Abstract
Background: Inflammatory response and inflammation-induced vascular hyper-permeability were established leading to the abnormalities of the pulmonary microenvironment in pre-metastasis stage of breast cancer. Ruyiping is a commonly used compound drug for clinical treatment of breast cancer metastasis, and Platycodon grandiflorum is mainly used to treat pulmonary inflammatory diseases. Therefore, this study used ruyiping combined with Platycodon grandiflorum (abbreviated as RP) to investigate their inhibitory effect on pre-metastatic microenvironment of lung in 4T1 tumor-bearing mice. Study Design and Methods: The permeability of lung tissue was detected by Evans blue method. The localization of S100A8/A9 in lung tissue was obtained by double-labeling immunofluorescence staining. The level of fibrinogen in pre-metastatic microenvironment of lung as well as the levels of pro-inflammatory factors (interleukin [IL]-1β and IL-6) and chemokines (CXCL2 and CXCL5) in bronchoalveolar lavage fluid was detected by ELISA (enzyme-linked immunosorbent assay). Results: From the experimental results, RP could protect the integrity of microvascular, inhibit the release of S100A8/A9, reduce the extravasation of fibrinogen, and decrease the expressions of IL-1β, IL-6, CXCL2, and CXCL5. Conclusions: RP could inhibit the extravasation of fibrinogen by protecting pulmonary vascular integrity and then interrupted its interaction with carcinoma in situ, thereby inhibiting the formation of inflammatory pre-metastatic microenvironment.
Keywords: breast cancer, lung metastasis, pre-metastatic microenvironment, traditional Chinese medicine
Introduction
Lung metastasis is the most common distal organ metastasis of breast cancer, and it is also the leading cause of death in patients.1 Recent studies showed that the microenvironment before metastasis played an important role in the process of tumor metastasis to the lungs.2,3 A new series of evidence on the “pretransfer microenvironment theory” suggested that the interactions between tumor cells and the target organ microenvironment had occurred before the tumor cells reached the target organ, and that primary tumors secreted a variety of cytokines and chemokines, which recruited hematopoietic progenitor cells, tumor-associated macrophages, and bone marrow-derived cells into the lungs to participate in the formation of the pretransfer microenvironment.4,5
The pre-metastatic environment has several characteristics, including angiogenesis, vascular permeability, extracellular matrix remodeling, chronic inflammation, and immunosuppression, which together make the lung more conducive to tumor cell colonization and promote metastasis.2 Chronic inflammation was an important driver of tumor metastasis, which recruited bone marrow–derived cells and circulating tumor cells to the distal target organs.6,7 Our previous experiments confirmed that inflammatory response and inflammation-induced vascular hyperpermeability was established leading to abnormalities of the pulmonary microenvironment.8 Therefore, improving the microenvironment before metastasis by relieving inflammation may be a new way to inhibit the occurrence of lung metastasis in breast cancer.
Platycodon grandiflorum (PG) is a common medicinal and edible plant. Its roots are widely used in traditional Chinese medicine, mainly for the treatment of lung diseases such as phlegm elimination, cough relief, and inflammation reduction. PG contains many active ingredients, such as steroidal saponins, flavonoids, phenolic acids, and sterols, of which saponins as the main active compounds have good antioxidant, anti-inflammatory, and anti-apoptosis pharmacological effects.9-11 In addition, our previous in vitro experiments showed platycodin D, the main triterpene saponin of PG, inhibited S100A8/A9-induced inflammatory response in 4T1 cells by suppressing the expression of interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α (TNF-α) via inhibition of nuclear factor κB (NF-κB) signaling pathways.12 Ruyiping (RYP) is a commonly used compound traditional Chinese drug for clinical treatment of breast cancer metastasis in our hospital. RYP consists of 5 Chinese herbal medicines, which are 12 g Iphigenia indica Kunth, 12 g Curcuma zedoary, 12 g nidus vespae, 12 g semen coicis, and 9 g akebia fruit. Therefore, this study used RYP combined with PG (abbreviated as RP) to investigate their inhibitory effect on mice before metastasis of breast cancer.
Materials and Methods
Cell Culture
Mouse breast cancer 4T1 cells were purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in 10% fetal bovine serum (Gibco-Invitrogen, New York, NY) containing 1640 medium (with 100 U/mL penicillin and 100 µg/mL streptomycin added) at 37°C in a 5% CO2 humidified incubator.
