Abstract
Objectives: The clinical effect of traditional Chinese medicine (TCM) on survival in patients with advanced lung adenocarcinoma treated with first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) is a major concern and requires more evidence from large-scale clinical studies. Materials and Methods: This population-based cohort study used the Taiwan National Health Insurance Research Database to enroll patients between 2006 and 2012 who had newly diagnosed locally advanced and metastatic lung adenocarcinoma treated with first-line gefitinib or erlotinib. Survival was tracked until 2013. The patients were separated into TCM users and nonusers, and Cox regression models were applied to determine the association between the use of TCM and the survival of patients. Results: A total of 1988 patients receiving first-line gefitinib or erlotinib for the treatment of EGFR-mutated advanced lung adenocarcinoma, with the exclusion of TCM users after tumor progression, were included in this cohort study. Compared with TCM nonuse, TCM use for ≥180 days was associated with a significantly decreased risk of mortality by 68% (adjusted hazard ratio [HR], 0.32 [95% CI, 0.21-0.50], P < .0001). Compared with TCM nonuse, TCM use for ≥180 days was associated with a significantly decreased risk of disease progression by 59% (adjusted HR, 0.41 [95% CI, 0.29-0.58], P < .0001). Conclusion: This cohort study suggests that adjunctive TCM therapy could improve overall survival and progression-free survival in patients with advanced lung adenocarcinoma treated with first-line TKIs. Future randomized, controlled trials are required to validate these findings.
Keywords: advanced lung adenocarcinoma, EGFR-TKI, traditional Chinese medicine, National Health Insurance Research Database, overall survival, progression-free survival
Introduction
The leading cause of cancer deaths worldwide is lung cancer, which is categorized as small cell lung cancer (SCLC) and non–small cell lung cancer (NSCLC). The subtypes of NSCLC include adenocarcinoma, squamous cell (epidermoid) carcinoma, large cell (undifferentiated) carcinoma, and other subtypes. Nowadays, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have become first-line therapy for advanced NSCLC with EGFR mutations because of dramatic tumor shrinkage and improved progression-free survival (PFS) compared with standard chemotherapy.1-7
The EGFR TKIs gefitinib and erlotinib were approved by the Food and Drug Administration (FDA) in May 2003 and November 2004, respectively. According to Taiwan National Health Insurance (NHI) payment regulations since 2006, first-line TKIs, including gefitinib and erlotinib, are covered for patients with locally advanced lung adenocarcinoma (stage IIIB) and metastatic lung adenocarcinoma (stage IV) with EGFR mutations. Usually, patients receiving EGFR-TKIs do not receive other cancer treatments at the same time except for radiation therapy for brain or bone metastases. If patients with brain metastases agree to radiation therapy, they will receive brain radiation therapy. Patients with bone metastases may receive radiotherapy to relieve bone pain. If resistance occurs after first-line TKIs, they will undergo surgery, radiotherapy, or chemotherapy as second-line treatment. If second-line treatment fails, they may return to receiving TKIs as third-line treatment.
Three surveys found that the prevalence of EGFR mutations in NSCLC patients was approximately 10% in Europe, 17% in the United States, and 78.8% in East Asia.8-10 The PIONEER study in 2014 was the first to confirm a high frequency of EGFR mutations (51.4%) in Asian patients with lung adenocarcinoma, with a rate of 62.1% in Taiwan.11 An epidemiological study in 2015 reported that the EGFR mutation rate among patients with treatment-naïve lung adenocarcinoma in Taiwan was 55.4%, with the main mutations being del 19 (44.8%) and L858R point mutations (47.9%).12
Traditional Chinese medicine (TCM) is one of the most common complementary and alternative medicine therapies for lung cancer in Taiwan. According to the conceptual framework of TCM, cancer arises when the body constitution becomes imbalanced, which leads to accumulation of toxins and heat and blood stasis and eventually produces carcinogenic factors.13 A recent investigation of Taiwan National Health Insurance Research Database (NHIRD) from 2001 to 2009 on the utilization among adult cancer patients revealed that most of the lung cancer patients were TCM nonusers (n = 58 168), and a small portion of them were TCM users (n = 6870).14
Although several studies indicate that TCM facilitates the treatment of lung cancer,15-17 there are few large-scale clinical analyses of the effect of TCM on disease prognosis in advanced lung adenocarcinoma treated with EGFR-TKIs. Because of the high rate of EGFR mutations in lung adenocarcinoma in Taiwan, it is important to investigate whether a combination of TCM and EGFR-TKIs is beneficial to patients with advanced lung adenocarcinoma with EGFR mutations.
Materials and Methods
Research Database
The cohort data came from the population-based Taiwan NHIRD, which includes the Registry of Catastrophic Illnesses Patient Database (RCIPD). In Taiwan, the NHI is an obligatory program for all residents, and therefore the NHIRD is a detailed health care database that covers almost the whole population of 23.7 million.
We used the databases to gather information about admissions and outpatient visits from the RCIPD, both of which contained information on patient characteristics such as sex, age, admission date, discharge date, dates of visits, and discharge diagnoses or outpatient visit diagnoses (made according to the International Classification of Diseases, Ninth Revision [ICD-9] classification). The data also included information on patient prescriptions, including the names of the prescribed medications, dosage, duration, initiation date, and total expenditure.
Following rigorous secrecy guidelines according to personal electronic data protection rules, the National Health Research Institutes of Taiwan maintain an anonymous database of NHI reimbursement data that is adequate for research. This study was approved by the Ethics Review Board of Chang Gung Memorial Hospital, Chia-Yi Branch, Taiwan. The data were analyzed anonymously, and the requirement for informed consent was waived by the institution’s review board.
Study Population
This study recruited patients who were diagnosed with lung cancer according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM code 162.x), between January 1, 2006, and December 31, 2012, and survival was tracked until December 31, 2013. The date of initiation of first-line therapy with EGFR-TKIs (including erlotinib and gefitinib) was defined as the index date. The endpoint of observation was death or withdrawal from the NHIRD.
Patients with other cancers that were diagnosed before lung cancer or that coexisted with lung cancer were excluded. To confirm TKIs as first-line therapy, we excluded patients who underwent surgery, chemotherapy, or radiation therapy before TKI treatment. To determine the effects of TCM on TKIs, we also excluded patients who used TCM after tumor progression. Although cytologic findings cannot be obtained from the NHIRD, we could identify patients with advanced lung adenocarcinoma with EGFR mutations as those who had received EGFR-TKI treatment according to Taiwan NHI payment regulations.
TCM Exposure
In Taiwan, TCM is covered by the NHI and is a generally established form of medical treatment. TCM treatment under the NHI is prescribed by board-certified Chinese medical physicians according to TCM syndrome differentiations. The NHIRD is the only computerized reimbursement database in the world that stores longitudinal prescription data for both TCM and Western medicine. This makes the NHIRD an optimal platform to determine the efficacy of TCM in reducing the risks of death and disease progression for patients with advanced lung adenocarcinoma treated with first-line EGFR-TKIs.
