Abstract
This nonrandomized controlled trial determined the effects of Phoenix dactylifera palm date (Ajwa) intake on the number of infections and hospitalizations associated with fever, neutropenia, and mortality of pediatric cancer patients admitted between 2008 and 2017 to King Abdulaziz University Hospital (Jeddah, Saudi Arabia). Patients were eligible to be enrolled if they fulfilled the inclusion criteria, were not allergic to Ajwa, and were not enrolled in another study. Of 200 screened patients, 56 were included and 144 were excluded. Of the 56, 26 agreed to take Ajwa, and 30 served as controls. Both groups were assessed based on infection rates, frequency of hospital admissions for fever and neutropenia, and mortality rate. Background information regarding demographics, clinicopathological data, and treatment options was documented. Supplementation of Ajwa significantly reduced hospital admissions (for fever-associated neutropenia) and infections (P = .009 and P < .001, respectively). Off-treatment did not significantly differ between the Ajwa and control groups. The Ajwa group had a better survival rate in comparison to the non-Ajwa group (stratified log-rank P = .005), where the main cause of death of patients in the non-Ajwa group was disease progression associated with infections (77%). In summary, Ajwa intake during the standard treatment of pediatric cancer patients improved their treatment outcome.
Keywords: Phoenix dactylifera Ajwa, integrative, controlled study, diet, flavonoids, polyphenols, pediatric cancer patients
Introduction
Dates have always been a main source of energy for people living in the Arabian Peninsula, with essential diet components being dates and milk. The use of Phoenix dactylifera L, medicinal plants, and spices in the treatment of various diseases has been reported by the Prophet Mohammed.1,2 Dates are highly nutritious and made up of the following substances: fibers, carbohydrates, vitamins, salts and minerals, fatty acids, and amino acids.3
There are many varieties of palm trees, and the type of date used in this study is the Phoenix dactylifera known as Ajwa, which belongs to family Arecaceae.4 It is a soft, dark brown date that grows mainly in the Madinah region of Saudi Arabia and is known for its antimicrobial, anticancer, anti-inflammatory, and antioxidant properties and many other efficacies in protecting against diseases. In addition, it has the potential to increase levels of various blood components such as red blood cells, hemoglobin, reticulocytes, and platelet counts and has hematopoietic activity in Wistar rats.5 Ajwa has similar potential to commercially available drugs such as naproxen, aspirin, and ibuprofen based on its cyclooxygenase inhibitory effects.6
It has been reported that aqueous extracts of dates have antioxidant, antimicrobial and antimutagenic activities,7-11 and dates are considered as a good source of antioxidants,12 with high levels of polyphenols as compared with other dried fruits.13 Phenolic compounds and carotenoids found in dates provide them with their antioxidant activities to neutralize free radicals.14,15 Phoenix dactylifera are known to contain a wide range of phenolic compounds, including p-coumaric, ferulic, and sinapic acids; flavonoids; and procyanidins.7,16 In addition, other studies indicated that Ajwa also contains 13 flavonoid glycosides of luteolin, quercetin, and apigenin at various levels of their maturity.15,17 Research showed that dates have a strong potential for scavenging of free radicals and as such limit cancer progression and development.14,18
The anti-inflammatory, antihyperlipidemic, and antioxidant activities of bioactive compounds in Ajwa and Ajwa seed extract have been reviewed.6,19 The total polyphenols content of water extracts of Ajwa was the highest as compared with other kinds of dates.20 In a study to evaluate the anticancer activities of Libyan dates, it was reported that glucan, which is extracted from dates, had an anticancer effect related to the existence of (1-3) β-d-glucan linkages.21 In another study, dates demonstrated antilymphoma activity.15
Pediatric patients with cancer are immunocompromised, and as such, they are susceptible to many microbial infections that can have a detrimental effect on their life and the outcome of their treatment. For this reason, this study was undertaken to evaluate the effects of Ajwa in the diet as integrative therapy on the microbial infections for pediatric cancer patients admitted to the Pediatric Hematology/Oncology unit at King Abdulaziz University Hospital, King Abdulaziz University, in Jeddah, Saudi Arabia. This is the first clinical trial with Ajwa as a supplement to standard of care in pediatric patients with cancer.
