Abstract
This study aimed to evaluate the effect of lignin-derived polyphenolic composition BP-C3 on the efficacy and hematological toxicity of cyclophosphamide (CPA). Male and female Swiss-H derived mice bearing benzo[a]pyrene-induced soft tissue sarcomas were treated with CPA 300 mg/kg, BP-C3 75 mg/kg, or a combination. Tumor growth inhibition in male mice treated with CPA, BP-C3, or a combination of CPA and BP-C3 was significant and corresponded to 78%, 45%, and 82%, respectively, on day 21 after CPA administration on day 0. In female mice, tumor growth inhibition was 58%, −11%, and 35% when treated with CPA, BP-C3, or a combination of CPA and BP-C3, respectively. CPA administration resulted in significant hematological toxicity evidenced by a decreased white blood cell count on day 4 (2.43 ± 1.77 × 109/L in male mice and 1.19 ± 0.71 × 109/L in female mice) and anemia development on day 7 (6.55 ± 1.74 × 1012/L in male mice and 5.89 ± 2.24 × 1012/L in female mice). The red blood cell count measured on day 7 in animals treated with the combination of BP-C3 and CPA constituted 7.12 ± 1.17 × 1012/L and 7.36 ± 2.07 × 1012/L for male and female mice, respectively. The results of our study demonstrate the antitumor activity of BP-C3 in male mice bearing soft tissue sarcomas. Neither the antitumor activity nor the hematological toxicity of CPA were significantly influenced by BP-C3. A less pronounced effect of CPA on RBC count is demonstrated when this agent is given jointly with BP-C3.
Keywords: polyphenolic composition BP-C3, soft tissue sarcoma, chemotherapy, toxicity, blood count, cyclophosphamide
Introduction
Plant polyphenols, due to their various biological effects, are considered as preparations for concomitant and direct therapy of cancer patients.1 Non-nitrogenous polyphenolic substances include hydrolytic lignin derivatives, which have diverse biological action including immune-stimulatory and anticancer effects. The mechanism of action is proposed to be linked to the free radical scavenging and to cytotoxic activity against some cancer cell lines but still the exact molecular mechanism of action is not well understood.2 In our previous studies, the ability of hydrolytic lignin derivatives to reduce the toxic effects of chemotherapy and radiation was shown and was linked to their protective effect on the hematopoiesis and intestinal mucosa.3,4 Plant polyphenols are under investigation as a means of reducing the toxicity of chemotherapy, in particular of cyclophosphamide (CPA).5,6 CPA is the most widely used alkylating agent in the treatment of hematological malignancies7,8 and solid tumors, especially for breast cancer in anthracycline- or taxane-based combined regimens.9,10 The main toxicity of this agent is related to the hematopoietic system and relatively little to non-hematopoietic organs.11 We aimed to assess the influence of polyphenolic composition BP-C3 on the effectiveness and toxicity of the CPA. As a model for the study, we selected soft tissue sarcomas induced by subcutaneous administration of benzo[a]pyrene (BP).12 This was to reduce the effect of a single tumor on the results, which is known to occur in the case of transplantable tumor models. These tumors were shown to be sensitive to CPA,13 which allows an evaluation of this model.
Materials and Methods
Materials
A 0.5% aqueous solution of BP-C3 was kindly provided for the studies by Nobel Ltd (Saint Petersburg, Russia). The polyphenolic composition is a modified geroprotective composition, BP-C3, that comprises lignin-derived polyphenolic complex.14 The composition contains several components in the following ratio per dry weight: BP-Cx-1 99.51%, iron(II) 0.20%, selenium(IV) 0.002%, ascorbic acid 0.20%, and retinol 0.081%. BP-Cx-1 (RD Innovation ApS, Copenhagen, Denmark) is a water-soluble derivate of lignin from conifer trees.15 Polyphenolic components, nominally flavonoids, sapogenins, and phenanthrenes, were identified as the major carriers of the biological activity of BP-Cx-1 through characterization of its molecular composition with Fourier transform ion cyclotron resonance mass spectrometry and subsequent identification of possible active components by searching for molecular matches in silico in ChEMBL.16 The modification of BP-C3 was necessary to provide a higher dose of BP-Cx-1 in the current study while keeping level of microelements and vitamins supply the same as in the product previously studied.17
Benzo[a]pyrene (Fluka) was used for the induction of soft tissue sarcomas.
The cyclophosphamide is Endoxan 200 mg from batch 6E116A (Baxter Oncology GmbH, Halle [Westfalen], Germany). Purified water was used as a solvent and as the placebo.
Ethics Statement
Experimental animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition. Protocols were approved by the Local Ethics Committee of the N.N. Petrov Research Institute of Oncology (Protocol No. 11; dated July 21, 2016). Experimental animals found in moribund condition and also the remaining animals were euthanized at the end of the studies with the CO2 inhalation method.
Animals
Swiss-H-derived Rappolovo albino (SHR) mice of both sexes weighing 18 to 22 g (Rappolovo Animal Facility, Leningrad region, Russia) were used in the studies. The animals were acclimated for at least 10 days prior to the commencement of the experiments. The animals were maintained on a 12:12 light-dark cycle at 21 ± 2°C with 50 ± 20% average humidity and with ad libitum access to tap water and PK120 laboratory diet (Laboratorkorm Ltd, Moscow, Russia). Animals were observed once daily.
Study 1: Assessment of CPA Efficacy and Hematological Toxicity
SHR mice were subcutaneously injected with 2 mg of BP dissolved in 0.1 mL of peach oil (Pharmaceutical Factory of Saint Petersburg OJSC, Saint Petersburg, Russia). After 15 weeks, animals that developed tumors of approximately 5 mm in diameter were selected and randomized into 1 of 4 single-sex study groups (designated as day −1) and treated with CPA or sham injection on day 0 plus BP-C3 or placebo for 21 days, starting on day 1. Control animals (N = 10 males, N = 11 females) were given a sham intraperitoneal injection of 0.9% sodium chlorine and purified water by gavage as a placebo. Animals in group 2 (N = 9 males, N = 10 females) were dosed with 200 mg/kg CPA once intraperitoneally and gavage by placebo. Group 3 (N = 10 males, N = 11 females) was treated with the sham injection and BP-C3 75 mg/kg by gavage daily. Group 4 (N = 10 males, N = 11 females) was given CPA and BP-C3. The study was terminated on day 21.
The length and width of tumor nodes in mm were measured twice a week. Volume of the tumor (cm3) was calculated with the following formula:
where a is the longest linear size in mm and b is the longest transverse to “a” linear size of a node in mm.
The efficacy of the therapy was assessed by tumor growth inhibition (TGI) calculated with the following formula:
where Vc is the average volume of tumors in the Control group, and Ve is the average volume of tumors in an Experimental group.
