Abstract
Objective
To determine the impact of cardiorespiratory fitness (FIT) on survival in relation to the obesity paradox in patients with systolic heart failure (HF).
Patients and Methods
We studied 2066 patients with systolic HF (body mass index [BMI] ≥18.5 kg/m2) between April 1, 1993 and May 11, 2011 (with 1784 [86%] tested after January 31, 2000) from a multicenter cardiopulmonary exercise testing database who were followed for up to 5 years (mean ± SD, 25.0±17.5 months) to determine the impact of FIT (peak oxygen consumption <14 vs ≥14 mL O2 • kg−1 • min−1) on the obesity paradox.
Results
There were 212 deaths during follow-up (annual mortality, 4.5%). In patients with low FIT, annual mortality was 8.2% compared with 2.8% in those with high FIT (P<.001). After adjusting for age and sex, BMI was a significant predictor of survival in the low FIT subgroup when expressed as a continuous (P=.03) and dichotomous (<25.0 vs ≥25.0 kg/m2) (P=.01) variable. Continuous and dichotomous BMI expressions were not significant predictors of survival in the overall and high FIT groups after adjusting for age and sex. In patients with low FIT, progressively worse survival was noted with BMI of 30.0 or greater, 25.0 to 29.9, and 18.5 to 24.9 (log-rank, 11.7; P=.003), whereas there was no obesity paradox noted in those with high FIT (log-rank, 1.72; P=.42).
Conclusion
These results indicate that FIT modifies the relationship between BMI and survival. Thus, assessing the obesity paradox in systolic HF may be misleading unless FIT is considered.
Being classified as overweight or obese is known to adversely impact left ventricular (LV) geometry and hypertrophy and to have adverse effects on LV systolic and, especially, diastolic function.1–3 Overweight and obesity increase the prevalence of heart failure (HF).1,4 However, despite the adverse effects of obesity on risk factors for HF and prevalence of HF, many studies, and even large meta-analyses, have reported that once cardiovascular (CV) diseases, including HF, become established, overweight and obese individuals seem to have a better overall clinical prognosis, which has been termed the “obesity paradox.”1,5–11 Some investigators have suggested that the obesity paradox may be partly explained by confounding factors.12–14 Cardiorespiratory fitness (FIT) is strongly related to prognosis in healthy individuals and in cohorts withCVdiseases.15–18 Many studies have reported the importance of FIT and other cardiopulmonary exercise testing (CPX) variables in predicting prognosis in HF.19–22 In fact, the classic cutoff point for peak oxygen consumption () of 14 mL O2 • kg−1 • min−1 proposed by Mancini et al23 is still frequently used to classify patients with HF into low- and high-risk groups. In cohorts of patients with coronary heart disease (CHD), in which a strong obesity paradox has also been noted,24–27 we and others have found that an obesity paradox was not present in cohorts with high levels of FIT.28–30 To our knowledge, the impact of FIT on the obesity paradox has not been assessed in a cohort with systolic HF.
Using a multicenter CPX database, we assessed the impact of FIT, using the standard peak cutoff point of 14 mL O2 • kg−1 • min−1, on survival in normal-weight, overweight, and obese patients with systolic HF to determine whether FIT affects the obesity paradox in HF.
PATIENTS AND METHODS
This study was a multicenter analysis of patients with HF from the CPX laboratories at San Paolo Hospital, Milan, Italy; LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina; Stanford University, Palo Alto, California; VA Palo Alto Health Care System, Palo Alto, California; Brigham and Women’s Hospital, Boston, Massachusetts; and Virginia Commonwealth University, Richmond. All the patients included in this analysis were clinically referred for CPX to determine heart transplantation/device implantation candidacy or functional capacity. A total of 2066 patients with systolic HF were included in the present analysis. Tests were conducted between April 1, 1993 and May 11, 2011, with 1784 (86%) of the tests conducted after January 31, 2000. The inclusion criteria consisted of a diagnosis of HF31 and evidence of LV systolic dysfunction by 2-dimensional echocardiography (ie, LV ejection fraction <50%) obtained within 1 month of data collection. Underweight individuals with a body mass index (BMI) (calculated as the weight in kilograms divided by the height in meters squared) below the lower end of the normal-weight threshold according to BMI (ie, <18.5) were excluded from the analysis. All the participants completed a written informed consent form, and institutional review board approval was obtained from each institution.