Patients who received combined TCM therapy for ≥30 days were classified as TCM users, and those treated for <30 days were classified as TCM nonusers.18 Furthermore, to observe a dose-response relationship, we separated TCM users into 2 groups: users of TCM for 30 to 179 days and users of TCM for ≥180 days.
Potential Confounders
We identified some comorbidities as potential confounding risk factors for lung cancer, which included the following diagnoses recorded during the study period: tuberculosis (ICD-9 codes 010-012), hypertension (ICD-9 codes 401-405), diabetes mellitus (ICD-9 codes 249-250), hyperlipidemia (ICD-9 code 272), coronary artery disease (CAD; ICD-9 codes 410-419), heart failure (ICD-9 code 428), stroke (ICD-9 codes 430-438), chronic obstructive pulmonary disease (COPD; ICD-9 codes 491, 492, 496), and liver cirrhosis (ICD-9 codes 571.2, 571.5, 571.6).19 We also considered sociodemographic characteristics, namely age, sex, monthly insurance income, and level of urbanization20 in the model. Treatments consisting of surgery, chemotherapy, or radiation after tumor progression were also included in our study.
Overall Survival and Progression-Free Survival
Overall survival (OS) was defined as the time from the initiation of first-line EGFR-TKIs to death from any cause or withdrawal from the NHIRD. Progression-free survival (PFS) was defined as the time from the initiation of first-line EGFR-TKIs to the time that we withdraw the EGFR-TKIs or other kind of cancer therapy.
Each patient with advanced lung adenocarcinoma treated with first-line EGFR-TKIs was requested to undergo imaging studies. Once the disease progressed, EGFR-TKIs were declined by the NHI, and other cancer treatments were censored in the NHIRD.
First-line EGFR-TKI responders were defined as patients who received first-line EGFR-TKI therapy for ≥90 days and the remaining patients were defined as non-responders. EGFR-TKI responders were also used as a surrogate for PFS.
Matched Cohort
To further examine the effect of TCM integration, we used propensity scores to estimate the probabilities of assigning a patient to use TCM, given background variables including age, sex, monthly insurance income, level of urbanization, and all comorbidities mentioned above. TCM users and nonusers were matched by using propensity scores at a ratio of 1:4. Overall, 985 insured adults (197 matched sets) were included in the matched cohort. The results of the analysis for both the study cohort and the matched cohort are presented in Tables 1 to 4 and Figures 1 and 2.
Table 1.
Baseline Patient Characteristics of the Study Cohort and the Matched Cohort.
| Study Cohort | Matched Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TCM (N = 217) | Non-TCM (N = 1771) | P | TCM (N = 197) | Non-TCM (N = 788) | P | |||||
| n | % | n | % | n | % | n | % | |||
| Age (years) | <.0001 | .6777 | ||||||||
| <65 | 124 | 57.14 | 654 | 36.93 | 104 | 52.79 | 429 | 54.44 | ||
| ≥65 | 93 | 42.86 | 1117 | 63.07 | 93 | 47.21 | 359 | 45.56 | ||
| Sex | .0177 | .7843 | ||||||||
| Male | 64 | 29.49 | 668 | 37.72 | 61 | 30.96 | 252 | 31.98 | ||
| Female | 153 | 70.51 | 1103 | 62.28 | 136 | 69.04 | 536 | 68.02 | ||
| Monthly insurance income (NT$) | .216 | .9791 | ||||||||
| 0 | 36 | 16.59 | 378 | 21.34 | 33 | 16.75 | 142 | 18.02 | ||
| 1-15 840 | 28 | 12.90 | 236 | 13.33 | 26 | 13.20 | 101 | 12.82 | ||
| 15 841-25 000 | 102 | 47.00 | 826 | 46.64 | 92 | 46.70 | 366 | 46.45 | ||
| >25 000 | 51 | 23.50 | 331 | 18.69 | 46 | 23.35 | 179 | 22.72 | ||
| Urbanization levela | .8588 | .9959 | ||||||||
| 1 (city) | 60 | 27.65 | 516 | 29.14 | 56 | 28.43 | 229 | 29.06 | ||
| 2 | 102 | 47.00 | 778 | 43.93 | 93 | 47.21 | 368 | 46.70 | ||
| 3 | 35 | 16.13 | 299 | 16.88 | 30 | 15.23 | 122 | 15.48 | ||
| 4 (village) | 20 | 9.22 | 178 | 10.05 | 18 | 9.14 | 69 | 8.79 | ||
| Comorbidities | ||||||||||
| Tuberculosis | .2559 | .7438 | ||||||||
| Yes | 7 | 3.23 | 88 | 4.97 | 7 | 3.55 | 32 | 4.06 | ||
| No | 210 | 96.77 | 1683 | 95.03 | 190 | 96.45 | 756 | 95.94 | ||
| Hypertension | .0004 | .8734 | ||||||||
| Yes | 98 | 45.16 | 1025 | 57.88 | 96 | 48.73 | 389 | 49.37 | ||
| No | 119 | 54.84 | 746 | 42.12 | 101 | 51.27 | 399 | 50.63 | ||
| Diabetes mellitus | .0051 | .8090 | ||||||||
| Yes | 37 | 17.05 | 456 | 25.75 | 37 | 18.78 | 154 | 19.54 | ||
| No | 180 | 82.95 | 1,315 | 74.25 | 160 | 81.22 | 634 | 80.46 | ||