Peripheral blood samples were collected on days 0, 4, 7, 14, and 21 into K3-EDTA tubes (MiniCollect) through a tail cut. Blood counts were performed using a Mindray BC-2800Vet Haematology Analyser (Shenzhen, PRC) within 1 to 2 hours after blood sampling.
Tumor tissue samples were obtained immediately after euthanasia on day 21, fixed with 10% neutral buffered formaldehyde (ErgoProduktshn Ltd, Saint Petersburg, Russia), dehydrated with isopropanol (ErgoProduktshn Ltd, Saint Petersburg, Russia), and paraffin-embedded and subsequently sliced into 5-µm sections for immunohistochemical evaluation. Slices were deparaffinized and placed into epitope retrieval solution (Agilent Technologies Denmark ApS, Glostrup, Denmark; pH 6.0) at 95°C for 30 minutes. Endogenous peroxidase activity was blocked using a 3% H2O2 solution for 15 minutes. The slices were further washed in phosphate-buffered saline and immunostained with primary anti-CD31 (77699s, Cell Signaling Technology Inc, Danvers, MA) or anti-PCNA (proliferating cell nuclear antigen, bs-0754r, Bioss Antibodies, Inc, Woburn, MA) rabbit polyclonal antibodies and further with an anti-rabbit horseradish peroxidase (HRP)-conjugated detection system (Agilent Technologies Denmark ApS EnVision+ System, HRP). Binding was visualized using 3,3″-diaminobenzidine, which produces a brown precipitate at the antigen site. Next, the slices were stained with Mayer’s hematoxylin (ErgoProduktshn Ltd, Saint Petersburg, Russia) with subsequent differentiation and bluing. The percentage of PCNA-expressing cells was counted. The relative area of CD31-positive microvessels per field was determined.
Study 2: Analysis of Lymphocyte Subpopulations in Peripheral Blood of Mice by Flow Cytometry
Forty 3-month-old SHR male mice were randomized into 4 groups: (1) Control (N = 10) with sham injection and purified water by gavage as a placebo; (2) CPA 300 mg/kg body weight intraperitoneally (N = 10); (3) BP-C3 75 mg/kg body weight (N = 10); and (4) CPA 300 mg/kg body weight + BP-C3 75 mg/kg body weight (N = 10).
The subpopulations of lymphocytes in the peripheral blood of mice were assessed on the 4th and 11th days after administration of CPA, as at these times there is a nadir of white blood cell (WBC) content and then recovery of their level.
An anti-mouse CD45 antibody labelled with APC-Cy7 (Catalogue No. 557659, BD Bioscience, San Jose, CA), anti-mouse CD19 antibody labelled with fluorescein isothiocyanate (Catalogue No. 557398, BD Bioscience), anti-mouse CD4 antibody labelled with PE-Cy5 (Catalogue No. 553050, BD Bioscience), anti-mouse CD3e antibody labelled with APC (Catalogue No. 553066, BD Bioscience), anti-mouse CD8a antibody labelled with PE-Cy7 (Catalogue No. 552877, BD Bioscience), and anti-mouse CD49b antibody labelled with PE (Catalogue No. 5295782, BD Bioscience) were used for flow cytometry. A solution of labelled monoclonal antibody (5 µL of each antibody) was placed into the test tube for cytofluorometric analysis (12 × 75 mm). Then, 100 µL of peripheral mouse blood sample was added and mixed with aid of a vortex shaker for 1 to 3 seconds. Red blood cells (RBCs) were lysed by FACS Lysing solution (10×) previously diluted by adding 9 volumes of distilled water to 1 volume of stock solution. Sample preparation was performed by BD FACS Lyse/Wash Assistant running (Complete protocol), which includes 20 minutes of incubation, RBC lysis by the addition of 1 mL of working FACS Lysing Solution to each test tube, and a washout cycle by CellWASH solution. A subpopulation of immunocompetent cells from the peripheral blood of mice was assessed with a flow cytofluorimeter (BD FACS Canto II, BD Biosciences, BD FACSDiva v8.0.1 software). The relative number (%) of lymphocytes, T-lymphocytes, B-lymphocytes, T-helpers, cytotoxic T-lymphocytes, natural killer cells, and natural killer T cells were counted, and the immunoregulatory index was estimated. To calculate the absolute number of immunocompetent cells (109/L), a 2-platform analysis system was used, and the calculation was performed using the results from the hematological analyzer.
Statistical Data Treatment
SPSS 16.0 software package and Graph Pad Prism 6.0 were used to evaluate the data. The results were assessed by a 2-way analysis of variance with Fisher’s least significant difference test and by a Kruskal-Wallis test with Dunn’s multiple comparisons posttest.
Results
Results of Study on Assessment of CPA Efficacy and Hematological Toxicity
All tumors were polymorphous cell sarcomas consisting mainly of spindle-shaped cells and a small number of multinucleated cells. The effect of gender factor on tumor growth was evaluated; and as it was found to be significant (P = .009 MANCOVA Pillai’s Trace), all results were analyzed separately for males and females.
Data on the dynamics of average body weight are given in Table 1 for males and in Table 3 for females.
Table 1.
Dynamics of Average Body Weight (g) of Male Mice Bearing Soft Tissue Sarcomas Treated With Cyclophosphamide (CPA) and BP-C3a.
| Group | Study Day |
|||||
|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 12 | 17 | 21 | |
| 1. Control | 41.4 ± 1.1 | 43.1 ± 1.0 | 44.0 ± 1.1 | 45.1 ± 1.2 | 46.0 ± 1.7 | 47.2 ± 2.4 |
| 2. CPA 200 mg/kg | 39.3 ± 1.0 | 37.5 ± 0.9**,# | 37.0 ± 1.2***,## | 36.8 ± 1.8***,### | 37.6 ± 2.0***,### | 38.2 ± 2.4***,### |
| 3. BP-C3 75 mg/kg | 42.2 ± 0.9 | 41.2 ± 1.6 | 41.9 ± 1.8 | 44.1 ± 1.6 | 45.9 ± 1.5 | 46.7 ± 1.4 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 41.3 ± 0.8 | 37.3 ± 0.9**,# | 37.7 ± 0.7***,## | 39.1 ± 0.8***,### | 39.4 ± 0.7***,### | 39.1 ± 1.0***,### |
Ordinary 2-way analysis of variance with Fisher’s least significant difference test. Values are presented as the means ± standard errors of mean.
Versus Control group, ** – P < .01, *** – P < .001.
Versus BP-C3 group, # – P < .05, ## – P < .01, ### – P < .001.
Table 3.