CPX Procedures
Symptom-limited CPX was performed on all the participants, and pharmacologic therapy was maintained during CPX. Progressive CPX protocols were used at all the centers, and ventilatory expired gas analysis was performed using a metabolic cart (CPX-D and Ultima from Medgraphics; Vmax 29 from SensorMedics; or TrueOne 2400 from ParvoMedics). Before each test, the equipment was calibrated in standard fashion using reference gases. Minute ventilation (VE), , and carbon dioxide output (VCO2) were acquired breath-by-breath and were averaged over 10-second intervals. Peak and peak respiratory exchange ratio were expressed as the highest 10-second averaged sample obtained during the last 20 seconds of testing. The VE and VCO2 values, acquired from the initiation of exercise to peak, were input into spreadsheet software (Microsoft Excel; Microsoft Corp) to calculate the VE/VCO2 slope via least squares linear regression (y = mx + b, where m indicates slope). The BMI was calculated for each patient on the day of CPX. Weight was obtained on a calibrated scale after the removal of shoes, jewelry, etc. Height was determined from the medical record and was confirmed by the patient.
End Points
Patients were followed up for death from any cause via medical record review for up to 5 years after CPX. Patients were followed up by the HF programs at their respective institutions, providing a high likelihood that all events were captured. External means of tracking events, such as the Social Security Death Index, were not used in the present study. Transplantation and LV assist device implantation were considered censored events.
Statistical Analyses
A statistical software package (SPSS, version 19.0; SPSS Inc) was used to perform all the analyses. Continuous and categorical data are reported as mean ± SD and frequency as percentages. Independent t tests were used to assess differences in baseline and CPX variables according to low FIT vs high FIT, which was dichotomized according to a peak threshold of 14 mL O2 • kg−1 • min−1 Oneway analysis of variance assessed differences among baseline and CPX variables according to a BMI classification of 18.5 to 24.9, 25.0 to 29.9, or 30.0 or greater in the low and high FIT subgroups. The Tukey honestly significant difference was used to determine differences in subgroups at one-way analysis of variance P<.05. Categorical data were assessed by the χ2 test according to low vs high FIT and by BMI category within the FIT subgroups. Life tables were used to determine annual mortality rates. Cox regression analysis was used to assess the prognostic value of BMI, as a continuous and dichotomous variable, in the overall group and in low and high FIT subgroups after adjusting for age and sex. Cox regression analysis was also used to assess the prognostic value of peak according to the threshold of 14 mL O2 • kg−1 • min−1 Kaplan-Meier analysis was used to calculate survival in patients according to a BMI classification of 18.5 to 24.9, 25.0 to 29.9, or 30.0 or greater. This analysis was performed in the overall group and in the low and high FIT subgroups. The log-rank test was used to assess differences in overall survival between BMI categories within the low and high FIT subgroups. P<.05 was considered statistically significant for all tests.
RESULTS
Table 1 lists differences in key baseline and CPX variables according to low vs high FIT. Except for angiotensin-converting enzyme inhibitor use, all the other variables of interest were statistically significantly different. Patients with high FIT were younger and were more likely to be male; to have a nonischemic HF diagnosis; to have a lower BMI, New York Heart Association class, and VE/VCO2 slope; and to have a higher LV ejection fraction. Peak respiratory exchange ratio was also slightly, but statistically significantly, higher in the high FIT group. Table 2 lists differences in key baseline and CPX variables according to BMI classification in the low and high FIT subgroups.
TABLE 1.
Differences in Key Baseline and CPX Variables According to Aerobic Capacity
| Variable | Low FIT (n=801) | High FIT (n= 1265) | P value |
|---|---|---|---|
| Age (y), mean ± SD | 57.5±13.1 | 55.6±14.5 | .003 |
| Male sex, No. (%) | 576 (72) | 1050 (83) | <.001 |
| BMI, mean ± SD | 29.8±6.6 | 28.0±5.2 | <.001 |
| NYHA class, mean ± SD | 2.8±0.74 | 2.2±0.80 | <.001 |
| HF ischemic etiology, No. (%) | 352 (44) | 455 (36) | <.001 |
| LVEF (%), mean ± SD | 26.0±9.8 | 30.1±10.1 | <.001 |
| Prescribed ACE inhibitor, No. (%) | 505 (63) | 759 (60) | .01 |
| Prescribed β-blocker, No. (%) | 609 (76) | 834 (66) | <.001 |
| Peak RER, mean ± SD | 1.10±0.15 | 1.11±0.13 | .02 |
| VE/VCO2 slope, mean ± SD | 39.1±10.9 | 30.9±6.7 | <.001 |
ACE = angiotensin-converting enzyme; BMI = body mass index; CPX = cardiopulmonary exercise testing; FIT = cardiorespiratory fitness (low: <14 mL O2 · kg−1 · min−1; high: ≥14 mL O2 · kg−1 · min−1); HF = heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; RER = respiratory exchange ratio; = oxygen consumption; VE/VCO2 = minute ventilation/carbon dioxide production.