Experimental Animals
Female BALB/c mice, aged 5 weeks, were purchased from SLAC Laboratory Animal Co Ltd (Shanghai, China) and raised throughout under standard conditions (24 ± 2°C temperature, 50 ± 10% relative humidity, and a 12 hour-light/12 hour-dark cycle) in specific-pathogen–free level in the Department of Experimental Animal Science, Fudan University. The mice were allowed to acclimate for 1 week before inoculation and were fed with clean water and food until the day before sacrifice. All animal experiments were conducted following the animal experimental guidelines set by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chinese Medicine Prescription and Dosage
RP consisted of 6 kinds of traditional Chinese medicines, which were 12 g Iphigenia indica Kunth, 12 g Curcuma zedoary, 12 g nidus vespae, 12 g semen coicis, 9 g akebia fruit, and 6 g P grandiflorum. All of the above Chinese medicines came from the Chinese Medicine Department of Longhua Hospital (Shanghai, China).
Animal Grouping
The mice were divided into 4 groups, which were the blank group, tumor group, low-dose group, and high-dose group, with 5 mice in each group. According to the equivalent dose of human and mouse,13 the dosage in low-dose group was 5.67 g/kg/day, and that of high-dose group was 22.68 g/kg/day. The blank group and tumor group were fed with clean water of the same volume. The administration began on the day after inoculation, and ended before the execution.
Pre-Metastatic Model of Breast Cancer
The 4T1 cells in logarithmic growth period were selected and the cell suspension concentration was adjusted to 2 × 106 cell/mL. Then the cells were inoculated 0.1 mL per mouse on the fat pad of the fourth nipple on the right side of BALB/c mice in aseptic condition. Based on previous results, it was determined that the time for pre-metastatic model of breast cancer was 14 days.8,12
Detection of Permeability of Mouse Lung Tissue by Evans Blue (EB) Method
The mice in each group were injected with 20 µg/µL EB (Sigma-Aldrich, St Louis, MO) in the tail vein, and the injection volume was 200 µL. The eyes, ears, and limbs of the mice immediately turned blue after the injection. After 4 hours, the mice were intraperitoneally injected with pentobarbital sodium. The right ventricle was injected with 10 mL of normal saline to wash the lungs. The left lung was weighed and immersed in the formamide solution at a ratio of 100 mg lung weight/3 mL formamide. The tissue homogenate was extracted in a water bath at 60°C for 16 hours, centrifuged at 12 000g for 10 minutes, and the absorbance value was measured at 620 nm using a microplate reader (Synergy H1, BioTek).
Detection of Localization and Expression of S100A8/A9 in Mouse Lung Tissue by Immunofluorescence
After 14 days of inoculation, mice were anesthetized with 5 mg/mL pentobarbital sodium intraperitoneally, and the anesthetic dosage was 50 mg/kg. The left lung was infused with 4% formaldehyde for 5 minutes. After perfusion, the lung tissue was fixed in 4% formaldehyde. The tissue was embedded and cut into slices. The slices were roasted at 60°C for 1 hour, dewaxed with xylene for 30 minutes, rinsed with gradient ethanol, and incubated with 3% H2O2 for 10 minutes. After the slices were blocked by bovine serum albumin, they were incubated overnight with S100A8/A9 primary antibody (1:100, Abcam, Cambridge, UK) overnight at 4°C. The slices were added to Alexa Fluor 488 fluorescent secondary antibody (1:200, Yeasen, Shanghai, China) and incubated for 60 minutes at 37°C. Finally, the slides were stained with DAPI (Beyotime Biotechnology, Jiangsu, China) and observed by the LSM 800 confocal laser scanning microscopy (Zeiss, Germany).
Detection of Fibrinogen Expression in Mouse Lung Tissue by ELISA (Enzyme-Linked Immunosorbent Assay)
Treatment of lung tissue and detection of fibrinogen were performed according to the procedures of the instructions (Abcam, Cambridge, UK). Briefly, 150-mg (wet weight) lung tissue was added with 500 µL precooled 1* cell extraction buffer. The homogenate was incubated on ice for 20 minutes and centrifuged at 18 000g for 20 minutes at 4°C. The supernatant was collected and the protein concentration was measured by the BCA method (Beyotime Biotechnology). The supernatant was diluted to the appropriate concentration with 1* cell extraction buffer PTR. Fifty microliters of standards and samples were added to each well. Fifty microliters of antibody cocktail were added and incubated at room temperature for 1 hour. Each well was washed 3 times with wash buffer PT, 100 µL of TMB substrate was added, and incubated for 10 minutes in the dark. Hundred microliters of stop solution was added and the optical density was determined using a microplate reader (Synergy H1) set to 450 nm.
Collection of Bronchoalveolar Lavage Fluid (BALF)
The 18 G indwelling needle was inserted into the trachea and lavaged with 400 µL of frozen saline for 3 times. The obtained lavage fluid was centrifuged at 1000g for 10 minutes at 4°C. Finally, the supernatant is stored for protein detection. Total protein in BALF was determined by BCA Kit (Beyotime Biotechnology).
