Abstract
This meta-analysis evaluates the clinical evidence for the addition of traditional Chinese medicines (TCMs) to 5-fluorouracil (5-FU)-based regimens for colorectal cancer (CRC) in terms of tumor response rate (TRR). Five electronic databases were searched for randomized controlled trials of 5-FU-based chemotherapy combined with TCMs compared to the same 5-FU-based regimen. Forty-five randomized controlled trials were involved in this study, and all the data were analyzed by Stata software (version 14.0). Our results suggested that the TRR of the group with TCMs combined with 5-FU-based regimens was higher than that in the group with 5-FU regimens alone (risk ratio [RR] 1.36 [1.25-1.49], I2 = 0%). Furthermore, both nonoral administration (RR 1.51 [1.29-1.76], I2 = 0%) and oral administration (RR 1.31 [1.18-1.45], I2 = 0%) of TCMs showed benefits to the CRC treatment. Further sensitivity analysis of specific plant-based TCMs found that fuling, sheshecao, banzhilian, eshu, baizhu, huangqi, yiyiren, and dangshen had significantly higher contributions to the results of the risk ratio. Therefore, TCMs may have the potential to improve the efficacy of 5-FU-based chemotherapy for CRC.
Keywords: 5-fluorouracil, colorectal cancer, traditional Chinese medicine, meta-analysis
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer and the third leading cause of cancer mortality worldwide.1 Despite the advancement in clinical oncology, approximately 25% of patients present with metastases on diagnosis and almost 50% of patients with CRC will develop metastases,2 contributing to the low survival rates reported for CRC. Chemotherapy is still the main treatment for metastatic and local late-stage CRC; however, side effects and resistance to chemotherapy have shown the limitations of current chemotherapy and led to the search for alternative treatments.
5-Fluorouracil (5-FU) is a pyrimidine analogue that incorporates into the DNA molecule to suppress thymidylate synthase.3 This subsequently inhibits the synthesis of pyrimidine thymidine required for DNA replication so that actively dividing cancerous cells will undergo apoptosis.3 So far, 5-FU-based chemotherapy remains the backbone of CRC treatment, and it has been demonstrated that the use of combination regimens of 5-FU with cytotoxic agents, such as 5-FU combination plus either irinotecan (FOLFIRI), oxaliplatin (FOLFOX), or capecitabine (CAPOX or XELOX), have significantly improved the survival of patients with advanced CRC.4
Traditional Chinese medicines (TCMs), which emphasize overall coordination of the environment inside and outside the human body, have been widely used for complementary treatment of cancer patients including CRC in China.5 Application of TCMs as an adjuvant cancer therapy has been reported to enhance the efficacy of chemotherapy and to help reduce adverse effects of anticancer drugs.6 However, whether the TCMs combined with 5-FU-based regimens may be more effective than 5-FU-based chemotherapy alone is still uncertain.
In this article, we compared the combination of 5-FU-based chemotherapy plus TCMs with 5-FU regimens alone in terms of tumor response rate (TRR) for CRC. As a result, our results suggested that the TRR of the group with TCMs combined with 5-FU-based regimens is remarkably higher than that in the group with 5-FU regimens alone. This meta-analysis provides evidence that TCMs could have the potential to improve the efficacy of 5-FU-based chemotherapy for CRC.
Methods
We have searched PubMed, EMBASE, Cochrane CENTRAL, China Academic Journals (CNKI), and Chinese Science and Technology Journals (CQVIP) databases for relevant articles from their inceptions to July 2018. The keywords are the following: (a) Disorder: colorectal cancer and related terms; (b) Intervention: fluorouracil (5-FU), herbal medicine, traditional medicine, and related terms; and (c) Study type: randomized controlled trial and related terms. The test arm and the control arm of the randomized controlled trials that are involved in the meta-analyses were fluorouracil regimens combined with TCM intervention and fluorouracil regimen alone, respectively. According to the administration type of the TCMs, the studies were divided into 2 groups: oral administration group and non-oral (injection products) administration group. All CRC cases in each study were confirmed by histopathological examination. The screening and review were performed by 2 independent reviewers searching the literature and extracting data independently.
According to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, tumor response criteria included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The primary clinical outcome was TRR. CR and PR were included in data pooling as TRR. Relative risk (RR) estimates were calculated using the Stata software application (version 14.0; StataCorp, College Station, TX). Methods were based on Cochrane Handbook 5.1.0. Subgroup analyses based on heterogeneity between trials used the χ2-based Q statistic and were considered statistically significant at a P value less than .05 or I2 ≥ 50%. In the presence of heterogeneity, data were analyzed in a random-effects model; otherwise, a fixed-effects model was used. A statistical test resulting in a P value less than .05 was considered to indicate a statistically significant difference.
Studies with zero events were included to avoid overestimation of effect. When the same outcome was reported by more than 10 studies, publication bias was assessed using a Begg funnel plot.
Results
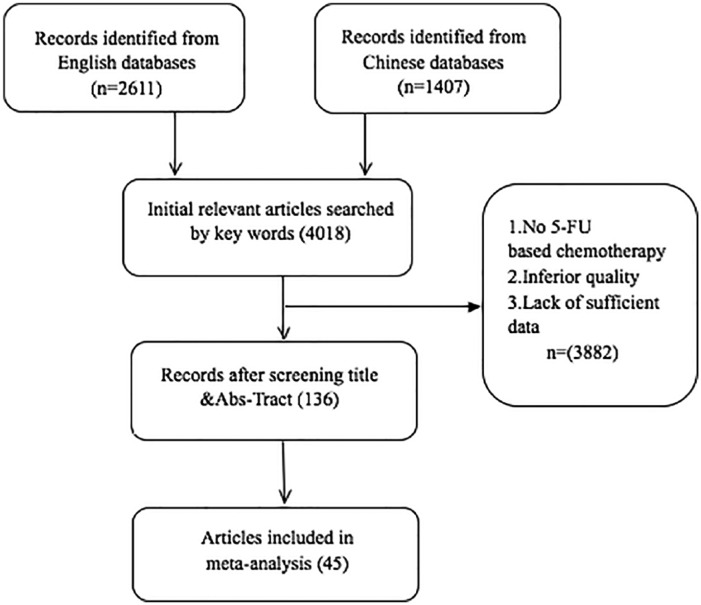
There were 4018 potential relevant citations that were located according to the search strategy. Following screening, 45 studies were included in the meta-analysis (Figure 1).7-51 All studies met the requirements of 5-FU regimen combined with TCM intervention versus 5-FU regimen alone. The TRR was provided as well. These 45 studies were classified as oral administration group (37 studies) and non-oral administration group (8 studies), which enrolled 2986 participants with 1544 participants in the experimental groups and 1442 participants in the control groups. All the clinical characteristics of the participants are summarized in Table 1, including the sample size, dosage and duration of TCM intervention, and dose and cycles of 5-FU regimen, among others.
Figure 1.
Flow diagram of the search and selection process of randomized controlled trials of 5-FU regimens combined with TCM for CRC.
Table 1.