Methods
Study Population
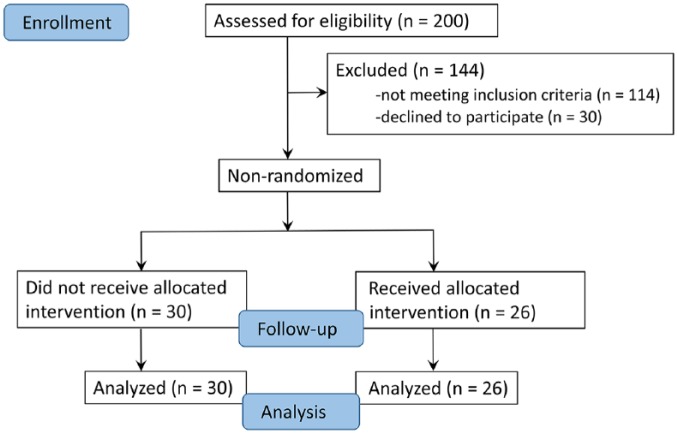
This study was a nonrandomized controlled trial of pediatric cancer patients attending King Abdulaziz University Hospital from 2008 to 2017. The study was conducted in accordance with and after approval from the ethical committee at our university (King Abdulaziz University). The pediatric patients’ guardians signed the consent form on their behalf. See Figure 1 for the screening for this study among the pediatric cancer patients. A total of 200 patients were screened. Patients were eligible to be enrolled in the study if the patient or the patient’s family consented to be involved and if the patient fulfilled the inclusion criteria, had no allergy to Ajwa, and was not enrolled in another study (Table 1). Of the 200 patients, 56 were included and 144 were excluded. Of those 56 patients, 26 (46.43%) agreed to take Ajwa and 30 (53.57%) served as controls and did not take Ajwa. Ajwa dates were purchased from a local market in Jeddah, KSA, and were organic in nature.
Figure 1.
Details of the Ajwa study among pediatric patients with cancer at King Abdulaziz University Hospital between 2008 and 2017.
Table 1.
Ajwa Study Inclusion and Exclusion Criteria for Pediatric Cancer Patients.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Agrees to intake of Ajwa | Refuses intake of Ajwa |
| Not participating in another study | Participating in another study |
| Signed the consent to participate in the study | Refused to sign the consent to participate in the study |
| Eligible for treatment even in advanced stage or first relapse | Terminal or palliative status |
In the Ajwa group, patients were given one piece of Ajwa with breakfast as a test dose daily. If they did not show any allergic reactions, Ajwa intake was increased to 3 pieces daily, taken with their daily breakfast for the whole duration of the standard treatment, including chemotherapy. The average weight of Ajwa intake was 7.33 ± 0.399 g. Patients were managed during chemotherapy and in between courses of chemotherapy per febrile neutropenia guidelines, with partial septic screening being done with every episode. Antibiotics were given during febrile neutropenia episodes per guidelines of the febrile neutropenia management protocol. Regular Ajwa intake was followed during the whole treatment period. Patients were administered Ajwa while they were undergoing chemotherapy/radiotherapy for the whole course of treatment under the supervision of the attending physician. While at home, parents monitored the Ajwa intake daily for their children. Study group patients were observed for the frequency of infections, febrile neutropenia episodes, number of days of hospitalization for supportive care of infections, and any treatment-related morbidity.
Bacteriological and Fungal Analyses
Bacterial isolates from blood were analyzed using an automated blood culture system (Bact/Alert, Organon Teknika Corp, Durham, NC). Positive samples were cultured on different selective media, including blood agar, chocolate agar, MacConkey agar, and Sabouraud dextrose agar [SDA]; (Saudi Prepared Media Laboratories, Riyadh, KSA). The inoculated plates were incubated in an ordinary incubator for a period of 18 to 24 hours (MacConkey agar and SDA at 35°C-37°C); however, in the case of cultures on blood agar or chocolate agar, they were incubated at 35°C to 37°C in a 5% to 10% CO2 incubator. Culture bottles positive for yeast cells were subcultured on SDA and incubated in an ordinary incubator for 18 to 24 hours at 35°C to 37°C.22
Primary organism identification was done with matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) on a VITEK mass spectrometer system (BioMerieux, Inc, Marcy l’Etoile, France). MALDI-TOF-MS was used as a rapid method for bacterial and fungal identification from microbial cultures.23 Final organism identification (bacterial and fungal) and antimicrobial susceptibility testing (AST) were done using a Vitek-2 system (BioMerieux, Inc). Further identification, and AST and minimum inhibitory concentration (MIC) for antimicrobial activity were also carried out using the Vitek-2 system according to the manufacturer’s protocols.24,25 According to CLSI guidelines that we follow in our Microbiology Lab regarding identification and AST of the isolated organisms from different specimens, yeast is completely identified with complete antifungal susceptibility in blood, sterile body fluid, and tissue samples, but isolated yeast of samples from other body sites such as sputum, midstream urine, and superficial wound are identified as yeast or Candida albicans or nonalbicans, and further identification and susceptibility is on physician request. The already isolated and identified Candida nonalbicans from blood samples of patients included in this study with antifungal susceptibility were C parapsiliosis and C dublinensis.
