 had available HRCT percent emphysema data and were used in most analyses (Fig 1). Baseline characteristics and respiratory outcomes for the 1,564 participants analyzed are presented in Table 1. The participants were 54% male, 82% white, had a median 45 pack-year smoking history, a postbronchodilator FEV1 of 70% predicted, and a Dlco of 66% predicted.
Table 1.
Characteristics of the Study Population
| Characteristic | Analysis (N = 1,564) |
|---|---|
| Age, y | 68 (62-74) |
| Male | 841 (54%) |
| Black | 281 (18%) |
| Education | |
| High school, no diploma | 117 (7%) |
| High school graduate or GED | 379 (24%) |
| Some college or technical school, no degree | 467 (30%) |
| College or technical school graduate (bachelor's or associate degree) | 437 (28%) |
| Master's or doctoral degree | 164 (10%) |
| BMI, kg/m2 | 28 (24-31) |
| Obese (BMI ≥ 30 kg/m2) | 519 (33%) |
| Anemia | 197 (13%) |
| Diabetes | 249 (16%) |
| Congestive heart failure | 60 (4%) |
| Sleep apnea | 280 (18%) |
| Pack-years of smoking | 45 (34-63) |
| Smoking status | |
| Former smoker | 484 (31%) |
| Current-to-former smokera | 191 (12%) |
| Current smoker | 889 (57%) |
| FEV1 % predicted | 70 (51-89) |
| ≤ 50% | 378 (24%) |
| Dlco % predicted | 66 (50-82) |
| ≤ 50% | 388 (25%) |
| KCO % predicted | 73 (58-87) |
| ≤ 50% | 246 (16%) |
| CAT | 13 (7-20) |
| SGRQ | 28 (13-45) |
| SF-36 Physical Function | 42 (33-50) |
| SF-36 Mental | 55 (46-60) |
| 6MWD, feet | 1,280 (1,000-1,512) |
| %LAA-950 | 4 (1-14) |
| LAA-950 > 5% | 741 (47%) |
| Resting Spo2, % | 96 (94-97) |
Data are expressed as median (interquartile range) or No. (%) as appropriate. 6MWD = 6-min walk distance; CAT = COPD Assessment Test; Dlco = diffusing capacity of the lung for carbon monoxide; GED = General Education Diploma; KCO = gas transfer efficiency coefficient; %LAA-950 = percent low attenuation areas at or below –950 Hounsfield units consistent with emphysema; SF-36 = Medical Outcomes Study Short Form 36; SGRQ = St. George’s Respiratory Questionnaire; Spo2= oxygen saturation.
Participants who went from being current smokers at the phase I visit to former smokers by the phase II visit.
Participants were moderately symptomatic, with a median CAT score of 13, SGRQ of 28, SF-36 Physical Function of 42, and 6MWD of 1,280 feet. There was a high prevalence of emphysema among the participants with available HRCT data, with 741 (47%) exhibiting > 5% LAA-950 according to CT scan. A moderate negative correlation was observed between Dlco and %LAA-950, with a Spearman correlation coefficient of –0.6 (P < .001) (e-Fig 1).
The baseline characteristics and outcomes for the total 1,806 participants and participants with missing CT data are provided in e-Table 1. The group with missing CT data reported lower percent predicted FEV1 and Dlco, increased morbidity according to symptom and quality of life scores (CAT, SGRQ, and SF-36 Physical Function), and decreased 6MWD than those with CT data.
Association Between Dlco and Respiratory Outcomes, Independent of FEV1 and Percent Emphysema
In multivariable models, lower percent predicted Dlco was associated with increased COPD morbidity, independent of FEV1 and %LAA-950 (Table 2). These findings were noted in multiple domains, including symptoms and quality of life (CAT, SGRQ, and SF-36 Physical Function), exercise performance (6MWD), and severe COPD exacerbations (Table 3). For example, a decrease of 10% predicted Dlco was associated with a decrease in 6MWD of 45.35 feet, demonstrating diminished exercise performance. The magnitude of association was similar to that of FEV1. The independent effect of Dlco tended to be lower than the effect of FEV1 for all other outcomes but was still in clinically relevant ranges. For example, a decrease in Dlco of 10% predicted was associated with approximately a 14% higher rate of hospitalization for COPD exacerbation compared with the 27% higher hospitalization rate for a decrease in FEV1 of 10% predicted. The results were not appreciably different when %LAA-950 was not included as a covariate (e-Table 2). Similar results were observed when percent predicted KCO was examined (e-Tables 3, 4).