TABLE 2.
Differences in Key Baseline and CPX Variables According to BMI Classification in Aerobic Capacity Subgroupsa
| Low FIT (n=801) |
High FIT (n=1265) |
|||||
|---|---|---|---|---|---|---|
| Variable | BMI 18.5–24.9 (n=192) | BMI 25.0–29.9 (n=275) | BMI ≥30.0 (n=334) | BMI 18.5–24.9 (n=378) | BMI 25.0–29.9 (n=493) | BMI ≥30.0 (n=394) |
| Age (y), mean ± SD | 60.4±13.6 | 58.6±12.8 | 54.8±12.5b | 55.3±16.5 | 56.9±14.0 | 54.3±13.1c |
| Male sex (%) | 65 | 78d | 70 | 72e | 88 | 86 |
| BMI, mean ± SD | 22.6±1.7f | 27.5±1.5f | 35.8±5.6f | 22.5±1.7f | 27.4±1.4f | 34.1±4.1f |
| NYHA class, mean ± SD | 2.7±0.80 | 2.8±0.68 | 2.7±0.75 | 2.3±0.71 | 2.2±0.83 | 2.2±0.84 |
| HF ischemic etiology, No. (%) | 90 (47) | 135 (49) | 127 (38)g | 132 (35) | 207 (42)h | 118 (30) |
| LVEF (%), mean ± SD | 25.7±10.8 | 26.2±9.3 | 26.0±9.7 | 30.3±10.8 | 30.7±9.7 | 29.2±9.8 |
| Prescribed ACE inhibitor, No. (%) | 125 (65) | 176 (64) | 203 (61) | 234 (62) | 276 (56)i | 244 (62) |
| Prescribed β-blocker, No. (%) | 134 (70) | 206 (75) | 271 (81)j | 241 (64) | 315 (64) | 272 (69) |
| Peak RER, mean ± SD | 1.10±0.15 | 1.10±0.16 | 1.10±0.15 | 1.12±0.14 | 1.12±0.13 | 1.11±0.13 |
| Peak (mL O2 · kg−1 · min−1), mean ± SD | 10.9±2.3 | 11.5±2.1k | 10.8±2.3 | 22.3±8.2l | 21.1±6.4m | 19.5±5.1 |
| VE/VCO2 slope, mean ± SD | 43.7±12.3n | 38.6±9.7 | 36.9±10.2 | 32.5±7.7o | 30.9±6.6p | 29.5±5.6 |
ACE = angiotensin-converting enzyme; BMI = body mass index; CPX = cardiopulmonary exercise testing; FIT = cardiorespiratory fitness (low: <14 ml O2 · kg−1 · min−1; high: ≥14 mL O2 · kg−1 · min−1); HF = heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; RER = respiratory exchange ratio; VE/VCO2 = minute ventilation/carbon dioxide production; = oxygen consumption.
The BMI ≥30.0 group significantly less than the BMI 25.0 to 29.9 (P<.001) and BMI 18.5 to 24.9 (P<.001) groups.
The BMI ≥30.0 group significantly less than the BMI 25.0 to 29.9 (P=.02) group.
The BMI 25.0 to 29.9 group significantly greater than the BMI 18.5 to 24.9 (P<.001) and BMI ≥30.0 (P<.001) groups.
The BMI 18.5 to 24.9 group significantly less than the BMI 25.0 to 29.9 (P<.001) and BMI ≥30.0 (P<.001) groups.
All the BMI groups are significantly different in the low and high FIT groups (P<.001).
The BMI ≥30.0 group significantly less than the BMI 25.0 to 29.9 (P<.001) and BMI 18.5 to 24.9 (P=.01) groups.
The BMI 25.0 to 29.9 group significantly greater than the BMI 18.5 to 24.9 (P=.008) and BMI ≥30.0 (P<.001) groups.
The BMI 25.0 to 29.9 group significantly less than the BMI 18.5 to 24.9 (P=.009) and BMI ≥30.0 (P=.02) groups.
