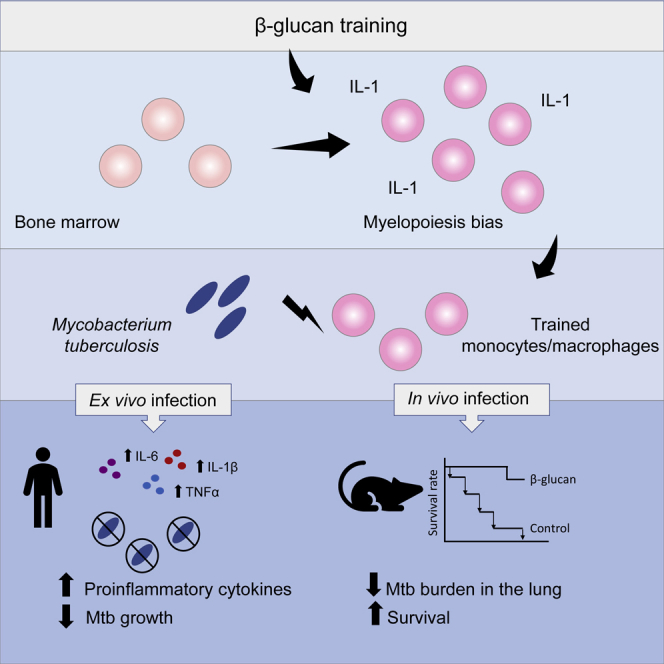
Summary
β-glucan is a potent inducer of epigenetic and functional reprogramming of innate immune cells, a process called “trained immunity,” resulting in an enhanced host response against secondary infections. We investigate whether β-glucan exposure confers protection against pulmonary Mycobacterium tuberculosis (Mtb) infection. β-glucan induces trained immunity via histone modifications at gene promoters in human monocytes, which is accompanied by the enhanced production of proinflammatory cytokines upon secondary Mtb challenge and inhibition of Mtb growth. Mice treated with β-glucan are significantly protected against pulmonary Mtb infection, which is associated with the expansion of hematopoietic stem and progenitor cells in the bone marrow and increased myelopoiesis. The protective signature of β-glucan is mediated via IL-1 signaling, as β-glucan shows no protection in mice lacking a functional IL-1 receptor (IL1R−/−). The administration of β-glucan may be used as a novel strategy in the treatment of mycobacterial infections and possibly as an adjuvant to improve anti-tuberculosis vaccines.
Keywords: trained immunity, β-glucan, tuberculosis, Mycobacterium tuberculosis, epigenetics, monocytes, IL-1, innate immune memory
Graphical Abstract
Highlights
-
•
β-glucan induces protective trained immunity in human monocytes infected with Mtb
-
•
β-glucan induces protective trained immunity in mice infected with Mtb
-
•
β-glucan-mediated protection against Mtb is dependent on IL-1 signaling
-
•
β-glucan increases expansion of hematopoietic progenitors and myelopoiesis via IL-1
Moorlag et al. show that β-glucan induces protective trained immunity in human monocytes and in mice infected with virulent Mtb. β-glucan-mediated protection against Mtb is dependent on IL-1 signaling and is associated with the expansion of hematopoietic stem and progenitor cells in the bone marrow and increased myelopoiesis.
Introduction
The innate immune system has the ability to generate memory responses after infections or vaccinations to mount rapid and enhanced immune responses upon exposure to secondary infections. Innate immune memory, also known as trained immunity, is not specific and can heighten protection upon reinfection with the same or unrelated pathogens (Netea et al., 2011, Netea et al., 2016). Genome-wide epigenetic reprogramming resulting in an upregulated transcriptomic profile of genes involved in the antimicrobial program of monocytes and macrophages underlies this phenomenon (Arts et al., 2018, Saeed et al., 2014). These functional changes are present for prolonged periods of time, and the signatures of enhanced immune responses have been observed for up to 1 year after the initial induction of trained immunity (Kleinnijenhuis et al., 2012).
β-Glucan is part of the cell wall of fungi and yeasts and known for decades to have immunomodulating effects boosting immunity against cancer and various infections (Vetvicka, 2011). During the last few years, it was shown that β-glucan is also a strong inducer of trained immunity. Human monocytes that are pre-exposed to β-glucan exhibit an enhanced immune response upon re-stimulation with various microbial ligands in vitro (Quintin et al., 2012). Furthermore, in vivo animal studies demonstrated that treatment with β-glucan offers macrophage-mediated protection from subsequent challenge with pathogens, including Candida albicans and Staphylococcus aureus (Bistoni et al., 1986, Quintin et al., 2012). Considering the short lifespan of myeloid cells in the circulation, the mechanism responsible for the long-lasting protective effects of β-glucan was initially unclear. However, a recent study by Mitroulis et al. (2018) revealed that β-glucan not only induces trained immunity in mature monocytes and macrophages but it also alters the functional program of hematopoietic progenitors in the bone marrow, which likely accounts for the prolonged generation of trained myeloid cells in the circulation. Similar adaptations at the level of the bone marrow have been observed for other inducers of trained immunity such as bacille Calmette-Guerin (BCG) vaccine (Kaufmann et al., 2018) and a high-fat diet (Christ et al., 2018).
Macrophages play a crucial role in host defense against Mycobacterium tuberculosis (Mtb) infection, the causative agent of tuberculosis (TB) (Behar et al., 2010, Divangahi and Behr, 2018, McClean and Tobin, 2016). Since β-glucan induces trained immunity in macrophages, we hypothesized that β-glucan may enhance protection against a virulent strain of Mtb. Earlier studies reported a decreased burden of BCG bacilli in mice treated with β-glucan (Hetland et al., 1998), and in line with these findings, a subsequent study found that β-glucan inhibited growth of Mtb strain H37Rv in peritoneal macrophages isolated from mice (Hetland and Sandven, 2002). However, if and how β-glucan-induced trained immunity provides protection against virulent Mtb infection is incompletely understood. In addition, our understanding of the potential protective impact of β-glucan on host defense against TB is extremely limited in humans. A study performed in human macrophages found no effect of β-glucan on the growth of a virulent strain of Mtb (H37Rv) (Betz et al., 2011). However, in this study, the time between β-glucan treatment and Mtb infection in macrophages was 30 min, whereas a trained immunity phenotype only develops in macrophages after at least a couple of days after an initial stimulus (Bekkering et al., 2016).
In this study, we investigated whether β-glucan-induced trained immunity protects against infection with the virulent strain of Mtb (H37Rv) in human monocytes and in a mouse model of aerosol Mtb infection. Here, we show that β-glucan induces a more open chromatin status and global changes in gene expression that enhances antimicrobial immunity of human monocytes against Mtb infection in vitro. We next show that the treatment of mice with β-glucan significantly augments protection as well as survival against pulmonary Mtb infection in vivo. In addition, we demonstrate that the protective effect of β-glucan is dependent on interleukin-1 (IL-1) signaling and is associated with the expansion of hematopoietic stem cells (HSCs) and an increase in myelopoiesis.
Results
β-Glucan-Trained Human Monocytes Are Armed with Antimicrobial Cytokines to Prevent the Growth of Mtb
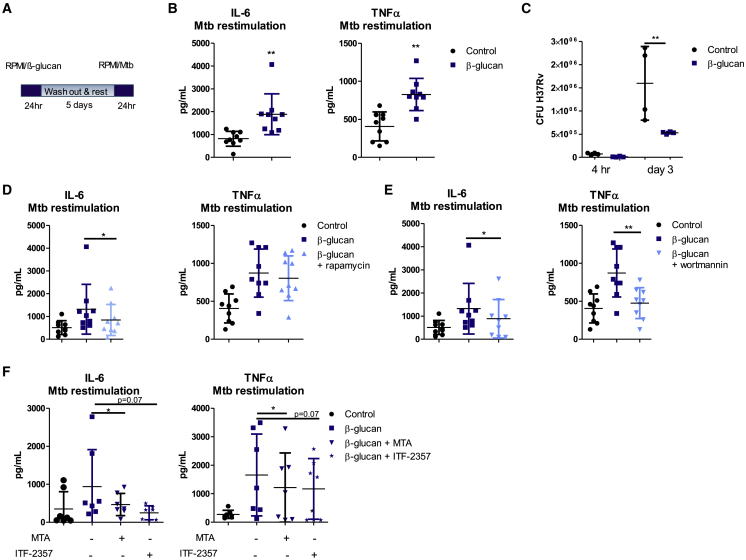
β-Glucan has been demonstrated to increase cytokine production upon secondary stimulation with various bacteria (Quintin, 2019). Therefore, we tested whether training of human monocytes with β-glucan in vitro increases the innate immune response upon secondary stimulation with heat-killed Mtb. To this end, monocytes from healthy volunteers were stimulated with RPMI control medium or β-glucan. Cells were washed after 24 h, incubated for 5 days, and re-stimulated on day 6 with heat-killed Mtb or control medium (Figure 1A). Pre-incubation of monocytes with β-glucan increased the concentration of IL-6, tumor necrosis factor α (TNF-α), and intracellular IL-1β upon stimulation with Mtb on day 6 (Figures 1B and S1). Next, we investigated whether β-glucan-induced trained immunity would enhance the anti-mycobacterial capacity of human monocytes against virulent M. tuberculosis H37Rv. Human monocytes were trained with β-glucan, and at day 6, cells were infected with Mtb (MOI 1) and the growth of Mtb was assessed 3 days after infection. The number of Mtb colony-forming units (CFUs) was significantly decreased in β-glucan-treated cells compared to the control, indicating an enhanced anti-mycobacterial capacity of monocytes treated with β-glucan (Figure 1C).