Table 2.
Reductions in Dlco and FEV1 Are Associated With Increased COPD Morbidity
| Outcome | Dlco % Predicted |
FEV1 % Predicted |
||
|---|---|---|---|---|
| Regression Coefficient (95% CI) | P Value | Regression Coefficient (95% CI) | P Value | |
| CAT score | 0.53 (0.33 to 0.73) | < .001 | 1.16 (0.96 to 1.36) | < .001 |
| SGRQ score | 1.67 (1.23 to 2.1) | < .001 | 2.94 (2.58 to 3.31) | < .001 |
| Activity | 2.58 (1.74 to 3.41) | < .001 | 3.99 (3.48 to 4.51) | < .001 |
| Impact | 1.3 (0.86 to 1.74) | < .001 | 2.25 (1.86 to 2.64) | < .001 |
| Symptom | 1.23 (0.70 to 1.76) | < .001 | 3.33 (2.93 to 3.72) | < .001 |
| SF-36 Physical Function | –0.89 (–1.18 to –0.6) | < .001 | –1.29 (–1.58 to –1.00) | < .001 |
| SF-36 Mental | 0.03 (–0.37 to 0.42) | .900 | –0.01 (–0.31 to 0.31) | .954 |
| 6MWD, feet | –45.35 (–58.21 to –32.48) | < .001 | –43.26 (–48.36 to –38.15) | < .001 |
| Resting Spo2, % | –0.22 (–0.38 to –0.05) | .012 | –0.19 (–0.24 to –0.14) | < .001 |
Associations are per 10% decrease in percent predicted Dlco or percent predicted FEV1. Models (N = 1,564) include age, sex, BMI category, ethnicity, education, smoking pack-years, smoking status, anemia status, diabetes status, congestive heart failure status, sleep apnea status, %LAA-950 (emphysema), percent predicted FEV1, and percent predicted Dlco. See Table 1 legend for expansion of abbreviations.
Table 3.
Reductions in Dlco and FEV1 Are Associated With Increased COPD Exacerbations
| Outcome | Dlco % Predicted |
FEV1 % Predicted |
||
|---|---|---|---|---|
| Rate Ratio (95% CI) | P Value | Rate Ratio (95% CI) | P Value | |
| Any exacerbations | 1.05 (0.97 to 1.12) | .231 | 1.22 (1.19 to 1.27) | < .001 |
| Moderate exacerbations | 1.01 (0.93 to 1.10) | .759 | 1.20 (1.16 to 1.27) | < .001 |
| Severe exacerbations | 1.14 (1.05 to 1.20) | < .001 | 1.27 (1.20 to 1.33) | < .001 |
Associations are per 10% decrease in percent predicted Dlco or percent predicted FEV1. Exacerbation models (N = 1,806) include age, sex, ethnicity, BMI categories, education, pack-years, smoking status, diabetes status, anemia status, congestive heart failure status, sleep apnea status, emphysema as a categorical variable (missing data, ≤ 5% LAA-950, and > 5% LAA-950), percent predicted Dlco, and percent predicted FEV1. Any exacerbation refers to all episodes requiring antibiotics or steroids, moderate refers to only those episodes not resulting in an ED visit or hospitalization, and severe refers to those requiring an ED visit or hospitalization. See Table 1 legend for expansion of abbreviations.