The BMI ≥30.0 group significantly greater than the BMI 25.0 to 29.9 (P=.02) and BMI 18.5 to 24.9 (P<.001) groups.
The BMI 25.0 to 29.9 group significantly greater than the BMI 18.5 to 24.9 (P=.03) and BMI ≥30.0 (P=.001) groups.
The BMI 18.5 to 24.9 group significantly greater than the BMI 25.0 to 29.9 (P=.02) and BMI ≥30.0 (P<.001) groups.
The BMI 25.0 to 29.9 group significantly greater than the BMI ≥30.0 group (P=.002).
The BMI 18.5 to 24.9 group significantly greater than the BMI 25.0 to 29.9 (P<.001) and BMI ≥30 (P<.001) groups.
The BMI 18.5 to 24.9 group significantly greater than the BMI 25.0 to 29.9 (P=.002) and BMI ≥30 (P<.001) groups.
The BMI 25.0 to 29.9 group significantly greater than the BMI ≥30.0 group (P=.004).
Participants were tracked for a mean ± SD of 25.0±17.5 months. Of the 1854 patients who were classified as surviving, 46% (n=853), 40% (n=742), and 14% (n=259) were tracked for less than 24 months, 24 to 48 months, and 49 to 60 months, respectively. There were 212 deaths during the 5-year tracking period. Annual mortality for the overall group was 4.5%. There were 128 deaths in the low FIT group, equating to annual mortality of 8.2%. There were 84 deaths in the group with high peak , equating to annual mortality of 2.8%. After adjusting age and sex, BMI was a significant predictor of survival in the low FIT subgroup when expressed as a continuous (hazard ratio, 0.97; 95% CI, 0.94–0.99; P=.03) and dichotomous (<25.0 vs ≥25.0) (hazard ratio, 0.61; 95% CI, 0.42–0.89; P=.001) variable. Dichotomous expression of BMI using a less than 30.0 vs 30.0 or greater threshold was not prognostically significant in the low FIT group (P=.09). Moreover, continuous and dichotomous BMI expressions were not significant predictors of survival in the overall group and in the high FIT subgroup after adjusting for age and sex (continuous: P=.07; </≥25.0: P=.70; </≥25.0: P=.20). Peak was a significant prognostic marker in the less than 14 mL O2 • kg−1 • min−1 subgroup (hazard ratio, 0.84; 95% CI, 0.78–0.90; P<.001) and in the 14 mL O2 • kg−1 • min−1 or greater subgroup (hazard ratio, 0.90; 95% CI, 0.85–0.95; P<.001).
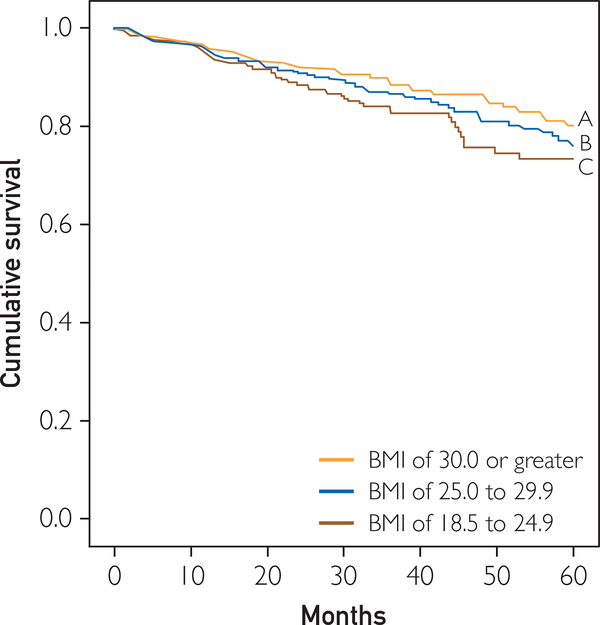
Figures 1 to 3 illustrate survival characteristics according to the traditional normal-weight, overweight, and obese BMI classifications. In the overall group, there was a slight trend toward better survival with higher BMI (log-rank, 4.8; P=.09). Differences in survival were significant in the low FIT group only, which had progressively worse survival with lower BMI (log-rank, 11.7; P=.003). However, the group with high FIT had excellent survival regardless of BMI.
FIGURE 1.