Figure 1.
β-Glucan Training Increases Antimicrobial Activity of Human Monocytes against M. tuberculosis
(A) Schematic representation of the in vitro training model.
(B) Human monocytes were trained with β-glucan for 24 h and re-stimulated with heat-killed M. tuberculosis at day 6. IL-6 and TNF-α production was measured in the supernatants (means ± SDs, n = 9, ∗∗p < 0.01, Wilcoxon signed-rank test). See also Figure S1.
(C) Monocytes were trained with β-glucan and infected with virulent M. tuberculosis H37Rv at MOI 1 for 4 h. Mtb CFUs were quantified at 4 h and 3 days post-infection (means ± SDs, n = 4, ∗∗p < 0.01, 2-way ANOVA).
(D and E) Monocytes were incubated for 24 h with culture medium or β-glucan, with or without an inhibitor of mTOR-glycolysis (rapamycin) (D) or a PI3K inhibitor (wortmannin) (E). After re-stimulation with M. tuberculosis, differences in IL-6 and TNF-α production were assessed between β-glucan-trained monocytes and monocytes that were trained with β-glucan in the presence of rapamycin orwortmannin (means ± SDs, n = 9, ∗p < 0.05, Wilcoxon signed-rank test).
(F) Monocytes were stimulated for 24 h with culture medium or β-glucan in the presence or absence of the histone methyltransferase inhibitor MTA or the histone deacetylase inhibitor ITF-2357. On day 6, cells were re-stimulated with M. tuberculosis for 24 h and the difference in cytokine production was assessed between monocytes that were trained in the presence or absence of epigenetic inhibitors (means ± SDs, n = 7, ∗p < 0.05, Wilcoxon signed-rank test).
As previous work demonstrated that an Akt/mammalian target of rapamycin (mTOR)-induced metabolic shift from oxidative phosphorylation toward glycolysis is essential for trained immunity (Cheng et al., 2014), we assessed the role of this pathway using an inhibitor of mTOR (rapamycin) (Figure 1D) and Akt-phosphatidylinositol 3-kinase (PI3K) (wortmannin) (Figure 1E). Inhibition of PI3K/Akt in β-glucan-treated monocytes reduced both IL-6 and TNF-α responses, whereas inhibition of mTOR by rapamycin only significantly reduced IL-6 production after Mtb re-stimulation. Hence, we observed only a small effect of rapamycin on TNF-α in trained macrophages after Mtb re-stimulation that we envision was due to biological variation. These data confirmed the importance of this pathway for the increased cytokine production in β-glucan-induced trained human monocytes after Mtb re-stimulation.
β-Glucan Induces Epigenetic Modifications in Genes Involved in Anti-mycobacterial Host Defense
We have previously shown that β-glucan induces genome-wide epigenetic reprogramming of monocytes (Quintin et al., 2012). To assess whether the increased cytokine responses upon Mtb re-stimulation are the result of epigenetic modifications, we treated monocytes from healthy volunteers with the histone methyltransferase inhibitor MTA (5′deoxy-5′methylthio-adenosine) and the histone deacetylase inhibitor ITF2357 before and during β-glucan training. In the presence of MTA, the in vitro effects of training, which were assessed by IL-6 and TNF-α production, were significantly reduced and a similar trend was observed with ITF2357, although it did not reach statistical significance (p = 0.07) (Figure 1F). These data collectively suggest that the reprogramming of monocytes by β-glucan was mediated epigenetically.
We next investigated the effect of β-glucan on genes that are important for anti-mycobacterial immunity by macrophages. A previous genome-wide small interfering RNA (siRNA) screen identified genes important for anti-Mtb host defense (Kumar et al., 2010), and we assessed their expression in monocytes exposed to β-glucan from a previously published study (GSE85243) (Novakovic et al., 2016). While monocyte-to-macrophage differentiation under the influence of human plasma in the in vitro system already induces the activation of some anti-mycobacterial genes, these effects were more pronounced after β-glucan training, with RNA sequencing (RNA-seq) analysis revealing that β-glucan-induced genes were involved in anti-Mtb host defense mediators (Figure S2A). To investigate whether the differentially expressed genes are associated with β-glucan-induced epigenetic changes, we next performed genome-wide chromatin immunoprecipitation sequencing (ChIP-seq) data of epigenetic changes in histone 3 lysine 27 acetylation (H3K27ac) and histone 3 lysine 4 trimethylation (H3K4me3) in β-glucan-trained monocytes, which were generated in a previous study (GSE85245) (Novakovic et al., 2016). Subsequent analysis revealed that the observed transcriptional changes induced by β-glucan were associated with epigenetic modifications at the promoter regions of the genes via these two histone marks (Figure S2B).
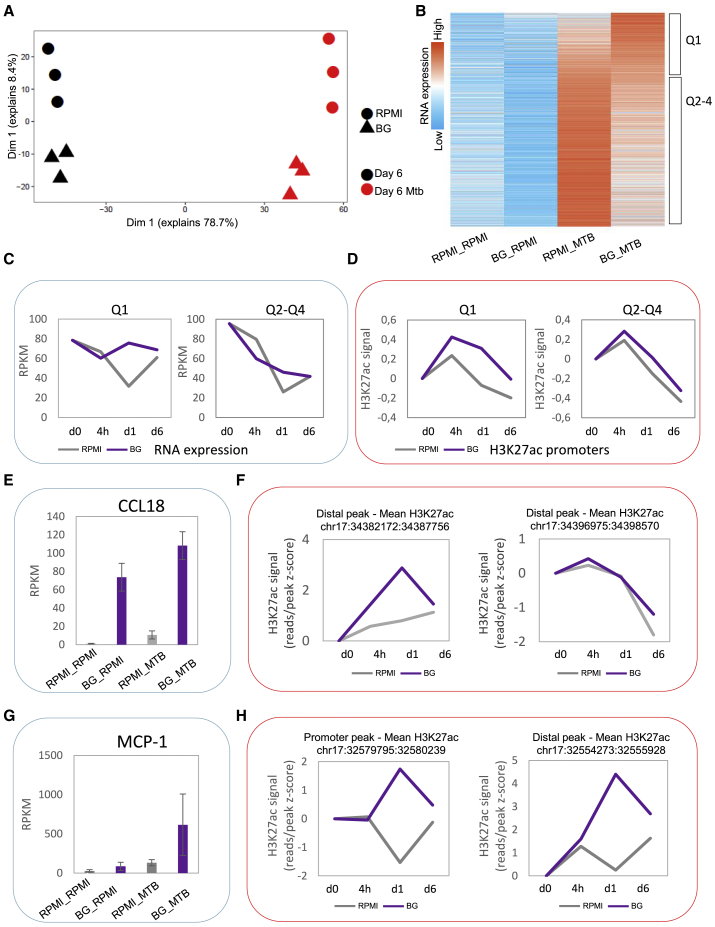
To further explore how β-glucan-induced training generates responses against Mtb, we performed an RNA-seq analysis of human monocytes that were trained with β-glucan or incubated in RPMI control medium and exposed to heat-killed Mtb for 4 h on day 6. Principal-component analysis (PCA) (Figure 2A) and unsupervised hierarchical clustering analysis (Figure 2B; Table S1) revealed that β-glucan reprograms the transcriptomic profile of cells exposed to Mtb. In total, 2,105 genes were differentially expressed in β-glucan-treated cells compared to the control (p < 0.05, fold change [FC] > 1.5, reads per kilobase million [RPKM] > 5, n = 3). Genes that were upregulated (trained) after Mtb exposure in β-glucan-treated cells (indicated by Q1 [quartile 1] in Figure 2B) showed increased levels of gene expression during the first 24 h after β-glucan stimulation compared to the control (Figure 2C) (GSE85243) (Novakovic et al., 2016). In line with our previous observation, these differences in gene expression were associated with large differences in the levels of H3K27ac at the promoter sites of trained genes 24 h after β-glucan exposure (Figure 2D) (GSE85245) (Novakovic et al., 2016).
Figure 2.
β-Glucan Training Alters the Transcriptomic Profile of Human Monocytes Exposed to Mtb
(A) PCA of RNA-seq data of human monocytes that were trained with β-glucan and exposed to heat-killed M. tuberculosis for 4 h on day 6 (p < 0.05, FC > 1.5, RPKM > 5, n = 3).
(B) Heatmap of 2,105 genes that are differentially expressed between β-glucan-treated cells and controls upon exposure to Mtb. The first quartile (Q1) indicates 526 genes that are upregulated (trained) after Mtb exposure in β-glucan-trained monocytes compared to the control. Non-trained genes (1,579 genes) are indicated by Q2–Q4.
(C) Average gene expression of genes in Q1 and average expression of non-trained genes (Q2–Q4).
(D) Average H3K27ac signal at promoter sites of genes that are present in Q1 and average signal at non-trained genes (Q2–Q4).
(E–H) RNA expression of CCL18 (E) and MCP-1 (G) in control versus β-glucan-treated cells upon RPMI/Mtb exposure. H3K27ac signal at promoter/distal regions of CCL18 (F) and MCP-1 (H) upon β-glucan exposure.