| Hyperlipidemia | .0775 | .9724 | ||||||||
| Yes | 63 | 29.03 | 621 | 35.06 | 60 | 30.46 | 241 | 30.58 | ||
| No | 154 | 70.97 | 1150 | 64.94 | 137 | 69.54 | 547 | 69.42 | ||
| CAD | .061 | .7675 | ||||||||
| Yes | 49 | 22.58 | 507 | 28.63 | 47 | 23.86 | 196 | 24.87 | ||
| No | 168 | 77.42 | 1264 | 71.37 | 150 | 76.14 | 592 | 75.13 | ||
| Heart failure | .0051 | .8686 | ||||||||
| Yes | 8 | 3.69 | 166 | 9.37 | 8 | 4.06 | 30 | 3.81 | ||
| No | 209 | 96.31 | 1605 | 90.63 | 189 | 95.94 | 758 | 96.19 | ||
| Stroke | .0003 | .6892 | ||||||||
| Yes | 21 | 9.68 | 349 | 19.71 | 21 | 10.66 | 92 | 11.68 | ||
| No | 196 | 90.32 | 1422 | 80.29 | 176 | 89.34 | 696 | 88.32 | ||
| COPD | .0036 | .6865 | ||||||||
| Yes | 9 | 4.15 | 183 | 10.33 | 9 | 4.57 | 31 | 3.93 | ||
| No | 208 | 95.85 | 1588 | 89.67 | 188 | 95.43 | 757 | 96.07 | ||
| Liver cirrhosis | .5130 | .8067 | ||||||||
| Yes | 4 | 1.84 | 23 | 1.30 | 3 | 1.52 | 14 | 1.78 | ||
| No | 213 | 98.16 | 1748 | 98.70 | 194 | 98.48 | 774 | 98.22 | ||
| Surgery | .9573 | 1.0000 | ||||||||
| Yes | 19 | 8.76 | 157 | 8.87 | 18 | 9.14 | 72 | 9.14 | ||
| No | 198 | 91.24 | 1614 | 91.13 | 179 | 90.86 | 716 | 90.86 | ||
| CT/RT | .0569 | .9719 | ||||||||
| CT + RT | 58 | 26.73 | 389 | 21.96 | 50 | 25.38 | 206 | 26.14 | ||
| CT | 64 | 29.49 | 436 | 24.62 | 55 | 27.92 | 229 | 29.06 | ||
| RT | 21 | 9.68 | 236 | 13.33 | 21 | 10.66 | 80 | 10.15 | ||
| No CT or RT | 74 | 34.10 | 710 | 40.09 | 71 | 36.04 | 273 | 34.64 | ||
| EGFR-TKI response | <.0001 | .6708 | ||||||||
| Responder | 193 | 88.94 | 1219 | 68.83 | 173 | 87.82 | 683 | 86.68 | ||
| Nonresponder | 24 | 11.06 | 552 | 31.17 | 24 | 12.18 | 105 | 13.32 | ||
| TKI | .2423 | .4322 | ||||||||
| Gefitinib | 212 | 97.70 | 1702 | 96.10 | 192 | 97.46 | 759 | 96.32 | ||
| Erlotinib | 5 | 2.30 | 69 | 3.84 | 5 | 2.54 | 29 | 3.68 | ||
| Third-line EGFR-TKI | ||||||||||
| Gefitinib-CT-erlotinib | 55 | 25.35 | 273 | 15.42 | .0002 | 47 | 23.86 | 143 | 18.15 | .0692 |
| Erlotinib-CT-gefitinib | 0 | 0.00 | 2 | 0.11 | 1.0000 | 0 | 0.00 | 0 | 0.00 | 0 |
| RT | .7459 | .9472 | ||||||||
| Yes | 79 | 36.41 | 625 | 35.29 | 71 | 36.04 | 286 | 36.29 | ||
| No | 138 | 63.59 | 1146 | 64.71 | 126 | 63.96 | 502 | 63.71 | ||
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CT, chemotherapy; EGFR, epidermal growth factor receptor; RT, radiotherapy; TCM, traditional Chinese medicine; TKI, tyrosine kinase inhibitor.
Urbanization levels in Taiwan are divided into 4 strata according to previous research, with level 1 referring to the most urbanized communities and level 4 to the least urbanized communities.20
Table 4.
Adjusted Cox Proportional Hazards Model Analysis of Mortality for the Commonly Used TCMs in Patients With Advanced Lung Adenocarcinoma Treated With First-Line EGFR-TKIs in the Study Cohort and the Matched Cohort.
| TCM Name | Study Cohort | Matched Cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| Adjusteda | Adjusteda | |||||||
| HR | 95% CI | P | HR | 95% CI | P | |||
| Fritillaria thunbergii | 0.44 | 0.26 | 0.74 | .0023 | 0.37 | 0.21 | 0.66 | .0007 |
| Oldenlandia diffusa | 0.60 | 0.39 | 0.92 | .0192 | 0.60 | 0.38 | 0.96 | .0312 |
| Platycodon grandiflorum | 0.24 | 0.10 | 0.57 | .0014 | 0.20 | 0.08 | 0.50 | .0005 |
| Prunus armeniaca | 0.52 | 0.27 | 1.01 | .0542 | 0.57 | 0.29 | 1.13 | .1059 |
| Astragalus membranaceus | 0.52 | 0.27 | 1.01 | .0522 | 0.50 | 0.25 | 1.01 | .0546 |
| Xiang Sha Liu Jun Zi Tang | 0.58 | 0.31 | 1.09 | .0883 | 0.61 | 0.32 | 1.15 | .1244 |
| Xiao Chai Hu Tang | 0.47 | 0.21 | 1.07 | .0707 | 0.57 | 0.25 | 1.29 | .1764 |
| Mai Men Dong Tang | 0.54 | 0.27 | 1.08 | .0830 | 0.62 | 0.31 | 1.26 | .1852 |
| Sheng Mai San | 0.64 | 0.30 | 1.35 | .2427 | 0.59 | 0.26 | 1.34 | .2095 |
| Bai He Gu Jin Tang | 0.43 | 0.22 | 0.84 | .0128 | 0.29 | 0.13 | 0.62 | .0015 |
Abbreviations: EGFR, epidermal growth factor receptor; HR, hazard ratio; TCM, traditional Chinese medicine; TKI, tyrosine kinase inhibitor.
Adjusted for age, sex, insurance income, urbanization, comorbidities, surgery, chemotherapy/radiotherapy, and EGFR-TKI response.
Figure 1.
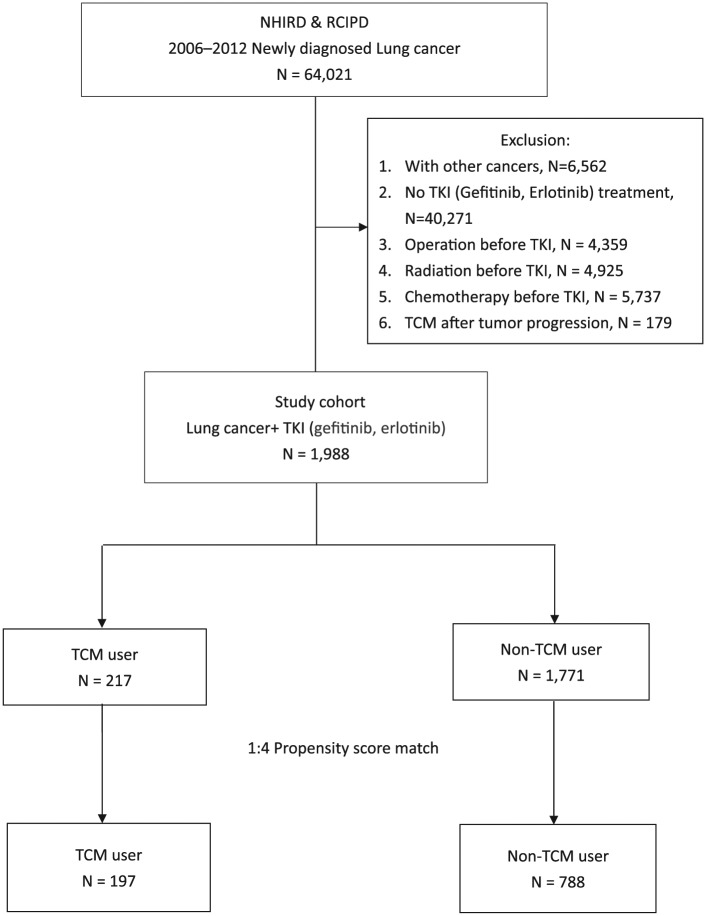
Flowchart of the patient enrollment process of the study cohort and the matched cohort. Abbreviations: NHIRD, Taiwan National Health Insurance Research Database; NSCLC, non–small cell lung cancer; TCM, traditional Chinese medicine; TKI, tyrosine kinase inhibitor.