Characteristics of Randomized Controlled Trials of Traditional Chinese Medicines (TCMs) Combined With Fluorouracil-Based Regiments for Colorectal Cancer (CRC).
| First Author (Year) | Sample Size T/C; Gender (M) T/C; Age T/C | TNM (T/C); KPS/ECOG | TCM Intervention; Dosage and Duration | Fluorouracil (5-FU) Regimen; Dose, Cycles (T/C) | Risk of Bias (SG, AC, BPt, BOA [Obj.], IOD, SOR) |
|---|---|---|---|---|---|
| Li W (2005) | 15/15; 15/15; 62.26 ± 10.40/64.00 ± 9.99 | IV (all); ACRC; KPS ≥ 60 | Jianpihuashiquyufang decoction, 300 mL, bid, for 8 weeks | FOLFOX: Ox. 130 mg/m2, 2 hours ID, CF 200 mg/m2, ivgtt, days 1-2, 5-FU 500 mg/m2, ivgtt, days 1-2, 4 weeks/cycle (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Yuan D (2010) | 15/15; 18/12; 63.80 ± 7.223/57.13 ± 9.613 | IV (all); ACRC; KPS ≥ 60 | Jianpihuashiquyufang decoction, 200 mL, bid, for 2 mouths | FOLFOX: Ox. 130 mg/m2, 2 hours ID, day 1, CF 100 mg/m2, ivgtt, days 1-5, 5-FU 500 mg/m2, ivgtt, days 1-5, 4 weeks/cycle (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Zhu L (2012) | 20/20; 17/15; 59.20 ± 12.34/56.40 ± 7.843 | III: 5/7; IV: 15/13; KPS ≥ 60 | Jianpihuashiquyufang decoction, 200 mL, bid, for 6 weeks | FOLFIRI: CPT-11 180 mg/m2, 90 minutes, day 1, CF 200 mg/m2, 2 hours, ivgtt, day 1, 5-FU 400 mg/m2, ivgtt, day 1, 2400-3000 mg/m2, 46 hours, 2 weeks/cycles, for 3 cycles | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Yu Q (2010) | 20/20; 12/13; 61.70 ± 7.63/60.6 ± 8.37 | III: 6/7; IV: 14/13; KPS ≥ 60 | Jianpihuayujiedufang decoction, 300 mL, bid, for 6 weeks | FOLFOX: Ox. 130 mg/m2, 2 hours ID, day 1, CF 200 mg/m2, ivgtt, days 1-5, 5-FU 300 mg/m2, ivgtt 4 h, days 1-5, 21 days/cycle, up to 2 cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Wang Z (2010) | 28/26; 11/11; 54.28 ± 8.21/54.15 ± 6.26 | II: 4/3; IIIB: 6/4; IIIC: 7/9; IV: 10/10; KPS ≥ 60 | Qilianfuzheng capsule, 1.2 g, tid, long period | OLF: Ox. 150-200 mg/m2, ivgtt, day 1, LV 300 mg/m2, ivgtt, days 2-5, 5-FU 500-750 mg/m2, ivgtt, days 1-5, 3 weeks/cycle, for 2 cycle (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Huang L (2015) | 21/20; 20/17; 56.52 ± 9.42/56.25 ± 9.93 | III: 4/5; IV: 17/15; KPS ≥ 60 | Qingrezaoshifa decoction, tid, for 3 weeks | FOLFOX: Ox. 85 mg/m2, 2 hours ivgtt, day 1, CF 200 mg/m2, 2 hours ivgtt, days 1-2, 5-FU 400 mg/m2, ivgtt, days 1-2; 5-FU 600 mg/m2, ivgtt, 22 hours, days 1-2; 2 weeks/cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Li X (2011) | 30/30; 17/18; 51.66 ± 8.2/50.39 ± 8.8 | IIIA: 5/3; IIIB: 8/10; IIIC: 10/6; IV: 7/11; KPS ≥ 60 | Shuangxian capsule, 2 pills, tid, till the experiment end | FOLFOX: Ox. 85 mg/m2, 2 hours ivgtt, day 1, CF 200 mg/m2, days 1-2, 5-FU 400 mg/m2, ivgtt, days 1-2; 5-FU 600 mg/m2, ivgtt, 22 hours, days 1-2; 2 weeks/cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Yu Z (2016) | 43/43; 24/25; 54.59 ± 7.96/54.85 ± 7.94 | IV (all); ACRC; NS | Fufangkushen injection, 40 mL per day, qd, for 15 days | FOLFOX: Ox. 85 mg/m2, 2 hours ID, day 1, CF 200 mg/m2, ivgtt, days 1-2, 5-FU 400 mg/m2, ivgtt, days 1-2, 14 days/cycle, up to 4 cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Zhou J (2013) | 41/43; 27/25; 53.8 ± 8.1/58.3 ± 5.2 | ACRC; NS | Fufangkushen injection, 20 mL per day, qd, for days 1-7 | FOLFOX: Ox. 85 mg/m2, 2 hours ID, day 1, CF 200 mg/m2, ivgtt, days 1-2, 5-FU 400 mg/m2, ivgtt, days 1-2, 14 days/cycle, up to 4 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Xin L (2014) | 55/55; 37/39; 63.6 ± 9.2 (med.) | III: 35/33; IV: 20/22; KPS ≥ 60 | Huachansu injection, 20 mL, ivgtt, days 1-7 | Cisplatin injection, 40 mg, days 1-3, 5-FU 500 mg/m2, ivgtt, days 3-7, repeated administration/2 days, 3 times/cycle, for 3 cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Zhu K (2009) | 25/25; 17/20; 57.24 ± 9.04/56.76 ± 9.33 | II: 17/11; III: 7/10; IV: 1/4; KPS ≥ 60 | Jianpihuashifang decoction, 300 mL, bid, for 8 weeks | FOLFOX: Ox 85 mg/m2, 2 hours ID, day 1, LV 200 mg/m2, ID, 2 hours, days 1-2, 5-FU 400-600 mg/m2, ivgtt, days 1-2, 14 days/cycle, for 4 cycles (all) | SG: L, AC: L, BPt: L BOA: L, IOD: L, SOR: L |
| Zhao Y (2007) | 23/20; 14/11; 59.00 ± 10.03/58.35 ± 9.09 | IV (all); KPS ≥ 60 | Jianpihuashifang decoction, 300 mL, bid, 21 days/cycle, for 2 weeks | FOLFOX: Ox. 130 mg/m2, 2 hours ID, day 1, LV 200 mg/m2, ID, 2 hours, days 1-2, 5-FU 300 mg/m2, ivgtt, 14 days/cycle, for 2 cycle (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Zeng D (2009) | 35/32; 25/21; NS | ACRC; KPS ≥ 70 | Shenyi capsule, 40 mg, bid, 30 days/cycle | FOLFOX: Ox. 85 mg/m2, 2 hours ivgtt, day 1, LV 50 mg/m2, 2 hours ivgtt, days 1-2, 5-FU 400 mg/m2, iv, 600 mg/m2, ivgtt for 22 hours, days 1-2, 14 days/cycle, for 4 cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Zhang Z (2009) | 20/20; 9/13; 61.70 ± 9.00/56.30 ± 12.54 | IV (all); KPS ≥ 60 | Jianpihuashifang decoction, 400 mL, bid, for 8 weeks | OLF: Ox. 130 mg/m2, 2 hours ID, day 1, CF 200 mg/m2, ivgtt, days 1-5, 5-FU 500 mg/m2, ivgtt, days 1-5, 21 days/cycle, up to 2 cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Lao G (2012) | 30/30; 21/23; 35.1 ± 20.2/36.7 ± 20.1 | II: 5/7; III: 15:14; IV: 10/9; KPS ≥ 60 | Jianpijiedu decoction, one decoction per day, 21 days/cycle, for 2 cycles | FOLFOX: Ox. 130 mg/m2, ID, day 1, LV 200 mg/m2, ID, day 1, 5-FU 500 mg, bolus, 2400 mg/m2 day 1, then 3000 mg/m2, ID, 48 hours; days 1-2, 21 days/cycle, for 2 cycles (all) | SG: L, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Liang Q (2009) | 76/76; 50/51; 53/52 (med.) | III: 45/46; IV: 31/30; KPS ≥ 60 | Shenqifuzheng injection, 250 mL/day, ID, 3 weeks/cycle, for 2 cycles | FOLFOX: Ox. 130 mg/m2, 2 hours, ID, day 1, LV 200 mg/m2, 2 hours, ID, day 1, 5-FU 500 mg, bolus, day 1, then 3000 mg/m2, ID, for 48 hours; 3 weeks/cycle, for 2 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Liu H (2009) | 36/34; 16/18; 50.2 (mean) | ACRC (all); KPS ≥ 60 | Kang ‘ai fangyi pian, one decoction per day, 21 days/cycle, for 3 cycles | FOLFOX: Ox. 130 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-5, 5-FU 300 mg, ID, days 1-5, 21 days/cycle, 3 cycles (all) | SG: L, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Zou B (2007) | 34/27; 29/22; 53/54.3 (mean) | IV (all); KPS ≥ 60 | Gubenkang’ai decoction, one decoction per day, for 6 weeks | FOLFOX: Ox. 135 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 2400 mg/m2, ID, for 48 hours, day 1, 21 days/cycle, for 2 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| You J (2010) | 30/30; 14/13; range; 30-75/31-74 | III: 8/9; IV: 22/21; KPS ≥ 50 | WD-3 decoction, 50 mL, tid,4 weeks/cycle, for 4 cycles | FOLFOX: Ox. 125 mg/m2, ID, day 1, LV 100 mg/m2, ID, days 1-5, 5-FU 500 mg/m2, ID, days 1-5, 4 weeks/cycle, for 4 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Zeng C (2013) | 30/30; 39/19; 54.3 ± 6.3/53.2 ± 6.6 | III: 20/12; IV: 41/18; KPS ≥ 60 | Fuzhengxiaoji decoction, one decoction per day, 14 days/cycle, for 4-6 cycles | FOLFOX: Ox. 85 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 360-500 mg/m2 bolus, 600 mg/m2, ID, for 22 hours, days 1-2, 14 days/cycle, for 4-6 weeks (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Shi Y (2007) | 15/15; 6/7; 57.4 ± 12.0/56.9 ± 12.8 | II: 5/4; III: 10/11; KPS ≥ 60 | Jianpiqinglihuoxuefang decoction, bid, for 2 mouths | FOLFOX: Ox. 130 mg/m2, 2 hours ID, CF 200 mg/m2, ivgtt, days 1-5, 5-FU 500 mg/m2, ivgtt, days 1-5, 3 weeks/cycle (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Dai L (2008) | 30/30; 20/17; 59.02 ± 10.03/58.35 ± 9.09 | IV (all); ACRC; KPS ≥ 60 | Jianpiquzhuoxiaojifang decoction,100 mL, bid, for 3 weeks | FOLFIRI: CPT-11 180 mg/m2, ivgtt, day 1, CF 200 mg/m2, 2 hours ivgtt, days 1-2, 5-FU 400 mg/m2, ivgtt, days 1-2, 2 weeks/cycles, for 2 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Wang B (2014) | 30/30; NS; 56.7 ± 12.1 | I: 24; II: 36; III: 22; IV: 8; KPS ≥ 60 | Junzifuzhengfang decoction, 50 mL, bid, more than 30 days | FOLFOX: Ox. 85 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 400 mg/m2 bolus, 600 mg/m2, ivgtt for 22 hours, days 1-2, 14 days/cycle, for 12 cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Yu H (2011) | 20/20; 12/11; 60.10 ± 9.40/59.95 ± 9.60 | IIIB: 9/8; IIIC: 9/11; IV: 2/1; KPS ≥ 60 | Qilianfuzheng capsule, 1.2 g, tid, 3 weeks/cycle, for 2 cycles | OLF: Ox. 130 mg/m2, day 1, LV 200 mg/m2, ivgtt, days 1-5, 5-FU 750 mg/m2, ivgtt, days 1-5, 3 weeks/cycle, for 2 cycle (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Wang H (2007) | 34/33; 47 (all); 55 (mean all) | ACRC; KPS ≥ 60 | Delisheng injection, 40-60 mL, ID per day,15 days/cycle, for 3 cycles | FOLFOX: Ox. 130 mg/m2, 2 hours ID, day 1, LV 200 mg/m2, 2 hours ID, days 1-5, 5-FU 500 mg/m2, ID, days 1-5, 3 wk/cycle, for 3 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Yang C (2007) | 50/50; 29/27; 51.36 ± 10.58/53.48 ± 9.35 | ACRC; KPS ≥ 60 | Jianpikangfu tablets, 6 g, tid, for 4 wk | FOLFOX: Ox. 135 mg/m2, ID, day 1, LV 100 mg/m2, ID, days 1-5, 5-FU 425 mg/m2, ID, day 1, for 4 weeks (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Wang H (2008) | 34/34; 20/22; 52.58 ± 8.12/51.11 ± 7.72 | IV: 34/34; KPS ≥ 50 | Yiqiguoxiebuchang decoction, one decoction per day, for 3 mouths | FOLFOX: Ox. 85 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 500 mg bolus day 1, 5-FU 2500 mg/m2, ID, for 48 hours, 21 days/cycle, for 4 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Zhang Q (2006) | 38/30; 35 (all); 54.8 (mean all) | ACRC; KPS ≥ 60 | Yiqihuoxue formula, one decoction per day, 21 days/cycle, for 3 cycles | FOLFOX: Ox. 125 mg/m2 ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 500 mg/m2 bolus, days 1-2, 2000 mg/m2, ID, for 72 hours, 21 days/cycle, for 3 cycles (all) | SG: L, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Xu Y (2006) | 32/20; 21/12; 53/51 (med) | IV: 32/20; KPS ≥ 60 | Fuzhenghuayujiedusanjie formula, one decoction per day, for 6 months | FOLFOX: Ox. 100 mg/m2, ID, days 1-8, 5-FU 500 mg bolus day 1, 5-FU 250 mg/m2, ID, days 1-15, or plus HCPT 10 mg/m2, ID, days 1-5, 30 days/cycle, for 6 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Wang D (2012) | 49/49; 58 (all); 54.5 (mean, all) | ACRC (all); KPS ≥ 60 | Unnamed multi-TM formula, one decoction per day, duration NS | FOLFOX: Ox. 120 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-5, 5-FU 300 mg/m2, ID, days 1-5, 21 days/cycle, NS | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Qin Y (2011) | 36/37; NS; NS | ACRC; NS | Fuzhenguban decoction, one decoction per day, 21 days/cycle, for 2 cycles | FOLFOX: Ox. 135 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 2400 mg/m2, ID, for 48 hours; 21 days/cycle, for 2 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: H, SOR: H |
| Song W (2012) | 20/20; 12/13; 56.4 ± 9.1/48.3 ± 8.2 (all); 72.2 (med. all) | ACRC; KPS ≥ 70 | Xiaoliuhuajichangfang II, one decoction per day, 21 days/cycle, for 2 cycles | FOLFOX: Ox. 135 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 2400 mg/m2, ID, for 48 hours; 21 days/cycle, for 2 cycles (all) | SG: L, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Tao C (2013) | 74/74; 51/50; 60.1 ± 7.9/60.4 ± 8.9 | ACRC; KPS ≥ 60 | Co-kushen injection, 15 mL per day, ID, started 14 days before chemotherapy, 5 wk/cycle, for 1 cycle | FOLFOX: Ox. 135 mg/m2, ID, day 1, LV 200 mg/m2, ID, 2 hours, days 1-5, 5-FU 500 mg/kg, ID, 8-10 hours, days 1-5, 3 wk/cycle, for 1 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Wan H (2013) | 30/30; 35 (all); 51.7 (mean, all) | III: 40 (all); IV: 20 (all); KPS ≥ 60 | Kushen injection 40 mL, ID, and Huangqi injection 20 mL, ID, per day, 10 days/cycle, for 6 cycles | FOLFOX: Ox. 100 mg/m2, ID, day 1, LV 200 mg/m2, ID, 5-FU 15-20 mg/kg, ID, days 1-5, 21 days/cycle, for 6 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Zhou J (2011) | 34/34; 22/20; 51.2/52.5 | II: 14/13; III: 16/11; IV: 6/5; KPS ≥ 60 | Fuzhengjianpi decoction, one decoction per day, for 8 weeks | FOLFOX: Ox. 130 mg/m2, ID, day 1, LV 100 mg/m2, ID, days 1-5, 5-FU 500 mg/m2, ID, days 1-5, 28 days/cycle, for 2 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Kono T (2013) | 27/23; NS; 67/61 (mean) | NS; ECOG 0-1 | TJ-107 