Urine samples were cultured on blood agar and MacConkey agar only; stool samples were cultured on selenite broth medium for 6 hours, and subcultured on xylose lysine deoxycholate agar medium; respiratory samples and swabs from different sources such as wounds, and superficial and deep skin lesions were processed and cultured on blood agar, MacConkey agar, or chocolate agar plates. All plates were incubated at 35°C to 37°C in an ordinary incubator for 18 to 24 hours; then, the organism’s identification and AST were determined using the Vitek-2 system.24,25
Serological Diagnosis of Varicella-Zoster
Identification of IgM against varicella-zoster virus (VZV) was carried out with ELISA (NovaTec, Immundiagnostica, GmbH, NovaLisa, Dietzenbach, Germany) according to the manufacturer’s instructions. In all, 100 µL of each control was dispensed into the respective wells. Then, 100 µL of diluted sample was dispensed into its respective well and the last well (A1) for the substrate blank, which had no reagents. All wells were incubated at 37°C for 1 hour. Then, the contents of the wells were aspirated, and the wells were washed 3 times. A total of 100 µL of VZV anti-IgM conjugate was dispensed into all wells except for A1 and was incubated at room temperature. This was followed by aspirating the contents of the wells and washing them 3 times. Also, 100 µL of 3,3′,5,5′-tetramethylbenzidine substrate solution was dispensed into all wells and incubated for 15 minutes at room temperature in the dark; then 100 µL of stop solution was added into all wells followed by measurement of sample absorbance at wavelengths of 450/620 nm within 30 minutes with the ELISA microwell plate reader. The sample was considered positive if its absorbance value was higher than 10% over the cutoff value.26
Statistical Analyses
Categorical data are described as frequencies and percentages, whereas continuous data are described as means and SDs. To assess the relationship between demographic and clinicopathological variables and the outcomes (infection rates and positive cultures, hospital admissions for febrile neutropenia, prolonged hospital admissions, off treatment, and mortality), the t-test, ANOVA, and χ2 tests were performed. The effect of Ajwa intake and mortality is presented as relative risk, absolute risk reduction (ARR), and number needed to treat (NNT) together with 95% CIs. Kaplan-Meier survival curves and the log-rank test were used to analyze mortality, stratified by Ajwa intake. Hazard ratio for Ajwa intake group compared with the non-Ajwa group was estimated using Cox regression. Data were analyzed using Stata version 13.0 (Stata Corp, College Station, TX), where tests were 2-sided, with 95% CI, and P < .05 was considered significant. All analyses were based on the intention-to-treat.
Results
Study Population
The study group was half male and half female, with a mean age of 9.1 ± 4.1 years. The majority (41%) of the patients came from Western Asia, 32% from Southeast Asia, 18% from Africa, and 9% from Southern Asia. A total of 68% of the patients had hematological cancer, and 32% had nonhematological cancer, including brain, Wilms, and neuroblastoma tumors. Most of the patients (73%) were at advanced stages, and 82% were not on radiotherapy (Table 2).
Table 2.
Demographics and Clinicopathological Characteristics of the Study Patients.
| Variables | Ajwa Intake |
P Value | |
|---|---|---|---|
| Yes, n (%) | No, n (%) | ||
| Gender | |||
| Male | 14 (54) | 17 (57) | .786 |
| Female | 12 (46) | 13 (43) | |
| Ethnicity | |||
| Sothern Asian | 2 (40) | 3 (60) | .502 |
| Southeastern Asian | 8 (44) | 10 (56) | |
| Western Asian | 11 (48) | 12 (52) | |
| African | 2 (20) | 8 (80) | |
| Type of cancer | |||
| Hematological | 17 (45) | 21 (55) | .418 |
| Nonhematological | 6 (33) | 12 (67) | |
| Relapse of cancer | |||
| Yes | 5 (56) | 4 (44) | .335 |
| No | 18 (38) | 29 (62) | |
| Stage of cancer | |||
| Early stage | 8 (53) | 7 (47) | .259 |
| Advanced stage | 15 (37) | 26 (63) | |
| Radiotherapy statusa | |||
| Yes | 5 (50) | 5 (50) | .527 |
| No | 18 (39) | 28 (61) | |
All patients were on chemotherapy, whereas radiotherapy was applied based on the chemotherapy protocol. Mean age ± SD = 9.1 ± 4.1 years.
Table 3 shows the number of infections with and without Ajwa supplementation. Staphylococcus aureus (methicillin-sensitive Staphylococcus aureus), Enterococcus faecium, E faecalis, and coagulase-negative Staphylococcus were significantly lower in the Ajwa group than in the non-Ajwa group.
Table 3.
Effect of Ajwa Intake on Infection Rate From Bacteria, Yeasts, Fungi, and Viruses.