Severe Impairment of FEV1 and Dlco Worsens Outcomes, Independent of FEV1 and Percent Emphysema
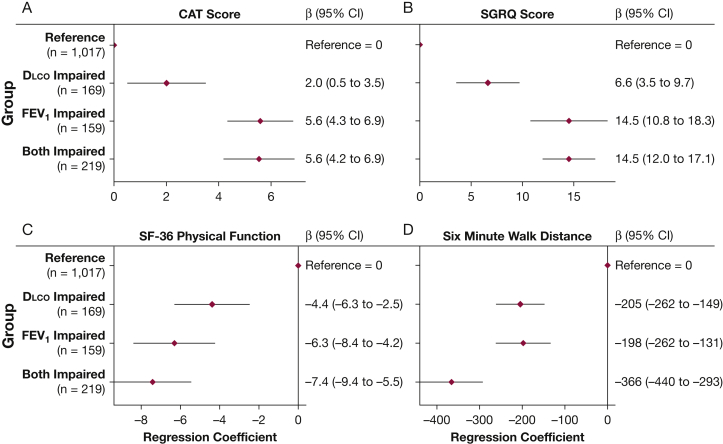
To further characterize the independent and combined effects of Dlco and FEV1 abnormalities, we created four categories of participants based on the presence or absence of severe impairment in FEV1 and Dlco, defined by a value ≤ 50% predicted. In adjusted analyses that included %LAA-950 and other potential confounders, severe impairment in either FEV1 alone, Dlco alone, or both FEV1 and Dlco was associated with worse CAT, SGRQ, SF-36 Physical Function, and 6MWD compared with the reference (P < .05 for all comparisons) (Fig 2). In addition, outcomes reflective of physical functioning, most evidently 6MWD, show a pattern in which those with severe impairment in both FEV1 and Dlco displayed significantly worse outcomes than impairment in FEV1 or Dlco alone (P < .05 for 6MWD).
Figure 2.
Severe impairment in Dlco in isolation and in combination with severe impairment in FEV1 is associated with increased COPD morbidity. Association between categories of percent predicted FEV1 and Dlco with (A) COPD Assessment Test score, (B) St. George’s Respiratory Questionnaire score, (C) Medical Outcomes Study Short Form 36 Physical Function, and (D) 6-min walk distance in feet. Groups are defined as reference (FEV1 and Dlco both > 50% predicted), Dlco impaired (FEV1 > 50% and Dlco ≤ 50%), FEV1 impaired (FEV1 ≤ 50% and Dlco > 50%), and both impaired (FEV1 and Dlco ≤ 50%). Models (N = 1,564) are adjusted for age, sex, BMI categories, ethnicity, education, smoking pack-years, smoking status, anemia status, diabetes status, congestive heart failure status, sleep apnea status, and %LAA-950 (emphysema). See Figure 1 legend for expansion of abbreviations.
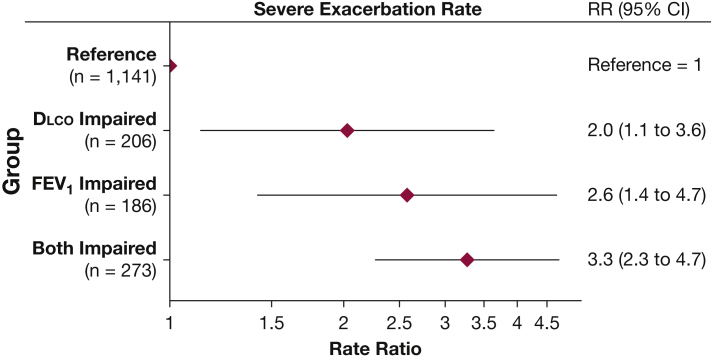
All groups with severe impairment in FEV1, Dlco, or both reported increased exacerbation rates compared with the reference group (e-Fig 2). Similar to the findings for 6MWD, combined severe reduction in FEV1 and Dlco tended to exhibit a higher rate of severe exacerbations compared with isolated impairment in either individual measure (Fig 3).
Figure 3.