Dynamics of Average Body Weight (g) of Female Mice Bearing Soft Tissue Sarcomas Treated With Cyclophosphamide (CPA) and BP-C3a.
| Group | Study Day |
|||||
|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 12 | 17 | 21 | |
| 1. Control | 31.5 ± 0.9 | 32.7 ± 1.2 | 32.8 ± 1.3 | 33.8 ± 1.5 | 34.0 ± 0.9 | 34.9 ± 0.9 |
| 2. CPA 200 mg/kg | 31.7 ± 0.9 | 29.9 ± 1.0 | 28.7 ± 0.8**,# | 29.7 ± 0.6**,# | 29.8 ± 0.6**,# | 30.0 ± 1.2**,## |
| 3. BP-C3 75 mg/kg | 30.8 ± 1.0 | 31.5 ± 0.9 | 32.4 ± 1.0 | 33.5 ± 1.2 | 34.6 ± 1.5 | 35.5 ± 2.2 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 32.6 ± 0.7 | 29.3 ± 0.8* | 28.9 ± 1.0**,# | 29.7 ± 1.4**,# | 30.8 ± 0.7**,# | 32.7 ± 0.7 |
Ordinary 2-way analysis of variance with Fisher’s least significant difference test. Values are presented as the means ± standard errors of mean.
Versus Control group, * – P < .05, ** – P < .01.
Versus BP-C3 group, # – P < .05, ## – P < .01.
In male mice from groups 2 and 4 treated with CPA, an approximately 10% decrease of the initial value of average body weight was observed between days 4 and 7 (Table 1). The weight was stable at other periods. The average body weight in the Control and BP-C3 groups increased during the observation period, likely due to the growth of tumors (Table 2).
Table 2.
Tumor Volume in Male Mice Bearing Soft Tissue Sarcomas, cm3 (Tumor Growth Inhibition, %)a.
| Group | Animal No. | Study Day |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 12 | 17 | 21 | ||
| 1. Control | 10 | 0.22 ± 0.05 | 0.64 ± 0.12 | 0.98 ± 0.20 | 1.65 ± 0.33 | 2.67 ± 0.55 | 2.89 ± 0.79 |
| 2. CPA 200 mg/kg | 9 | 0.25 ± 0.08 (−11%) | 0.28 ± 0.09 (56%) | 0.30 ± 0.10 (69%)* | 0.29 ± 0.10 (83%)***,# | 0.41 ± 0.16 (85%)***,## | 0.64 ± 0.24 (78%)***,# |
| 3. BP-C3 75 mg/kg | 10 | 0.31 ± 0.13 (−42%) | 0.64 ± 0.21 (1%) | 0.92 ± 0.31 (6%) | 1.11 ± 0.38 (33%) | 1.51 ± 0.56 (44%)** | 1.60 ± 0.22 (45%)** |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 10 | 0.27 ± 0.07 (−20%) | 0.37 ± 0.08 (42%) | 0.35 ± 0.08 (64%)* | 0.34 ± 0.08 (79%)***,# | 0.43 ± 0.13 (84%)***,## | 0.53 ± 0.14 (82%)***,## |
Abbreviation: CPA, cyclophosphamide.
Ordinary 2-way analysis of variance with Fisher’s least significant difference test. Values are presented as the means ± standard errors of mean.
Versus Control group, * – P < .05, ** – P < .01, *** – P < .001.
Versus BP-C3 group, # – P < .05, ## – P < .01.
The average tumor volume in male mice from the Control arm increased progressively. The administration of CPA resulted in a TGI of 69% to 85%, which was significant on days 7 to 21. A TGI of 44% to 45% was achieved between days 17 and 21 in the group treated with BP-C3 alone. The combined administration of CPA and BP-C3 resulted in a TGI of 64% to 84%. Thus, the addition of BP-C3 to the CPA regimen did not provide an additive or synergistic effect in male mice.
A decrease in the average body weight of up to 11% from the initial value was noted between days 4 and 7 in female mice of groups 2 and 4 treated with CPA. Thereafter, the average body weight was at about the initial level (Table 3). The increase in the average body weight in Control and BP-C3 groups matched the tumor volume growth shown in Table 4.
Table 4.
Tumor Volume in Female Mice Bearing Soft Tissue Sarcomas, cm3 (Tumor Growth Inhibition, %)a.
| Group | Animal No. | Study Day |
|||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 12 | 17 | 21 | ||
| 1. Control | 11 | 0.26 ± 0.05 | 0.65 ± 0.13 | 0.95 ± 0.22 | 1.61 ± 0.48 | 1.63 ± 0.41 | 2.42 ± 0.62 |
| 2. CPA 200 mg/kg | 10 | 0.38 ± 0.10 (−43%) | 0.39 ± 0.10 (40%) | 0.43 ± 0.10 (55%) | 0.41 ± 0.12 (74%)**,## | 0.49 ± 0.11 (70%)**,### | 1.01 ± 0.33 (58%)***,### |
| 3. BP-C3 75 mg/kg | 11 | 0.36 ± 0.08 (−36%) | 0.69 ± 0.19 (−7%) | 0.98 ± 0.27 (−2%) | 1.61 ± 0.39 (0%) | 2.20 ± 0.52 (−35%) | 2.70 ± 0.62 (−11%) |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 11 | 0.43 ± 0.14 (−61%) | 0.50 ± 0.14 (22%) | 0.42 ± 0.11 (55%) | 0.51 ± 0.11 (68%)**,## | 0.72 ± 0.13 (56%)*,### | 1.58 ± 0.36 (35%)*,## |
Abbreviation: CPA, cyclophosphamide.
Ordinary 2-way analysis of variance with Fisher’s least significant difference test test. Values are presented as the means ± standard errors of mean.
Versus Control group, * – P < .05, ** – P < .01, *** – P < .001.
Versus BP-C3 group, ## – P < .01, ### – P < .001.
CPA administered to the female mice of group 2 resulted in a TGI of 58% to 74%, and the effect was significant on days 12 to 21. BP-C3 had no significant effect on the tumor growth rate. A TGI of 35% to 68% on days 12 to 21 (Table 4) was achieved in animals treated with a combination of BP-C3 and CPA. The effect was similar to that obtained with CPA alone. A notably smaller TGI was obtained for female mice than for male mice treated with CPA alone.
The hematological toxicity of CPA was assessed by blood counts performed during the study. The data are presented in Table 5 for male mice and in Table 6 for female mice. The development of tumors was associated with absolute leucocytosis increase, mainly due to an increased granulocyte count. The WBC count increased in male mice from the Control arm from 14.32 ± 4.19 × 109/L on day 0 to 36.25 ± 16.11 × 109/L (P < .05) on day 21. During the same period, the granulocyte count increased from 7.64 ± 3.91 × 109/L (P < .05) to 25.50 ± 12.98 × 109/L (P < .05). This increase indicates the development of a tumor-associated chronic inflammatory response of the immune system. Treatment with CPA resulted in a significant drop in the WBC count by day 4 (2.43 ± 1.77 × 109/L, P < .05 vs 12.70 ± 4.69 × 109/L on day 0). It was restored by day 7 (17.14 ± 11.98) and remained unchanged until day 21 (14.68 ± 10.71 × 109/L). A lower WBC count in the CPA group at the end of the study could be explained by the significant TGI and a much less pronounced tumor-associated inflammation in this group compared with the Control arm. Interestingly, increased levels of both WBCs (15.77 ± 11.28 × 109/L on day 0, 26.53 ± 15.54 × 109/L on day 21) and granulocytes (9.79 ± 9.78 × 109/L on day 0, 19.30 ± 12.00 × 109/L on day 21) were registered in the BP-C3 group during the observation period, though the changes did not reach the level of statistical significance when compared with either initial levels or to the Control arm at the same time points. The changes in WBC and granulocyte counts observed in the BP-C3 arm can be ascribed to the tumor growth inhibition activity or to the immunomodulatory and/or anti-inflammatory activity of this agent. Anemia, which developed by day 7 in animals treated with CPA (RBC = 6.55 ± 1.74 × 1012/L), was later normalized (RBC = 9.03 ± 0.55 × 1012/L on day 21), exceeding the level in the Control arm (RBC = 7.38 ± 2.14 × 1012/L on day 21, P < .05). The platelet count increased in response to CPA from day 7 (2867 ± 936 × 109/L, P < .05) to day 21 (2002 ± 988 × 109/L), compared with the initial value on day 0 (1324 ± 327 × 109/L).