| Non-Ajwa Group |
Ajwa Group |
P Value | |||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| Klebsiella pneumoniae | 0.1 ± 0.4 | 0-2 | 0.1 ± 0.3 | 0-1 | .734 |
| K pneumoniae (ESBL) | 0.06 ± 0.2 | 0-1 | 0.04 ± 0.2 | 0-1 | .782 |
| K oxytoca | 0.1 ± 0.2 | 0-1 | 0 | 0 | .237 |
| Escherichia coli | 0.2 ± 0.5 | 0-2 | 0.2 ± 0.4 | 0-1 | .967 |
| Escherichia coli (ESBL) | 0.2 ± 0.5 | 0-2 | 0.1 ± 0.3 | 0-1 | .490 |
| Proteus mirabilis | 0.03 ± 0.2 | 0-1 | 0.04 ± 0.2 | 0-1 | .798 |
| Spice organismsa | 0.1 ± 0.4 | 0-2 | 0.1 ± 0.3 | 0-1 | .734 |
| Pseudomonas aeruginosa | 0.04 ± 0.2 | 0-1 | 0 | 0 | .234 |
| Pseudomonas species | 0.03 ± 0.2 | 0-2 | 0 | 0 | .4 |
| Acinetobacter baumannii | 0.1 ± 0.3 | 0-1 | 0.2 ± 0.5 | 0-2 | .633 |
| Stenotrophomonas maltophilia | 0.3 ± 0.8 | 0-3 | 0 | 0 | .109 |
| Coagulase-negative Staphylococcus | 1.6 ± 1.8 | 0-8 | 0.2 ± 0.4 | 0-1 | .001b |
| Staphylococcus aureus (MSSA) | 0.2 ± 0.6 | 0-2 | 0 | 0 | .043b |
| Staphylococcus aureus (MRSA) | 0.2 ± 0.4 | 0-2 | 0.04 ± 0.2 | 0-1 | .281 |
| Enterococcus faecalis | 0.1 ± 0.3 | 0-1 | 0 | 0 | .086 |
| Enterococcus faecium | 0.2 ± 0.4 | 0-1 | 0 | 0 | .018b |
| Streptococcus viridans group | 0.1 ± 0.4 | 0-2 | 0.04 ± 0.2 | 0-1 | .412 |
| Streptococcus agalactiae | 0.1 ± 0.4 | 0-2 | 0 | 0 | .263 |
| Streptococcus pneumoniae | 0.03 ± 0.2 | 0-1 | 0 | 0 | .4 |
| Candida albicans | 0.1 ± 0.3 | 0-2 | 0 | 0 | .409 |
| Candida species | 0.4 ± 1.4 | 0-8 | 0.04 ± 0.2 | 0-1 | .214 |
| Aspergillus species | 0.04 ± 0.2 | 0-1 | 0 | 0 | .234 |
| Varicella zoster | 0.03 ± 0.2 | 0-1 | 0 | 0 | .4 |
Abbreviations: ESBL, extended-spectrum β-lactamase; MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
Spice organisms are Serratia, Morganella, Providentia, Enterobacter, Citrobacter spp, and Proteus vulgaris group. There are inducible chromosomal β-lactamases that are intrinsically resistant to penicillins, and narrow-spectrum cephalosporins and third-generation cephalosporins are not recommended because rapid resistance can develop quickly during therapy.
P < .05.
The effect of Ajwa intake on infection rate is listed in Table 4, showing a decrease after Ajwa intake in the infection rate for most of the organisms. The effect of Ajwa intake on infection rates and frequency of hospital admissions related to fever and neutropenia is shown in Table 5. Patients in the Ajwa group had significantly lower infection rates compared with patients in the non-Ajwa group (P < .001). Similarly, hospital admissions related to fever and neutropenia were lower in Ajwa patients compared with non-Ajwa patients (P = .009).
Table 4.
Infection Rates Before and After Ajwa Intake Among 12 Randomly Screened Pediatric Oncology Patients.
| Before Ajwa Intake |
After Ajwa Intake |
|||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| Klebsiella pneumoniae | 0.6 ± 0.9 | 0-3 | 0.2 ± 0.4 | 0-1 |
| K pneumoniae (ESBL) | 0.8 ± 2.3 | 0-8 | 0.1 ± 0.3 | 0-1 |
| K oxytoca | — | — | — | - |
| Escherichia coli | 0.7 ± 1.0 | 0-3 | 0.4 ± 0.5 | 0-1 |
| Escherichia coli (ESBL) | 0.4 ± 0.9 | 0-3 | 0.3 ± 0.5 | 0-1 |
| Proteus mirabilis | 0.3 ± 0.9 | 0-3 | 0.1 ± 0.3 | 0-1 |
| Spice organisms | 0.2 ± 0.4 | 0-1 | 0.2 ± 0.4 | 0-1 |
| Pseudomonas aeruginosa | 1.5 ± 4.6 | 0-16 | 0 | 0 |
| Pseudomonas species | 0.1 ± 0.3 | 0-1 | 0 | 0 |
| Acinetobacter baumannii | 0.4 ± 0.8 | 0-2 | 0.3 ± 0.7 | 0-2 |
| Stenotrophomonas maltophilia | — | — | — | - |
| Coagulase negative Staphylococcus | 1.8 ± 4.8 | 0-17 | 0.3 ± 0.5 | 0-1 |
| Staphylococcus aureus (MSSA) | 0.2 ± 0.6 | 0-3 | 0 | 0 |
| Staphylococcus aureus (MRSA) | 0.1 ± 0.3 | 0-1 | 0.1 ± 0.3 | 0-1 |
| Enterococcus faecalis | 0.3 ± 0.5 | 0-1 | 0 | 0 |
| Enterococcus faecium | 0.4 ± 1.4 | 0-5 | 0 | 0 |
| Streptococcus viridans group | 0.1 ± 0.3 | 0-1 | 0.1 ± 0.3 | 0-1 |
| Streptococcus agalactiae | — | — | — | - |
| Streptococcus pneumoniae | — | — | — | - |
| Candida albicans | 0.3 ± 1.2 | 0-4 | 0 | 0 |
| Candida species | 0.2 ± 0.4 | 0-1 | 0.1 ± 0.3 | 0-1 |
| Aspergillus species | 0.1 ± 0.3 | 0-1 | 0.1 ± 0.3 | 0-1 |
| Varicella zoster | — | — | ||
Abbreviations: MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
Table 5.