Severe impairment in Dlco in isolation and in combination with severe impairment in FEV1 is associated with higher severe COPD exacerbation rates. Association between categories of FEV1 and Dlco and self-reported rate of severe exacerbations. Groups are defined as reference (FEV1 and Dlco both > 50% predicted), Dlco impaired (FEV1 > 50% and Dlco ≤ 50%), FEV1 impaired (FEV1 ≤ 50% and Dlco > 50%), and both impaired (FEV1 and Dlco ≤ 50%). Model is adjusted for age, sex, ethnicity, BMI categories, smoking pack-years, smoking status, anemia status, diabetes status, congestive heart failure status, sleep apnea status, and emphysema as a categorical variable (missing, ≤ 5% LAA-950, > 5% LAA-950), with N = 1,806. Severe refers only to those exacerbations requiring an ED visit or hospitalization. RR = rate ratio. See Figure 1 legend for expansion of other abbreviations.
Discussion
In this large, well-characterized cohort of individuals with COPD, lower Dlco was associated with increased measures of COPD morbidity across multiple domains, including increased respiratory symptoms, worse health-related quality of life, reduced exertional capacity, and increased rate of exacerbations for COPD. These findings were independent of spirometry and of emphysema analysis conducted using quantitative CT imaging. Severe reduction in Dlco was associated with worsening of all outcomes, and the combination of a severely reduced FEV1 and Dlco showed greater impairment than either FEV1 or Dlco alone for physical function outcomes and severe exacerbations. In the context of efforts to further classify patients with COPD by using multidimensional tools, these findings suggest that Dlco offers additional clinically valuable information that extends beyond what is captured by using spirometry and CT assessments of emphysema.
Current evaluation of phenotypes in COPD has moved away from the use of spirometry alone, with increasing data suggesting that spirometry is insufficient in predicting symptoms, quality of life, or exacerbation frequency.3, 4 One method of phenotyping uses CT identification of emphysema and, hitherto, emphysema has been well documented as being associated with impairment in Dlco. Pathologic, radiographic, and physiologic studies have shown a correlation between increasing emphysema and a reduction in Dlco, such that Dlco measurement may have utility in identifying patients with emphysema.7, 8, 22, 23, 24 Although we found moderate correlation of Dlco and emphysema, variability in Dlco was not fully explained by CT-confirmed emphysema; in models adjusting for emphysema, impairment in Dlco exhibited independent associations with worse outcomes in COPD, suggesting that Dlco is independently informative and may help define a subgroup of patients at increased risk for adverse health outcomes.
The results highlight the potential for Dlco to provide insight into patients with COPD at risk for limitations in physical function. Previous studies that have evaluated Dlco in the context of cardiopulmonary exercise testing have found that Dlco was associated with dyspnea and exercise intolerance in smokers with mild obstruction, as well as those with moderate to severe lung function impairment.25, 26, 27, 28 The current study extends these findings in a large COPD cohort to include an association between Dlco and functional exercise performance (indicated by 6MWD) and self-reported physical functioning (SGRQ activity, SF-36 Physical Function). These indicators suggest an association between Dlco and functional limitations that contribute to the overall burden of morbidity in the daily life of patients with COPD.
Identification of patients at risk for frequent or severe exacerbations is a priority given the links between COPD exacerbations and mortality,29 as well as the high degree of health-care utilization and associated costs.30 To date, the most reliable predictor of future COPD exacerbations is a history of exacerbations in the previous year.31, 32, 33 It is notable that in a systematic review including 27 models to predict COPD exacerbations, Dlco was not considered as a predictor in any of the models.31 Our results show that severe impairments in Dlco are independently associated with increased rates of COPD exacerbations, with a particularly strong association between Dlco and severe exacerbations requiring an ED visit or hospitalization. These findings add to those of previous small studies that have shown diminished gas transfer and gas transfer efficiency among frequent exacerbators.14, 15 Of note, the associations reported here are of Dlco and COPD exacerbations in the previous year and are therefore cross-sectional in nature. The COPDGene data are not currently available to investigate the ability of Dlco to predict future exacerbations, but the findings reported here suggest that this is an important next step.