Goshajinkigan; or placebo was administered orally, tid, before each meal (7.5 g/day) for 26 weeks | FOLFOX: Ox. 85 mg/m2, ID, day 1, LV 200 mg/m2, ID, 5-FU 400 mg bolus, followed by 2400 mg/m2, ID 46 hours, 14 days/cycle, for 8 cycles or more (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Jiang G (2013) | 32/31; 18/18; 53.2 ± 12.4/53.1 ± 12.8 | ACRC; NS | Unnamed multi-TM formula, one decoction per day, for 8 weeks | FOLFOX: Ox. 135 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 400 mg bolus, days 1-2, 2400-3600 mg/m2, ID 48 hours, 14 days/cycle, for 4 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Cao B (2013) | 60/60; 32/33; 55.2 ± 13.3/58.8 ± 13.7 | IV (all); ECOG 0-1 | Yiqizhuyu decoction (YZD), one decoction per day orally administered for up to 48 weeks | FOLFOX: Ox. 85 mg/m2, 2 hours ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 400 mg/m2 bolus, 600 mg/m2, ID, 22 hours, days 1-2, 14 days/cycle, up to 24 cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Chen X (2005) | 47/46; 31/32; 54/53 (med.) | II: 11/12; III: 21/20; IV: 15/14; KPS ≥ 60 | Composite salviae dripping pill, 25 mg/pill, 10 pills/day, tid, for 3 cycles or more | FOLFOX: Ox. 130 mg/m2, 2 hours ID, day 1, LV 200 mg/m2, 2 hours ID, 5-FU 500 mg bolus, then 3000 mg/m2 ID for 48 hours, 3 wk/cycle for 3 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Hu A (2006) | 28/22; 18/14; 49.3 ± 4.5/48.5 ± 4.3 | IV (all); KPS ≥ 50 | Treatment with 4 different TM decoctions according to symptom differentiation; one decoction per day, for more than 30 days | FOLFOX: Ox. 130 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-2, 5-FU 2400 mg/m2, ID, 46 hours, 21 days/cycle, 2 cycles (all) | SG: U, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
| Xu Y (2010) | 61/60; 38/37; 53/52 (mean) | ACRC; KPS ≥ 70 | Jiangnilin decoction, bid, 2 weeks/cycle, for 8-10 cycles | FOLFOX: Ox. 85 mg/m2, day 1, CF 200 mg/m2, 2 hours ivgtt, days 1-2, 5-FU 400 mg/m2, ivgtt, days 1-2; 5-FU 600 mg/m2, ivgtt, 22 hours, days 1-2; 2 weeks/cycles, for 8-10 cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Wang J (2010) | 26/26; 11/14; 53.26/51.79 (mean) | ACRC; NS | Banmaosuanna vitamin B6 injection, 0.5 mg, qd | FOLFOX: Ox. 85 mg/m2, ivgtt, day 1, LV 200 mg/m2, ivgtt, days 1-2, 5-FU 400 mg/m2, iv, 600 mg/m2, ivgtt, for 22 hours, days 1-2, 14 days/cycle, for 12 cycles (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Ding Y (2007) | 20/20; 9/11; 58.00 ± 10.60/57 ± 10.19 | IV (all); ACRC; KPS ≥ 60 | Jianpihuoxuetongfufang decoction, 300 mL, bid, for 8 weeks | FOLFOX: Ox. 130 mg/m2, 2 hours ID, day 1, CF 100 mg/m2, ivgtt, days 1-5, 5-FU 500 mg/m2, ivgtt, 3 weeks/cycle (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Li P (2010) | 26/25; 8/18; 51/54 (med) | IV (all); ACRC; KPS ≥ 60 | Jianpihuashifang decoction, 300 mL, bid, 3 weeks/cycle, for 2 cycles | OLF: Ox. 130 mg/m2, 2 hours ID, day 1, LV 100 mg/m2, ivgtt, days 1-5, 5-FU 400 mg/m2, ivgtt 4-5 hours, days 1-5, 3 weeks/cycle, for 2 cycle (all) | SG: L, AC: L, BPt: L, BOA: L, IOD: L, SOR: L |
| Liu J (2005) | 43/21; 23/10; 61.52 ± 10.12/60.11 ± 9.78 | IV: all; KPS ≥ 50 | Jianpihuoxue formula, one decoction per day, 30 days/cycle, 3 cycles | FOLFOX: Ox. 150 mg/m2, ID, day 1, LV 200 mg/m2, ID, days 1-5, 5-FU 500 mg, ID, days 1-5, 30 days/cycle, for 3 cycles (all) | SG: L, AC: U, BPt: H, BOA: L, IOD: L, SOR: L |
Abbreviations: T, treatment group; C, control group; M, male; N, number; NS, not stated; ID, intravenous drip; TRR, tumor response rate; TNM, cancer staging system (“T” for tumor, denotes the extent of the intestinal wall; “N” for lymphatic node, the amount of lymphatic node involvement; and “M” for the metastasis); KPS, Karnofsky Performance Status; ECOG, Eastern Cooperative Oncology Group Performance Status; TM, traditional medicine; 5-FU, 5-fluorouracil; LV, leucovorin; Ox, oxaliplatin; HCPT, hydroxycamptothecine; FOLFOX, Ox. + 5-FU + LV; ACRC, advanced colorectal cancer; bid, twice per day; tid, thrice per day; qd, once per day; wk, week; mth, mouth; med, median. Risk of Bias Categories: SG, sequence generation; AC, allocation concealment; BPt, blinding of participants/personnel; BOA (obj), blinding of outcome assessment (objective outcome measure, ie, TRR); IOD, incomplete outcome data; SOR, selective outcome reporting. Risk of Bias Judgements; L, low risk; U, unclear risk; H, high risk.
Methodological Assessment
All studies stated the use of randomization, so risk of bias of sequence generation (SG) was judged as “low.” Allocation concealment (AC) and blinding of participants (BPt) were only described in 2 studies (Kono T, Cao B) and these were judged “low risk,” since a placebo control had been used for the TCM. The other studies did not describe procedures for AC, so were judged “unclear risk” for AC, while BPt was “high risk” since the additional therapy of TCM was difficult to conceal. Blinding of outcome assessors (BOA) for TRR was not mentioned. Blinding of participants is difficult to achieve in cancer trials. Six studies (Qin Y, Xu Y, Wang Z, You J, Xu Y, Dai L) reported that they had participants dropping out or lost to follow-up; thus, these missing data were not treated as “intent to treat.” So these were judged as “high risk” of attrition bias. Studies that had the same numbers of participants at inception as in the outcome reports were judged as “low risk” of incomplete outcome data (IOD; Table 1).
As for the selective outcome reporting (SOR), only when the objectives and outcome measures stated in the method section were all reported in the results section, was the study judged as “low risk.” The 47 studies showed symmetry for TRR in the funnel plot, suggesting the risk of publication bias was low.
Meta-Analysis of Tumor Response
The RECIST criteria were used to evaluate TRR in 47 studies. Meta-analyses were conducted for CR and TRR. When RR ≥1 (IV model, fixed, 95% confidence interval), it favors the test group. Three groups were divided for meta-analyses: total (47 studies); non-oral group (8 studies) and oral administration group (39 studies).