Effect of Ajwa Intake on Infection Rates and Frequency of Hospital Admissions Related to Fever and Neutropenia.
| Ajwa Group, Mean ± SD | Non-Ajwa Group, Mean ± SD | P Value | |
|---|---|---|---|
| Infectionsa | 1.1 ± 1.8 | 5.1 ± 3.7 | <.001 |
| Hospital admissions F/Nb | 5 ± 5.5 | 17.1 ± 20.7 | .009 |
Number of positive cultures for the entire period of treatment.
F/N, fever and neutropenia per year.
In analyses adjusted for sex, age, ethnicity, diagnosis, type of cancer, relapse, and radiotherapy status, the association between prolonged admission and being off treatment to Ajwa is displayed in Table 6. Ajwa intake was associated with decreased odds ratio of prolonged admission (adjusted odds ratio = 2.6; 95% CI = 0.7-0.9; P = .002). Table 6 shows that patients on Ajwa as a supplement to their ongoing conventional therapy had a better survival rate as compared with controls (P = .005).
Table 6.
Demographics and Clinicopathological Characteristics According to Survival Rates Among the Sample Patients.
| Variables | Survival Status |
P Value | |
|---|---|---|---|
| Living, n (%) | Dead, n (%) | ||
| Gender | |||
| Male | 22 (51) | 6 (46) | .752 |
| Female | 21 (49) | 7 (54) | |
| Ethnicity | |||
| Sothern Asian | 4 (80) | 1 (20) | .558 |
| Southeastern Asian | 15 (83) | 3 (17) | |
| Western Asian | 18 (78) | 5 (22) | |
| African | 6 (60) | 4 (40) | |
| Type of cancer | |||
| Hematological | 31 (82) | 7 (18) | .217 |
| Nonhematological | 12 (67) | 6 (33) | |
| Relapse of cancer | |||
| Yes | 8 (89) | 1 (11) | .348 |
| No | 35 (74) | 12 (26) | |
| Stage of cancer | |||
| Early stage | 14 (93) | 1 (7) | .076 |
| Advanced stage | 29 (70) | 12 (29) | |
| Radiotherapy status | |||
| Yes | 6 (60) | 4 (40) | .165 |
| No | 37 (80) | 9 (20) | |
Of the 30 participants in the non-Ajwa group, 13 died. This is in contrast to the Ajwa group, where all survived. The main causes of death for the 13 deceased patients were disease progression associated with infection (76.9%), infection (7.8%), cardiac failure (7.7%), and disease progression (7.6%). Ajwa intake was associated with decreased relative risk of death (adjusted odds ratio = 0.12; 95% CI = 0.02 to 0.86; P = .01); ARR = 0.23 (95% CI = 0.14) to 0.5; and NNT = 3.1 (95% CI = 2 to 7.3). Patients on Ajwa had a significantly lower mortality, with log-rank P = .005 (hazard ratio = 0.1, 95% CI = 0.01-0.74, P = .024; Figure 2).
Figure 2.
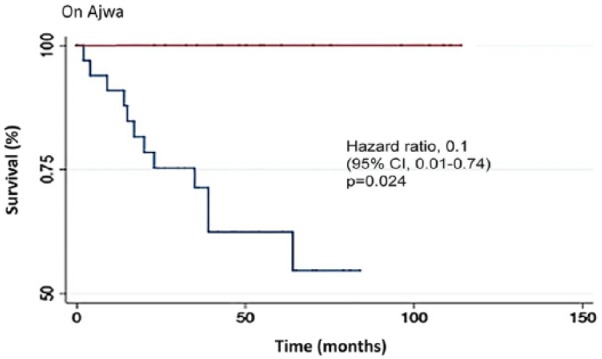
Kaplan-Meier curves for overall survival among the study sample stratified by Ajwa.
Discussion
The use of chemotherapy and radiotherapy in the treatment of pediatric cancer patients results in adverse side effects as well as bacterial infections, which may be recurrent and will compromise the outcome of the treatment.27 Diet supplementation with Ajwa dates is an adjunct treatment that showed enhanced efficacy of the conventional treatment. The dates may have antileukemia and/or antimicrobial activities. One of the most serious problems that cancer patients suffer from is the antimicrobial resistance of certain organisms. Such a resistance may lead in many instances to the demise of the patient. Research on Phoenix dactylifera has shown that it plays a significant role in the prevention and/or treatment of bacterial, fungal, and viral diseases.28
The current study did not examine the bioactive ingredients responsible for the antimicrobial activities of the dates achieved in correlation with blood levels of the bioactive ingredients. Additionally, eating the dates twice a day (3-5 dates) rather than what was done in the current study, where the date regimen was once a day in the morning with breakfast (3-5 dates), might shed light on attaining the same or greater benefits when given twice versus once a day.