Figure 2.
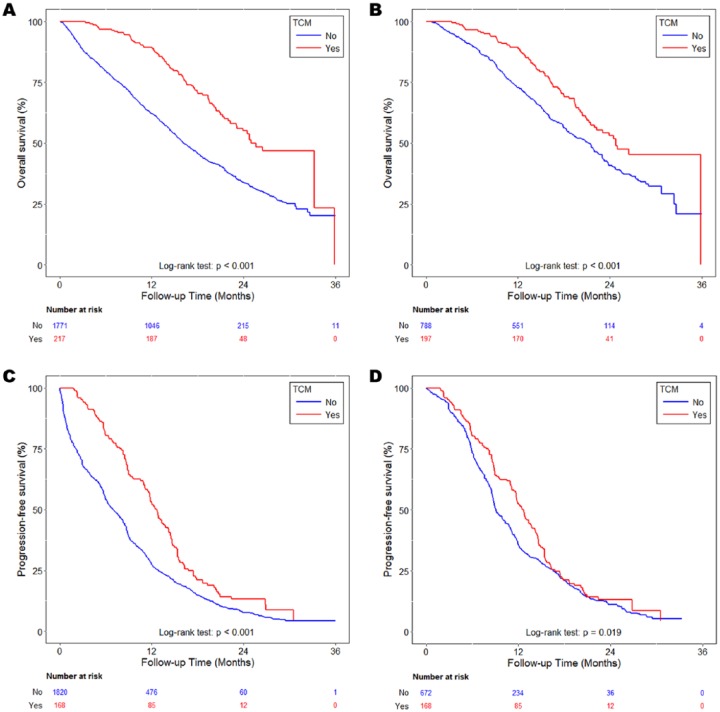
Kaplan-Meier curves of OS in patients with advanced lung adenocarcinoma treated with first-line EGFR-TKIs according to TCM usage during the follow-up period from the study cohort (A) and matched cohort (B) and PFS in patients with advanced lung adenocarcinoma treated with first-line EGFR-TKIs according to TCM usage during the follow-up period from the study cohort (C) and the matched cohort (D). Abbreviations: EGFR, epidermal growth factor receptor; OS, overall survival; PFS, progression-free survival; TCM, traditional Chinese medicine; TKI, tyrosine kinase inhibitor.
Statistical Analysis
The distribution of baseline characteristics, the proportions of comorbidities, treatments after tumor progression, the proportions of first-line EGFR-TKI responses, the proportions of first-line EGFR-TKI, and the proportions of third-line EGFR-TKI were compared between the TCM nonusers and users in the study cohort and in the matched cohort.
We used the Kaplan-Meier method to estimate the cumulative probability of OS and PFS for TCM nonusers and users. The log-rank test was performed to compare the curves of OS and PFS between the groups. Finally, Cox proportional hazards models were used to calculate the hazard ratios (HRs) coexisting with 95% confidence intervals after adjustment for TCM usage, age, sex, monthly insurance income, level of urbanization, comorbidities, treatments after tumor progression, and first-line EGFR-TKI response. A 2-tailed P < .05 was considered to indicate a significant difference.
The study endpoint was all-cause mortality. Data from patients who were alive at the time of the last follow-up were censored at the date of withdrawal from the NHI or the end of the study period, whichever came first. To verify the dose-response relationship of TCM use with mortality and disease progression, we treated the TCM use category as a continuous variable to calculate the p value of the linear trend. All analyses were conducted with SAS statistical software (version 9.4; SAS Institute, Cary, NC, USA).
Results
A total of 64 021 patients were newly diagnosed with lung cancer in the RCIPD of the NHIRD from 2006 to 2012. Of these, 6562 patients were excluded because of other cancers existing before or coexisting with lung cancer. Another 40 271 patients were excluded because they did not receive gefitinib or erlotinib. Patients who had undergone surgery (n = 4359), radiotherapy (n = 4925), or chemotherapy (n = 5737) before TKI treatment were also excluded. Another 179 patients were excluded who had used TCM after tumor progression. The remaining 1988 patients received gefitinib or erlotinib for locally advanced and metastatic lung adenocarcinoma with EGFR mutations. The number of patients who were TCM users was 217 (10.9%), whereas 1771 patients (89.1%) were TCM nonusers. After using propensity scores with a ratio of 1:4, the numbers of TCM users and TCM nonusers were 197 and 788, respectively (Figure 1).
The mean age of both TCM users and nonusers was 63.7 years. In the matched cohort, patient baseline characteristics did not differ significantly between TCM users and nonusers (Table 1).
Overall Survival
For evaluation of OS, the mean follow-up time was 18.7 months for TCM users and 13.9 months for TCM nonusers. A total of 1134 deaths occurred during the 7-year period.
Multivariate analysis showed that men had a significantly higher risk of mortality than women (adjusted HR, 1.54 [95% CI, 1.26-1.89] for men, P < .0001). Compared with TCM nonuse, TCM use for ≥180 days was associated with a significantly decreased risk of mortality by 68% (adjusted HR, 0.32 [95% CI, 0.21-0.50], P < .0001). Although TCM use between 30 and 179 days was associated with a nonsignificantly lower risk of mortality (adjusted HR, 0.80 [95% CI, 0.60-1.06], P = .1182), we can still conclude that the longer the duration of TCM usage, the lower the mortality rate. A dose-response relationship was observed between TCM use and survival (Table 2).
Table 2.
Adjusted Cox Proportional Hazards Model Analysis of Mortality in Patients With Advanced Lung Adenocarcinoma Treated With First-Line EGFR-TKIs According to TCM Usage During the Follow-up Period in the Study Cohort and the Matched Cohort.