Table 5.
Blood Counts for Male Mice Bearing Soft Tissue Sarcomas*.
| Study Day |
|||||
|---|---|---|---|---|---|
| 0 | 4 | 7 | 14 | 21 | |
| WBC, 10 9 /L | |||||
| 1. Control | 14.32 ± 4.19 | 19.01 ± 7.65 | 20.43 ± 9.17 | 25.80 ± 10.09c | 36.25 ± 16.11c |
| 2. CPA 200 mg/kg | 12.70 ± 4.69 | 2.43 ± 1.77a,b,c | 17.14 ± 11.98 | 18.62 ± 8.81 | 14.68 ± 10.71a |
| 3. BP-C3 75 mg/kg | 15.77 ± 11.28 | 15.13 ± 9.54 | 16.56 ± 12.28 | 14.98 ± 4.13 | 26.53 ± 15.54 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 16.87 ± 4.00 | 1.10 ± 0.55a,b,c | 17.33 ± 17.44 | 14.19 ± 9.81a | 12.27 ± 5.75a,b |
| Lymph, 10 9 /L | |||||
| 1. Control | 6.12 ± 1.95 | 6.33 ± 2.14 | 5.81 ± 3.42 | 6.54 ± 1.35 | 9.50 ± 3.77c |
| 2. CPA 200 mg/kg | 5.36 ± 1.95 | 1.39 ± 1.21a,b,c | 3.26 ± 2.11a,b | 5.60 ± 2.70 | 3.95 ± 3.06a |
| 3. BP-C3 75 mg/kg | 5.43 ± 2.16 | 5.38 ± 2.50 | 4.86 ± 2.79 | 3.88 ± 0.50 | 6.43 ± 3.48a |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 6.34 ± 2.02 | 0.73 ± 0.25a,b,c | 2.69 ± 1.77b,c | 3.09 ± 1.39a,c | 2.63 ± 1.06a,b,c |
| Gran, 10 9 /L | |||||
| 1. Control | 7.64 ± 3.91 | 11.97 ± 7.56 | 13.89 ± 7.70 | 18.30 ± 9.73c | 25.50 ± 12.98c |
| 2. CPA 200 mg/kg | 6.71 ± 3.00 | 0.90 ± 0.68a,b | 13.24 ± 10.59 | 12.05 ± 6.16 | 9.88 ± 7.04a |
| 3. BP-C3 75 mg/kg | 9.79 ± 9.78 | 9.24 ± 7.69 | 11.07 ± 10.55 | 10.60 ± 3.84 | 19.30 ± 12.00 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 9.93 ± 4.47 | 0.46 ± 0.24a,b,c | 13.98 ± 15.20 | 10.43 ± 8.22 | 9.08 ± 4.86a,b |
| Mon, 10 9 /L | |||||
| 1. Control | 0.56 ± 0.18 | 0.71 ± 0.33 | 0.73 ± 0.31 | 0.96 ± 0.47 | 1.25 ± 0.47c |
| 2. CPA 200 mg/kg | 0.63 ± 0.36 | 0.14 ± 0.14a,c | 0.64 ± 0.46 | 0.97 ± 0.58 | 0.85 ± 1.03 |
| 3. BP-C3 75 mg/kg | 0.54 ± 0.36 | 0.51 ± 0.30 | 0.63 ± 0.42 | 0.50 ± 0.18 | 0.80 ± 0.41 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 0.60 ± 0.16 | 0.08 ± 0.09a,b,c | 0.66 ± 0.58 | 0.68 ± 0.44 | 0.56 ± 0.35a |
| RBC, 10 12 /L | |||||
| 1. Control | 9.04 ± 1.03 | 8.19 ± 2.12 | 8.03 ± 1.74 | 8.68 ± 1.18 | 7.38 ± 2.14c |
| 2. CPA 200 mg/kg | 8.63 ± 0.85 | 7.11 ± 1.61b,c | 6.55 ± 1.74a,b,c | 9.24 ± 0.38 | 9.03 ± 0.55a,b |
| 3. BP-C3 75 mg/kg | 8.71 ± 1.11 | 8.88 ± 1.73 | 8.74 ± 1.14 | 8.68 ± 0.49 | 6.90 ± 1.17c |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 8.91 ± 0.85 | 7.69 ± 1.12c | 7.12 ± 1.17b,c | 9.19 ± 0.44 | 9.26 ± 0.75a,b |
| PLT, 10 9 /L | |||||
| 1. Control | 1246 ± 581 | 1164 ± 909 | 1048 ± 885 | 1309 ± 1496 | 457 ± 318 |
| 2. CPA 200 mg/kg | 1324 ± 327 | 1166 ± 373 | 2867 ± 936a,b,c | 3001 ± 875a,b,c | 2002 ± 988a,b |
| 3. BP-C3 75 mg/kg | 1407 ± 461 | 1594 ± 720 | 1704 ± 853 | 732 ± 390 | 370 ± 142c |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 1292 ± 473 | 1018 ± 260 | 2752 ± 808a,b,c | 3292 ± 690a,b,c | 2213 ± 997a,b,c |
Abbreviations: WBC, white blood cell; CPA, cyclophosphamide; Lymph, lymphocytes; Gran, granulocytes; Mon, monocytes; RBC, red blood cell; PLT, platelets.
Values are presented as the means ± SDs. Data were treated by 2-way analysis of variance with Fisher’s least significant difference test.
P < .05 versus day 0 in the same group.
P < .05 versus Control group.
P < .05 versus BP-C3 group.
Table 6.