Assessment of the top upregulated genes upon Mtb exposure in β-glucan-trained cells revealed various chemokines, including CCL18, CCL2, CCL23, and CCL8, and CXCL5 and CXCL10 (Figures 2E and F; Table S1), which have been shown to play a key role in Mtb infection (Slight and Khader, 2013). Of the 275 genes involved in the regulation of Mtb survival in human macrophages (Kumar et al., 2010), 27 were differentially expressed in β-glucan-trained cells exposed to Mtb as compared to non-trained cells stimulated with Mtb (Table S1). In addition, genome-wide association studies (GWAS) performed in human cohorts have revealed various genes that play a key role in susceptibility or resistance to TB. The public database ClinVar (NCBI) (https://www.ncbi.nlm.nih.gov/clinvar) contains 21 different genes that have been found to affect TB outcomes, including interferon-gamma (IFNG), monocyte chemoattractant protein (MCP)-1/CCL2, CD209/DC-SIGN, and Toll-like receptor 1 (TLR1) (Barreiro et al., 2006, Barreiro et al., 2012, Dittrich et al., 2015, Thye et al., 2009). Among the genes listed in the ClinVar database, various genes (e.g., MCP-1) display increased H3K27ac levels and enhanced expression upon Mtb exposure in β-glucan-trained cells compared to the control (Figures 2G and H). In conclusion, β-glucan-induced epigenetic modifications leading to enhanced gene transcription of host factors involved in the immune response of the macrophage against Mtb may enhance their protective capacity against mycobacteria.
β-Glucan Training Protects Mice against Mtb via an IL-1-Dependent Mechanism
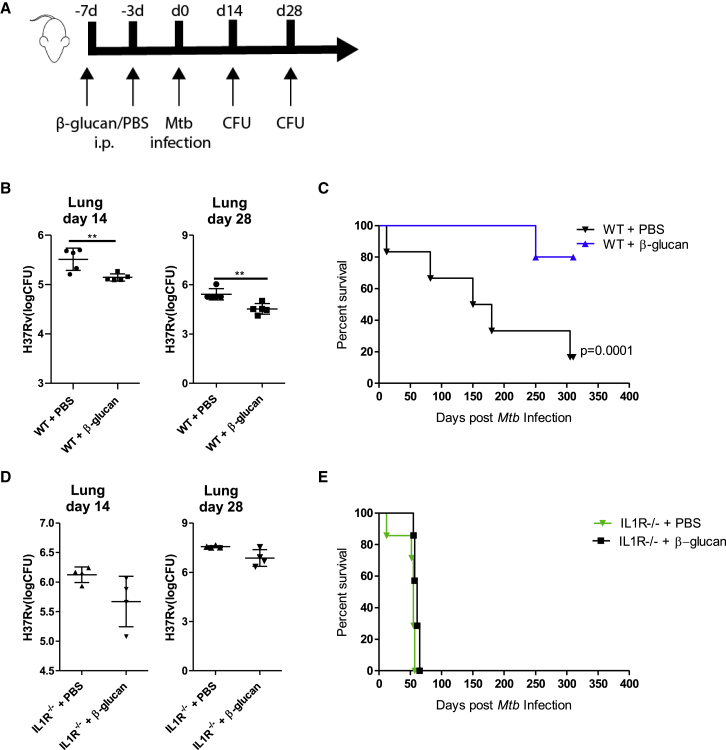
Considering the potentiating effects of β-glucan on anti-mycobacterial genes, we next investigated whether training with β-glucan protects mice from pulmonary infection with virulent Mtb. To this end, mice received an intraperitoneal injection of β-glucan or PBS-control on day 0 and day 3, followed by aerosolized infection with Mtb (∼50 H37Rv) on day 7 (Figure 3A). Mice treated with β-glucan demonstrated significantly lower Mtb burden in the lung compared to PBS-control mice (Figure 3B). In addition, the survival of β-glucan-treated mice was significantly increased in comparison to PBS-control mice (Figure 3C).
Figure 3.
Training with β-Glucan Enhances Resistance of Mice to Mtb Infection via an IL-1-Dependent Mechanism
(A) WT and IL1R−/− mice were treated with β-glucan or PBS at day 0 and day 3, followed by aerosolized infection with Mtb (∼50 H37Rv) at day 7.
(B–E) Bacterial burden in the lungs after 14 and 28 days of aerosol infection (means ± SDs, n = 4–5 mice per group, unpaired t test) in WT (B) and IL1R−/− mice (D). Survival of WT (C) and IL1R−/− mice (E) (n = 6–8 mice per group, log-rank test).
Since previous studies indicated an important role for IL-1 signaling in trained immunity (Arts et al., 2018, Christ et al., 2018, Mitroulis et al., 2018), and the IL-1 pathway is required for the control of Mtb infection (Fremond et al., 2007, Mayer-Barber et al., 2014, Sugawara et al., 2001, Yamada et al., 2000), we hypothesized that IL-1 signaling is essential for β-glucan-mediated protection against Mtb. To test this hypothesis, we treated IL1R−/− or wild-type (WT) control mice with β-glucan or PBS and subsequently infected them with Mtb. The protection capacity of β-glucan was absent in IL1R−/− mice infected with Mtb (Figures 3D and 3E), demonstrating that IL-1 signaling is required for β-glucan-mediated protection against Mtb.
β-Glucan Induces Expansion of Hematopoietic and Myeloid Progenitors through IL-1
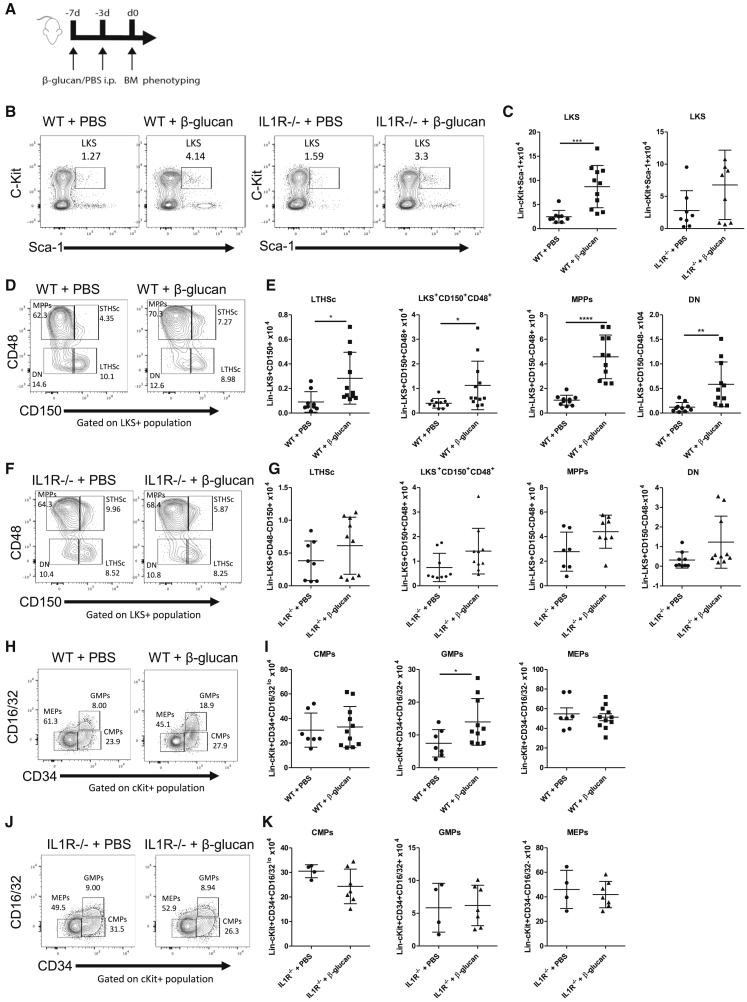
Our group has previously demonstrated that the presence of BCG in the bone marrow leads to the reprogramming of HSCs with a unique bias in myelopoiesis and subsequently the generation of protective trained monocytes and macrophages against Mtb infection (Kaufmann et al., 2018). β-Glucan training has also been shown to reprogram hematopoietic progenitors in the bone marrow toward myelopoiesis and generation of trained immunity (Mitroulis et al., 2018). As the protective effect of β-glucan was absent in IL1R−/− mice, we investigated whether this effect was associated with differences in the subsets of progenitors residing in the bone marrow. WT and IL1R−/− mice were treated with β-glucan or PBS at day 0 and day 3, followed by analysis of the bone marrow at day 7 (Figure 4A). β-Glucan induced a significant increase in the number of HSCs (LKS cells [Lineage−cKit+Sca1+]). Specifically, the long-term HSCs (LTHSCs; LKS+CD150+CD48−) and multipotent progenitors (MPPs; LKS+CD48+CD150−) were increased compared to PBS-control mice (Figures 4B–4E). In addition, this increase was observed in the number of LKS+CD150+CD48+ cells and the number of double negatives (DNs; LKS+CD48−CD150−) (Figure 4E). In contrast, we found no significant differences in HSCs and progenitor cells between β-glucan and PBS injection in IL1R−/− mice (Figures 4B, 4C, 4F, and 4G), suggesting that in the absence of IL-1 signaling, β-glucan has no impact on the expansion and proliferation of HSCs.
Figure 4.
Administration of β-Glucan Induces Expansion of HSPCs and Promotes Myelopoiesis in an IL-1-Dependent Manner
(A) WT and IL1R−/− mice were treated with β-glucan or PBS at day 0 and day 3. The expansion of cellular subpopulations was assessed in bone marrow 7 days post-β-glucan immunization.
(B and C) Representative fluorescence-activated cell sorting (FACS) plots (B) and total cell counts of LKS (C).
(D–G) Representative FACS plots (D and F) and total cell counts (E and G) of LTHSc, LKS+CD150+CD48+ cells, MPPs, and DN in WT (D and E) and IL1R−/− mice (F and G).