Kaplan-Meier analysis according to body mass index (BMI) in the overall group. Log-rank, 4.8; P=.09. Line A shows patients with a BMI of 30.0 or greater. Of 728 patients, 546, 166, and 72 were still alive and tracked at 1, 3, and 5 years, respectively; 63 died. Line B shows patients with a BMI of 25.0 to 29.9. Of 768 patients, 577, 181, and 89 were still alive and tracked at 1, 3, and 5 years, respectively; 81 died. Line C shows patients with a BMI of 18.5 to 24.9. Of 570 patients, 395, 128, and 51 were still alive and tracked at 1, 3, and 5 years, respectively; 68 died.
FIGURE 3.
Kaplan-Meier analysis according to body mass index (BMI) in the high cardiorespiratory fitness group (oxygen consumption ≥14 mL O2 • kg−1 • min−1). Log-rank, 1.72; P=.42. Line A shows patients with a BMI of 30.0 or greater. Of 394 patients, 322, 101, and 54 were still alive and tracked at 1, 3, and 5 years, respectively; 22 died. Line B shows patients with a BMI of 25.0 to 29.9. Of 493 patients, 381, 117, and 60 were still alive and tracked at 1, 3, and 5 years, respectively; 36 died. Line C shows patients with a BMI of 18.5 to 24.9. Of 378 patients, 288, 93, and 43 were still alive and tracked at 1, 3, and 5 years, respectively; 26 died.
DISCUSSION
This study has 3 important findings. First, these results provide support for an obesity paradox in patients with systolic HF referred for CPX. Second, these results demonstrate the important role of FIT to impact prognosis in patients with systolic HF. Third, to our knowledge and for the first time in this chronic CV population, we demonstrate that unlike patients with high-risk systolic HF with low levels of FIT(peak <14 mL O2 • kg−1 • min−1), those with high FIT (peak 14 mL O2 • kg−1 • min−1), albeit well below age- and sex-predicted normal values, have a good prognosis and do not demonstrate any evidence of an obesity paradox. The findings of the present study are consistent with those of previous investigations in CHD cohorts,28–30 demonstrating that FIT also affects the obesity paradox.
Obesity has numerous adverse effects on hemodynamic and CV structure and function, which have been reviewed in detail elsewhere.1,2 Obesity has adverse effects on systolic and diastolic LV function,1–3 and overweight and obesity seem to predispose to the development of HF.1,4,32 In a study of 5881 Framingham Heart Study participants, Kenchaiah et al4 found that during 14-year follow-up, for every 1-U increase in BMI, the risk of HF increased by 5% in men and by 7% in women. In fact, in this large epidemiologic cohort, a greater increase in the risk of HF was observed across all the categories of BMI. Other studies also support the association of obesity, especially more severe obesity, with the development of HF.1,32
However, despite the known effects of obesity on systolic and diastolic LV function and the epidemiologic data demonstrating a strong link between obesity and HF, many studies have now found that overweight and obese patients with HF have a better prognosis.1,5–10 In fact, we previously reported a favorable prognosis using high BMI and high percentage of body fat in patients with advanced systolic HF.6 Recently, investigators have found that patients with HF with higher BMI and higher waist circumference had the best prognosis in advanced HF.33,34 In a meta-analysis of 9 observational studies including nearly 30,000 patients with HF followed up, on average, for almost 3 years, Oreopoulos et al7 found that compared with individuals without elevated BMI, overweight and obese patients with HF had reductions in CV mortality (−19% and −40%, respectively) and all-cause mortality (−16% and −33%, respectively). Likewise, in an analysis of BMI and in-hospital survival in more than 100,000 decompensated patients with HF, high BMI was associated with lower mortality rates; for every 5-U increase in BMI, the risk of mortality was lowered by 10%.8
Numerous studies indicate the powerful impact of FIT in predicting prognosis in healthy cohorts and in those with CV diseases, including CHD and HF.15–18,20,21 Clearly, many studies and meta-analyses have found that FIT is a potent predictor of prognosis in patients with advanced HF.20,21,35 In fact, the traditional cutoff point for peak of 14 mL O2 • kg−1 • min−1 proposed by Mancini et al23 is still frequently used to divide patients with systolic HF into low- and high-risk subgroups. The present study found that FIT remains an important prognostic marker when the cohort was dichotomized according to a threshold of 14 mL O2 • kg−1 • min−1, further highlighting the importance of this measure in patients with systolic HF. Thus, irrespective of other characteristics, improvement of FIT should be considered a primary treatment goal in this chronic disease population.