Detection of Chemokine (C-X-C motif) Ligand 2 (CXCL2) and CXCL5 Expression in BALF by ELISA
Chemokines including CXCL2 and CXCL5 in BALF were determined using ELISA kits (R&D System, Minneapolis, MN) according to the instructions. For mouse CXCL2, a 96-well microplate was coated with 100 µL per well of the capture antibody. The plate was sealed and incubated overnight at room temperature. The plate was blocked by adding 300 µL of reagent diluent and incubated for 1 hour. Hundred microliters of samples or standards was added and incubated for 2 hours. Hundred microliters of the detection antibody was added and incubated for 2 hours. Hundred microliters of streptavidin-horseradish peroxidase was added and incubated for 20 minutes. Hundred microliters of substrate solution was added and incubated for 20 minutes. Fifty microliters of stop solution was added, and the optical density was determined using a microplate reader (Synergy H1) set to 450 nm.
For mouse CXCL5 immunoassay, 50 µL of assay diluent RD1W was added to each well. Fifty microliters of standards, control, or samples were added and incubated at room temperature for 2 hours. After being aspirated and washed, 100 µL of mouse CXCL5 conjugate was added and incubated for 2 hours. Hundred microliters of substrate solution was added and incubated for 30 minutes. Hundred microliters of stop solution was added and the optical density was determined using a microplate reader (Synergy H1) set to 450 nm.
Detection of IL-1β and IL-6 Expression in BALF by ELISA
The expression levels of IL-1β and IL-6 in BALF were measured using ELISA kits (Beyotime Biotechnology) according to the instructions. Hundred microliters of standards and samples were added and incubated at 37°C for 2 hours. Hundred microliters of labeled antibody working solution was added and incubated at 37°C for 1 hour. Hundred microliters of streptavidin-horseradish peroxidase was added and incubated for 30 minutes. Ninety microliters of TMB substrate solution was added and incubated at 37°C for 20 minutes. Fifty microliters of stop solution was added and the optical density was determined using a microplate reader (Synergy H1) set to 450 nm.
Statistics
The experiments were repeated 3 times or as indicated by special instructions. The results were expressed as mean ± standard deviation. The data were processed by SPSS18.0 statistical software and the differences among and between groups were analyzed with 1-way analysis of variance followed by Dunnett’s post hoc test. P < .05 was considered statistically significant.
Results
RP Protected the Integrity of Pulmonary Vasculature in Pre-Metastatic Microenvironment of Lung
The level of EB leakage represents the permeability of pulmonary capillaries. The greater the amount of EB leakage, the higher the vascular permeability, the worse the integrity of the blood vessels, and the more severe the vascular damage. As can be seen from Figure 1, compared with the blank group, the EB leakage of the tumor group increased significantly, about 3.8 times that of the blank group, indicating that the integrity of the pulmonary vasculature has been impaired before breast cancer metastasis. The RP low-dose group had no significant improvement on EB leakage (P > .05), but the integrity of pulmonary capillaries was effectively protected after treatment of RP high-dose (P < .05).
Figure 1.
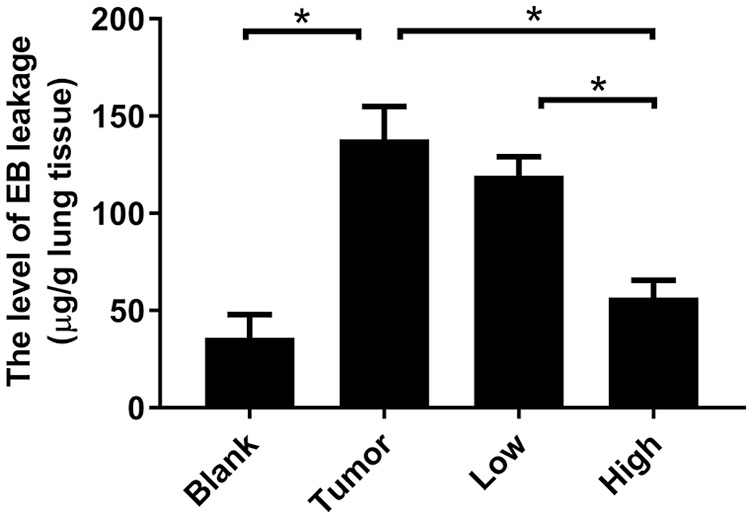
RP reduced the level of EB leakage in pre-metastatic microenvironment of lung. The mice were injected with 200 µL of 20 µg/µL EB solution 4 hours before the lung tissue was removed. The lung homogenate was extracted with formamide and the absorbance at a 620 nm was measured. The level of EB leakage in the lung tissue was calculated from the standard curve. Each bar represents mean ± SD (n = 5), and P values were obtained with 1-way ANOVA followed by Dunnett’s post hoc test. *P < .05.