(H–K) Expansion of CMPs, GMPs, and MEPs was assessed in the bone marrow at day 7 post-β-glucan immunization. Representative FACS plots (H and J) for the frequencies of myeloid progenitors and total cell counts of CMPs, GMPs, and MEPs (I and K) in WT (H and I) and IL1R−/− mice (J and K) are shown (means ± SDs, n = 4–7 mice per group, ∗p < 0.05, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001 unpaired t test).
See Figure S3 for gating strategy.
We next determined the effect of β-glucan on myeloid progenitors. While the number of granulocyte-monocyte progenitors (GMPs; cKit+CD34+CD16/32+) was significantly increased in β-glucan-treated mice compared to PBS-control mice, there was no statistical difference in multipotent common myeloid progenitor (CMP; cKit+CD34+CD16/32lo) cells or megakaryocyte-erythrocyte progenitor (MEP; cKit+CD34−CD16/32−) cells (Figures 4H, 4I, and S3). In sharp contrast, in IL1R−/− mice there was no significant difference in the number of myeloid progenitor subsets between β-glucan or PBS-control mice (Figures 4J and 4K). These findings demonstrate that β-glucan induced the expansion of HSCs and myeloid progenitors via IL-1 signaling. Furthermore, these data suggest that the protection of the β-glucan-IL-1 axis against Mtb infection may be mediated via the reprogramming of HSCs and their bias toward myelopoiesis and generation of trained immunity.
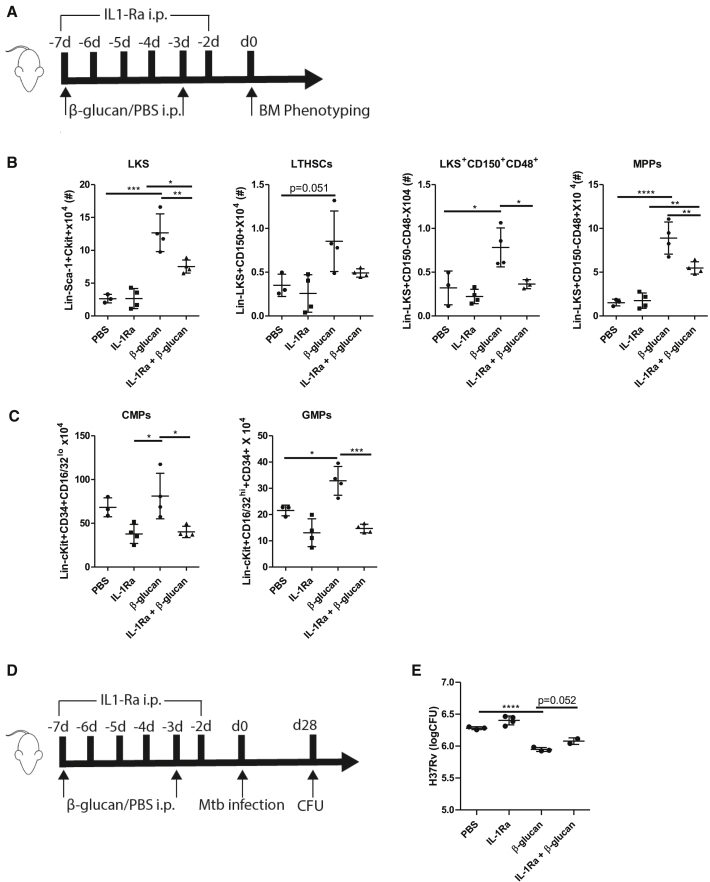
To further investigate a direct link between IL-1-mediated HSCs training and the progression of pulmonary infection, we inhibited IL-1 signaling by the IL-1 receptor antagonist (IL-1Ra; anakinra) before Mtb infection. Briefly, WT mice were treated with β-glucan at days 0 and 3 and received daily IL-1Ra until day 6 (Figure 5A). Similar to our findings in IL1R−/− mice, WT mice treated with β-glucan and IL-1Ra showed a significant reduction in myelopoiesis (Figures 5B and 5C). More specifically, the inhibition of IL-1Ra in β-glucan-trained WT mice partially reduced the expansion of LKS, LTHSC, LKS+CD150+CD48+ cells, and MPPs. Next, we assessed the impact of impaired IL-1 signaling on the β-glucan-induced protective effects against Mtb in vivo (Figure 5D). While the inhibition of IL-1 signaling before Mtb infection had no impact on pulmonary bacterial growth in the lung in control mice, the inhibition of IL-1 in β-glucan-treated mice showed an increase in pulmonary bacterial burden (p = 0.052) 4 weeks after Mtb infection (Figure 5E). These data collectively indicate that β-glucan promotes myelopoiesis in an IL-1-dependent manner that enhances protection against pulmonary Mtb infection.
Figure 5.
β-Glucan Fails to Induce Myelopoiesis in the Presence of an IL-1R Antagonist
(A) WT mice were treated with β-glucan or PBS at day 0 and day 3 along with IL-1Ra antagonist administered intraperitoneally (i.p.) at day 0 and consecutively until day 6.
(B and C) Expansion of cellular subpopulations was assessed in bone marrow 7 days post-β-glucan immunization. Total cell counts of LKS, LT-HSC, LKS+CD150+CD48+ cells, MPPs (B), and CMPs and GMPs (C) are shown (means ± SDs, n = 4 mice per group, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.00001, 1-way ANOVA).
(D) WT mice were treated with β-glucan or PBS at day 0 and day 3 along with IL-1Ra antagonist administered i.p. at day 0 and consecutively until day 6, followed by aerosolized infection with Mtb (∼50 H37Rv) at day 7.
(E) Bacterial burden in the lungs after 28 days of aerosol infection (means ± SDs, n = 4–5 mice per group, ∗∗∗∗p < 0.00001, 1-way ANOVA).
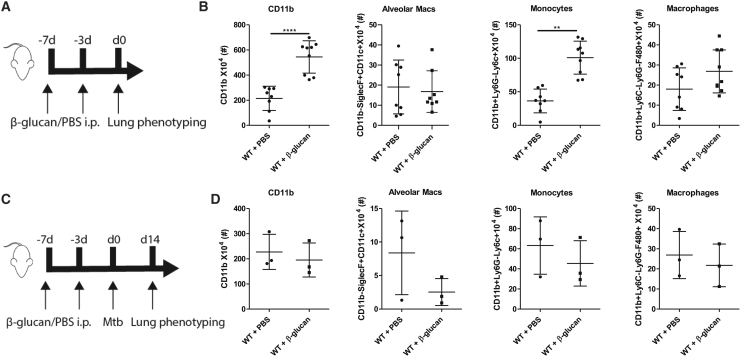
To determine the effects of β-glucan training on different cellular subpopulations in the lung, we immunophenotyped the immune cells isolated from the lungs of mice 7 days after β-glucan treatment (Figure 6A and S4). We observed a significant increase in myeloid cell subsets—in particular, monocytes and granulocytes (Figure 6B)—and there was no difference in the number of alveolar macrophages. We next assessed whether the observed changes in cell subsets were also present in the lungs of mice treated with β-glucan following Mtb infection at day 14 (Figure 6C). No differences were observed in recruited or residential cells in the lungs of β-glucan-trained mice infected with Mtb. These data suggest that while the number of recruited pulmonary monocytes and macrophages was increased 7 days after β-glucan treatment, there was no additional increase 14 days post-Mtb infection.
Figure 6.
β-Glucan Induces an Increase in Myeloid Cell Subsets in the Lungs of Mice
(A) WT mice were treated with β-glucan or PBS at day 0 and day 3. Cellular subpopulations were assessed in the lungs 7 days post-β-glucan immunization.
(B) Frequency of CD11b, alveolar macrophages, monocytes, and interstitial macrophages 7 days post-β-glucan immunization (means ± SDs, n = 7–8 mice per group).
(C) WT mice were treated with β-glucan or PBS at day 0 and day 3, followed by aerosolized infection with Mtb (∼50 H37Rv) at day 7. Cellular subpopulations were assessed in the lungs 14 days post-Mtb infection.
(D) Frequency of CD11b, alveolar macrophages, monocytes, and interstitial macrophages 14 days post-Mtb infection (means ± SDs, n = 3 mice per group, ∗∗p < 0.01 and ∗∗∗∗p < 0.0001 unpaired t test).
See Figure S4 for gating strategy.