The independent association between Dlco and COPD morbidity may be indicative of underlying pathophysiologic injury that cannot be wholly accounted for by airflow obstruction or emphysema. In general, the physiologic mechanisms by which COPD can impair Dlco are through decreased diffusivity across the alveolar capillary membrane, or through decreased pulmonary vascular volume associated with gas-exchanging units. In previous studies partitioning Dlco into membrane diffusivity and pulmonary vascular volume in COPD, a demonstrable decrease in both measures was reported.23, 34, 35 This outcome has been attributed to airspace destruction and associated capillary membrane destruction secondary to emphysema, with resultant decreased gas exchange surface area. Although this mechanism likely contributes to the association detected, we postulate that pulmonary vascular injury, including vascular thickening, distal vessel pruning, and capillary rarefaction reflected in the Dlco measurement, is playing a role. Pulmonary vascular volume impairment has been observed independent of and even prior to development of emphysema in COPD23, 36, 37, 38, 39, 40 and is associated with worse outcomes.41, 42, 43, 44 We postulate that one of the drivers of the independent association between Dlco and COPD morbidity is subclinical pulmonary vascular injury, an area that warrants further study in patients with COPD.
This study is limited in part by the cross-sectional nature and low frequency of the exacerbation events, curtailing discussion regarding prediction of future exacerbations. These results are a reflection, however, of a frequent exacerbator phenotype, and the associations with Dlco noted here help characterize this subgroup. In creating categorical subgroups, dichotomization of FEV1 and Dlco above and below 50% predicted was conducted to represent severe impairment in neither, either, or both values. This analysis shows the independent and combined influences of each measure on outcomes and was not designed to formally evaluate threshold effects. To describe independent associations of Dlco, we used quantitative CT assessment of emphysema; however, there is the possibility that Dlco is a marker for microscopic emphysema below the level of detection by current CT measures. This theory deserves further exploration to fully understand the pathophysiology of Dlco in COPD but does not detract from the notion that Dlco offers information beyond readily available and currently utilized clinical tools. Further characterization of individuals for the presence or absence of pulmonary hypertension was unavailable, but assessment of alternative causes for pulmonary vascular disease, including sleep apnea and congestive heart failure, were included in the analyses. In addition, body plethysmography, which would account for hyperinflation and its impact on COPD morbidity, was unavailable in this cohort. Finally, the results of this study represent those based on testing in a clinical research setting, and generalizability may be limited due to variability in adherence to quality control procedures of Dlco measurements in clinical testing.45 However, the results presented offer evidence to support future investigations surrounding the clinical utility of Dlco under real-world conditions.
Conclusions
The current study showed that Dlco measurement, a readily available, frequently obtained test, provides clinically relevant information beyond spirometry and CT evidence of emphysema. Although many multidimensional prognostic models assessing mortality and morbidity do not include Dlco, findings from this study suggest that inclusion of Dlco in such models should be considered. Specifically, Dlco measurement may provide information regarding functional status and identify subgroups of patients who display diminished exercise performance or frequent exacerbations that are incompletely captured by existing indices. Furthermore, it may provide a window into the interactions between vascular and pulmonary physiology, an area that should be further investigated. Future studies investigating the utility of Dlco in prognostic indices of morbidity and mortality, the relationship between Dlco and vascular disease in COPD, and the implications of longitudinal changes in Dlco are warranted. Dlco offers clinically important information beyond that obtained from spirometry and radiography and should be considered in characterizing and managing patients with COPD.