| Variables | Study Cohort | Matched Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Death Events | Adjusted | Death Events | Adjusted | |||||||
| HR | 95% CI | P | HR | 95% CI | P | |||||
| TCM (days) | ||||||||||
| <30 | 1049 | 1.00 | Reference | 396 | 1.00 | Reference | ||||
| 30-179 | 61 | 0.82 | 0.63 | 1.07 | .1470 | 57 | 0.80 | 0.60 | 1.06 | .1182 |
| ≥180 | 24 | 0.37 | 0.24 | 0.55 | <.0001 | 22 | 0.32 | 0.21 | 0.50 | <.0001 |
| Age (years) | ||||||||||
| <65 | 402 | 1.00 | Reference | 263 | 1.00 | Reference | ||||
| ≥65 | 732 | 1.26 | 1.09 | 1.46 | .0015 | 212 | 1.04 | 0.83 | 1.29 | .7494 |
| Sex | ||||||||||
| Male | 472 | 1.53 | 1.34 | 1.73 | <.0001 | 170 | 1.54 | 1.26 | 1.89 | <.0001 |
| Female | 662 | 1.00 | Reference | 305 | 1.00 | Reference | ||||
| Monthly insurance income (NT$) | ||||||||||
| 0 | 263 | 1.00 | Reference | 99 | 1.00 | Reference | ||||
| 1-15 840 | 162 | 0.99 | 0.81 | 1.21 | .9350 | 62 | 0.66 | 0.47 | 0.91 | .0121 |
| 15 841-25 000 | 519 | 0.87 | 0.75 | 1.02 | .0843 | 211 | 0.66 | 0.51 | 0.84 | .0009 |
| >25 000 | 190 | 0.69 | 0.57 | 0.84 | .0003 | 103 | 0.54 | 0.40 | 0.73 | <.0001 |
| Urbanization levela | ||||||||||
| 1 (city) | 331 | 0.85 | 0.68 | 1.05 | .1372 | 136 | 0.83 | 0.58 | 1.19 | .3028 |
| 2 | 492 | 0.87 | 0.70 | 1.07 | .1727 | 225 | 0.91 | 0.65 | 1.27 | .5748 |
| 3 | 198 | 0.97 | 0.77 | 1.23 | .8255 | 73 | 0.97 | 0.66 | 1.42 | .8606 |
| 4 (village) | 113 | 1.00 | Reference | 41 | 1.00 | Reference | ||||
| Comorbidities (yes/no) | ||||||||||
| Tuberculosis | 60 | 1.07 | 0.82 | 1.40 | .6115 | 22 | 1.22 | 0.79 | 1.90 | .3719 |
| Hypertension | 651 | 1.05 | 0.91 | 1.21 | .4943 | 218 | 0.92 | 0.74 | 1.14 | .4192 |
| Diabetes mellitus | 307 | 1.23 | 1.06 | 1.43 | .0055 | 99 | 1.36 | 1.06 | 1.74 | .0164 |
| Hyperlipidemia | 390 | 0.90 | 0.78 | 1.03 | .1310 | 134 | 0.82 | 0.65 | 1.03 | .0864 |
| CAD | 331 | 0.92 | 0.79 | 1.07 | .2784 | 118 | 1.05 | 0.83 | 1.34 | .6775 |
| Heart failure | 111 | 1.07 | 0.87 | 1.33 | .5196 | 15 | 0.89 | 0.52 | 1.52 | .6565 |
| Stroke | 238 | 1.14 | 0.98 | 1.34 | .0898 | 58 | 1.28 | 0.95 | 1.72 | .1103 |
| COPD | 125 | 0.94 | 0.77 | 1.15 | .5441 | 18 | 0.92 | 0.56 | 1.52 | .7532 |
| Liver cirrhosis | 17 | 1.20 | 0.74 | 1.95 | .4618 | 8 | 0.94 | 0.46 | 1.92 | .8554 |
| Surgery | ||||||||||
| Yes | 119 | 0.98 | 0.81 | 1.19 | 0.8363 | 54 | 0.95 | 0.70 | 1.28 | 0.7183 |
| No | 1015 | 1.00 | Reference | 421 | 1.00 | Reference | ||||
| CT/RT | ||||||||||
| CT + RT | 289 | 0.73 | 0.62 | 0.87 | .0003 | 150 | 1.06 | 0.82 | 1.36 | .6549 |
| CT | 235 | 0.66 | 0.56 | 0.78 | <.0001 | 116 | 0.82 | 0.63 | 1.06 | .1287 |
| RT | 199 | 1.17 | 0.98 | 1.41 | .0874 | 71 | 2.17 | 1.60 | 2.93 | <.0001 |
| No CT or RT | 411 | 1.00 | Reference | 138 | 1.00 | Reference | ||||
| 1st-line EGFR-TKI response | ||||||||||
| Responder | 658 | 0.26 | 0.23 | 0.30 | <.0001 | 380 | 0.33 | 0.26 | 0.42 | <.0001 |
| Nonresponder | 476 | 1.00 | Reference | 95 | 1.00 | Reference | ||||
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CT, chemotherapy; EGFR, epidermal growth factor receptor; HR, hazard ratio; RT, radiotherapy; TCM, traditional Chinese medicine; TKI, tyrosine kinase inhibitor.
Urbanization levels in Taiwan are divided into 4 strata according to previous research, with level 1 referring to the most urbanized communities and level 4 to the least urbanized communities.20
Patients with a higher monthly insurance income had a significantly lower risk of mortality, and patients with a lower monthly insurance income had a significantly higher risk of mortality (adjusted HR, 0.66 [95% CI, 0.47-0.91] for NT$ 1-15 840, P = .0121; adjusted HR, 0.66 [95% CI, 0.51-0.84] for NT$ 15 841-25 000, P = .0009; adjusted HR, 0.54 [95% CI, 0.40-0.73] for NT$ >25 000, P < .0001).
Diabetes mellitus, one of the comorbidities, was found to increase mortality significantly (adjusted HR, 1.36 [95% CI, 1.06-1.74], P = .0164). Radiation therapy after disease progression or simultaneously for brain metastases or bone metastases increased mortality significantly in comparison with patients who did not undergo chemotherapy or radiation therapy (adjusted HR, 2.17 [95% CI, 1.60-2.93], P < .0001). Compared with nonresponders to first-line EGFR-TKI, TKI responders had a significantly decreased risk of mortality by 67% (adjusted HR, 0.33 [95% CI, 0.26-0.42], P < .0001) (Table 2).
Progression-Free Survival
For evaluation of PFS, the mean follow-up time was 12.5 months for TCM users and 8.3 months for TCM nonusers.
Multivariate analysis showed that men had a significantly higher risk of disease progression than women (adjusted HR, 1.29 [95% CI, 1.09-1.52] for men, P = .0035). Compared with TCM nonuse, TCM use for ≥180 days was associated with a significantly decreased risk of disease progression by 59% (adjusted HR, 0.41 [95% CI, 0.29-0.58], P < .0001). Although TCM use between 30 and 179 days was associated with a nonsignificantly lower risk of disease progression (adjusted HR, 0.91 [95% CI, 0.74-1.14], P = .4150), we can still conclude that the longer the duration of TCM usage, the lower the rate of disease progression. A dose-response relationship was observed between TCM use and PFS (Table 3).
Table 3.