β-Glucan Training Induces Transcriptomal Changes in IL-1 Family Genes via Epigenetic Reprogramming of Human Monocytes
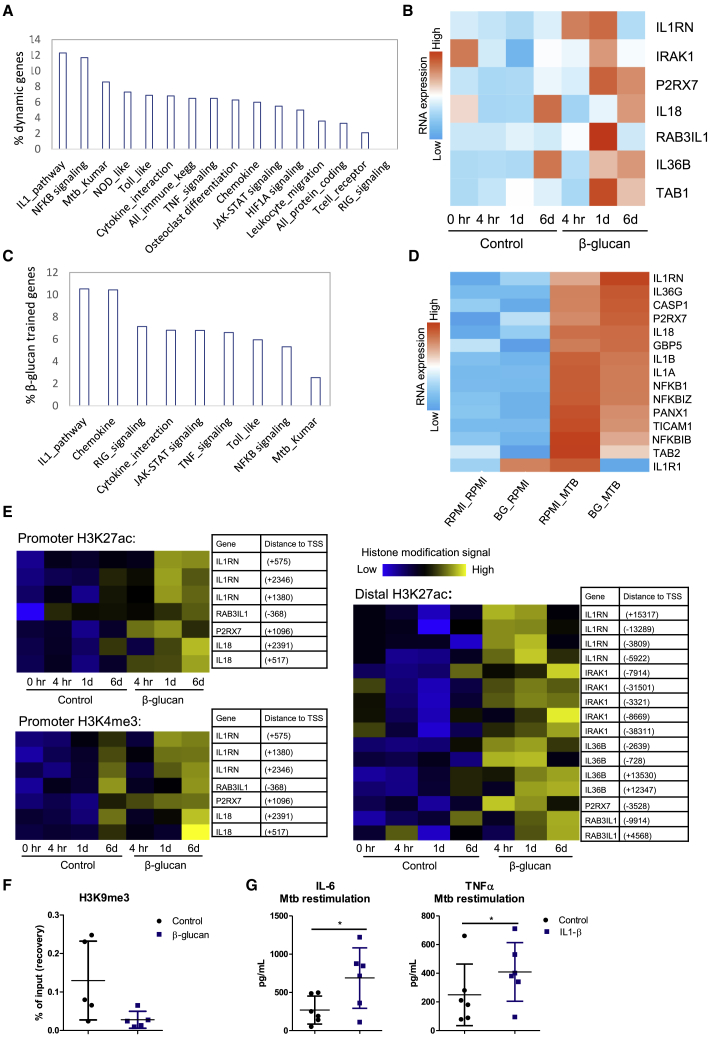
As the protective effect of β-glucan against Mtb infection requires IL-1 signaling in a murine model of TB, we next investigated the direct impact of β-glucan on the IL-1 pathway in human monocytes (GSE85243) (Novakovic et al., 2016). We measured the enrichment of specific immune-related Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in genes upregulated 24 h upon β-glucan exposure. Pathway analysis of the 663 genes that were upregulated after β-glucan exposure indicated that the IL-1 pathway was the most enriched, with 12.3% of genes in this pathway induced by β-glucan, compared to 6.5% and 3.3% of all immune-related KEGG and protein-coding genes, respectively (Figure 7A). RNA-seq analysis demonstrated that genes such as ILRN, IL36B, and IL18, all key members of the IL-1 family, are markedly upregulated after 24 h of β-glucan treatment (Figures 7B and S5). In line with these findings, pathway analysis revealed that of the genes that were upregulated after Mtb exposure in β-glucan-trained cells compared to the control, the IL-1 pathway was the most enriched, followed by chemokines (Figure 7C). Various IL-1 family genes were differentially expressed upon Mtb stimulation in β-glucan-trained cells (Figure 7D), supporting the critical role of the IL-1 pathway in the response to Mtb infection.
Figure 7.
β-Glucan Alters the Transcriptional and Epigenetic Profile of IL-1 Family Genes
(A) Percentage of genes in a pathway that are upregulated in human monocytes 24 h after β-glucan exposure (total of 663 genes).
(B) Heatmap showing RNA expression of 7 IL-1 family genes induced by exposure to β-glucan compared to the control. Monocytes were incubated with either culture medium or β-glucan. mRNA expression was assessed before incubation, 4 h, 24 h, and 6 days after incubation. See also Figure S5.
(C) Enrichment of pathways in genes upregulated after exposure to heat-killed M. tuberculosis in β-glucan-trained cells.
(D) Heatmap showing RNA expression of IL-1 family genes upon Mtb exposure on day 6 in β-glucan-trained cells compared to the control.
(E) Heatmap of the changes in H3K27ac and H3K4me3 in β-glucan-trained monocytes and control at 4 h, 1 day, and 6 days after the start of incubation near IL-1 family genes shown in (B).
(F) Human monocytes were stimulated for 24 h with culture medium or β-glucan. Chromatin was fixated on day 6 and ChIP-qPCR was performed. H3K9me3 was determined at the promoter of IL1B (means ± SDs, n = 5). See Table S2 for primers used.
(G) Human monocytes were incubated with culture medium or IL-1β for 24 h. On day 6, cells were re-stimulated with heat-killed M. tuberculosis and levels of IL-6 and TNF-α were assessed (means ± SDs, n = 6, ∗p < 0.05, Wilcoxon signed-rank test).
To test whether these transcriptional changes were associated with epigenetic modifications, we next assessed the levels of H3K27ac and H3K4me3 4 h, 24 h, and 6 days after β-glucan exposure (GSE85245) (Novakovic et al., 2016) (Figure 7E). The levels of both H3K27ac and H3K4me3 in the promoter and distal region of IL-1 genes were increased in β-glucan-treated monocytes compared to the control. In addition, ChIP-qPCR experiments revealed reduced levels of the repressor mark H3K9me3 at the promotor site of IL1B upon β-glucan training (Figure 7F). As the histone modifications are associated with changes in the transcriptomic profile of β-glucan-treated monocytes, it is most likely that these epigenetic mechanisms are involved in the regulation of the transcription of the IL-1 gene family.
Since IL-1 signaling was critical for both β-glucan-induced trained immunity in human monocytes in vitro and protection in a murine model of Mtb infection in vivo, we next assessed whether the training of human monocytes with IL-1β leads to the enhanced production of cytokines during re-stimulation with heat-killed Mtb. The levels of both IL-6 and TNF-α were significantly increased in monocytes that were trained with IL-1β and re-stimulated with Mtb (Figure 7G), further supporting the importance of IL-1 in β-glucan-induced protection against Mtb.
Discussion
It has been recently shown that β-glucan induces trained immunity, generating long-term functional changes in monocytes and macrophages, which subsequently result in increased protection against various microbial infections (Quintin et al., 2012). In the present study, we demonstrate for the first time that β-glucan-induced trained immunity confers protection against virulent Mtb via the IL-1 signaling pathway. First, we demonstrate that β-glucan-induced trained immunity enhances the production of proinflammatory cytokines in human monocytes challenged with heat-killed Mtb; the observed increase in cytokine production capacity was the result of epigenetic reprogramming and mediated via the PI3K/Akt/mTOR pathway (Cheng et al., 2014). Second, we show that mice treated with β-glucan revealed significantly lower lung bacterial burden after aerosol Mtb infection. Most important, β-glucan-treated mice infected with Mtb demonstrated remarkably enhanced survival, which was dependent on IL-1 signaling.
As previous findings have demonstrated the importance of IL-1 signaling in the induction of trained immunity (Christ et al., 2018, Mitroulis et al., 2018), we studied the role of IL-1 signaling in β-glucan-induced protection against Mtb in IL1R−/− mice and in mice treated with IL-1Ra. In line with a central role of the IL-1 pathway for the induction of trained immunity, the protective effect of β-glucan against Mtb was abolished when IL-1 signaling was impaired, confirming the importance of IL-1 in the establishment of protective trained immunity. In addition, these findings demonstrated for the first time the crucial role of IL-1 signaling in β-glucan-induced protection against Mtb.
Consistent with previous studies, we showed that the administration of β-glucan induces IL-1-dependent adaptations in hematopoietic progenitors in the bone marrow, promoting the expansion of subsets of HSCs and myelopoiesis (Christ et al., 2018, Kaufmann et al., 2018, Mitroulis et al., 2018, Patchen and MacVittie, 1983). While the induction of myeloid cell proliferation by β-glucan was already described by Patchen and MacVittie (1983) nearly 4 decades ago, only recently was the crucial role of IL-1 in this process demonstrated. A study by Mitroulis et al. (2018) showed that β-glucan administration in mice increased the cell-cycle progression of LT-HSCs, glycolysis, and the expansion of hematopoietic stem and progenitor cells HSPCs and GMPs. β-Glucan-dependent effects were abrogated when IL-1 signaling was inhibited by IL-1Ra. Ex vivo treatment of isolated progenitor cells with IL-1β enhanced glycolysis, demonstrating a direct link between IL-1β and metabolic alterations in progenitor cells (Mitroulis et al., 2018). The importance of reprogramming at the level of bone marrow progenitors in trained immunity and the identification of IL-1 as a key mediator in this process was further supported by several other studies (Christ et al., 2018, Kaufmann et al., 2018). One study showed that a Western-type diet induces trained immunity in myeloid cells, which was reduced when IL-1 signaling was inhibited (Christ et al., 2018). Kaufmann et al. (2018) showed that the adoptive transfer of bone marrow-derived macrophages from BCG-trained mice into Rag1−/− mice enhanced protection against Mtb in vivo, indicating that trained HSCs generate macrophages with enhanced protective capacity. These studies therefore strongly support our hypothesis of the role of IL-1 for the protective effects induced by β-glucan, including at the level of myeloid progenitor cells in the bone marrow.
As monocytes in the circulation are short-lived, reprogramming at the level of precursor cells in the bone marrow is thought to be responsible for the long-term functional changes in circulating myeloid cells. However, the duration of the protective effect induced by β-glucan is unknown. Here, we showed that β-glucan treatment at 7 and 3 days before Mtb infection resulted in increased protection against TB. Mitroulis et al. (2018) reported sustained changes in the protective response for at least 28 days after β-glucan administration. As an increased understanding about the duration of the effect is crucial for the design of proper vaccination strategies, future studies are required to identify the duration of the protective effect mediated by β-glucan. In addition, future studies should investigate the effects of β-glucan on the function of alveolar macrophages, as these are crucial cells for host defense against mycobacteria (Huang et al., 2018), and recent studies demonstrated that they can be targeted for training (Yao et al., 2018).