Total Group
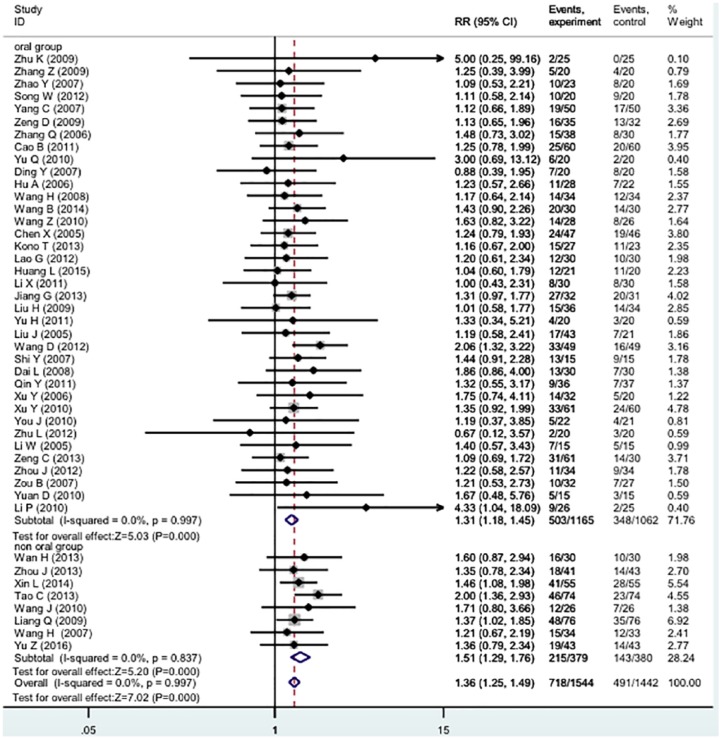
In the 47 studies (n = 3066, Table 1), the test groups showed significantly improved TRR (RR 1.38 [1.26-1.50], I2 = 0%; Figure 2).
Figure 2.
Forest plot of meta-analysis of tumor response rate of TCM plus 5-FU-based regimens versus 5-FU-based regimens alone.
Non-Oral Group
Five different injection products (Kushen or Fufangkushen, Huachansu, Shenqifuzheng, Delisheng, Banmaosuanna vitamin B6) were tested in 8 studies (n = 795; Table 1). There were significant improvements for TRR (RR 1.51 [1.29-1.76], I2 = 0%). The TRR funnel plot was symmetric.
Oral Administration Group
In 39 studies (n = 2307), TCMs were administered orally as decoctions, capsules, or tablets. The pooled TRR showed significant improvement (RR 1.33 [1.19-1.47], I2 = 0%).
The Effects of Multi-Ingredient TCM in the Oral Administration Group
As is well known, the TCM formulae usually have different names; however, their main components are considered to have similarity. In order to identify the most comparable subgroups of studies and potential synergistic effects, we made a series of planned sensitivity analyses. Only the TCMs with significant RR results for tumor response have been reported in our analyses.
Level 1: Single TCMs
Ninety-two ingredients in the formulae have been included in this review. Among them, there are 35 ingredients that have been used in 3 or more formulae. The Chinese name in pin yin of each ingredient was used to stand for the TCMs.
According to their frequency of use in formulae, TCMs were listed as follows: Huangqi (n = 28), baizhu (n = 27), yiyiren (n = 27), fuling (n = 26), dangshen (n = 22), sheshecao (n = 20), baijiangcao (n = 12), eshu (n = 11), and banzhilian (n = 10).
Then, the RR values were calculated, which were listed in descending order in Table 2. The pooled RR values were divided into 2 groups. The RR values of the first group were equal to or greater than the RR results of the total pool. In the second group, the RR values were less than the RR results of the total pool.
Table 2.
Effects of Specific Orally Administered Traditional Chinese Medicines (TCMs) on Tumor Response.
| Level | Traditional Chinese Medicine | RR | 95% CI | No. of Studies | I2 % |
|---|---|---|---|---|---|
| 1 | Baishao | 1.644 | 1.108-2.440 | 5 | 0.0% |
| 1 | Kusheng | 1.643 | 1.267-2.130 | 4 | 0.0% |
| 1 | Baijiangcao | 1.485 | 1.056-2.089 | 12 | 0.0% |
| 1 | Muxiang | 1.459 | 1.172-1.815 | 5 | 7.2% |
| 1 | Rensheng | 1.439 | 1.181-1.754 | 5 | 15.7% |
| 1 | Banzhilian | 1.404 | 1.178-1.673 | 10 | 0.0% |
| 1 | Hongteng | 1.404 | 1.178-1.673 | 8 | 0.0% |
| 1 | Sheshecao | 1.403 | 1.209-1.629 | 20 | 0.0% |
| 1 | Xianhecao | 1.401 | 1.046-1.876 | 10 | 0.0% |
| 1 | Huangqi | 1.364 | 1.212-1.534 | 28 | 0.0% |
| 1 | Eshu | 1.357 | 1.066-1.729 | 11 | 0.0% |
| 1 | Baizhu | 1.352 | 1.181-1.549 | 27 | 0.0% |
| 1 | Dangsheng | 1.348 | 1.168-1.556 | 22 | 0.0% |
| 1 | Fuling | 1.347 | 1.171-1.550 | 26 | 0.0% |
| 1 | Yiyiren | 1.329 | 1.159-1.524 | 27 | 0.0% |
| 1 | Banxia | 1.319 | 1.041-1.672 | 7 | 0.0% |
| 1 | Shanyao | 1.259 | 0.867-1.828 | 6 | 0.0% |
| 2 | Sheshecao + banzhilian | 1.774 | 1.203-2.617 | 11 | 0.0% |
| 2 | Sheshecao + eshu | 1.658 | 1.205-2.282 | 7 | 0.0% |
| 2 | Baizhu + huangqi | 1.624 | 1.286-2.050 | 12 | 0.0% |
| 2 | Yiyiren + sheshecao | 1.592 | 1.259-2.01 | 12 | 0.0% |
| 2 | Huangqi + sheshecao | 1.516 | 1.272-1.808 | 15 | 0.0% |
| 2 | Dangsheng + banzhilian | 1.514 | 1.207-1.898 | 7 | 0.0% |
| 2 | Baizhu + banzhilian | 1.504 | 1.183-1.913 | 8 | 0.0% |
| 2 | Eshu + xianhecao | 1.473 | 0.917-2.365 | 5 | 0.0% |
| 2 | Baizhu + eshu | 1.465 | 1.054-2.036 | 7 | 0.0% |
| 2 | Yiyiren + eshu | 1.427 | 1.053-1.934 | 7 | 0.0% |
| 2 | Huangqi + eshu | 1.414 | 1.096-1.823 | 9 | 0.0% |
| 2 | Dangsheng + fuling | 1.410 | 1.172-1.696 | 16 | 0.0% |
| 2 | Baizhu + fuling | 1.391 | 1.195-1.619 | 23 | 0.0% |
| 2 | Dangshen + eshu | 1.386 | 0.968-1.984 | 5 | 0.0% |
| 2 | Baizhu + yiyiren | 1.355 | 1.169-1.571 | 23 | 0.0% |
| 2 | Baizhu + dangsheng | 1.336 | 1.145-1.560 | 20 | 0.0% |
| 2 | Eshu + banzhilian | 1.237 | 0.837-1.827 | 8 | 44.7% |
| 3 | Baizhu + dangsheng + eshu | 1.686 | 1.114-2.554 | 4 | 0.0% |
| 3 | Yiyiren + huangqi + eshu | 1.606 | 1.117-2.309 | 6 | 0.0% |
| 3 | Baizhu + dangsheng + xianhecao | 1.538 | 0.828-2.859 | 4 | 0.0% |
| 3 | Yiyiren + huangqi + baijiangcao | 1.555 | 1.040-2.324 | 5 | 0.0% |
| 3 | Baizhu + dangsheng + baijiangcao | 1.501 | 1.038-2.170 | 6 | 0.0% |
| 3 | Dangsheng + fuling + baijiangcao | 1.501 | 1.038-2.170 | 6 | 0.0% |
| 3 | Baizhu + dagsheng + banzhilian | 1.463 | 1.167-1.834 | 8 | 0.0% |
| 3 | Fuling + yiyiren + sheshe | 1.438 | 1.196-1.729 | 14 | 0.0% |
| 3 | Baizhu + dangsheng + sheshecao | 1.420 | 1.151-1.753 | 12 | 0.0% |
| 3 | Fuling + yiyiren + eshu | 1.415 | 0.990-2.021 | 6 | 0.0% |
| 3 | Baizhu + dangsheng + fuling | 1.374 | 1.168-1.616 | 19 | 0.0% |
| 3 | Baizhu + dangsheng + yiyiren | 1.399 | 1.177-1.663 | 18 | 0.0% |
| 3 | Dangsheng + fuling + yiyiren | 1.390 | 1.172-1.648 | 18 | 0.0% |
| 3 | Dangsheng + fuling + sheshecao | 1.389 | 1.162-1.661 | 14 | 0.0% |
| 3 | Dangsheng + fuling + eshu | 1.386 | 0.968-1.984 | 5 | 0.0% |