Adjusted Cox Proportional Hazards Model Analysis of PFS in Patients With Advanced Lung Adenocarcinoma Treated With First-Line EGFR-TKIs According to TCM Usage During the Follow-up Period in the Study Cohort and the Matched Cohort.
| Variables | Study Cohort | Matched Cohort | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PD Events | Adjusted | PD Events | Adjusted | |||||||
| HR | 95% CI | P | HR | 95% CI | P | |||||
| TCM (days) | ||||||||||
| <30 | 1599 | 1.00 | Reference | 568 | 1.00 | Reference | ||||
| 30-179 | 101 | 0.88 | 0.72 | 1.09 | .2393 | 101 | 0.91 | 0.74 | 1.14 | .4150 |
| ≥180 | 37 | 0.42 | 0.30 | 0.58 | <.0001 | 37 | 0.41 | 0.29 | 0.58 | <.0001 |
| Age (years) | ||||||||||
| <65 | 701 | 1.00 | Reference | 417 | 1.00 | Reference | ||||
| ≥65 | 1036 | 0.93 | 0.83 | 1.04 | .1921 | 289 | 0.88 | 0.73 | 1.06 | .1709 |
| Sex | ||||||||||
| Male | 658 | 1.17 | 1.05 | 1.30 | .004 | 228 | 1.29 | 1.09 | 1.52 | .0035 |
| Female | 1079 | 1.00 | Reference | 478 | 1.00 | Reference | ||||
| Monthly insurance income (NT$) | ||||||||||
| 0 | 357 | 1.00 | Reference | 104 | 1.00 | Reference | ||||
| 1-15 840 | 241 | 1.06 | 0.89 | 1.25 | .5163 | 92 | 0.99 | 0.74 | 1.32 | .9364 |
| 15 841-25 000 | 815 | 0.91 | 0.80 | 1.04 | .1731 | 347 | 0.86 | 0.68 | 1.08 | .1830 |
| >25 000 | 324 | 0.66 | 0.56 | 0.78 | <.0001 | 163 | 0.69 | 0.53 | 0.89 | .0048 |
| Urbanization levela | ||||||||||
| 1 (city) | 493 | 0.87 | 0.73 | 1.05 | .1418 | 176 | 0.68 | 0.51 | 0.91 | .0092 |
| 2 | 783 | 0.86 | 0.72 | 1.01 | .0707 | 328 | 0.67 | 0.51 | 0.87 | .0024 |
| 3 | 292 | 0.94 | 0.78 | 1.13 | .5064 | 127 | 0.79 | 0.59 | 1.07 | .1246 |
| 4 (village) | 169 | 1.00 | Reference | 75 | 1.00 | Reference | ||||
| Comorbidities (yes/no) | ||||||||||
| Tuberculosis | 81 | 1.10 | 0.88 | 1.38 | .4055 | 17 | 0.73 | 0.45 | 1.21 | .2204 |
| Hypertension | 971 | 1.12 | 0.99 | 1.25 | .0642 | 325 | 1.02 | 0.85 | 1.22 | .8571 |
| Diabetes mellitus | 425 | 1.04 | 0.92 | 1.17 | .5745 | 108 | 1.06 | 0.84 | 1.34 | .6104 |
| Hyperlipidemia | 591 | 0.87 | 0.78 | 0.98 | .0175 | 211 | 0.85 | 0.71 | 1.03 | .0978 |
| CAD | 475 | 0.98 | 0.86 | 1.10 | .7079 | 156 | 1.10 | 0.89 | 1.36 | .3828 |
| Heart failure | 154 | 1.25 | 1.05 | 1.50 | .0142 | 30 | 1.28 | 0.87 | 1.90 | .2143 |
| Stroke | 319 | 1.02 | 0.90 | 1.17 | .7258 | 68 | 0.92 | 0.70 | 1.22 | .5775 |
| COPD | 165 | 1.01 | 0.85 | 1.20 | .9471 | 31 | 0.83 | 0.56 | 1.23 | .3606 |
| Liver cirrhosis | 26 | 1.37 | 0.93 | 2.03 | .1141 | 15 | 1.81 | 1.07 | 3.06 | .0266 |
| Surgery | ||||||||||
| Yes | 106 | 1.50 | 1.22 | 1.84 | <.0001 | 24 | 1.68 | 1.10 | 2.57 | .0173 |
| No | 1631 | 1.00 | Reference | 682 | 1.00 | Reference | ||||
| CT/RT | ||||||||||
| CT + RT | 447 | 2.62 | 2.28 | 3.02 | <.0001 | 207 | 4.87 | 3.90 | 6.07 | <.0001 |
| CT | 494 | 1.91 | 1.68 | 2.17 | <.0001 | 233 | 3.07 | 2.50 | 3.76 | <.0001 |
| RT | 257 | 3.00 | 2.56 | 3.52 | <.0001 | 81 | 4.58 | 3.46 | 6.07 | <.0001 |
| No CT or RT | 539 | 1.00 | Reference | 185 | 1.00 | Reference | ||||
| 1st-line EGFR-TKI response | ||||||||||
| Responder | 1,165 | 0.04 | 0.04 | 0.05 | <.0001 | 654 | 0.04 | 0.03 | 0.06 | <.0001 |
| Nonresponder | 572 | 1.00 | Reference | 52 | 1.00 | Reference | ||||
Abbreviations: CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CT, chemotherapy; EGFR, epidermal growth factor receptor; HR, hazard ratio; PD, progressive disease; PFS, progression-free survival; RT, radiotherapy; TCM, traditional Chinese medicine; TKI, tyrosine kinase inhibitor.
Urbanization levels in Taiwan are divided into 4 strata according to previous research, with level 1 referring to the most urbanized communities and level 4 to the least urbanized communities.20
Monthly insurance income >NT$ 25 000 was associated with a significantly decreased risk of disease progression (adjusted HR, 0.69 [95% CI, 0.53-0.89], P = .0048). Urbanization levels of 1 (city) and 2 were associated with lower risks of disease progression (adjusted HR, 0.68 [95% CI, 0.51-0.91] for level 1, P = .0092; adjusted HR, 0.67 [95% CI, 0.51-0.87] for level 2, P = .0024). Liver cirrhosis, one of the comorbidities, was found to increase the rate of disease progression significantly (adjusted HR, 1.81 [95% CI, 1.07-3.06], P = .0266).
In comparison with patients who did not undergo chemotherapy or radiation therapy, patients who received chemotherapy with radiotherapy, chemotherapy, and radiotherapy were found to increase the rate of disease progression significantly (adjusted HR, 4.87 [95% CI, 3.90-6.07] for chemotherapy with radiotherapy, P < .0001; adjusted HR, 3.07 [95% CI, 2.50-3.76] for chemotherapy, P < .0001 and adjusted HR, 4.58 [95% CI, 3.46-6.07] for radiotherapy, P < .0001). Compared with nonresponders to first-line EGFR-TKI, TKI responders had a significantly decreased risk of disease progression by 96% (adjusted HR, 0.04 [95% CI, 0.03-0.06], P < .0001) (Table 3).
Probabilities of OS and PFS
Figure 2A-D illustrate the results of the Kaplan-Meier analysis in the study cohort and the matched cohort for the probabilities of OS and PFS in patients with advanced lung adenocarcinoma treated with first-line gefitinib or erlotinib according to TCM usage. Improvement in OS and PFS displayed a progressive dose-response relationship in the study cohort and the matched cohort. The log-rank test indicated a significant difference over the Kaplan-Meier curve of OS (P < .001) and PFS (P = .019) in the matched cohort.