To validate the role of IL-1 in β-glucan-induced protection in human monocytes, we analyzed genome-wide RNA-seq and ChIP-seq data of human monocytes exposed to β-glucan. Using an unbiased approach, we observed that many genes affected by β-glucan are genes involved in the IL-1 signaling pathway. More specifically, we found that β-glucan increases the expression of several members of the IL-1 family via the induction of epigenetic changes. As epigenetic reprogramming in monocytes and macrophages has already occurred after β-glucan treatment and the number of the recruited monocytes and macrophages are increased in advance of Mtb infection, both of these mechanisms contribute to increased protection and survival when the host is exposed to Mtb. Furthermore, training with IL-1β itself enhanced the production of proinflammatory cytokines upon stimulation with heat-killed Mtb, which is supported by early animal studies demonstrating a protective effect of IL-1β injection given before inoculation with a lethal infection (van der Meer et al., 1988).
TB remains a major global health problem, with 1.4 million deaths and ∼10 million new cases annually (World Health Organization, 2018). Undoubtedly, an effective vaccine is essential to control TB, and yet recent reports in The Lancet (Tameris et al., 2013) and the New England Journal of Medicine (Nemes et al., 2018) revealed the failure of large randomized human vaccine trials in infants and adults, respectively. These results underscore the urgent need for innovative approaches to generate a high-efficacy vaccine against TB. Epidemiological studies indicate that innate immunity may make a crucial contribution to the eradication of Mtb, as up to 20%–25% of Mtb-exposed individuals clear an infection before the development of adaptive immunity (Koeken et al., 2019, Verrall et al., 2014). The IL-1 family of cytokines may play a key role in this process (Fremond et al., 2007, Sugawara et al., 2001, Yamada et al., 2000). These findings suggest that a strategy to enhance the anti-mycobacterial function of innate immunity is a promising approach to developing a high-efficacy TB vaccine. The potential of this strategy is supported by recent studies demonstrating the power of BCG in generating protective trained immunity against TB (Kaufmann et al., 2018) and how IL-1β-mediated trained immunity provides protection against experimental virus infection (Arts et al., 2018). We also envision that the induction of protective trained immunity in individuals suffering from latent TB may reduce the risk for the progression to active disease; trained immunity also induces an increase in TNF-α production, known to be a strong immune factor against disease reactivation. Further studies are required to investigate the potential prophylactic and therapeutic effects of β-glucan administration against TB.
In conclusion, we show that β-glucan epigenetically reprograms human monocytes, leading to a phenotype characterized by a unique IL-1 signature and anti-mycobacterial properties. β-Glucan-treated mice were protected against pulmonary Mtb infection. While both β-glucan and BCG reprogram HSCs to induce trained immunity, BCG reprogramming of HSCs was dependent on IFNγ signaling (Kaufmann et al., 2018), while we found that β-glucan reprogramming of HSCs was mediated via IL-1 signaling, which was also required for protection against Mtb infection. Considering the safety of β-glucan in a human clinical trial (Lehne et al., 2006), our results strongly suggest the potential clinical implications of β-glucan for both prophylactic and therapeutic use in TB.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Fixable Viability Stain eFluor501 | eBioscience | Cat#65-0863-18 |
| anti-CD16/32 (clone 93) | eBioscience | RRID: AB_467134 |
| anti-Ter-119 biotin-conjugated | BD Biosciences | RRID: AB_394985 |
| anti-CD11b biotin-conjugated (clone M1/70) | BD Biosciences | RRID: AB_394773 |
| anti-CD5 biotin-conjugated (clone 53-7.3) | BD Biosciences | RRID: AB_394557 |
| anti-CD4 biotin-conjugated (clone RM4-5) | BD Biosciences | RRID: AB_394581 |
| anti-CD8a biotin-conjugated (clone 53-6.7) | BD Biosciences | RRID: AB_394567 |
| anti-CD45R biotin-conjugated (clone RA3-6B2) | BD Biosciences | RRID: AB_394616 |
| anti-Ly6G/C biotin-conjugated (clone RB6-8C5) | BD Biosciences | RRID: AB_394641 |
| Streptavidin – APC-Cy7 | eBioscience | RRID: AB_394641 |
| anti-c-Kit – APC (clone 2B8) | eBioscience | RRID: AB_469430 |
| anti-Sca-1 – PE-Cy7 (clone D7) | eBioscience | RRID: AB_469668 |
| anti-CD150 – eFluor450 (clone mShad150) | eBioscience | RRID: AB_2574045 |
| anti-CD48 – PerCP-eFluor710 (clone HM48-1) | BD Biosciences | RRID: AB_396724 |
| anti-Flt3 – PE (clone A2F10.1) | BD Biosciences | RRID: AB_395079 |
| anti-CD34 – FITC (clone RAM34 | eBioscience | RRID: AB_465020 |
| anti-CD16/32 AF700 (clone 93 | eBioscience | RRID: AB_467134 |
| anti-CD11b-Pacific blue (M1/70) | BD Biosciences | RRID: AB_394491 |
| anti-CD11c-PEcy7 (N418) | BD Biosciences | Cat#565451 |
| Siglec-F-PECSF594 (E50–2440) | BD Biosciences | RRID: AB_2687994 |
| F4/80-APC (BM8) | eBioscience | RRID: AB_469452 |
| Ly6C-FITC (HK1.4) | BD Biosciences | BD Bioscience |
| Ly6G-PerCPefluor710 (1A8) | eBioscience | RRID: AB_2573892 |
| Rabbit polyclonal anti-H3K9me3 | Diagenode | pab-193-050, C15410193 |
| Bacterial and Virus Strains | ||
| Mycobacterium tuberculosis H37Rv | ATCC | ATCC Number: 27294 |
| Heat-killed Mycobacterium tuberculosis | Gift | H27Rv |
| Chemicals, Peptides, and Recombinant Proteins | ||
| β−glucan (β−1,3-(D)-glucan) | Professor David Williams, College of Medicine, Johnson City, USA | NA |
| Recombinant human Interleukin-1Ra | Sobi | Kineret® Anakinra |
| Recombinant human Interleukin-1β | R&D systems | Cat#201-LB-005 |
| Lipopolysaccharide | Sigma-Aldrich | From E. coli serotype 055:B5, L2880 |
| Protease inhibitor cocktail | Sigma-Aldrich | Cat#P8465 |
| BSA | Wisent | Cat#800-195-EG |
| 5′-Deoxy-5′-(methylthio) adenosine (MTA) | Sigma-Aldrich | Cat#D5011 |
| Percoll | Sigma-Aldrich | Cat#P1644 |
| Ficoll-Paque | GE Healthcare | Cat#17-1440-03 |
| Roswell Park Memorial Institute medium (RPMI) | Invitrogen | Cat#22406031 |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | Cat#A7030 |
| iScript reverse transcriptase | Bio-Rad | Cat#1708840 |
| TRIzol reagent | Life Technologies | Cat#15596018 |
| SYBR Green | Applied Biosciences | Cat#4368708 |
| 16% Formaldehyde | Fisher Scientific | Cat#28908, 11835835 |
| Critical Commercial Assays | ||
| Human IL-1β ELISA | R&D systems | Cat#DY201 |
| Human TNFα ELISA | R&D systems | Cat#DY210 |
| Human IL-6 ELISA | Sanquin | Cat#M1916 |
| iScript cDNA Synthesis Kit | Bio-Rad | Cat#1708891 |
| MinElute PCR purification Kit | QIAGEN | Cat#28006 |
| DNase I | QIAGEN | Cat#79254 |
| Rneasy Mini Kit | QIAGEN | Cat#74106 |
| Deposited Data | ||
| β-glucan in vitro trained monocytes restimulated with Mtb RNA-seq data | This paper | GEO: GSE141656 |
| RNA-seq data of control and β-glucan-trained monocytes | Novakovic et al., 2016 | GEO: GSE85243 |
| ChIP sequencing data of control and β-glucan-trained monocytes | Novakovic et al., 2016 | GEO: GSE85245 |
| Experimental Models: Organisms/Strains | ||
| BALB/c female mice | Charles River Laboratories | 028 |
| IL1R−/− mice in BALB/c background | Gift from Dr. Salman Qureshi, McGill University, Montreal, Canada | NA |
| Oligonucleotides | ||
| Primers for qPCR, see Table S2 | This paper | NA |
| Software and Algorithms | ||
| FACSDiva Software | BD Biosciences | SCR_001456 |
| FlowJo software v.10 | Tree Star | SCR_000410 |
| GraphPad Prism v. 5.03 | Graphpad Software | https://www.graphpad.com |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Mihai Netea at the Radboud University Medical Center, Nijmegen, the Netherlands (mihai.netea@radboudumc.nl).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Raw data files of the RNA-sequencing analysis on β-glucan-trained/control monocytes exposed to Mtb/RPMI have been deposited in the NCBI Gene Expression Omnibus under accession number GEO: GSE141656.
Experimental Model and Subject Details
Mice
Six- to 10-week-old BALB/c female mice were purchased from Charles River Laboratories. IL1R−/− mice in BALB/c background were a kind gift from Dr. Salman Qureshi (McGill University). All animal studies were conducted in accordance with the guidelines of and approved by the Animal Research Ethics Board of McGill University. Mice were housed under SPF conditions with ad libitum access to food and water and were randomly assigned to experimental groups.
Healthy volunteers
PBMCs were isolated from buffy coats obtained from healthy volunteers (both females and males) after giving written informed consent (Sanquin Bloodbank, Nijmegen, the Netherlands).