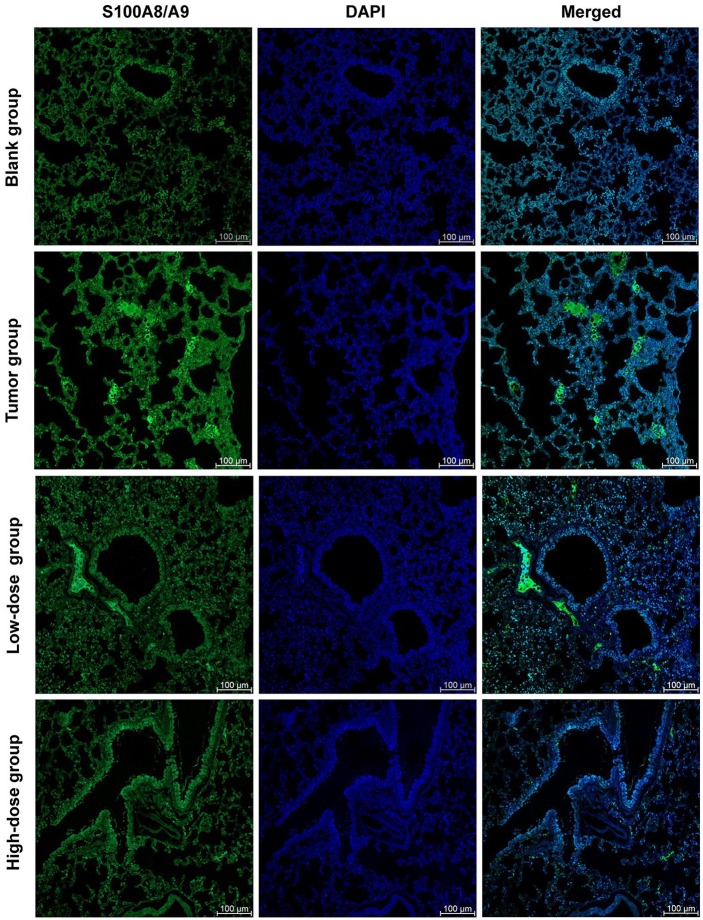
RP Reduced the Release of S100A8/A9 in Pre-Metastatic Microenvironment of Lung
In the current study, we attempted to investigate 100A8/A9 as it has been identified as a potential upstream participant in the inflammatory process. From the experimental results, we found that S100A8/A9 was released into the extracellular space in the lung tissue of 4T1 tumor-bearing mice (Figure 2). However, the extracellular S100A8/A9 in the lung tissue of the blank mice was at a low level (Figure 2). These findings indicated that S100A8/A9 was actually actively increased and released into the extracellular space during the pre-metastatic phase of breast cancer. Our previous studies have found that the inflammatory response causes abnormal changes in the lung microenvironment before the metastasis.8 In the present study, the S100A8/A9 complex was released and exerted its proinflammatory effect, which is consistent with our previous conclusions.
Figure 2.
RP reduced the release of S100A8/A9 in pre-metastatic microenvironment of lung. The localization of S100A8/A9 in lung tissue was obtained by double-labeling immunofluorescence staining of paraffin sections of lung tissue. Imaging shown is representative of 3 experiments with similar results. Green, S100A8/A9; Blue, nucleus; Scale bar, 100 µm.
After intervention with RP, we found that the low-dose group of RP did not reduce the release of S100A8/A9 in lung tissue (Figure 2). However, from the high-dose group, the effect of the administered intervention began to appear. The high-dose group demonstrated an obvious reduction effect of S100A8/A9 release (Figure 2).
RP Reduced the Expression Level of Fibrinogen in Pre-Metastatic Microenvironment of Lung
It can be seen from the experimental results (Figure 3) that the expression level of fibrinogen in the lung tissue of the mice inoculated with 4T1 was about 4 times that of the blank control group, and there was a statistically significant difference (P < .05). As an important inflammatory marker, fibrinogen indicated that the lung of the tumor-bearing mouse was already in an inflammatory state before the lung metastasis, which is very attractive for circulating tumor cells.14
Figure 3.