The antibacterial efficacy of Ajwa has been reported in the literature. For instance, methanol and acetone extracts of leaves and pits from Phoenix dactylifera resulted in inhibition of growth of Fusarium species and Gram-positive and Gram-negative bacteria.29-31 In a study on the antimicrobial activity of extracts of Phoenix dactylifera against Klebsiella pneumoniae and Escherichia coli, results showed positive effects, and its use resulted in reducing the side effects associated with methylprednisolone. Those extracts also had antibacterial effects against enteric bacteria such as Enterococcus faecalis, which are considered a main source of infection in immunocompromised individuals such as leukemia patients.32
The antibacterial activity of Phoenix dactylifera could be related to the composition of the different parts of the plant, which include agents known for their antibacterial properties such as alkaloids, flavonoids, and tannins. In a study by Perveen et al,33 the antibacterial activity of crude water, methanol, and acetone extracts of pits and seeds of Phoenix dactylifera were investigated against 7 pathogenic bacteria, including Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Streptococcus pyogenes, Pseudomonas aeruginosa, Enterococcus faecalis, and Shigella flexeneri. Both methanol and acetone extracts of pits and seeds had good antibacterial activity against all bacteria except Enterococcus faecalis. However, the water extract had very little effect against the test bacteria. The pit extracts of Phoenix dactylifera showed better antibacterial effect than seed extracts. The most sensitive strain was Streptococcus pyogenes as determined with MIC after extraction of the seeds and pits with either methanol or acetone.33
In a study by Al-Daihan and Bhat34 using aqueous, methanol, and acetone extracts of leaves, seeds, fruits, and bark of the date tree, results showed a potent effect against both Gram-positive (Staphylococcus aureus, Streptococcus pyogenes) and Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa) as measured using a disc diffusion method. Data indicated that any extract from different parts of the plant had an antibacterial potential. Less efficacy was noted in aqueous extracts as compared with methanol and acetone extracts. Extracts using the parts that are exposed to light, such as the fruit and leaf, had better antibacterial activity than extracts of those not exposed to light. The best results for bacterial inhibition were noted with acetone fruit extract and methanol leaf extract, with best efficacy noted against Staphylococcus aureus and Escherichia coli, respectively. Phytochemical analysis indicated that carbohydrates and alkaloids were found in all parts of the tree and flavonoids, steroids, saponins, and tannins were only present in certain specific parts of the tree.34
It has been also documented that pits are more effective than other antibiotics, and it is reported to be a result of differences in resistance of bacteria. Methanolic pits extract of Phoenix dactylifera also has activity against Escherichia coli and K pneumoniae.8,35
Ajwa has also been investigated as an antifungal and found to have an effect. Flavonoids, tannins, alkaloids, and coumarins were detected in the rachis extracts of 8 date palm Phoenix dactylifera cultivars analyzed with phytochemical screening and bioautography against Fusarium oxysporum f sp albedinis (Foa). The best antifungal activity was mostly obtained with dichloromethane extract of the cultivar “Bent-Cherk” rachis followed by the ethyl acetate extract of the cultivar “Rotbi” rachis.36 Bokhari and Perveen30 evaluated the antifungal potential of water, methanol, and acetone extract of leaves and pits of Phoenix dactylifera against 7 pathogenic fungi (F oxysporum, Fusarium sp, F solani, Aspergillus flavus, Alternaria alternata, Alternaria sp, and Trichoderma sp) using the standard agar well diffusion and dilution methods. Methanol extract showed the most potency against Alternaria alternata, whereas water extracts had no effects on the growth of these fungi. Methanolic extracts had a more potent antifungal activity against all the test fungi except for Aspergillus flavus as compared with acetone extracts. The results strongly demonstrated that 2 tested varieties of Phoenix dactylifera extracts had pronounced antifungal properties. Variations in the inhibition noted may be related to the levels of the phytochemicals present in those 2 varieties.30
Studies conducted by us show that antioxidants such as vitamin C, l-lysine, and green tea extract epigallocatechin-3-gallate play an important role in the induction of apoptosis in both HTLV-1 positive and negative malignant T-lymphocytes.7,37,38 In addition, we showed that synergism occurs among these antioxidants and results in better efficacy.37 Other published work indicated that plant phenolic compounds such as flavonoids are potent antioxidants and have anticancerous potential.39,40
The use of Ajwa fruits as a supplement in the treatment regimens of leukemia patients warrants further investigations. Large-scale clinical trials are required to elucidate this novel finding.
Conclusions
Patients with cancer are immunocompromised and as such are susceptible to many infections, which will have an impact on treatment. In this study, Ajwa was used as integrative or adjunct therapy together with standard treatment. This adjunct therapy is safe, effective, and affordable and helped improve the outcomes of the standard therapy.