Acknowledgments
Author contributions: M. C. M. contributed to the conception, design of the study, data analysis, interpretation, and preparation of the manuscript, and is the guarantor of the paper. A. B. contributed to data analysis, interpretation, and manuscript writing. R. J. H. contributed statistical support and performed all statistical analyses on the data. N. R. M., R. L. J., G. K., W. W. S, C. P. H., R. P. B., R. C., M. K. H., J. P., R. G. B., B. J. M., and R. A. W. contributed to data interpretation and revision of the manuscript. All authors reviewed and approved the manuscript prior to submission for publication.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: N. R. M. reports site Principal Investigator for the National Institutes of Health-funded COPDGene study and consultant to Breathe Technologies, Ventec, and InspiRx Pharma, and that none of these have conflicting interests with the manuscript. C. P. H. reports research grants from Novartis and Boehringer Ingelheim; and consulting fees from AstraZeneca, Novartis, and 23andMe. R. P. B. served on the advisory boards (GlaxoSmithKline, Boehringer Ingelheim, and Mylan) and received research grants (GlaxoSmithKline and Boehringer Ingelheim) not related to the manuscript, and these activities have not influenced his work on the manuscript. R. C. reports institutional grant funding from GlaxoSmithKline, Boehringer Ingelheim, Astellas, and AstraZeneca; consulting for Astellas and Regeneron; advisory boards for Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, and MedImmune; speakers bureau for GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim; and stock ownership in Inogen. M. K. H. reports consulting for GlaxoSmithKline, Boehringer Ingelheim, and AstraZeneca; and research support from Novartis and Sunovion. R. G. B. reports grant funding from the COPD Foundation relating to and outside of this study. B. J. M. reports funding from the National Heart, Lung, and Blood Institute for the COPDGene study; grants and medical advisory boards from Boehringer Ingelheim, GlaxoSmithKline, AstraZeneca, and Sunovion; Data and Safety Monitoring Board fees from Spiration and Shire/Baxalta; CME personal fees from WebMD, National Jewish Health, American College of Chest Physicians, Projects in Knowledge, Hybrid Communications, SPIRE Learning, Ultimate Medical Academy, Catamount Medical, Eastern Pulmonary Society, Catamount Medical Communications, Medscape, Eastern VA Medical Center, Academy Continued Healthcare Learning, and Mt. Sinai Medical Center; royalties from UpToDate; medical advisory boards from Novartis, Phillips, Third Pole, Science 24/7, and Vernoa; and grants from Pearl, outside the submitted work. M. C. M. reports royalties from UpToDate for authorship and has no conflicts of interest related to the manuscript. None declared (A. B., R. J. H., R. L. J., G. K., W. W. S., J. P., R. A. W.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Tables and e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Heart, Lung, and Blood Institute [T32 HL007534-36, U01 HL089897, and U01 HL089856]. The Genetic Epidemiology of COPD (COPDGene) study (NCT00608764) is also supported by the COPD Foundation through contributions made to an Industry Advisory Committee comprising AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Sunovion.
Supplementary Data
References
- 1.Wheaton A.G., Cunningham T.J., Ford E.S., Croft J.B. Employment and activity limitations among adults with chronic obstructive pulmonary disease—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(11):289–295. [PMC free article] [PubMed] [Google Scholar]
- 2.Kochanek K.D., Murphy S., Xu J., Arias E. Mortality in the United States, 2016. NCHS Data Brief. 2017;293:1–8. [PubMed] [Google Scholar]
- 3.Han M.K., Muellerova H., Curran-Everett D. Implications of the GOLD 2011 disease severity classification in the COPDGene cohort. Lancet Respir Med. 2013;1(1):43–50. doi: 10.1016/S2213-2600(12)70044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agusti A., Calverley P.M.A., Celli B. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11 doi: 10.1186/1465-9921-11-122. 122-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelmeier C.F., Criner G.J., Martinez F.J. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 6.Jones P.W. Health status and the spiral of decline. COPD. 2009;6(1):59–63. doi: 10.1080/15412550802587943. [DOI] [PubMed] [Google Scholar]