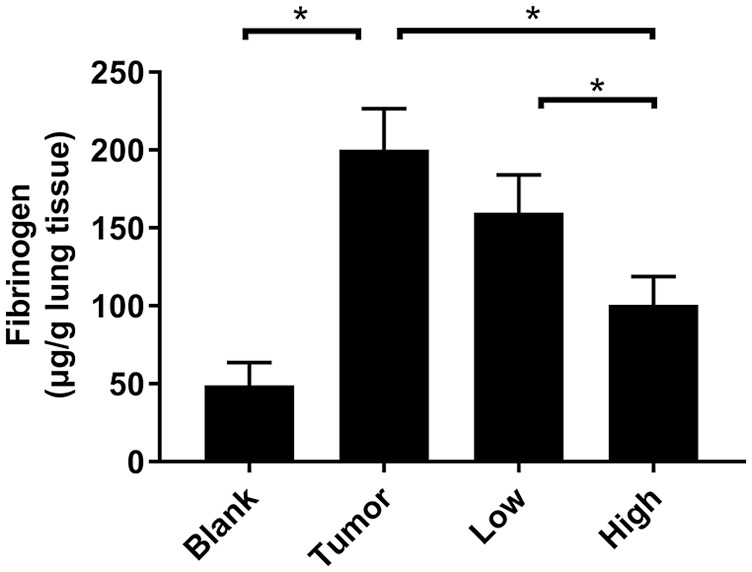
RP reduced the expression level of fibrinogen in pre-metastatic microenvironment of lung. The expression level of fibrinogen was detected by ELISA. Each bar represents mean ± SD (n = 5), and P values were obtained with 1-way ANOVA followed by Dunnett’s post hoc test.*P < .05.
After RP administration, the expression level of fibrinogen in lung tissue decreased (Figure 3). The low-dose group showed a decreasing trend but no statistical difference (P > .05). The expression level of fibrinogen in the high-dose group of RP decreased significantly (P < .05).
RP Reduced the Expression Level of Total Protein in BALF
The expression level of total protein in BALF can also reflect the permeability of lung tissue. Compared with the blank group, the protein expression of BALF in the tumor group was increased by 2.5 times (Figure 4). After RP administration, the total protein content in BALF decreased, especially in the high-concentration group (P < .05; Figure 4). This indicated that RP significantly inhibited protein leakage in lung tissue before lung metastasis in tumor-bearing mice, and reduced pulmonary vascular permeability. This result was consistent with the results of the EB leakage experiment.
Figure 4.
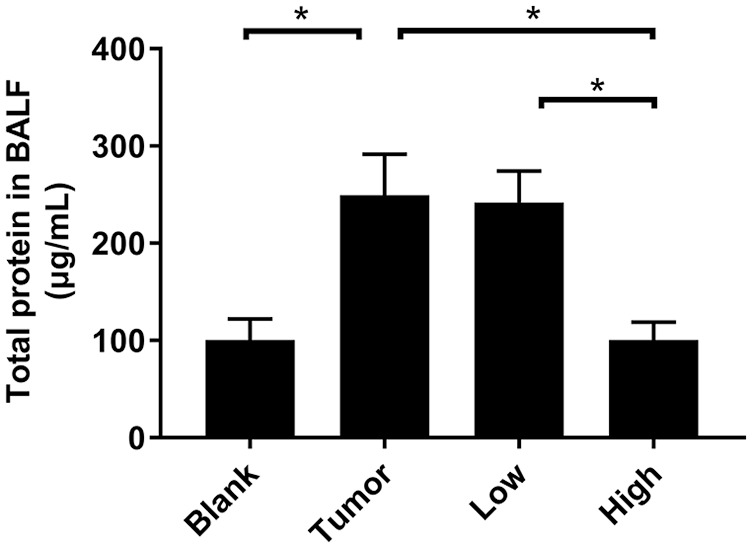
RP reduced the expression level of total protein in BALF. The whole lung of the mice was lavaged, the BALF was collected, and the total protein concentration in the BALF was measured by the BCA method. Each bar represents mean ± SD (n = 5), and P values were obtained with 1-way ANOVA followed by Dunnett’s post hoc test. *P < .05.
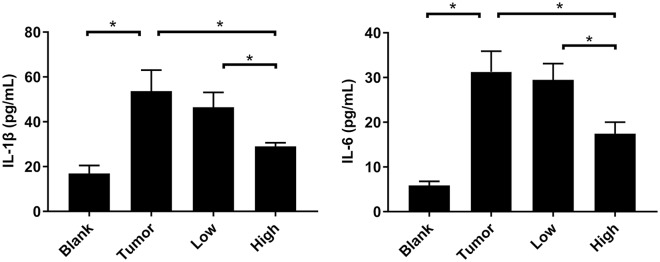
RP Reduced the Expression Levels of IL-1β and IL-6 in BALF
The inflammation-related factors IL-6 and IL-1β played an important role in the formation of the pre-metastatic microenvironment and tumor metastasis. IL-6 controlled the morphological changes of tumor cells, participated in epithelial-mesenchymal transition, and affected the migration and invasion of tumor cells.15 IL-1β was one of the most important members of the IL-1 family. It mediated the important process of inflammation and was also one of the important components in the inflammatory microenvironment.16 From the results of the ELISA experiment (Figure 5), we could see that compared with the blank group, the IL-6 and IL-1β in BALF of the tumor-bearing mice were significantly increased (P < .05). After the intervention of RP administration, the expression levels of these 2 proteins decreased (Figure 5). The change in the RP low-dose group was still not obvious (P > .05), but the expression levels of these 2 proteins in the RP high-dose group were markedly decreased (P < .05).