| 3 | Yiyiren + huangqi + sheshecao | 1.386 | 1.168-1.643 | 15 | 0.0% |
| 4 | Baizhu + dangsheng + fuling + Eshu | 1.692 | 0.942-3.040 | 3 | 0.0% |
| 4 | Dangsheng + fuling + yiyiren + baijiangcao | 1.690 | 1.080-2.643 | 5 | 1.4% |
| 4 | Dangsheng + fuling + yiyiren + banzhilian | 1.610 | 1.197-2.166 | 6 | 0.0% |
| 4 | Baizhu + dangsheng + fuling + huangqi | 1.428 | 1.168-1.748 | 18 | 0.0% |
| 4 | Baizhu + dangsheng + fuling + sheshecao | 1.420 | 1.151-1.753 | 12 | 0.0% |
| 4 | Dangsheng + fuling + yiyiren + sheshecao | 1.411 | 1.172-1.699 | 13 | 0.0% |
| 4 | Baizhu + dangsheng + fuling + yiyiren | 1.390 | 1.172-1.648 | 18 | 0.0% |
| 5 | b + d + f + y + banzhilian | 1.806 | 1.305-2.499 | 6 | 0.0% |
| 5 | d + f + y + h + banzhilian | 1.734 | 1.245-2.416 | 5 | 0.0% |
| 5 | b + d + f + y + baijiangcao | 1.690 | 1.080-2.643 | 5 | 1.4% |
| 5 | d + f + y + h + baijiangcao | 1.598 | 1.019-2.505 | 4 | 0.0% |
| 5 | b + d + f + y + xianhecao | 1.538 | 0.828-2.859 | 4 | 0.0% |
| 5 | d + f + y + h + sheshecao | 1.528 | 1.189-1.964 | 9 | 8.1% |
| 5 | b + d + f + y + eshu | 1.461 | 0.963-2.215 | 4 | 0.0% |
| 5 | d + f + y + h + eshu | 1.461 | 0.963-2.215 | 4 | 3.4% |
| 5 | b + d + f + y + sheshecao | 1.420 | 1.151-1.753 | 17 | 0.0% |
| 5 | b + d + f + y + huangqi | 1.393 | 1.139-1.703 | 18 | 0.0% |
| 6 | b + d + f + y + h + hongteng | 1.930 | 1.259-2.961 | 3 | 0.0% |
| 6 | b + d + f + y + h + banzhilian | 1.734 | 1.245-2.416 | 5 | 0.0% |
| 6 | b + d + f + y + h + baijiangcao | 1.598 | 1.019-2.505 | 4 | 8.1% |
| 6 | b + d + f + y + h + eshu | 1.461 | 0.963-2.215 | 4 | 0.0% |
| 6 | b + d + f + y + h + sheshecao | 1.458 | 1.155-1.841 | 9 | 0.0% |
| 6 | b + d + f + y + h + xianhecao | 1.500 | 0.733-3.068 | 3 | 27.8% |
| 7 | b + d + f + y + h + s + banzhilian | 1.734 | 1.245-2.416 | 5 | 3.4% |
| 7 | b + d + f + y + h + s + baijiangcao | 1.598 | 1.019-2.505 | 4 | 8.1% |
| 7 | b + d + f + y + h + s + xianhecao | 1.500 | 0.733-3.068 | 3 | 0.0% |
| 7 | b + d + f + y + h + s + eshu | 1.461 | 0.963-2.215 | 4 | 0.0% |
Abbreviations: RR, risk ratio for tumor response; 95% CI, 95% confidence interval; I2 %, measure of heterogeneity; 5. b + d + f + y (baizhu + dangsheng + fuling + yiyiren); 6. d + f + y + h (dangsheng + fuling + yiyiren + huangqi); 6. b + d + f + y + h (baizhu + dangsheng + fuling + yiyiren + huangqi); 7. b + d + f + y + h + s (baizhu + dangsheng + fuling + yiyiren + huangqi + baihuasheshecao).
The first group included 16 TCMs: baishao (n = 5), kusheng (n = 4), baijiangcao (n = 12), muxiang (n = 5), rensheng (n = 5), banzhilian (n = 10), hongteng (n = 8), sheshecao (n = 20), xianhecao (n = 10), huangqi (n = 28), eshu (n = 11), baizhu (n = 27), dangshen (n = 22), fuling (n = 26), yiyiren (n = 27), and banxia (n = 7). In the second group there was only one TCM, shanyao, which had a lower value than the RR of the total pool (Table 2).
Level 2: Combinations of 2 TCMs
In this level, there are 17 pairs of TCMs from group 1 or 2 above. Among them, the RR values of 16 pairs were shown to be equal to or greater than the total pool, including sheshecao + banzhilian (n = 11), sheshecao + eshu (n = 7), baizhu + huangqi (n = 12), yiyiren + sheshecao (n = 12), huangqi + sheshecao (n = 15), dangshen + banzhilian (n = 7), baizhu + banzhilian (n = 8), eshu + xianhecao (n = 5), baizhu + eshu (n = 7), yiyiren + eshu (n = 7), huangqi + eshu (n = 9), dangshen + fuling (n = 16), baizhu + fuling (n = 23), dangshen + eshu (n = 5), baizhu + yiyiren (n = 23), and baizhu + dangshen (n = 20). Only one pair was lower than the RR of the total pool (Table 2).
Level 3: Combinations of 3 TCMs
In this level, there are 16 significant pairs from level 2 that were combined with other TCMs that showed significant RRs at level 1. The RR values of all pairs in this level were greater than the total pool, as follows: baizhu + dangshen + eshu (n = 4), yiyiren +huangqi +eshu (n = 6), baizhu +dangshen + xianhecao (n = 4), yiyiren + huangqi + baijiangcao (n = 5), baizhu + dangshen + baijiangcao (n = 6), dangshen + fuling + baijiangcao (n = 6), baizhu + dangshen + banzhilian (n = 8), fuling + yiyiren +sheshe (n = 14), baizhu + dangshen + sheshecao (n = 12), fuling + yiyiren + eshu (n = 6), baizhu +dangshen + fuling (n = 19), baizhu + dangshen + yiyiren (n = 18), dangshen + fuling + yiyiren (n = 18), dangshen + fuling + sheshecao (n = 14), dangshen + fuling + eshu (n = 5), and yiyiren + huangqi + sheshecao (n = 15; Table 2).
Levels 4 to 7: Combinations of 4 to 7 TCMs
The significant combinations from levels 4 to 7 were further combined. As a result, the RR values of levels 4 to 7 were significantly higher than the total pool (Table 2).
TCMs Potential Synergistic Effects Selection
Compared with TCMs alone, 15 TCM pairs showed higher RR values and potential synergistic effects, including sheshecao + banzhilian (n = 11), sheshecao + eshu (n = 7), baizhu + huangqi (n = 12), yiyiren + sheshecao (n = 12), huangqi + sheshecao (n = 15), dangshen + banzhilian (n = 7), baizhu + banzhilian (n = 8), eshu + xianhecao (n = 5), baizhu + eshu (n = 7), yiyiren + eshu (n = 7), huangqi + eshu (n = 9), dangshen + fuling (n = 16), baizhu + fuling (n = 23), dangshen + eshu (n = 5), and baizhu + yiyiren (n = 23).