Based on our results, it would be prudent to consider the use of Ajwa as an adjunct treatment to reduce microbial infections, which could result in better outcomes with conventional treatment in those pediatric patients who are undergoing cancer treatment. It is likely that the adverse effects of conventional treatment will be reduced with Ajwa as adjuvant treatment. Using Ajwa is likely to be a safe, effective, and affordable approach of supplementation in pediatric cancer management. Ajwa dates are a natural, safe nutritional substance, and we recommend regular Ajwa intake with standard cancer treatment as an integrative approach.
Acknowledgments
The authors would like to acknowledge all comprehensive medical staff at King Abdulaziz University Hospital. Sincere thanks to the clinical dietitian.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Dar-ul-Iman Healing. Foods of the prophet (s.a.w.s). http://chishti.org/foods_of_the_prophet.htm. Accessed May 8, 2018.
- 2. Marwat SK, Khan MA, Fazal u-R, Bhatti IU. Aromatic plant species mentioned in the Holy Qura’n and Ahadith and their ethnomedicinal importance. Pak J Nutr. 2009;8:1472-1479. [Google Scholar]
- 3. Al-Shahib W, Marshall RJ. The fruit of the date palm: its possible use as the best food for the future? Int J Food Sci Nutr. 2003;54:247-259. [DOI] [PubMed] [Google Scholar]
- 4. Al-Mssallem IS, Hu S, Zhang X, et al. Genome sequence of the date palm Phoenix dactylifera L. Nat Commun. 2013;4:2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Onuh SN, Ukaejiofo EO, Achukwu PU, Ufelle SA, Okwuosa CN, Chukwuka CJ. Haemopoietic activity and effect of crude fruit extract of Phoenix dactylifera on peripheral blood parameters. Int J Biol Med Res. 2012;3:1720-1723. [Google Scholar]
- 6. Zhang CR, Aldosari SA, Vidyasagar PSPV, Nair KM, Nair MG. Antioxidant and anti-inflammatory assays confirm bioactive compounds in Ajwa date fruit. J Agric Food Chem. 2013;61:5834-5840. [DOI] [PubMed] [Google Scholar]
- 7. Hamad I, AbdElgawad H, Al Jaouni S, et al. Metabolic analysis of various date palm fruit (Phoenix dactylifera L.) cultivars from Saudi Arabia to assess their nutritional quality. Molecules. 2015;20:13620-13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saddiq AA, Bawazir AE. Antimicrobial activity of date palm (Phoenix dactylifera) pits extracts and its role in reducing the side effect of methyl prednisolone on some neurotransmitter content in the brain, hormone testosterone in adulthood. Acta Hort. 2010;882:665-690. doi: 10.17660/ActaHortic.2010.882.74 [DOI] [Google Scholar]
- 9. Vayalil PK. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae). J Agric Food Chem. 2002;50:610-617. [DOI] [PubMed] [Google Scholar]
- 10. Mohamed DA, Al-Okbi SY. In vitro evaluation of antioxidant activity of different extracts of Phoenix dactylifera L. Fruits as functional foods. Dtsch Lebensmitt Rundsch. 2005;101:305-308. [Google Scholar]
- 11. Ragab AR, Elkablawy MA, Sheik BY, Baraka HN. Antioxidant and tissue-protective studies on Ajwa extract: dates from Al-Madinah Al-Munawarah, Saudi Arabia. J Environ Anal Toxicol. 2013;3:163. [Google Scholar]
- 12. Zineb G, Boukouada M, Djeridane A, Saidi M, Yousfi M. Screening of antioxidant activity and phenolic compounds of various date palm (Phoenix dactylifera) fruits from Algeria. Mediterr J Nutr Metab. 2012;5:119-126. [Google Scholar]
- 13. Vinson JA, Zubik L, Bose P, Samman N, Proch J. Dried fruits: excellent in vitro and in vivo antioxidants. J Am Coll Nutr. 2005;24:44-50. [DOI] [PubMed] [Google Scholar]
- 14. Al-Farsi M, Alasalvar C, Morris A, Baron M, Shahidi F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J Agric Food Chem. 2005;53:7592-7599. [DOI] [PubMed] [Google Scholar]
- 15. Biglari F, AlKarkhi AFM, Easa AM. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem. 2008;107:1636-1641. [Google Scholar]
- 16. Gu L, Kelm MA, Hammerstone JF, et al. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J Agric Food Chem. 2003;51:7513-7521. [DOI] [PubMed] [Google Scholar]
- 17. Hong YJ, Tomas-Barberan FA, Kader AA, Mitchell AE. The flavonoid glycosides and procyanidin composition of Deglet Noor dates (Phoenix dactylifera). J Agric Food Chem. 2006;54:2405-2411. [DOI] [PubMed] [Google Scholar]
- 18. Guo C, Yang J, Wei J, Li Y, Xu J, Jiang Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res. 2003;23:1719-1726. [Google Scholar]
- 19. Khan TJ, Kuerban A, Razvi SS, et al. In vivo evaluation of hypolipidemic and antioxidative effect of “Ajwa” (Phoenix dactylifera L.) date seed-extract in high-fat diet-induced hyperlipidemic rat model. Biomed Pharmacother. 2018;107:675-680. [DOI] [PubMed] [Google Scholar]