Many investigators have suggested that the obesity paradox may at least partly be explained by confounding factors.12–14 Certainly, we and others have suggested that in cohorts with CHD, patients with high levels of FIT did not seem to have an obesity paradox.28–30 On the other hand, in higher-risk patients with CHD with low levels of FIT, a strong obesity paradox was present, with overweight and obese individuals having a better prognosis compared with their lean counterparts with low FIT. To our knowledge, the impact of FIT on the obesity paradox has not been assessed in patients with systolic HF. As previously found in patients with CHD, we found that FIT strongly mitigates the impact of overweight/obesity on prognosis in patients with systolic HF. In fact, in patients with HF with more preserved FIT (peak 14 mL O2 • kg−1 • min−1), the age- and sex-adjusted survival rate was equally good regardless of body composition. On the other hand, in patients with HF with low FIT (peak <14 mL O2 • kg−1 • min−1), overweight and obese patients had better age- and sex-adjusted survival rates than did their normal-weight counterparts, thus demonstrating an obesity paradox. Part of the explanation regarding the impact of FIT on survival and the obesity paradox in HF may simply be that patients with HF with high FIT have a good prognosis, regardless of body composition status. On the other hand, patients with HF with low FIT have a considerably worse prognosis, which is particularly noted in leaner patients, which likely represents a “lean paradox” as much as an “obesity paradox,” as we have discussed previously in CHD.26 Although we did not assess body composition other than BMI in this HF cohort, in recent studies in CHD we found that the most lean patients, those with either low BMI and low percentage of body fat26 or low body fat and low lean body mass,27 especially combined with low FIT,30 represent the group with particularly poor clinical prognoses. We recently reviewed the importance of muscular strength in predicting prognosis in patients with CV diseases.36 Although we did not assess muscular strength in the present HF cohort, it seems plausible that the leanest patients with HF, especially those with low FIT, may also have poor overall muscular fitness and strength, thus adversely affecting their survival. In fact, we recently reported in a small study of patients with HF with impaired LV ejection fraction that adiposity correlates with greater strength, which may explain some of the protection that obesity has in patients with HF with low FIT.37
There are several potential limitations to this study. First, we used a CPX database obtained from major centers, so the study cannot evaluate the obesity paradox in patients with HF not referred for CPX, including those unable to undergo CPX owing to clinical considerations. We did not have data on the location from which patients were referred, although we assume that most lived in the region where CPX took place. In addition, we evaluated body composition by BMI only, and we did not assess other parameters of adiposity (percentage of body fat, waist circumference, etc). Therefore, we were not able to assess peak adjusted for lean body mass, which may be a better indicator of FIT and prognosis in patients with HF with higher body fat, including women and overweight/obese patients with HF; neither did we assess the impact of other CPX variables on prognosis.22 We also assessed BMI at only one point in time (immediately before CPX), so similar to most studies assessing the obesity paradox, we cannot evaluate changes in weight and, especially, nonpurposeful weight loss, which is known to be associated with a poor prognosis.38,39 The present patients were not all tracked for the full 5-year tracking period; the mean ± SD follow-up was 25.0±17.5 months. This study is not powered to assess the impact of FIT in patients with severe degrees of obesity. However, we did not evaluate “underweight” patients with HF (BMI <18.5), who are generally known to have a poor prognosis, which may be viewed as a positive attribute. Finally, we did not have data on smoking status, cancer, use of cardiac defibrillators, doses of medications, etc, all factors that could also affect survival in patients with HF.
CONCLUSION
We believe that these data indicate that FIT influences the importance of BMI on HF prognosis. Using BMI to assess risk in patients with systolic HF may be misleading unless FIT is considered. Patients with systolic HF and levels of FIT meeting or surpassing the established threshold of 14 mL O2 • kg−1 • min−1 do not seem to have an obesity paradox. On the other hand, in patients with systolic HF and low levels of FIT, a strong obesity paradox is apparent, with overweight and obese patients having a better prognosis than their normal-weight counterparts.
FIGURE 2.
Kaplan-Meier analysis according to body mass index (BMI) in the low cardiorespiratory fitness group (oxygen consumption <14 mL O2 • kg−1 • min−1). Log-rank, 11.7; P=.003. Line A shows patients with a BMI of 30.0 or greater. Of 334 patients, 223, 64, and 17 were still alive and tracked at 1, 3, and 5 years, respectively; 41 died. Line B shows patients with a BMI of 25.0 to 29.9. Of 275 patients, 195, 63, and 27 were still alive and tracked at 1, 3, and 5 years, respectively; 45 died. Line C shows patients with a BMI of 18.5 to 24.9. Of 192 patients, 106, 34, and 7 were still alive and tracked at 1, 3, and 5 years, respectively; 42 died.