Blood Count for Female Mice Bearing Soft Tissue Sarcomas*.
| Study Day |
|||||
|---|---|---|---|---|---|
| 0 | 4 | 7 | 14 | 21 | |
| WBC, 10 9 /L | |||||
| 1. Control | 11.18 ± 3.77 | 14.30 ± 9.80 | 18.95 ± 14.04 | 17.44 ± 11.93 | 16.87 ± 12.71 |
| 2. CPA 200 mg/kg | 14.50 ± 9.96 | 1.19 ± 0.71a,b,c | 13.70 ± 12.92 | 16.12 ± 11.62 | 10.83 ± 5.75 |
| 3. BP-C3 75 mg/kg | 12.46 ± 6.90 | 10.68 ± 4.44 | 10.87 ± 4.79 | 17.64 ± 9.87 | 17.18 ± 8.93 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 13.53 ± 7.99 | 1.29 ± 0.71a,b,c | 9.71 ± 9.00a | 13.93 ± 10.09 | 17.62 ± 16.08 |
| Lymph, 10 9 /L | |||||
| 1. Control | 6.38 ± 2.20 | 7.93 ± 7.79 | 10.31 ± 9.72 | 7.69 ± 4.10 | 5.54 ± 1.45 |
| 2. CPA 200 mg/kg | 7.15 ± 4.87 | 0.91 ± 0.29a,c | 5.58 ± 8.78a | 3.80 ± 3.29 | 3.31 ± 1.04 |
| 3. BP-C3 75 mg/kg | 7.26 ± 4.72 | 5.04 ± 2.90 | 5.24 ± 2.41a | 7.04 ± 2.46 | 6.38 ± 2.42 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 6.82 ± 4.65 | 0.89 ± 0.40a,c | 3.93 ± 5.62a | 3.31 ± 2.44 | 6.02 ± 8.71 |
| Gran, 10 9 /L | |||||
| 1. Control | 4.4 ± 2.24 | 5.87 ± 4.43 | 7.96 ± 6.49 | 9.13 ± 8.13 | 9.29 ± 8.12 |
| 2. CPA 200 mg/kg | 6.67 ± 5.25 | 0.33 ± 0.31c | 7.57 ± 4.41 | 11.53 ± 8.56 | 7.01 ± 5.18 |
| 3. BP-C3 75 mg/kg | 4.76 ± 2.79 | 5.24 ± 2.50 | 5.19 ± 2.97 | 9.93 ± 7.61 | 10.08 ± 7.07 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 6.19 ± 4.79 | 0.44 ± 0.34a,c | 5.38 ± 3.67 | 9.73 ± 8.55 | 11.03 ± 10.38 |
| Mon, 10 9 /L | |||||
| 1. Control | 0.40 ± 0.12 | 0.50 ± 0.30 | 0.68 ± 0.55 | 0.63 ± 0.34 | 0.61 ± 0.49 |
| 2. CPA 200 mg/kg | 0.68 ± 0.65 | 0.06 ± 0.11a,c | 0.56 ± 0.42 | 0.79 ± 0.39 | 0.50 ± 0.20 |
| 3. BP-C3 75 mg/kg | 0.44 ± 0.31 | 0.40 ± 0.15 | 0.44 ± 0.26 | 0.67 ± 0.39 | 0.72 ± 0.46 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 0.52 ± 0.37 | 0.05 ± 0.07a,c | 0.40 ± 0.16 | 0.89 ± 0.85 | 0.57 ± 0.43 |
| RBC, 10 12 /L | |||||
| 1. Control | 9.45 ± 0.68 | 8.29 ± 2.05 | 8.03 ± 2.19 | 8.88 ± 1.15 | 8.47 ± 1.62 |
| 2. CPA 200 mg/kg | 8.38 ± 1.89 | 6.69 ± 2.18a,b,c | 5.89 ± 2.24a,b,c | 8.17 ± 1.20 | 8.34 ± 1.50 |
| 3. BP-C3 75 mg/kg | 9.25 ± 1.62 | 9.61 ± 0.60 | 9.01 ± 0.73 | 8.72 ± 1.61 | 8.82 ± 1.28 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 8.83 ± 1.55 | 7.23 ± 2.21b,c | 7.36 ± 2.07b | 8.74 ± 0.72 | 7.86 ± 1.68 |
| PLT, 10 9 /L | |||||
| 1. Control | 1184 ± 454 | 1056 ± 475 | 940 ± 553 | 1138 ± 661 | 647 ± 567 |
| 2. CPA 200 mg/kg | 1223 ± 598 | 1152 ± 631 | 2810 ± 506a,b,c | 3204 ± 773a,b,c | 1983 ± 469a,b,c |
| 3. BP-C3 75 mg/kg | 1272 ± 467 | 1226 ± 693 | 1037 ± 427 | 932 ± 523 | 1145 ± 686 |
| 4. CPA 200 mg/kg + BP-C3 75 mg/kg | 1142 ± 345 | 1179 ± 479 | 2477 ± 878a,b,c | 2498 ± 1007a,b,c | 1957 ± 984a,b,c |
Abbreviations: WBC, white blood cell; CPA, cyclophosphamide; Lymph, lymphocytes; Gran, granulocytes; Mon, monocytes; RBC, red blood cell; PLT, platelets.
Values are presented as the means ± SDs. Data were treated by 2-way analysis of variance with Fisher’s least significant difference test.
P < .05 versus Control group.
P < .05 versus BP-C3 group.
P < .05 versus day 0 in the same group.
Female mice had a less prominent increase in WBC count from 11.18 ± 3.77 × 109/L on day 0 to 16.87 ± 12.71 × 109/L on day 21, which was caused by an increase in the granulocyte count (4.4 ± 2.24 × 109/L on day 0; 9.29 ± 8.12 × 109/L on day 21, P < .05). In the group treated with CPA, WBC dropped significantly by day 4 (1.19 ± 0.71 × 109/L), but at later time points (10.83 ± 5.75 × 109/L on day 21), it did not differ from the initial level and the Control arm. In female mice, the administration of CPA resulted in anemia, which developed between day 4 and day 7. In this group, the RBC count was restored by day 14. The platelet count remained increased from day 7 to day 21.
The addition of BP-C3 to CPA did not alter the hematological toxicity of CPA, except for some amelioration of anemia observed both in male and female mice treated with CPA. Therefore, the most prominent RBC count decrease in mice treated with CPA was observed on day 7: 6.55 ± 1.74 × 1012/L in male mice and 5.89 ± 2.24 × 1012/L in female mice. The RBC count measured on day 7 in animals treated with the combination of BP-C3 and CPA constituted 7.12 ± 1.17 × 1012/L and 7.36 ± 2.07 × 1012/L for male and female mice, respectively. The differences did not reach the level of significance. In our previous studies, we have demonstrated the activity of BP-C3 against the hematological toxicity induced by 5-fluorouracil: BP-C3 prevented the development of anemia in SHR mice exposed to 5-fluorouracil.3
Results of Study on Analysis of Lymphocyte Subpopulations in Peripheral Blood of Mice by Flow Cytometry
Because the hematological toxicity of CPA was obtained with regard to WBC, we decided to study the effects of CPA and BP-C3 on subpopulations of lymphocytes in mice without tumors (Study #2). As an antitumor effect of the test agents was demonstrated in male mice, animals of this sex were used for Study #2. Results of Study #2 are presented in Table 7.