Method Details
Stimuli
β-glucan (β-1,3-(D)-glucan) was kindly provided by Professor David Williams (College of Medicine, Johnson City, USA) or purchased from Sigma (G-5011). Cultures of H37Rv Mycobacterium tuberculosis (Mtb) were grown to mid-log phase in Middlebrook 7H9 liquid medium (Difco, Becton Dickinson, East-Rutherford) supplemented with oleic acid/albumin/dextrose/catalase (OADC) (BBL, Becton Dickinson), washed three times in sterile saline solution, heat-killed and disrupted using a bead beater. Concentration was measured using a bicinchoninic acid (BCA) assay (Pierce, Thermo Scientific, Rockville).
PBMC/monocyte isolation and in vitro training experiments
Venous blood from the cubital vein of human healthy volunteers was drawn into 10 mL EDTA tubes (Monoject). PBMC isolation was performed by density centrifugation of blood diluted 1:1 in pyrogen-free PBS over Ficoll-Paque (GE healthcare, UK). Cells were washed twice in PBS and resuspended in RPMI culture medium (Roswell Park Memorial Institute medium, Invitrogen, CA, USA) supplemented with 50 μg/mL gentamicin (Centrafarm), 2 mM glutamax (GIBCO), and 1 mM pyruvate (GIBCO). Monocytes were separated by hyperosmotic density gradient centrifugation over Percoll (Sigma-Aldrich, St. Louis, MO, USA). 150-200 × 106 PBMCs were layered on top of a hyperosmotic Percoll solution (48,5% Percoll, 41,5% sterile H2O, 0.16M filter sterilized NaCl) and centrifuged for 15 minutes at 580 × g. The interphase layer was isolated, cells were washed with cold PBS and resuspended in RPMI. Percoll-isolated monocytes were counted and concentration was adjusted to 1x10ˆ6 cells/mL. A 100 μl volume was added to flat bottom 96-well plates (Corning, NY, USA) and let to adhere to polystyrene flat bottom plates for 1 hour at 37°C; subsequently cells were washed with warm PBS to achieve maximal purity. Monocytes were incubated either with culture medium (RPMI) only as a negative control or 1 μg/mL of β-glucan at 37°C (all in the presence of 10% pooled human serum). After 24 hr, cells were washed once with 200 μL warm PBS and incubated for 5 days in culture medium with 10% human pooled serum. Medium was changed once on day 3. On day 6, cells were restimulated with either 200 μL RPMI or heat-killed Mtb 10 μg/mL for 24 hr. Supernatants were collected and stored at −20°C until assayed. Intracellular amounts of pro-IL-1β were assessed after adding 200 μL of 0.5% Triton X in PBS to the adherent cells. In some experiments, cells were pre-incubated before stimulation for 1 hour with 10nM rapamycin (Sigma), 100 nM wortmannin, 1mM MTA (5′- deoxy-5′-methylthio-adenosine, Sigma) or the histone deacetylase inhibitor ITF2357 (Sigma). The concentrations used were highest non-toxic concentrations assessed in pilot experiments (not shown).
In vitro killing assay
Monocytes were isolated from human peripheral blood as described above and trained in vitro with β-glucan (1μg/ml) or incubated in RPMI medium only for 24 hr. Next, cells were washed and incubated in culture medium supplemented with 10% human pooled serum. After incubation for 6 days as described above, monocytes were harvested, counted and reseeded at 1x106 cells/ml in antibiotics-free media containing 10% human serum overnight. Cells were then infected with virulent M. tuberculosis H37Rv at MOI of 1 for 4 hr. Subsequently, cells were washed three times with PBS and either lysed directly (4 hr time point) or incubated in antibiotics-free media for 3 days. Serial dilutions of Mtb CFU in PBS+0.05% Tween 80 were enumerated on 7H10 agar plates.
Cytokine measurements
Cytokine production was assessed in supernatants using commercial ELISA kits for human IL-6 (Sanquin, Amsterdam, the Netherlands), IL-1β (R&D systems, MN, USA) and TNFα (R&D systems, MN, USA), in accordance with the manufacturer’s instructions.
mRNA extraction and RT-PCR
To assess IL-1β expression, 1 × 10ˆ6 monocytes were seeded in two flat bottom 96-well plates (Corning, NY, USA) and cells were let to adhere for 1 hour at 37°C. Next, cells were washed trice with warm PBS and incubated for 24 hr with culture medium or β-glucan. For RNA sequencing experiments cells were trained with β-glucan or incubated in RPMI medium as described above. On day 6, cells were stimulated for 4 hr with heat-killed Mtb 10 μg/mL or RPMI medium (control). To measure mRNA expression, mRNA was extracted by TRIzol (Life technologies) according to the manufacturer’s instructions. cDNA was synthesized using iScript reverse transcriptase (Invitrogen) and relative expression was determined using SYBR Green method (Invitrogen) on an Applied Biosciences Step-one PLUS qPCR machine. Primers are listed in Table S2. RNA sequencing was performed as described previously (Saeed et al., 2014).
In vitro assessment of specific histone marks
Monocytes were isolated from human volunteers and trained in vitro as described above. After 6 days, cells were detached from the plate with Versene and fixed in methanol-free 1% formaldehyde. Cells were sonicated using a Diagenode Bioruptor Pica sonicator and immunoprecipitation was performed using antibodies against H3K9me3 (Diagenode, Seraing, Belgium). DNA was purified using QIAGEN MinElute PCR purification Kit and processed further for qPCR analysis using the SYBR green method. Samples were analyzed by a comparative Ct method according to the manufacturer’s instructions. GAPDH was used as a negative control and ZNF as a positive control in experiments assessing H3K9me3. Primer sequences can be found in Table S2.
In vivo experiments
Injection of mice
Mice were injected intraperitoneally (i.p.) with 1 mg of β-glucan or pyrogen-free phosphate-buffered saline (PBS) alone on day 0 an day 3. IL-1 inhibition was performed by i.p. administration of IL-1Ra (Kineret (Anakinra), Sobi (Swedish Orphan Biovitrum AB), 10mg/kg/body weight per dose) (Iannitti et al., 2016) from day 0 till day 6. On day 0 and day 3, IL-1Ra was administered 8 hr after β-glucan injection.
Mice Mtb infection and CFU enumeration
Mice were either infected with ∼50 CFU of Mtb (H37Rv) in a nose-only aerosol exposure unit (Intox) 7 days after the first administration of β-glucan and survival was monitored. Infection dose was confirmed by enumerating the lung CFU one day after infection. For CFU enumeration, lungs were homogenized in 1 mL 7H9 broth (BD) supplemented with 0.2% glycerol (Wisent), 0.05% Tween80 (Fisher), and 10% ADC using OmniTip Plastic Homogenizer Probes (Omni International). Serial dilutions in PBS+0.05% Tween80 were plated on 7H10 agar plates with 10% OADC enrichment and PANTA (BD). Plates were then incubated at 37°C and counted after 21 days.
Flow Cytometry
Bone marrow cells (3 × 106 cells) after RBC lysis were stained with fixable viability stain eFluor501 (eBioscience) at the concentration of 1:1000 for 30 minutes (4°C). Subsequently, the cells were washed with PBS supplemented with 0.5% BSA (Wisent) and incubated with anti-CD16/32 (clone 93, eBioscience) at a concentration of 1:100 in PBS/0.5% BSA at 4°C for 10 minutes except for myeloid progenitors staining. The following antibodies were then used for staining: anti-Ter-119, anti-CD11b (clone M1/70), anti-CD5 (clone 53-7.3), anti-CD4 (clone RM4-5), anti-CD8a (clone 53-6.7), anti-CD45R (clone RA3-6B2), and anti-Ly6G/C (clone RB6-8C5), all biotin-conjugated (all BD Bioscience),were added at a concentration of 1:100 for 30 minutes at 4°C. Cells were subsequently washed with PBS/0.5% BSA. For staining of LKS, HSCs, and MPPs: Streptavidin –APC-Cy7 (eBioscience), anti-c-Kit – APC (clone 2B8, eBioscience), anti-Sca-1 – PE-Cy7 (clone D7, eBioscience), anti-CD150 –eFluor450 (clone mShad150, eBioscience), anti-CD48 – PerCP-eFluor710 (clone HM48-1, BD Bioscience), anti-Flt3 – PE (clone A2F10.1, BD Bioscience), and anti-CD34 – FITC (clone RAM34, eBioscience) (all 1:100) were added and incubated at 4°C for 30 minutes. For Myeloid progenitors: Streptavidin –APC-Cy7 (eBioscience), anti-c-Kit – APC (clone 2B8, eBioscience), anti-Sca-1 – PE-Cy7 (clone D7, eBioscience), anti-CD34 – FITC, anti-CD16/32 AF700 (clone 93, ebioscience) were added and incubated at 4°C for 30 minutes.
Innate cells staining: Lungs cells (3 × 106) isolated from single-cell suspension obtained from collagenase-digested lungs were subjected to RBC lysis and then stained with fixable viability stain eFluor501 (eBioscience) at the concentration of 1:1000 for 30 minutes (4°C). Subsequently, the cells were washed with PBS supplemented with 0.5% BSA (Wisent) and incubated with anti-CD16/32 (clone 93, eBioscience) at a concentration of 1:100 in PBS/0.5% BSA at 4°C for 10. After washing, cells were incubated with anti-CD11b-Pacific blue (M1/70), anti-CD11c-PE-Cy7 (N418), Siglec-F-PE-CF594 (E50–2440), F4/80-APC (BM8), Ly6C-FITC (HK1.4), Ly6G-PerCPefluor710 (1A8) at 4°C for 30 minutes.