When comparing the RR values of the combinations in levels 3 to 7 with each singe TCM in level 1, however, we found that the RR value of 2 combinations in level 3 is lower than the single TCMs, as follows: dangshen +fuling + sheshecao (n = 14) and yiyiren + huangqi + sheshecao (n = 15).
Discussion
TCM, as a complementary and alternative medicine, has been recorded in the Chinese medical literature for thousands of years and gradually became a commonly used treatment for cancer in China.52 Application of TCM as an adjuvant cancer therapy has been recognized as an important approach to enhance the efficacy of anticancer strategies or to reduce adverse effects of these treatments.53-55 5-FU is widely used in the treatment of a variety of cancer types, and 5-FU in combination with other chemotherapeutic agents has been demonstrated to improve the overall and disease-free survival of patients with resected stage III CRC.56-58 The study from Chen et al evaluated the clinical evidence for the addition of TCMs to oxaliplatin-based regimens for CRC in terms of tumor response rate and their results showed specific combinations of TCMs appeared to produce higher contributions to TRR than the TCMs individually.59 However, the relationship between the combination of 5-FU-based chemotherapy plus TCMs with 5-FU regimens alone in the treatment for CRC is still unclear.
To evaluate the clinical evidence for the addition of TCMs to 5-FU-based regimens for CRC in terms of TRR, our study reviewed 45 studies that were classified as oral administration group (37 studies) and non-oral administration group (8 studies) and enrolled 2986 participants with 1544 participants in the experiment groups and 1442 participants in the control groups. As a result, we demonstrated that the TRR of the group with oral TCMs or injection products (Kushen or Fufangkushen, Huachansu, Shenqifuzheng, Delisheng, Banmaosuanna vitamin B6) combined with 5-FU-based regimens was remarkably higher than that in the group with 5-FU regimens alone. Furthermore, both oral administration and injection of TCMs showed benefits to the CRC treatment. Further sensitivity analysis of specific plant-based TCMs found that fuling, sheshecao, banzhilian, eshu, baizhu, huangqi, yiyiren and dangshen produced significantly higher contributions to the results of RR value.
In conclusion, our study suggests that specific combinations of TCMs with 5-FU-based chemotherapy appear to produce higher contributions to TRR than 5-FU regimens alone. Notable among these is the combinations of fuling, sheshecao, banzhilian, eshu, baizhu, huangqi, yiyiren, and dangshen. Therefore, TCMs may have the potential to improve the efficacy of 5-FU-based chemotherapy for CRC.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from National Natural Science Foundation of China (Grant Nos. 81874380, 81672932, and 81730108), Zhejiang Provincial Natural Science Foundation of China for Distinguished Young Scholars (Grant No. LR18H160001), Zhejiang Province Medical Science and Technology Project (Grant No. 2017RC007), Key Project of Zhejiang Province Ministry of Science and Technology (Grant No. 2015C03055), Talent Project of Zhejiang Association for Science and Technology (Grant No. 2017YCGC002), Zhejiang Province Science and Technology Project of TCM (Grant No. 2019ZZ016), Key Project of Hangzhou Ministry of Science and Technology (Grant Nos. 20162013A07 and 20142013A63), and Zhejiang Provincial Project for the Key Discipline of Traditional Chinese Medicine (Grant No. 2017-XK-A09).
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [DOI] [PubMed] [Google Scholar]
- 2. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii1-iii9. [DOI] [PubMed] [Google Scholar]
- 3. Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-338. [DOI] [PubMed] [Google Scholar]
- 4. Sánchez-Gundin J, Fernández-Carballido AM, Martínez-Valdivieso L, Barreda-Hernández D, Torres-Suárez AI. New trends in the therapeutic approach to metastatic colorectal cancer. Int J Med Sci. 2018;15:659-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med. 2014;12:331-335. [DOI] [PubMed] [Google Scholar]
- 6. Chen Z, Wang P. Clinical distribution and molecular basis of traditional Chinese medicine ZHENG in cancer. Evid Based Complement Alternat Med. 2012;2012:783923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li W. The clinical study of the advanced colorectal cancer treatment with the Jianpi Huashi Quyu decoction combined with L-OHP+5-FU/CF. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2005. [Google Scholar]
- 8. Yuan D. Theoretical and clinical research of Jianpi Huashi Quyu Jiedu for the treatment of advanced colorectal cancer patients. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2010. [Google Scholar]
- 9. Zhu L. Jianpi Huashi Quyu Jiedu decotion combined with FOLFIRI regimen for the treatment of advanced colorectal cancer patients. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2012. [Google Scholar]
- 10. Yu Q. Clinical Study of the combination of Jianpi Quyu Jiedu decotion and Oxaliplatin-based Regimen for the treatment of colorectal cancer patients. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2010. [Google Scholar]
- 11. Wang Z. Clinical study of Qilian FuZheng Capsule combined with Chemotherapy for the treatment of advanced colorectal cancer patients. Shandong, China: Shandong University of Traditional Chinese Medicine; 2010. [Google Scholar]
- 12. Huang L. Clinical observation of Qingre Huashi Chinese herbs combined chemotherapy for the treatment of advanced colon cancer patients with Shire syndrome. Chendu, China: Chendu University of Traditional Chinese Medicine; 2015. [Google Scholar]
- 13. Li X. The Clinical Impact of “Shuangxian Capsule” combined with chemotherapy on immunological function of colorectal cancer patients. Luzhou, China: Luzhou Medical College; 2011. [Google Scholar]
- 14. Yu Z. Clinical observation of fufangkushegjian combined with FOLFOX4 in the treatment for the advanced colorectal carcinoma. Chin J Mod Drug Appl. 2016;10:173-174. [Google Scholar]
- 15. Zhou J. The clinical effect of compound matrine injection combined with oxaliplatin on the adjuvant chemotherapy of advanced colorectal cancer. Chin J Clin Oncol Rehabil. 2013;20:1141-1143. [Google Scholar]
- 16. Xin L. Clinical observation of cinobufacini combined with 5-fluorouracil in the treatment for intestinal tumor. China Pharmacy. 2014;25:3415-3416. [Google Scholar]
- 17. Zhu K. Studies on impact of Jianpi Huashi Fa combined with chemotherapy for the treatment of corectal cancer after a radical operation. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2009. [Google Scholar]
- 18. Zhao Y. Clinical Study of “Jianpi Yiqi fa” combined with chemotherapy on colorectal cancer patients. Shandong, China: Shandong University of Traditional Chinese Medicine; 2007. [Google Scholar]
- 19. Zeng D. Clinical study of Shengyi capsule combined with FOLFOX4 in the treatment for the advanced colorectal carcinoma. J Clin Med Pract. 2009;13(5):57-58. [Google Scholar]
- 20. Zhang Z. Clinical study of Jianpi Huashi Jiedu decoction with chemotherapy for the treatment of advanced colorectal cancer patients. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2009. [Google Scholar]
- 21. Lao G. Clinical research on treating advanced colorectal cancer with the Jianpi Jiedu decoction plus chemotherapy. Clin J Chin Med. 2012;4(17):1-3. [Google Scholar]
- 22. Liang QL, Pan DC, Xie JR. Effect of Shenqifuzheng injection combined with chemotherapy in the treatment for advanced colorectal carcinoma. Chin J Integr Tradit West Med. 2009;29(5):439-441. [PubMed] [Google Scholar]
- 23. Liu H, Sun MF. Clinical observation of Kang’ai Fangyi pill combined with chemotherapy in treating advanced colorectal carcinoma. Chin J Inform TCM. 2009;16(1):71-72. [Google Scholar]