Figure 5.
RP reduced the expression levels of IL-1β and IL-6 in BALF. The expression levels of IL-1β and IL-6 was detected by ELISA. Each bar represents mean ± SD (n = 5), and P values were obtained with 1-way ANOVA followed by Dunnett’s post hoc test. *P< .05.
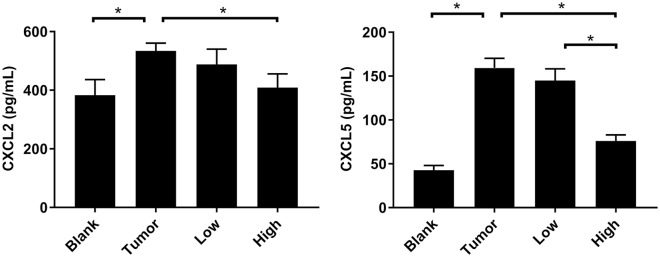
RP Reduced the Expression Levels of CXCL2 and CXCL5 in BALF
Compared with the blank group, the expressions of CXCL2 and CXCL5 in BALF of the tumor group were significantly increased (P < .05; Figure 6). After RP administration, the expression of CXCL2 and CXCL5 in BALF in the low-dose group was lower than that in the tumor group, but it was not statistically significant (P > .05; Figure 6). The high dose of RP had a much greater effect on CXCL2 and CXCL5 in BALF, and the expressions of these 2 chemokines were significantly reduced (P < .05; Figure 6).
Figure 6.
RP reduced the expression levels of CXCL2 and CXCL5 in BALF. The expression levels of CXCL2 and CXCL5 was detected by ELISA. Each bar represents mean ± SD (n = 5), and P values were obtained with 1-way ANOVA followed by Dunnett’s post hoc test. *P < .05.
Discussion
The development and metastasis of breast cancer is a complex process in which the interaction between tumors and distant metastatic target organs is complicated. Many researchers, including ourselves, have confirmed that the microenvironment in the lungs has changed before the lung metastasis of breast cancer.8,17,18 This change was to allow the circulating tumor cells to be retained, extravasate, and grow in the lung tissue better and more efficiently.
S100A8/A9 is significantly elevated in a variety of inflammatory processes and this protein complex has been used as a biomarker of inflammation for many years. Secreted S100A8/S100A9 proteins have also been implicated in cancer growth19,20 and in the establishment of a favorable environment for metastasis by promoting the migration of monocytes and tumor cells to metastatic sites.5,21 Once secreted into the extracellular space, S100A8/A9 acts as a chemical attractant, recruiting more inflammatory cells and producing an inflammatory microenvironment that promotes tumor development.22 Hiratsuka et al reported that the expression levels of S100A8 and S100A9 were higher in the lungs than in other organs (such as the liver and kidney) and that the higher levels were induced by primary tumors.5 Our previous studies found that the inflammatory role of extracellular S100A8/A9 induced abnormal microenvironment of lung tissue before metastasis.12 Those findings together with our findings suggested that S100A8/A9 might act as a guiding protein for cancer cells to metastasize to the lung.
In several chronic inflammatory disorders, S100A8/A9 could amplify pro-inflammatory responses by promoting leukocyte migration and inducing the release of cytokines and chemokines.22,23 IL-1β and IL-6 were important inflammatory biomarkers, which were also closely related to the development and metastasis of breast cancer. Our in vitro study demonstrated that the recombinant protein S100A8/A9 significantly mobilized the expressions of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α in 4T1 cells.12 A recent study provided evidence for a role of S100A8 and S100A9 in the secretion of IL-6, TNF-α, and IL-1β through the production of reactive oxygen species, which activates the transcription factor NF-κB in peripheral blood mononuclear cells.24 Both CXCL2 and CXCL5 are members of the chemokine CXC family, which have strong chemotactic capacities on neutrophils. This is because CXC receptor 2 is present on the surface of neutrophils, which has high affinity with CXCL2 and CXCL5.25 CXCL2 and CXCL5 would be produced when cells are stimulated by the inflammatory cytokine IL-1 or TNF-α.26,27 Therefore, we believe that RP could inhibit the release of S100A8/A9, thereby inhibiting the induction of chemokine factors (CXCL2 and CXCL5) for neutrophils, and ultimately avoiding the continuous amplification of the inflammatory response caused by the recruitment of immune cells to the site of inflammation.