- 20. Saleh E, Tawfik M, Abu-Tarbous H. Phenolic contents and antioxidant activity of various date palm (Phoenix dactylifera L.) fruits from Saudi Arabia. Food Nutr Sci. 2011;2:1134-1141. [Google Scholar]
- 21. Ishurd O, Kennedy JF. The anti-cancer activity of polysaccharide prepared from Libyan dates (Phoenix dactylifera L.). Carbohydr Polym. 2005;59:531-535. [Google Scholar]
- 22. Kocoglu ME, Bayram A, Balci I. Evaluation of negative results of BacT/Alert 3D automated blood culture system. J Microbiol. 2005;43:257-259. [PubMed] [Google Scholar]
- 23. Dubois D, Grare M, Prere MF, Segonds C, Marty N, Oswald E. Performances of the Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for rapid identification of bacteria in routine clinical microbiology. J Clin Microbiol. 2012;50:2568-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hata DJ, Hall L, Fothergill AW, Larone DH, Wengenack NL. Multicenter evaluation of the new VITEK 2 advanced colorimetric yeast identification card. J Clin Microbiol. 2007;45:1087-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wallet F, Loïez C, Renaux E, Lemaitre N, Courcol RJ. Performances of VITEK 2 colorimetric cards for identification of gram-positive and gram-negative bacteria. J Clin Microbiol. 2005;43:4402-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harper DR, Kangro HO, Heath RB. Serological responses in varicella and zoster assayed by immunoblotting. J Med Virol. 1988;25:387-398. [DOI] [PubMed] [Google Scholar]
- 27. Derradji H, Abou-El-Ardat K, Faraj K, Baatout S, Harakeh S. Antioxidants: a new approach to tackle radiation induced cancers. In: Sharma RK, Arora R. eds. Herbal Medicine: A Cancer Chemopreventive and Therapeutic Perspective. Delhi, India: Jaypee Brothers Medical Publishers; 2009:441-472. [Google Scholar]
- 28. Mallhi TH, Qadir MI, Ali M, Ahmad B, Khan YH, Rehman A. Review: Ajwa date (Phoenix dactylifera)—an emerging plant in pharmacological research. Pak J Pharm Sci. 2014;27:607-616. [PubMed] [Google Scholar]
- 29. Ammar NM, Abou El-Kassem LT, El-Sayed NH, Calabria LM, Mabry TJ. Flavonoid constituents and antimicrobial activity of date (Phoenix dactylifera L.) seeds growing in Egypt. Med Arom Plant Sci Biotech. 2009;3:1-5. [Google Scholar]
- 30. Bokhari NA, Perveen K. In vitro inhibition potential of Phoenix dactylifera L. extracts on the growth of pathogenic fungi. J Med Plant Res. 2012;6:1083-1088. [Google Scholar]
- 31. Jassim SA, Naji MA. In vitro evaluation of the antiviral activity of an extract of date palm (Phoenix dactylifera L.) pits on a Pseudomonas phage. Evid Based Complement Alternat Med. 2010;7:57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aamir J, Kumari A, Khan MN, Medam SK. Evaluation of the combinational antimicrobial effect of Annona Squamosa and Phoenix dactylifera seeds methanolic extract on standard microbial strains. Int Res J Biol Sci. 2013;2:68-73. [Google Scholar]
- 33. Perveen K, Bokhari NA, Soliman DAW. Antibacterial activity of Phoenix dactylifera L. leaf and pit extracts against selected gram negative and gram positive pathogenic bacteria. J Med Plants Res. 2012;6:296-300. [Google Scholar]
- 34. Al-daihan S, Bhat RS. Antibacterial activities of extracts of leaf, fruit, seed and bark of Phoenix dactylifera. Afr J Biotech. 2012;11:10021-10025. [Google Scholar]
- 35. Al-Judaibi A, Al-Zahrani A, Altammar KA, Ismail SB, Darweesh NT. Comparative study of antimicrobial activity of plant extracts from several regions of Asia. Am J Pharmacol Toxicol. 2014;9:139-147. [Google Scholar]
- 36. Boulenouar N, Marouf A, Cheriti A. Antifungal activity and phytochemical screening of extracts from Phoenix dactylifera L. cultivars. Nat Prod Res. 2011;25:1999-2002. [DOI] [PubMed] [Google Scholar]
- 37. Harakeh S, Abou-Khouzam R, Damanhouri GA, et al. Effects of nutrients on matrix metalloproteinases in human T-lymphotropic virus type 1 positive and negative malignant T-lymphocytes. Int J Oncol. 2014;45:2159-2166. [DOI] [PubMed] [Google Scholar]
- 38. Harakeh S, Diab-Assaf M, Khalife JC, et al. Ascorbic acid induces apoptosis in adult T-cell leukemia. Anticancer Res. 2007;27(1A):289-298. [PubMed] [Google Scholar]
- 39. Middleton E, Jr, Kandaswamy C. The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. In: Harborne JB, ed. The Flavonoids: Advances in Research Since 1986. London, England: Chapman & Hall; 1993:619-652. [Google Scholar]
- 40. Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152-159. [Google Scholar]