- 7.Gould G., Redpath A., Ryan M. Lung CT density correlates with measurements of airflow limitation and the diffusing capacity. Eur Respir J. 1991;4(2):141–146. [PubMed] [Google Scholar]
- 8.Nambu A., Zach J., Schroeder J. Relationships between diffusing capacity for carbon monoxide (DLCO), and quantitative computed tomography measurements and visual assessment for chronic obstructive pulmonary disease. Eur J Radiol. 2015;84(5):980–985. doi: 10.1016/j.ejrad.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Boutou A.K., Shrikrishna D., Tanner R.J. Lung function indices for predicting mortality in COPD. Eur Respir J. 2013;42(3):616–625. doi: 10.1183/09031936.00146012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neas L.M., Schwartz J. Pulmonary function levels as predictors of mortality in a national sample of US adults. Am J Epidemiol. 1998;147(11):1011–1018. doi: 10.1093/oxfordjournals.aje.a009394. [DOI] [PubMed] [Google Scholar]
- 11.Boushy S.F., Thompson H.K., Jr., North L.B., Beale A.R., Snow T.R. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1973;108(6):1373–1383. doi: 10.1164/arrd.1973.108.6.1373. [DOI] [PubMed] [Google Scholar]
- 12.Kanner R.E., Renzetti A.D., Jr., Stanish W.M., Barkman H.W., Jr., Klauber M.R. Predictors of survival in subjects with chronic airflow limitation. Am J Med. 1983;74(2):249–255. doi: 10.1016/0002-9343(83)90623-x. [DOI] [PubMed] [Google Scholar]
- 13.Traver G.A., Cline M.G., Burrows B. Predictors of mortality in chronic obstructive pulmonary disease. A 15-year follow-up study. Am Rev Respir Dis. 1979;119(6):895–902. doi: 10.1164/arrd.1979.119.6.895. [DOI] [PubMed] [Google Scholar]
- 14.Lee H.Y., Kim J.W., Lee S.H. Lower diffusing capacity with chronic bronchitis predicts higher risk of acute exacerbation in chronic obstructive lung disease. J Thorac Dis. 2016;8(6):1274–1282. doi: 10.21037/jtd.2016.04.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei X., Ma Z., Yu N. Risk factors predict frequent hospitalization in patients with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:121–129. doi: 10.2147/COPD.S152826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regan E.A., Hokanson J.E., Murphy J.R. Genetic Epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 18.Stanojevic S., Graham B., Cooper B., Thompson B., Carter K., Hall G. Global lung function initiative: reference equations for the transfer factor for carbon monoxide (TLCO) Eur Respir J. 2017;50(3) doi: 10.1183/13993003.00010-2017. [DOI] [PubMed] [Google Scholar]
- 19.Quanjer P.H., Stanojevic S., Cole T.J. Multi-ethnic reference values for spirometry for the 3-95 year age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Thoracic Society ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 21.Han M.K., Kazerooni E.A., Lynch D.A. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261(1):274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gevenois P.A., Vuyst P.D., Maertelaer V.D. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154(1):187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 23.Morrison N.J., Abboud R.T., Müller N.L. Pulmonary capillary blood volume in emphysema. Am Rev Respir Dis. 1990;141(1):53–61. doi: 10.1164/ajrccm/141.1.53. [DOI] [PubMed] [Google Scholar]
- 24.Morrison N.J., Abboud R.T., Ramadan F. Comparison of single breath carbon monoxide diffusing capacity and pressure-volume curves in detecting emphysema. Am Rev Respir Dis. 1989;139(5):1179–1187. doi: 10.1164/ajrccm/139.5.1179. [DOI] [PubMed] [Google Scholar]
- 25.Behnia M., Wheatley C., Avolio A., Johnson B. Influence of resting lung diffusion on exercise capacity in patients with COPD. BMC Pulmon Med. 2017;17(1):117. doi: 10.1186/s12890-017-0454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elbehairy A.F., Faisal A., Guenette J.A. Resting physiological correlates of reduced exercise capacity in smokers with mild airway obstruction. COPD. 2017;14(3):267–275. doi: 10.1080/15412555.2017.1281901. [DOI] [PubMed] [Google Scholar]