In the cohort, the 5 most commonly used herbs were Fritillaria thunbergii, Oldenlandia diffusa, Platycodon grandiflorum, Prunus armeniaca, and Astragalus membranaceus. The 5 most commonly used formulas were Xiang Sha Liu Jun Zi Tang, Xiao Chai Hu Tang, Mai Men Dong Tang, Sheng Mai San, and Bai He Gu Jin Tang. Among them, 3 herbs could significantly reduce mortality, including F thunbergii (adjusted HR, 0.37 [95% CI, 0.21-0.66], P = .0007), O diffusa (adjusted HR, 0.60 [95% CI, 0.38-0.96], P = .0312), and P grandiflorum (adjusted HR, 0.20 [95% CI, 0.08-0.50], P = .0005). One formula, Bai He Gu Jin Tang, could significantly reduce mortality (adjusted HR, 0.29 [95% CI, 0.13-0.62], P = .0015) (Table 4).
Discussion
Until now, there have been few clinical studies on the efficacy of adjunctive TCM for patients with advanced lung adenocarcinoma treated with first-line EGFR-TKIs. To our knowledge, our study is the first large-scale, nationwide cohort study to investigate the influence of adjunctive TCM therapy on OS and PFS in this patient population.
Our study concludes that integrative TCM can improve OS and PFS significantly in patients with advanced lung adenocarcinoma treated with first-line gefitinib or erlotinib. However, this finding seems inconsistent with that of another retrospective study from one hospital in Taiwan, which observed that among patients treated with EGFR-TKIs, PFS and OS were nonsignificantly better in TCM users than in TCM nonusers.21
There are some possible explanations for the different outcomes. First, the earlier study had a smaller sample size (34 patients receiving TCM and EGFR-TKIs at the same time) than our study (217 patients). Second, the earlier study did not exclude patients receiving Chinese herbal medicine treatment after their disease progressed to EGFR-TKI treatment. Third, their patients came from only one hospital in Taiwan, whereas our patients came from the NHIRD, which covers all of Taiwan. Fourth, their patients might have received TCM treatment in other hospitals or clinics, whereas we collected all patients from the NHIRD.
A pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive NSCLC demonstrated that the most common withdrawal adverse effects were skin toxicity, interstitial lung disease, hepatotoxicity, and diarrhea.22 Some patients with advanced lung adenocarcinoma treated with first-line EGFR-TKIs would choose TCM because of the adverse effects of TKIs, which may lead to TKI dose reduction, TKI discontinuation, TKI withdrawal, or worsening quality of life. Some of these patients may continue to take TKIs, since TCMs improve TKI toxicities. The number of TCM users excluded from out study after progression was 179. Some patients still chose TCM treatment after progression, and the survival of these patients needs to be analyzed.
Our study showed there were “dose-responsive” effects of TCM treatments. We had used time-dependent Cox proportional hazard model to adjust immortal time bias before, and the results showed that the adjusted hazard ratio for study cohort was 0.30 (95% CI 0.16-0.56) for TCM users with more than 14 days per 12 weeks. The results of time-dependent Cox proportional hazard model and Cox proportional hazard model were compatible so that there was no immortal time bias in our analysis.
Our study showed that 3 herbs, F thunbergii, O diffusa, and P grandiflorum, could significantly reduce mortality in patients with advanced lung adenocarcinoma treated with first-line EGFR-TKIs (gefitinib and erlotinib). These herbs may have the characteristics of relieving TKI adverse effects or improving OS and PFS. The bulb of F thunbergii is used as a mucoregulator and expectorant for treating airway inflammatory diseases in TCM. Extracts from the bulb of F thunbergii (verticine, ebeiedine, and suchengbeisine) can inhibit the gene expression and production of MUC5AC mucin from human airway epithelial cells.23 Another extract from the bulb of F thunbergii (peiminine) was found to inhibit colorectal cancer cell proliferation by inducing apoptosis and autophagy.24 O diffusa is a common anticancer herb in TCM. Its anticancer mechanisms include augmenting the macrophage oxidative burst, inhibiting tumor growth, inducing a significant increase in apoptosis, and significantly inhibiting lung metastases in an animal model without adverse effects.25,26 P grandiflorum is known for its immune modulation and antitumor effects in TCM. The aqueous extract from the root of P grandiflorum can decrease telomerase activity and downregulate Bcl-2 expression, thus inducing apoptosis and growth inhibition in human lung carcinoma cells.27 The aqueous extract from the root of P grandiflorum can also inhibit the adhesion of tumor cells to the basement membrane and activate natural killer cells, thus reducing the range of lung metastases.28 Another study showed that platycodin D can induce autophagy via PI3K/Akt/mTOR and MAPK signaling pathways in NSCLC cells.29
Most of the Chinese herbal formulas had no significant reduction of hazard ratio. It may because some of the formulas were used for decreasing the side effect of EGFR TKIs, relieving original symptoms of cancer patients or improving fatigue of the patients, not just for cancer therapies. For example, Xiang Sha Liu Jun Zi Tang may relieve diarrhea caused by EGFR TKIs, Xiao Chai Hu Tang may reduce gastrointestinal discomforts, Mai Men Dong Tang may subside cough, and Sheng Mai San may improve fatigue.
Our study showed that the longer the duration of TCM usage, the lower the rate of disease progression. Thus, integrated use of TKI and TCM resulted in better PFS than TKI alone. The combination of TKI and TCM may delay acquired resistance to TKI. Some studies also found that EGFR-TKI plus TCM compared with EGFR-TKI alone in patients with NSCLC could prolong PFS and median survival time, increase efficacy, and reduce toxicity.15,16 However, one case report mentioned that inappropriate use of TCM, including ginseng, Fomes fomentarius, Inonotus obliquus, Phellinus linteus, and selenium, could decrease sensitivity to gefitinib, while withdrawing these herbs restored sensitivity to gefitinib.30 Therefore, which TCM formulas and herbs are used would be the key to determining their efficacy.
There are several limitations to this study. First, the NHI program only pays for TCM treatment prescribed by Chinese medicine physicians, and not for over-the-counter TCM treatment. Thus, the use of TCM may be underestimated. A recent investigation of Taiwan NHIRD from 2001 to 2009 on the application among adult cancer patients demonstrated that TCM treatments could be separated into only Chinese herbal medicines (N = 69 086), only acupuncture or traumatology (N = 459), or together (N = 5057).14 This research reflects the current situation of Taiwan NHIRD that we are unable to know whether the patients receive moxibustion, manipulation, other TCM treatment, and over-the-counter TCM treatment or not from NHIRD. Second, there may not have been full compliance with prescriptions among TCM users, but we could still demonstrate improvement in OS and PFS from TCM application. Third, we did not compare dose reductions, delays, discontinuations, or decreasing starting doses of EGFR-TKIs. Fourth, we could not determine the subtype of EGFR mutation from the NHIRD, but we could confirm that patients with advanced lung adenocarcinoma had EGFR mutations from their EGFR-TKI treatment according to Taiwan NHI payment regulations. Fifth, it was unclear whether the patients had ever participated in other clinical trials before and after the EGFR-TKIs treatment. But patients participating in other clinical trials would not receive TCM at the same time except in TCM clinical trials. Finally, because the Registry of Catastrophic Illness Database of the NHIRD only records the date of death and not the cause of death, the effects of TCM on patients with advanced lung adenocarcinoma treated with first-line EGFR-TKIs dying of specific causes could not be analyzed.