It is now evident that S100A8/A9 was not only a biomarker for inflammatory conditions but also had a pathogenic function. S100A8/A9 could impair endothelial cell integrity and trigger endothelial cell apoptosis.28 Endothelial injury would cause increased vascular permeability and leukocyte adhesion and migration to the vessel wall intima. Indeed, we confirmed an increase in pulmonary vascular permeability by detecting leakage of EB in the lungs and total protein content in BALF.
In conditions of increased vascular permeability, leaky vessels allowed the extravasation of fibrinogen and exposure to lung tissues, where it was deposited as insoluble fibrin.29 Fibrinogen, a 340kDa glycoprotein that is mainly synthesized in liver and is transformed into fibrin through the effect of activated thrombin, plays an important role as a coagulation factor.30 Although fibrinogen is mostly secreted by hepatocytes, it is also secreted from the basolateral surface of alveolar epithelial cells in response to the induction of IL-6.31 Hyperfibrinogenemia is frequently observed in various malignancies and has been shown to play an important role in tumor progression, invasion, and distant metastasis.32 In fact, one of the possible metastasis-promoting mechanisms was due to a positive feedback loop between cancer-induced inflammation and fibrinogen expression levels. The increase in systemic inflammatory response caused by cancer progression greatly increased the expression level of fibrinogen, which in turn promoted the metastasis of cancer cells.30 Therefore, we hypothesized that RP could inhibit the extravasation of fibrinogen by protecting pulmonary vascular integrity and then interrupted its interaction with carcinoma in situ, thereby inhibiting the formation of the inflammatory pre-metastatic microenvironment.
However, it should be noted that our study has a limitation. Although we confirmed that traditional Chinese medicine formula RP improved the lung microenvironment in the pre-metastasis stage of breast cancer, we still lack the data on the inhibitory effects of RP on lung metastasis of breast cancer in animal models.
Conclusion
From the experimental results, we concluded that RP had a protective effect on the microenvironment of lung before breast cancer metastasis. It protected the integrity of the microvasculature, inhibited the release of S100A8/A9, reduced the extravasation of fibrinogen, and decreased the expression of pro-inflammatory factors (IL-1β and IL-6) and chemokines (CXCL2 and CXCL5), thereby inhibiting the promotion of inflammatory response to lung metastasis in breast cancer.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Natural Science Foundation of Shanghai (Nos. 17ZR1430700 and 17ZR1430900) and the 2018-2020 Three-Year Action Plan for Traditional Chinese Medicine Further Development in Shanghai (No. ZY (2018-2020)-CCCX-2002-10).
ORCID iD: Sheng Liu  https://orcid.org/0000-0003-0670-5389
https://orcid.org/0000-0003-0670-5389
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2. Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668-681. doi: 10.1016/j.ccell.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 3. Aguado BA, Bushnell GG, Rao SS, Jeruss JS, Shea LD. Engineering the pre-metastatic niche. Nat Biomed Eng. 2017;1:0077. doi: 10.1038/s41551-017-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maru Y. The lung metastatic niche. J Mol Med (Berl). 2015;93:1185-1192. doi: 10.1007/s00109-015-1355-2 [DOI] [PubMed] [Google Scholar]
- 5. Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369-1375. doi: 10.1038/ncb1507 [DOI] [PubMed] [Google Scholar]
- 6. Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77:3655-3665. doi: 10.1158/0008-5472.can-16-3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413-417. doi: 10.1038/nature16140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ye Y, Liu S, Wu C, Sun Z. TGFβ modulates inflammatory cytokines and growth factors to create premetastatic microenvironment and stimulate lung metastasis. J Mol Histol. 2015;46:365-375. doi: 10.1007/s10735-015-9633-4 [DOI] [PubMed] [Google Scholar]
- 9. Choi JH, Jin SW, Kim HG, et al. Saponins, especially platyconic acid A, from Platycodon grandiflorum reduce airway inflammation in ovalbumin-induced mice and PMA-exposed A549 cells. J Agric Food Chem. 2015;63:1468-1476. doi: 10.1021/jf5043954 [DOI] [PubMed] [Google Scholar]
- 10. Zhang W, Hou J, Yan X, et al. Platycodon grandiflorum saponins ameliorate cisplatin-induced acute nephrotoxicity through the NF-κB-mediated inflammation and PI3K/Akt/apoptosis signaling pathways. Nutrients. 2018;10:E1328. doi: 10.3390/nu10091328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin YC, Lin CH, Yao HT, et al. Platycodon grandiflorum (PG) reverses angiotensin II-induced apoptosis by repressing IGF-IIR expression. J Ethnopharmacol. 2017;205:41-50. doi: 10.1016/j.jep.2017.04.028 [DOI] [PubMed] [Google Scholar]