Abbreviations and Acronyms
- BMI
body mass index
- CHD
coronary heart disease
- CPX
cardiopulmonary exercise testing
- CV
cardiovascular
- FIT
cardiorespiratory fitness
- HF
heart failure
- LV
left ventricular
- VCO2
carbon dioxide output
- VE
minute ventilation
oxygen consumption
Footnotes
Data Previously Presented: These data were presented in part at the 2012 American Heart Association Annual Scientific Meeting in Los Angeles, CA.
Contributor Information
Carl J. Lavie, Department of Cardiovascular Diseases, John Ochsner Heart and Vascular Institute, Ochsner Clinical Schoole The University of Queensland School of Medicine, New Orleans, LA; Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge.
Lawrence P. Cahalin, Department of Physical Therapy, Leonard M. Miller School of Medicine, University of Miami, Miami, FL.
Paul Chase, Lebauer Cardiovascular Research Foundation, Greensboro, NC.
Jonathan Myers, Division of Cardiology, VA Palo Alto Healthcare System, Palo Alto, CA; Cardiovascular Medicine, Stanford University, Palo Alto, CA.
Daniel Bensimhon, Lebauer Cardiovascular Research Foundation, Greensboro, NC.
Mary Ann Peberdy, Department of Internal Medicine, Virginia Commonwealth University, Richmond.
Euan Ashley, Cardiovascular Medicine, Stanford University, Palo Alto, CA.
Erin West, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA.
Daniel E. Forman, Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA.
Marco Guazzi, Cardiology, I.R.C.C.S. Policlinico San Donato, University of Milano, San Donato Milanese, Italy.
Ross Arena, Division of Physical Therapy—Department of Orthopaedics and Rehabilitation and Division of Cardiology—Department of Internal Medicine, University of New Mexico School of Medicine, Albuquerque.
REFERENCES
- 1.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. [DOI] [PubMed] [Google Scholar]
- 2.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci. 2001; 321(14):225–236. [DOI] [PubMed] [Google Scholar]
- 3.Lavie CJ, Milani RV, Ventura HO, Cardenas GA, Mehra MR, Messerli FH. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. Am J Cardiol. 2007;100(9):1460–1464. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. [DOI] [PubMed] [Google Scholar]
- 5.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001; 38(3):789–795. [DOI] [PubMed] [Google Scholar]
- 6.Lavie CJ, Osman AF, Milani RV, Mehra MR. Body composition and prognosis in chronic systolic heart failure: the obesity paradox. Am J Cardiol. 2003;91(7):891–894. [DOI] [PubMed] [Google Scholar]
- 7.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156(1): 13–22. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC, Srikanthan P, Costanzo MR, Cintron GB, Lopatin M; ADHERE Scientific Advisory Committee and Investigators. An obesity paradox in acute heart failure: analysis of body mass index and inhospital mortality for 108,927 patients in the Acute Decompensated Heart Failure National Registry. Am Heart J. 2007;153(1):74–81. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004; 43(8):1439–1444. [DOI] [PubMed] [Google Scholar]
- 10.Lavie CJ, Mehra MR, Milani RV. Obesity and heart failure prognosis: paradox or reverse epidemiology? Eur Heart J. 2005; 26(1):5–7. [DOI] [PubMed] [Google Scholar]
- 11.Lavie CJ, Milani RV, Ventura HO, Romero-Corral A. Body composition and heart failure prevalence and prognosis: getting to the fat of the matter in the “obesity paradox.” Mayo Clin Proc. 2010;85(7):605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ades PA, Savage PD. The obesity paradox: perception vs knowledge. Mayo Clin Proc. 2010;85(2):112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das SR, Alexander KP, Chen AY, et al. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58(25): 2642–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavie CJ, Milani RV, Ventura HO. Impact of obesity on outcomes in myocardial infarction: combating the “obesity paradox.” J Am Coll Cardiol. 2011;58(25):2651–2653. [DOI] [PubMed] [Google Scholar]
- 15.Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009; 84(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DC, Sui X, Church TS, Lavie CJ, Jackson AS, Blair SN. Changes in fitness and fatness on the development of cardiovascular disease risk factors hypertension, metabolic syndrome, and hypercholesterolemia. J Am Coll Cardiol. 2012;59(7):665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arena R, Myers J, Guazzi M. The future of aerobic exercise testing in clinical practice: is it the ultimate vital sign? Future Cardiol. 2010;6(3):325–342. [DOI] [PubMed] [Google Scholar]