The outcome of our study suggests that adjunctive TCM can improve OS and PFS in patients with advanced lung adenocarcinoma treated with EGFR-TKIs. TCM may play an important, integrative role in cancer treatment. Further randomized controlled trials are needed to validate these observational discoveries.
Acknowledgments
The authors would like to thank the Health Information and Epidemiology Laboratory (CLRPG6G0041) of Chang Gung Memorial Hospital, Chiayi Branch, for their comments and assistance in data analysis. This study was based on a portion of data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health, and was managed by the National Health Insurance Research Institutes, Taiwan. The statistical results and conclusions presented in this paper do not represent those of the Bureau of National Health Insurance, Department of Health, or the National Health Insurance Research Institutes.
Footnotes
Author Contributions: Chia-Ling Li: conception of study design, interpretation of the data, literature review, and writing the manuscript. Te-Chun Hsia: conception of study design, interpretation of the data, and critical revision. Chia-Hsiang Li: conception of study design, interpretation of the data, and critical revision. Ko-Jung Chen: statistical analysis and interpretation of the data. Yao-Hsu Yang: statistical analysis, interpretation of the data, critical revision, and study supervision. Su-Tso Yang: conception of study design, interpretation of the data, critical revision, and study supervision.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957. [DOI] [PubMed] [Google Scholar]
- 2. Maemondo M, Inoue A, Kobayashi K, et al. ; North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-2388. [DOI] [PubMed] [Google Scholar]
- 3. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-246. [DOI] [PubMed] [Google Scholar]
- 4. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-742. [DOI] [PubMed] [Google Scholar]
- 5. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327-3334. [DOI] [PubMed] [Google Scholar]
- 6. Mitsudomi T, Morita S, Yatabe Y, et al. ; West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121-128. [DOI] [PubMed] [Google Scholar]
- 7. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213-222. [DOI] [PubMed] [Google Scholar]
- 8. Barlesi F, Mazieres J, Merlio JP, et al. ; Biomarkers France contributors. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet. 2016;387:1415-1426. [DOI] [PubMed] [Google Scholar]
- 9. Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol. 2010;28:4616-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu KH, Ho CC, Hsia TC, et al. Identification of five driver gene mutations in patients with treatment-naive lung adenocarcinoma in Taiwan. PLoS One. 2015;10:e0120852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy—from TCM theory to mechanistic insights. Planta Med. 2010;76:1118-1131. [DOI] [PubMed] [Google Scholar]
- 14. Kuo YT, Chang TT, Muo CH, et al. Use of complementary traditional Chinese medicines by adult cancer patients in Taiwan: a nationwide population-based study. Integr Cancer Ther. 2018;17:531-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang XB, Wu WY, Long SQ, Deng H, Pan ZQ. Effect of gefitinib plus Chinese herbal medicine (CHM) in patients with advanced non-small-cell lung cancer: a retrospective case-control study. Complement Ther Med. 2014;22:1010-1018. [DOI] [PubMed] [Google Scholar]
- 16. Liu ZL, Zhu WR, Zhou WC, et al. Traditional Chinese medicinal herbs combined with epidermal growth factor receptor tyrosine kinase inhibitor for advanced non-small cell lung cancer: a systematic review and meta-analysis. J Integr Med. 2014;12:346-358. [DOI] [PubMed] [Google Scholar]
- 17. Tang WR, Yang SH, Yu CT, et al. Long-term effectiveness of combined treatment with traditional Chinese medicine and Western medicine on the prognosis of patients with lung cancer. J Altern Complement Med. 2016;22:212-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang YH, Chen WC, Tsan YT, et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol. 2015;63:1111-1117. [DOI] [PubMed] [Google Scholar]
- 19. Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103:792-802. [DOI] [PubMed] [Google Scholar]
- 20. Liu CY, Hung YT, Chuang YL, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manag. 2006;4:1-22. [Google Scholar]
- 21. Hung HY, Tseng YH, Liao CM, et al. The efficacy of traditional Chinese herbal medicine in the treatment of EGFR mutated stage IV pulmonary adenocarcinoma patients who received first-line EGFR-TKI treatment. Integr Cancer Ther. 2017;16:126-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88:74-79. [DOI] [PubMed] [Google Scholar]
- 23. Kim EJ, Yoon YP, Woo KW, et al. Verticine, ebeiedine and suchengbeisine isolated from the bulbs of Fritillaria thunbergii Miq. inhibited the gene expression and production of MUC5AC mucin from human airway epithelial cells. Phytomedicine. 2016;23:95-104. [DOI] [PubMed] [Google Scholar]
- 24. Zheng Z, Xu L, Zhang S, et al. Peiminine inhibits colorectal cancer cell proliferation by inducing apoptosis and autophagy and modulating key metabolic pathways. Oncotarget. 2017;8:47619-47631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong BY, Lau BH, Jia TY, Wan CP. Oldenlandia diffusa and Scutellaria barbata augment macrophage oxidative burst and inhibit tumor growth. Cancer Biother Radiopharm. 1996;11:51-56. [DOI] [PubMed] [Google Scholar]
- 26. Gupta S, Zhang D, Yi J, Shao J. Anticancer activities of Oldenlandia diffusa. J Herb Pharmacother. 2004;4:21-33. [PubMed] [Google Scholar]
- 27. Park DI, Lee JH, Moon SK, et al. Induction of apoptosis and inhibition of telomerase activity by aqueous extract from Platycodon grandiflorum in human lung carcinoma cells. Pharmacol Res. 2005;51:437-443. [DOI] [PubMed] [Google Scholar]
- 28. Lee KJ, Kim JY, Choi JH, et al. Inhibition of tumor invasion and metastasis by aqueous extract of the radix of Platycodon grandiflorum. Food Chem Toxicol. 2006;44:1890-1896. [DOI] [PubMed] [Google Scholar]
- 29. Zhao R, Chen M, Jiang Z, et al. Platycodin-D induced autophagy in non-small cell lung cancer cells via PI3K/Akt/mTOR and MAPK signaling pathways. J Cancer. 2015;6:623-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hwang SW, Han HS, Lim KY, Han JY. Drug interaction between complementary herbal medicines and gefitinib. J Thorac Oncol. 2008;3:942-943. [DOI] [PubMed] [Google Scholar]