- 12. Ye Y, Pei L, Ding J, Wu C, Sun C, Liu S. Effects of platycodin D on S100A8/A9-induced inflammatory response in murine mammary carcinoma 4T1 cells. Int Immunopharmacol. 2019;67:239-247. doi: 10.1016/j.intimp.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 13. Chen Q. Methodology on Chinese Medicinal Pharmacology [in Chinese]. Beijing, China: People’s Medical Publishing House; 1994. [Google Scholar]
- 14. Ma C, Zhou Y, Zhou S, Zhao K, Lu B, Sun E. Preoperative peripheral plasma fibrinogen level is an independent prognostic marker in penile cancer. Oncotarget. 2017;8:12355-12363. doi: 10.18632/oncotarget.12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osuala KO, Sameni M, Shah S, et al. IL-6 signaling between ductal carcinoma in situ cells and carcinoma-associated fibroblasts mediates tumor cell growth and migration. BMC Cancer. 2015;15:584. doi: 10.1186/s12885-015-1576-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carmi Y, Dotan S, Rider P, et al. The role of IL-1β in the early tumor cell-induced angiogenic response. J Immunol. 2013;190:3500-3509. doi: 10.4049/jimmunol.1202769 [DOI] [PubMed] [Google Scholar]
- 17. Wu CF, Andzinski L, Kasnitz N, et al. The lack of type I interferon induces neutrophil-mediated pre-metastatic niche formation in the mouse lung. Int J Cancer. 2015;137:837-847. doi: 10.1002/ijc.29444 [DOI] [PubMed] [Google Scholar]
- 18. Hiratsuka S, Ishibashi S, Tomita T, et al. Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nat Commun. 2013;4:1853. doi: 10.1038/ncomms2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheikh AA, Vimalachandran D, Thompson CC, et al. The expression of S100A8 in pancreatic cancer-associated monocytes is associated with the Smad4 status of pancreatic cancer cells. Proteomics. 2007;7:1929-1940. doi: 10.1002/pmic.200700072 [DOI] [PubMed] [Google Scholar]
- 20. Ichikawa M, Williams R, Wang L, Vogl T, Srikrishna G. S100A8/A9 activate key genes and pathways in colon tumor progression. Mol Cancer Res. 2011;9:133-148. doi: 10.1158/1541-7786.mcr-10-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yan HH, Pickup M, Pang Y, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139-6149. doi: 10.1158/0008-5472.can-10-0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Srikrishna G. S100A8 and S100A9: new insights into their roles in malignancy. J Innate Immun. 2012;4:31-40. doi: 10.1159/000330095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simard JC, Cesaro A, Chapeton-Montes J, et al. S100A8 and S100A9 induce cytokine expression and regulate the NLRP3 inflammasome via ROS-dependent activation of NF-κB. PLoS One. 2013;8:e72138. doi: 10.1371/journal.pone.0072138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murphy PM, Baggiolini M, Charo IF, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145-176. [PubMed] [Google Scholar]
- 26. Guilloteau K, Paris I, Pedretti N, et al. Skin inflammation induced by the synergistic action of IL-17A, IL-22, oncostatin M, IL-1α, and TNF-α recapitulates some features of psoriasis. J Immunol. 2010;184:5263-5270. doi: 10.4049/jimmunol.0902464 [DOI] [PubMed] [Google Scholar]
- 27. Liu Y, Mei J, Gonzales L, et al. IL-17A and TNF-α exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro. J Immunol. 2011;186:3197-3205. doi: 10.4049/jimmunol.1002016 [DOI] [PubMed] [Google Scholar]
- 28. Viemann D, Barczyk K, Vogl T, et al. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood. 2007;109:2453-2460. doi: 10.1182/blood-2006-08-040444 [DOI] [PubMed] [Google Scholar]
- 29. Heinolainen K, Karaman S, D’Amico G, et al. VEGFR3 modulates vascular permeability by controlling VEGF/VEGFR2 signaling. Circ Res. 2017;120:1414-1425. doi: 10.1161/circresaha.116.310477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu X, Hu F, Yao Q, Li C, Zhang H, Xue Y. Serum fibrinogen levels are positively correlated with advanced tumor stage and poor survival in patients with gastric cancer undergoing gastrectomy: a large cohort retrospective study. BMC Cancer. 2016;16:480. doi: 10.1186/s12885-016-2510-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan HO, Simpson-Haidaris PJ. Cell type-specific differential induction of the human gamma-fibrinogen promoter by interleukin-6. J Biol Chem. 2006;281:12451-12457. doi: 10.1074/jbc.M600294200 [DOI] [PubMed] [Google Scholar]
- 32. Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41:960-970. doi: 10.1016/j.ctrv.2015.10.002 [DOI] [PubMed] [Google Scholar]