- 27.Kirby M., Owrangi A., Svenningsen S. On the role of abnormal DLCO in ex-smokers without airflow limitation: symptoms, exercise capacity and hyperpolarised helium-3 MRI. Thorax. 2013;68(8):752–759. doi: 10.1136/thoraxjnl-2012-203108. [DOI] [PubMed] [Google Scholar]
- 28.Farkhooy A., Janson C., Arnardóttir R.H., Malinovschi A., Emtner M., Hedenström H. Impaired carbon monoxide diffusing capacity is the strongest predictor of exercise intolerance in COPD. COPD. 2013;10(2):180–185. doi: 10.3109/15412555.2012.734873. [DOI] [PubMed] [Google Scholar]
- 29.Suissa S., Dell'Aniello S., Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ke X., Marvel J., Yu T.C. Impact of lung function on exacerbations, health care utilization, and costs among patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1689–1703. doi: 10.2147/COPD.S108967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerra B., Gaveikaite V., Bianchi C., Puhan M.A. Prediction models for exacerbations in patients with COPD. Eur Respir Rev. 2017;26(143) doi: 10.1183/16000617.0061-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurst J.R., Vestbo J., Anzueto A. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 33.Le Rouzic O., Roche N., Cortot A.B. Defining the “frequent exacerbator” phenotype in COPD: a hypothesis-free approach. Chest. 2018;153(5):1106–1115. doi: 10.1016/j.chest.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Liebow A.A. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis. 1959;80(1):67–93. doi: 10.1164/arrd.1959.80.1P2.67. [DOI] [PubMed] [Google Scholar]
- 35.Schulz U., Langwieler S., Riedel S., Schreiber J. Pulmonary capillary blood volume and membrane components of pulmonary diffusion capacity in patients with chronic obstructive bronchitis (COPD) Pneumologie (Stuttgart, Germany) 2014;68(4):266–269. doi: 10.1055/s-0034-1365056. [DOI] [PubMed] [Google Scholar]
- 36.Magee F., Wright J.L., Wiggs B.R., Paré P.D., Hogg J.C. Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax. 1988;43(3):183–189. doi: 10.1136/thx.43.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordasco E.M., Beerel F.R., Vance J.W., Wende R.W., Toffolo R.R. Newer aspects of the pulmonary vasculature in chronic lung disease: a comparative study. Angiology. 1968;19(7):399–407. doi: 10.1177/000331976801900703. [DOI] [PubMed] [Google Scholar]
- 38.Scarrow G.D. The pulmonary angiogram in chronic bronchitis and emphysema. Proc R Soc Med. 1965;58(9):684–687. doi: 10.1177/003591576505800907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muñoz-Esquerre M., López-Sánchez M., Escobar I. Systemic and pulmonary vascular remodelling in chronic obstructive pulmonary disease. PLOS One. 2016;11(4) doi: 10.1371/journal.pone.0152987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos S., Peinado V.I., Ramírez J. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19(4):632–638. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- 41.Estépar R.S.J., Kinney G.L., Black-Shinn J.L. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188(2):231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hueper K., Vogel-Claussen J., Parikh M.A. Pulmonary microvascular blood flow in mild chronic obstructive pulmonary disease and emphysema. The MESA COPD study. Am J Respir Crit Care Med. 2015;192(5):570–580. doi: 10.1164/rccm.201411-2120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells J.M., Iyer A.S., Rahaghi F.N. Pulmonary artery enlargement is associated with RV dysfunction and loss of blood volume in small pulmonary vessels in chronic obstructive pulmonary disease. Circ Cardiovasc Imaging. 2015;8(4) doi: 10.1161/CIRCIMAGING.114.002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimura K., Suzuki Y., Uto T., Sato J., Imokawa S., Suda T. Morphological changes in small pulmonary vessels are associated with severe acute exacerbation in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:1435–1445. doi: 10.2147/COPD.S107424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wise R.A., Teeter J.G., Jensen R.L. Standardization of the single-breath diffusing capacity in a multicenter clinical trial. Chest. 2007;132(4):1191–1197. doi: 10.1378/chest.07-0455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.