Table 7.
Subpopulations of Lymphocytes in the Peripheral Blood of Male Tumor-Free SHR Micea.
| Group | Units | WBC | Lymphocytes | T-Lymphocytes | B-Lymphocytes | T-Helpers CD4+ | T-Cytotoxics CD8+ | Natural Killer Cells | Natural Killer T Cells | CD4+/CD8+ |
|---|---|---|---|---|---|---|---|---|---|---|
| Day 4 after CPA injection | ||||||||||
| 1. Control | #, 109/L | 10.9 ± 1.1 | 5.9 ± 1.1 | 2.4 ± 0.5 | 1.9 ± 0.8 | 1.6 ± 0.4 | 0.72 ± 0.15 | 0.28 ± 0.05 | 0.33 ± 0.13 | 2.4 ± 0.5 |
| % | — | 53.0 ± 6.2 | 38.9 ± 2.6 | 43.0 ± 3.0 | 25.5 ± 2.5 | 12.6 ± 2.6 | 6.0 ± 2.1 | 5.2 ± 1.6 | ||
| 2. CPA 300 mg/kg | #, 109/L | 1.1 ± 0.4** | 0.7 ± 0.3** | 0.6 ± 0.2* | 0.017 ± 0.006* | 0.5 ± 0.2* | 0.09 ± 0.03* | 0.01 ± 0.00 | 0.02 ± 0.01*** | 5.9 ± 0.2* |
| % | — | 61.0 ± 3.3 | 91.6 ± 1.9b | 3.6 ± 1.8b | 77.5 ± 2.1b | 13.0 ± 0.5 | 1.9 ± 0.7 | 2.7 ± 0.3b | ||
| 3. BP-C3 75 mg/kg | #, 109/L | 9.3 ± 1.3 | 5.8 ± 1.1 | 2.1 ± 0.3 | 2.1 ± 0.2 | 1.7 ± 0.3 | 0.49 ± 0.10 | 0.16 ± 0.03 | 0.10 ± 0.02** | 3.5 ± 0.5 |
| % | — | 61.0 ± 3.1 | 38.9 ± 4.4 | 51.3 ± 4.7 | 29.2 ± 2.3 | 9.2 ± 1.8 | 3.0 ± 0.6 | 1.5 ± 0.3** | ||
| 4. CPA 300 mg/kg + BP-C3 75 mg/kg | #, 109/L | 1.2 ± 0.3** | 0.8 ± 0.2** | 0.7 ± 0.1* | 0.004 ± 0.002* | 0.6 ± 0.1 | 0.08 ± 0.02b | 0.01 ± 0.00 | 0.02 ± 0.00*** | 8.9 ± 0.9*** |
| % | — | 61.5 ± 4.4 | 95.3 ± 0.8*** | 1.0 ± 0.5*** | 84.3 ± 1.1*** | 9.9 ± 1.1 | 1.1 ± 0.1 | 2.4 ± 0.5* | ||
| Day 11 after CPA injection | ||||||||||
| 1. Control | #, 109/L | 8.3 ± 1.4 | 4.9 ± 1.0 | 2.6 ± 0.3 | 2.7 ± 0.6 | 1.6 ± 0.1 | 0.98 ± 0.30 | 0.15 ± 0.05 | 0.09 ± 0.02 | 2.1 ± 0.4 |
| % | — | 58.4 ± 2.9 | 54.9 ± 5.6 | 34.5 ± 6.6 | 35.8 ± 4.1 | 20.2 ± 5.1 | 3.2 ± 0.9 | 1.8 ± 0.4 | ||
| 2. CPA 300 mg/kg | #, 109/L | 10.7 ± 2.8 | 2.9 ± 1.0 | 1.7 ± 0.5 | 0.042 ± 0.033** | 1.4 ± 0.5 | 0.35 ± 0.11* | 0.67 ± 0.35 | 0.09 ± 0.03 | 4.6 ± 0.9 |
| % | — | 25.4 ± 2.9** | 62.4 ± 3.1 | 0.7 ± 0.2*** | 49.8 ± 4.2** | 12.3 ± 2.1 | 19.1 ± 3.1*** | 3.1 ± 0.3 | ||
| 3. BP-C3 75 mg/kg | #, 109/L | 8.4 ± 0.9 | 4.9 ± 0.5 | 2.3 ± 0.3 | 3.1 ± 0.8 | 1.7 ± 0.1 | 0.63 ± 0.14 | 0.07 ± 0.01 | 0.06 ± 0.01 | 3.5 ± 1.0 |
| % | — | 58.4 ± 3.3 | 46.9 ± 1.2 | 43.4 ± 0.9 | 36.0 ± 1.6 | 12.3 ± 1.9 | 1.5 ± 0.3 | 1.3 ± 0.1 | ||
| 4. CPA 300 mg/kg + BP-C3 75 mg/kg | #, 109/L | 19.2 ± 5.5** | 4.0 ± 1.2 | 2.7 ± 0.7 | 0.008 ± 0.004** | 2.2 ± 0.5 | 0.56 ± 0.17 | 0.68 ± 0.39 | 0.07 ± 0.04 | 5.4 ± 1.6* |
| % | — | 21.7 ± 2.3** | 70.9 ± 3.9** | 0.1 ± 0.0*** | 59.2 ± 5.9*** | 12.7 ± 2.0 | 13.1 ± 5.4** | 1.6 ± 0.6 | ||
Abbreviations: SHR, Swiss-H-derived Rappolovo albino; WBC, white blood cell; CPA, cyclophosphamide.
Values are presented as the means ± standard errors of mean.
Versus Control group, * – P < .05, ** – P < .01, *** – P < .001.
CPA administration caused a prominent decrease in subpopulations of lymphocytes in the peripheral blood and an increased CD4+/CD8+ ratio. Despite the WBC count being restored by day 11 after its prominent drop on day 4 after CPA administration, the level of B-lymphocytes was still decreased at this time point (Table 7). BP-C3 did not influence the subpopulations of peripheral blood lymphocytes, except for the natural killer T cell count, which dropped on day 4 and was restored by day 11.
CD31 Determination by Immunohistochemistry in Soft Tissue Sarcomas of Male Mice
On day 21, the relative average area of microvessels in tumors was 5.00 ± 1.65% in the Control group, 4.57 ± 1.45% in the CPA group, 5.94 ± 2.04% in the BP-C3 group, and 3.96 ± 1.49% in the CPA + BP-C3 group (Figure 1). CPA, administered alone, does not affect tumor microvasculature at the late time point. The course of administration of BP-C3 resulted in an increased microvessel area compared with the CPA group, but not when compared with the Control group. Interestingly, the combined administration of CPA with BP-C3 led to a prominent decrease in vascular density in the tumor.
Figure 1.