- 18.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009; 301(19):2024–2035. [DOI] [PubMed] [Google Scholar]
- 19.Chase P, Arena R, Myers J, et al. Relation of the prognostic value of ventilatory efficiency to body mass index in patients with heart failure. Am J Cardiol. 2008;101(3):348–352. [DOI] [PubMed] [Google Scholar]
- 20.Balady GJ, Arena R, Sietsema K, et al. ; American Heart Association Exercise, Cardiac Rehabilitation and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191–225. [DOI] [PubMed] [Google Scholar]
- 21.Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. 2008;13(2):245–269. [DOI] [PubMed] [Google Scholar]
- 22.Osman AF, Mehra MR, Lavie CJ, Nunez E, Milani RV. The incremental prognostic importance of body fat adjusted peak oxygen consumption in chronic heart failure. J Am Coll Cardiol. 2000;36(7):2126–2131. [DOI] [PubMed] [Google Scholar]
- 23.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–786. [DOI] [PubMed] [Google Scholar]
- 24.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–678. [DOI] [PubMed] [Google Scholar]
- 25.Lavie CJ, Milani RV, Artham SM, Patel DA, Ventura HO. The obesity paradox, weight loss, and coronary disease. Am J Med. 2009;122(12):1106–1114. [DOI] [PubMed] [Google Scholar]
- 26.Lavie CJ, De Schutter A, Patel D, Artham SM, Milani RV. Body composition and coronary heart disease mortality: an obesity or a lean paradox? Mayo Clin Proc. 2011;86(9):857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the “obesity paradox.” J Am Coll Cardiol. 2012;60(15):1374–1380. [DOI] [PubMed] [Google Scholar]
- 28.Goel K, Thomas RJ, Squires RW, et al. Combined effect of cardiorespiratory fitness and adiposity on mortality in patients with coronary artery disease. Am Heart J. 2011;161(3):590–597. [DOI] [PubMed] [Google Scholar]
- 29.McAuley PA, Kokkinos PF, Oliveira RB, Emerson BT, Myers JN. Obesity paradox and cardiorespiratory fitness in 12,417 male veterans aged 40 to 70 years. Mayo Clin Proc. 2010;85(2): 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAuley PA, Artero EG, Sui X, et al. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clin Proc. 2012;87(5):443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radford MJ, Arnold JM, Bennett SJ, et al. ; American College of Cardiology; American Heart Association Task Force on Clinical Data Standards; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Failure Society of America. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Failure Society of America. Circulation. 2005;112(12):1888–1916. [DOI] [PubMed] [Google Scholar]
- 32.Alpert MA, Terry BE, Mulekar P, et al. Cardiac morphology and left ventricular function in normotensive morbidly obese patients with and without congestive heart failure, and effect of weight loss. Am J Cardiol. 1997;80(6):736–740. [DOI] [PubMed] [Google Scholar]
- 33.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17(5):374–380. [DOI] [PubMed] [Google Scholar]
- 34.Lavie CJ, Ventura HO. Weighing in on obesity and the obesity paradox in heart failure. J Card Fail. 2011;17(5): 381–383. [DOI] [PubMed] [Google Scholar]
- 35.Poggio R, Arazi HC, Giorgi M, Miriuka SG. Prediction of severe cardiovascular events by VE/Vco2 slope versus peak Vo2 in systolic heart failure: a meta-analysis of the published literature. Am Heart J. 2010;160(6):1004–1014. [DOI] [PubMed] [Google Scholar]
- 36.Artero EG, Lese DC, Lavie CJ, et al. Effects of muscular strength on cardiovascular risk factors and prognosis. J Cardiopulm Rehabil Prev. 2012;32(6):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zavin A, Daniels K, Arena R, et al. Adiposity facilitates increased strength capacity in heart failure patients with reduced ejection fraction [published online June 26, 2012]. Int J Cardiol. 10.1016/j.ijcard.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arena R, Lavie CJ. The obesity paradox and outcome in heart failure: is excess bodyweight truly protective? Future Cardiol. 2010;6(1):1–6. [DOI] [PubMed] [Google Scholar]
- 39.Anker SD, Negassa A, Coats AJ, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361(9363):1077–1083. [DOI] [PubMed] [Google Scholar]