- 24. Zou BF, He L, Song HY, Xin SY. Gubenkang’ai decoction combined with chemotherapy in treating advanced colorectal carcinoma. Liaoning J Trad Chin Med. 2007;34(6):772-773. [Google Scholar]
- 25. You JL, Gong SX, Zhou L. WD-3 decoction combined with chemotherapy in the treated 30 cases of large intestine cancer in the advanced stage. J Nanjing Tradit Chin Med Univ. 2010;26(4):259-261. [Google Scholar]
- 26. Zeng C. Clinical observation of combine traditional Chinese and western medicine in the treatment for the colorectal carcinoma. Chin J Exp Tradit Med Formulae. 2013;19(8):335-337. [Google Scholar]
- 27. Shi Y. The clinical research of the Jianpi Qingli Huoxue decoction combined with chemotherapy for the treatment of colorectal cancer patients after Operation. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2007. [Google Scholar]
- 28. Dai L. Clinical study of “Jianpi Quzhuo Xiaoji decoction” combined with chemotherapy for the treatment of advanced colorectal cancer patients. Shandong, China: Shandong University of Traditional Chinese Medicine; 2012. [Google Scholar]
- 29. Wang B. The impact of Junzi Fuzheng decoction on reducing the toxicity of chemotherapy and enhancing the immunoligical function for the colorectal cancer patients after operation. Jilin, China: Jilin University; 2014. [Google Scholar]
- 30. Yu H. Clinical Study “Qilian fuzheng capsule” combined with chemotherapy for the treatment of colorectal cancer patients. Shandong, China: Shandong University of Traditional Chinese Medicine; 2011. [Google Scholar]
- 31. Wang H, Gao Y, Zhang Y. Effect of Delisheng injection plus OLF regimen in treatment of advanced colorectal carcinoma. Mod Oncol. 2007;15:703-704. [Google Scholar]
- 32. Yang CB, Yuan J, Zhu L. Clinical study on jianpikangfu pills for treatment of 50 cases of advanced colorectal cancer. Emerg Tradit Chin Med. 2007;16:1198-1199. [Google Scholar]
- 33. Wang H. The observation on efficacy of Yiqi Huoxue Buchang decoction with chemotherapy in patients with rectal cancer of postoperation. J Liaoning Univ Tradit Chin Med. 2008;10(5):81-82. [Google Scholar]
- 34. Zhang Q, Zhao W, Yu J, Wang X. Clinical study on advanced colorectal cancer treated by Yiqi Huoxue TCM combined with chemotherapy. Chin J Inform Tradit Chin. 2006;13(10):17-18. [Google Scholar]
- 35. Xu YQ, Xiao Y, Luo Y. Clinical study on Fuzhenghuayu jiedusanjie CM combined with chemotherapy treated 32 cases of advanced colorectal cancer. Jiangsu J Tradit Chin Med. 2006;27(11):41-42. [Google Scholar]
- 36. Wang D, Wang J, Yan CX. Advanced colorectal carcinoma treated by integrated traditional Chinese and Western medicine: a clinical efficacy observation of 49 cases. China Mod Doctor. 2012;50(13):147-148. [Google Scholar]
- 37. Qin YS, Zhou ZJ. Fuzheng guben decoction combined with chemotherapy in treatment of advanced colorectal cancer. J China TCM Inform. 2011;3(14):163. [Google Scholar]
- 38. Song WX, Zhang WW. Clinical study of combination of Xiaoliuhuaji Decoction II and chemotherapy on the treatment of advanced colorectal cancer. Jiangxi J Tradit Chin Med. 2012;44(2):13-14. [Google Scholar]
- 39. Tao CL, Xu JF. Co-Kushen injection combined with chemotherapy in treatment of advanced colorectal cancer. J Tradit Chin Med Pharm. 2013;19(11):42-44. [Google Scholar]
- 40. Wan HJ, Hong L, Bian SC. Kushen injection and Huangqi injection combined with FOLFOX in treatment of advanced colorectal cancer. North Pharm. 2013;10(4):26-27. [Google Scholar]
- 41. Zhou J. Observation of clinical efficacy of Fuzheng Jianpi decoction combined with chemotherapy in treatment of advanced colorectal cancer. Chin Arch Tradit Chin Med. 2011;29:2814-2816. [Google Scholar]
- 42. Kono T, Hata T, Morita S, et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (GONE): a phase 2, multicenter, randomized, double-blind, placebo-controlled trial of goshajinkigan to prevent oxaliplatin induced neuropathy. Cancer Chemother Pharm. 2013;72:1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jiang GS, Zhang G, Dan RW. Clinical curative effect observation of traditional Chinese and Western medicine combination for treatment of advanced colorectal cancer. Chin J Exp Tradit Med Formulae. 2013;19:323-325. [Google Scholar]
- 44. Cao B, Li ST, Li Z, Deng WL. Yiqi zhuyu decoction combined with FOLFOX-4 as first-line therapy in metastatic colorectal cancer. Chin J lntegr Med. 2011;17:593-599. [DOI] [PubMed] [Google Scholar]
- 45. Chen X, Liang Q, Li X, Zhang Y, Li J, Liang Z. Effect of composite salvia dropping pill combined with chemotherapy in patients with colorectal carcinoma. Chin J Surg Integr Tradit West Med. 2005;11:300-303. [Google Scholar]
- 46. Hu AM, Chuan Y, Li Z. The clinical efficacy of chemotherapy combined with Chinese traditional drugs for advanced colorectal cancer patients. Pract J Cancer. 2006;21:74-76. [Google Scholar]
- 47. Xu YX, Wang SL. Clinical observation of jiangnilin combined with chemotherapy treatment on advanced colorectal carcinoma. China Med Herald. 2010;7(3):84-85. [Google Scholar]
- 48. Wang J. The application of Banaosuvitamin 6 injection in the treatment for the advanced colorectal carcinoma. Mod Med Health. 2010;26:844-845. [Google Scholar]
- 49. Ding Y. The clinic research of Jianpi Huoxue Tongfu decoction combined with chemotherapy for the treatment of advanced colorectal cancer patients. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2007. [Google Scholar]
- 50. Liu J, Wang W, Zhou Y. Observation on therapeutic effect of Jianpi Huoxue herbs combined with chemotherapy in treating post-operational colonic cancer patients. Chin J lntegr Med. 2005;25:207-209. [PubMed] [Google Scholar]
- 51. Li P. Clinical impact Jianpi Jiedu Huayu Fa on the quality of life of advanced colorectal cancer patients. Nanjing, China: Nanjing University of Traditional Chinese Medicine; 2010. [Google Scholar]
- 52. Liu Z, Chen S, Cai J, et al. Traditional Chinese medicine syndrome-related herbal prescriptions in treatment of malignant tumors. J Tradit Chin Med. 2013;33(1):19-26. [DOI] [PubMed] [Google Scholar]
- 53. Zhao X, Dai X, Wang S, et al. Traditional Chinese medicine integrated with chemotherapy for stage II-IIIA patients with non-small-cell lung cancer after radical surgery: a retrospective clinical analysis with small sample size. Evid Based Complement Alternat Med. 2018;2018:4369027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang S, Long S, Xiao S, Wu W, Hann SS. Decoction of Chinese herbal medicine Fuzheng Kang-Ai induces lung cancer cell apoptosis via STAT3/Bcl-2/caspase-3 pathway. Evid Based Complement Alternat Med. 2018;2018:8567905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dong X, Jiang J. Association between cancer and utilization of traditional Chinese medicine in US. Chinese women: findings from the PINE study. Gerontol Geriatr Med. 2018;4:2333721418778199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939-944. [PubMed] [Google Scholar]
- 57. Aranda E, García-Alfonso P, Benavides M, et al. First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: phase II randomised MACRO2 TTD study. Eur J Cancer. 2018;101:263-272. [DOI] [PubMed] [Google Scholar]
- 58. Chen EY, Blanke CD, Haller DG, et al. A phase II study of celecoxib with irinotecan, 5-fluorouracil, and leucovorin in patients with previously untreated advanced or metastatic colorectal cancer. Am J Clin Oncol. 2018;14:1193-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen M, May BH, Zhou IW, Xue CC, Zhang AL. Meta-analysis of oxaliplatin-based chemotherapy combined with traditional medicines for colorectal cancer: contributions of specific plants to tumor response. Integr Cancer Ther. 2016;15:40-59. [DOI] [PMC free article] [PubMed] [Google Scholar]