CD31 expression in tumors. (Left) Microphotographs of histopathology sections of benzo[a]pyrene-induced soft tissue sarcomas (hematoxylin and diaminobenzidine stain; ×40 objective), day 21 after administration of cyclophosphamide. (Right) Relative area of CD31-positive microvessels. Data presented as means ± SDs and treated with Kruskal-Wallis test with Dunn’s multiple comparisons posttest.
PCNA Determination by Immunohistochemistry in Soft Tissue Sarcomas of Male Mice
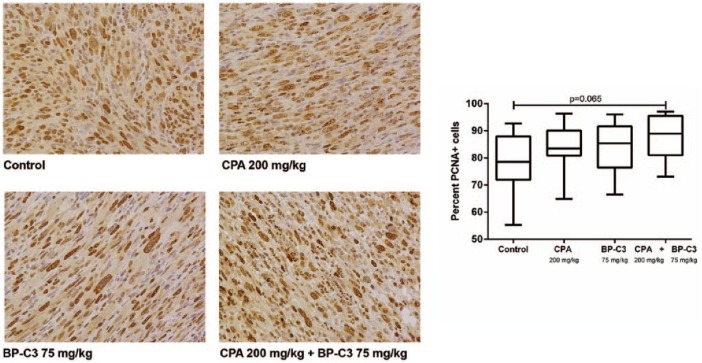
The mitotic activity of tumors assessed by a PCNA marker was high on day 21. The percent of PCNA+ cells was 78.21 ± 10.35% in the Control group, 83.69 ± 8.72% in the CPA group, 83.96 ± 8.59% in the BP-C3 group, and 87.56 ± 8.21% in the CPA + BP-C3 group (Figure 2). There was no significant difference between the groups except for the tendency for a slight increase in PCNA+ cells after the joint administration of CPA with BP-C3.
Figure 2.
PCNA (proliferating cell nuclear antigen) expression in tumors. (Left) Microphotographs of histopathology sections of benzo[a]pyrene-induced soft tissue sarcomas (hematoxylin and diaminobenzidine stain; ×40 objective), day 21 after administration of cyclophosphamide. (Right) Relative PCNA immunohistochemistry staining. Data presented as means ± SDs and treated with Kruskal-Wallis test with Dunn’s multiple comparisons posttest.
Discussion
Cyclophosphamide is widely used as an efficient anticancer drug, but it is also known to cause damage to normal cells. Polyphenolic compounds are under thorough investigation as possible agents for the mitigation of such toxicity. Propolis, a mixture of hundreds of polyphenols, ameliorates CPA toxicity due to the multiple biological effects it exerts, such as antiviral, antioxidant, anti-inflammatory, antiproliferative, antitumor, and immunomodulatory effects.18 The limitation of the study of such compounds is a lack of relation of the effect to the exact molecule in the complex compound. Small molecules like resveratrol are free from these shortcomings, and it was shown that resveratrol, a natural polyphenolic compound, is able to reduce CPA toxicity associated with increased organ-to-body weight ratios of the heart, kidney, and liver, accompanied by changes in serum creatine kinase, blood urea nitrogen, creatinine, alanine aminotransferase, and aspartate aminotransferase.6 Thus, the authors of the publication propose resveratrol as an adjuvant for CPA treatment to protect from the damage via antioxidant and anti-inflammatory mechanisms.
We evaluated the activity of BP-C3, a composition comprising polyphenolic polymer BP-Cx-1, toward toxicity induced by CPA in mice bearing soft tissue sarcomas and the effect of BP-C3 toward the sensitivity of soft tissue sarcomas to treatment with CPA. We demonstrated that the administration of BP-C3 together with CPA does not affect the antitumor activity of the latter. It is well known that ascorbic acid and retinol are used in cancer treatment. Their biological activity is apparently not relevant, as in the current study, their doses were only 1/100 and 1/3 of the dietary reference intakes and were lower than those reported in in vivo studies.19
Iron homeostasis is often disturbed in cancer, and it plays an important role in cell division, growth, and metastasis. Anemia is often developed due to iron retention by cancer cells and cytostatic damage to bone marrow. The pro-inflammatory microenvironment of tumor is suggested to be crucial for iron donation to tumor cells.20 In our study, CPA caused development of anemia in mice and administration of BP-C3 prevented it to some extent, though iron content in BP-C3 was low and corresponded to 1/8 of the dietary reference intake.
The anti-inflammatory properties of polyphenols are well known. For example, the protective effects of carnosic acid, a natural polyphenol, against hepatic inflammation induced by acetaminophen was reported through the inhibition of the NF-κB and p65 pathways and reduced expression of pro-inflammatory genes such as tumor necrosis factor-α, interleukin-1β, interleukin-6, and monocyte chemoattractant protein-1.21 A variety of flavonoid molecules are known to exhibit anti-inflammatory activity by means of antioxidative and radical scavenging activities, regulation of cellular activities of inflammation-related cells, modulation of the activity of enzymes involved in metabolism of arachidonic acid, and modulation of the production of pro-inflammatory molecules and/or of pro-inflammatory gene expression.22 Flavonoids are part of BP-C3 composition.
As the administration of BP-C3 alone prevented the tumor-associated development of chronic inflammation (ie, granulocytosis), we suggest that its ligand BP-Cx-1 could have had an effect on iron metabolism in the tumor microenvironment. Earlier, we reported this ligand, when used in another product, to have a protective effect on bone marrow progenitor cells of mice exposed to total body irradiation.4 Though the iron content in tumors was not investigated in the current study, it should be addressed in studies on tumors treated with polyphenols. In the present study, BP-C3 was less efficient in protecting laboratory animals from the hematological toxicity induced by CPA than in the previously reported study where BP-C3 was administered to animals treated with 5-fluorouracil.3
The results of our study confirm the earlier reported antitumor effect of polyphenolic compounds by demonstrating the antitumor activity of the lignin-derived polyphenolic composition BP-C3 in male mice with soft tissue sarcomas. BP-C3 does not have a significant effect on the proliferative rate of the soft tissue sarcomas, while the addition of BP-C3 to a CPA regimen allows a decrease in tumor vasculature. Our results are consistent with results reported from other studies of polyphenols in sarcoma models. Thus, antitumor, antioxidant, and immunoregulatory activities of the 40% ethanol eluent of polyphenols from pinecone of Pinus koraiensis (PPP-40) was evaluated in sarcoma-180-bearing mice,23 and the results revealed that PPP-40 exerts an effective antitumor activity by activating the mitochondrial apoptotic pathway and improving the antioxidant and immunoregulatory activities.
Conclusion
This study aimed to collect data on the protective effects of BP-C3 against the systemic side effects induced by CPA administered to the tumor-bearing animals. The results of our study demonstrate the antitumor activity of BP-C3 in male mice bearing BP-induced soft tissue sarcomas with a TGI of up to 45%. The lack of such an effect in female mice will need to be further investigated. Neither the antitumor activity nor the hematological toxicity of CPA was significantly influenced by BP-C3. A less pronounced effect of CPA on RBC count is demonstrated when this agent is given jointly with BP-C3. Our experimental data indicate some beneficial effects of BP-C3. More experimental data should be collected concerning the mechanism of action of BP-C3 to support further development of this agent for the amelioration of side effects induced by chemotherapeutic agents.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Russian Science Foundation (Grant Number 16-15-00142).
ORCID iD: Andrey V. Panchenko  https://orcid.org/0000-0002-5346-7646
https://orcid.org/0000-0002-5346-7646
References
- 1. Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:E686. doi: 10.3390/ijms19030686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barapatre A, Meena AS, Mekala S, Das A, Jha H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int J Biol Macromol. 2016;86:443-453. doi: 10.1016/j.ijbiomac.2016.01.109 [DOI] [PubMed] [Google Scholar]
- 3. Panchenko AV, Fedoros EI, Pigarev SE, et al. Effect of the polyphenol composition BP-C3 on haematological and intestinal indicators of 5-fluorouracil toxicity in mice. Exp Ther Med. 2018;15:3124-3132. doi: 10.3892/etm.2018.5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bykov VN, Drachev IS, Kraev SY, et al. Radioprotective and radiomitigative effects of BP-C2, a novel lignin-derived polyphenolic composition with ammonium molybdate, in two mouse strains exposed to total body irradiation. Int J Radiat Biol. 2018;94:114-123. doi: 10.1080/09553002.2018.1416204 [DOI] [PubMed] [Google Scholar]
- 5. Chakraborty M, Bhattacharjee A, Kamath JV. Cardioprotective effect of curcumin and piperine combination against cyclophosphamide-induced cardiotoxicity. Indian J Pharmacol. 2017;49:65-70. doi: 10.4103/0253-7613.201015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El-Sheikh AA, Morsy MA, Okasha AM. Inhibition of NF-κB/TNF-α pathway may be involved in the protective effect of resveratrol against cyclophosphamide-induced multi-organ toxicity. Immunopharmacol Immunotoxicol. 2017;39:180-187. doi: 10.1080/08923973.2017.1318913 [DOI] [PubMed] [Google Scholar]
- 7. Rao R, Shammo JM, Enschede SH, et al. The combination of fludarabine, cyclophosphamide, and granulocyte-macrophage colony-stimulating factor in the treatment of patients with relapsed chronic lymphocytic leukemia and low-grade non-Hodgkin’s lymphoma. Clin Lymphoma. 2005;6:26-30. [DOI] [PubMed] [Google Scholar]
- 8. Dimopoulos MA, Hamilos G, Zomas A, et al. Pulsed cyclophosphamide, thalidomide and dexamethasone: an oral regimen for previously treated patients with multiple myeloma. Hematol J. 2004;5:112-117. doi: 10.1038/sj.thj.6200326 [DOI] [PubMed] [Google Scholar]
- 9. Chrystal K, Cheong K, Harper P. Chemotherapy of small cell lung cancer: state of the art. Curr Opin Oncol. 2004;16:136-140. [DOI] [PubMed] [Google Scholar]
- 10. Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v8-v30. doi: 10.1093/annonc/mdv298 [DOI] [PubMed] [Google Scholar]
- 11. Boddy AV, Yule SM. Metabolism and pharmacokinetics of oxazaphosphorines. Clin Pharmacokinet. 2000;38:291-304. doi: 10.2165/00003088-200038040-00001 [DOI] [PubMed] [Google Scholar]
- 12. Vesnushkin GM, Plotnikova NA, Semenchenko AI, Anisimov VN. Dose-dependent inhibitory effect of melatonin on carcinogenesis induced by benzo[a]pyrene in mice. J Exp Clin Cancer Res. 2006;25:507-513. [PubMed] [Google Scholar]
- 13. Anikin IV, Tyndyk ML, Zabezhinskiĭ MA, Popovich IG, Anisimov VN, Pliss GB. Effect of epsilon-aminocaproic acid, cyclophosphamide and their combination on the growth of autochthonous sarcomas of mice induced by benzo(a)pyrene [in Russian]. Vopr Onkol. 2014;60:94-95. [PubMed] [Google Scholar]
- 14. Anisimov VN, Zabezhinskiĭ MA, Popovich IG, et al. Pharmacological geroprotective composition and method for preparing it. http://www.freepatent.ru/patents/2522547. Published July 20, 2014. Accessed July 5, 2018.
- 15. Shipov VP, Pigarev ES, Fedoros EI. Benzene polycarboxylic acid compounds and their use as drug. May 2017. https://patents.google.com/patent/US9644074/en. Accessed January 29, 2019.
- 16. Fedoros EI, Orlov AA, Zherebker A, et al. Novel water-soluble lignin derivative BP-Cx-1: identification of components and screening of potential targets in silico and in vitro. Oncotarget. 2018;9:18578-18593. doi: 10.18632/oncotarget.24990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anisimov VN, Popovich IG, Zabezhinski MA, et al. Polyphenolic drug composition based on benzenepolycarboxylic acids (BP-C3) increases life span and inhibits spontaneous tumorigenesis in female SHR mice. Aging (Albany NY). 2016;8:1866-1875. doi: 10.18632/aging.101024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akyol S, Gulec MA, Erdemli HK, Akyol O. Can propolis and caffeic acid phenethyl ester be promising agents against cyclophosphamide toxicity? J Intercult Ethnopharmacol. 2016;5:105-107. doi: 10.5455/jice.20160127024542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Carvalho Melo-Cavalcante AA, da Rocha Sousa L, Alencar MVOB, et al. Retinol palmitate and ascorbic acid: role in oncological prevention and therapy. Biomed Pharmacother. 2019;109:1394-1405. doi: 10.1016/j.biopha.2018.10.115 [DOI] [PubMed] [Google Scholar]
- 20. Jung M, Mertens C, Tomat E, Brüne B. Iron as a central player and promising target in cancer progression. Int J Mol Sci. 2019;20:E273. doi: 10.3390/ijms20020273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nguyen NU, Stamper BD. Polyphenols reported to shift APAP-induced changes in MAPK signaling and toxicity outcomes. Chem Biol Interact. 2017;277:129-136. doi: 10.1016/j.cbi.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 22. García-Lafuente A, Guillamón E, Villares A, Rostagno MA, Martínez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm Res. 2009;58:537-552. doi: 10.1007/s00011-009-0037-3 [DOI] [PubMed] [Google Scholar]
- 23. Yi J, Qu H, Wu Y, Wang Z, Wang L. Study on antitumor, antioxidant and immunoregulatory activities of the purified polyphenols from pinecone of Pinus koraiensis on tumor-bearing S180 mice in vivo. Int J Biol Macromol. 2017;94(pt A):735-744. doi: 10.1016/j.ijbiomac.2016.10.071 [DOI] [PubMed] [Google Scholar]