Quantification and Statistical Analysis
Statistics human in vitro experiments
Statistical parameters including the exact value of n, the definition of center, dispersion and precision measures (mean ± SD) and statistical significance are reported in the Figures and Figure Legends. Results were analyzed using Wilcoxon signed-rank test or Mann-Whitney U test where applicable. Calculations were performed in Graphpad prism 5 (CA, USA) and in figures, asterisks indicate statistical significance (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001). Data are shown as mean ± SD.
Statistics mice experiments
Calculations were performed in Graphpad prism 6.0.c. Statistical significance was calculated by t test or 2-way ANOVA, as indicated in the figure legends. Asterisks indicate statistical significance (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001). Data are shown as mean ± SD.
Statistics epigenetics
Dynamic genes and H3K27ac and H3K4me3 peaks were identified using DEseq with change in signal determined as fold change > 2.5 and padj < 0.05, with a mean RPKM > 1 for gene expression or mean reads/peak > 50 for histone modifications (Novakovic et al., 2016). Genes of interest were downloaded from Kyoto Encyclopedia of Genes and Genomes (KEGG) (Kanehisa and Goto, 2000) and enrichment of the IL-1 pathway was calculated using a chi squared test. H3K27ac and H3K4me3 peaks were assigned to the nearest transcription start site (TSS) within 1Mb using the GREAT tool (McLean et al., 2010).
Acknowledgments
M.G.N. was supported by a European Research Council (ERC) Advanced Grant (#833247) and a Spinoza grant from the Netherlands Organization for Scientific Research (NWO). M.D. is supported by a Canadian Institute of Health Research (CIHR) Foundation Grant (FDN-143273) and the Bill & Melinda Gates Foundation. M.D. holds a Fonds de la Recherche du Quebec-Sante (FRQS) Award and the Strauss Chair in Respiratory Diseases. N.K. is supported by a CIHR Fellowship. B.N. is supported by a National Health and Medical Research Council (NHMRC) CJ Martin Fellowship (#1072966). E.K. is supported by a FRQS Postdoctoral Fellowship.
Author Contributions
Conceptualization, M.G.N. and M.D.; Methodology, S.J.C.F.M.M., N.K., T.J., B.N., and E.K.; Investigation, S.J.C.F.M.M., N.K., B.N., and E.K.; Writing – Original Draft, S.J.C.F.M.M.; Writing – Review & Editing, N.K., M.D., and M.G.N.; Supervision, M.D. and M.G.N.
Declaration of Interests
The authors declare no competing interests.
Published: May 19, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.107634.
Contributor Information
Maziar Divangahi, Email: maziar.divangahi@mcgill.ca.
Mihai G. Netea, Email: mihai.netea@radboudumc.nl.
Supplemental Information
Genes induced by Mtb exposure on day 6 in RPMI- or β-glucan-treated human monocytes with a fold change of at least 1,5 and p < 0.05.
References
- Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23:89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Barreiro L.B., Neyrolles O., Babb C.L., Tailleux L., Quach H., McElreavey K., Helden P.D., Hoal E.G., Gicquel B., Quintana-Murci L. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med. 2006;3:e20. doi: 10.1371/journal.pmed.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro L.B., Tailleux L., Pai A.A., Gicquel B., Marioni J.C., Gilad Y. Deciphering the genetic architecture of variation in the immune response to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. 2012;109:1204–1209. doi: 10.1073/pnas.1115761109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar S.M., Divangahi M., Remold H.G. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat. Rev. Microbiol. 2010;8:668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkering S., Blok B.A., Joosten L.A., Riksen N.P., van Crevel R., Netea M.G. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016;23:926–933. doi: 10.1128/CVI.00349-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz B.E., Azad A.K., Morris J.D., Rajaram M.V.S., Schlesinger L.S. β-Glucans inhibit intracellular growth of Mycobacterium bovis BCG but not virulent Mycobacterium tuberculosis in human macrophages. Microb. Pathog. 2011;51:233–242. doi: 10.1016/j.micpath.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect. Immun. 1986;51:668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.C., Quintin J., Cramer R.A., Shepardson K.M., Saeed S., Kumar V., Giamarellos-Bourboulis E.J., Martens J.H., Rao N.A., Aghajanirefah A. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ A., Günther P., Lauterbach M.A.R., Duewell P., Biswas D., Pelka K., Scholz C.J., Oosting M., Haendler K., Baßler K. Western Diet Triggers NLRP3-Dependent Innate Immune Reprogramming. Cell. 2018;172:162–175.e14. doi: 10.1016/j.cell.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich N., Berrocal-Almanza L.C., Thada S., Goyal S., Slevogt H., Sumanlatha G., Hussain A., Sur S., Burkert S., Oh D.Y. Toll-like receptor 1 variations influence susceptibility and immune response to Mycobacterium tuberculosis. Tuberculosis (Edinb.) 2015;95:328–335. doi: 10.1016/j.tube.2015.02.045. [DOI] [PubMed] [Google Scholar]
- Divangahi M., Behr M.A. Cracking the Vaccine Code in Tuberculosis. Am. J. Respir. Crit. Care Med. 2018;197:427–432. doi: 10.1164/rccm.201707-1489PP. [DOI] [PubMed] [Google Scholar]
- Fremond C.M., Togbe D., Doz E., Rose S., Vasseur V., Maillet I., Jacobs M., Ryffel B., Quesniaux V.F. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 2007;179:1178–1189. doi: 10.4049/jimmunol.179.2.1178. [DOI] [PubMed] [Google Scholar]
- Hetland G., Sandven P. beta-1,3-Glucan reduces growth of Mycobacterium tuberculosis in macrophage cultures. FEMS Immunol. Med. Microbiol. 2002;33:41–45. doi: 10.1111/j.1574-695X.2002.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Hetland G., Løvik M., Wiker H.G. Protective effect of beta-glucan against mycobacterium bovis, BCG infection in BALB/c mice. Scand. J. Immunol. 1998;47:548–553. doi: 10.1046/j.1365-3083.1998.00350.x. [DOI] [PubMed] [Google Scholar]
- Huang L., Nazarova E.V., Tan S., Liu Y., Russell D.G. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J. Exp. Med. 2018;215:1135–1152. doi: 10.1084/jem.20172020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannitti R.G., Napolioni V., Oikonomou V., De Luca A., Galosi C., Pariano M., Massi-Benedetti C., Borghi M., Puccetti M., Lucidi V. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016;7:10791. doi: 10.1038/ncomms10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M., Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann E., Sanz J., Dunn J.L., Khan N., Mendonça L.E., Pacis A., Tzelepis F., Pernet E., Dumaine A., Grenier J.C. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell. 2018;172:176–190.e19. doi: 10.1016/j.cell.2017.12.031. [DOI] [PubMed] [Google Scholar]
- Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A., Ifrim D.C., Saeed S., Jacobs C., van Loenhout J., de Jong D., Stunnenberg H.G. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeken V., Verrall A.J., Netea M.G., Hill P.C., van Crevel R. Trained innate immunity and resistance to Mycobacterium tuberculosis infection. Clin. Microbiol.I Infect. 2019;25:1468–1472. doi: 10.1016/j.cmi.2019.02.015. [DOI] [PubMed] [Google Scholar]
- Kumar D., Nath L., Kamal M.A., Varshney A., Jain A., Singh S., Rao K.V.S. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell. 2010;140:731–743. doi: 10.1016/j.cell.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Lehne G., Haneberg B., Gaustad P., Johansen P.W., Preus H., Abrahamsen T.G. Oral administration of a new soluble branched β-1,3-D-glucan is well tolerated and can lead to increased salivary concentrations of immunoglobulin A in healthy volunteers. Clin. Exp. Immunol. 2006;143:65–69. doi: 10.1111/j.1365-2249.2005.02962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber K.D., Andrade B.B., Oland S.D., Amaral E.P., Barber D.L., Gonzales J., Derrick S.C., Shi R., Kumar N.P., Wei W. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClean C.M., Tobin D.M. Macrophage form, function, and phenotype in mycobacterial infection: lessons from tuberculosis and other diseases. Pathog. Dis. 2016;74:ftw068. doi: 10.1093/femspd/ftw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C.Y., Bristor D., Hiller M., Clarke S.L., Schaar B.T., Lowe C.B., Wenger A.M., Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitroulis I., Ruppova K., Wang B., Chen L.S., Grzybek M., Grinenko T., Eugster A., Troullinaki M., Palladini A., Kourtzelis I. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell. 2018;172:147–161.e12. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes E., Geldenhuys H., Rozot V., Rutkowski K.T., Ratangee F., Bilek N., Mabwe S., Makhethe L., Erasmus M., Toefy A. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N. Engl. J. Med. 2018;379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Quintin J., van der Meer J.W. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355–361. doi: 10.1016/j.chom.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Netea M.G., Joosten L.A., Latz E., Mills K.H., Natoli G., Stunnenberg H.G., O’Neill L.A., Xavier R.J. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic B., Habibi E., Wang S.Y., Arts R.J.W., Davar R., Megchelenbrink W., Kim B., Kuznetsova T., Kox M., Zwaag J. β-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell. 2016;167:1354–1368.e14. doi: 10.1016/j.cell.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchen M.L., MacVittie T.J. Temporal response of murine pluripotent stem cells and myeloid and erythroid progenitor cells to low-dose glucan treatment. Acta Haematol. 1983;70:281–288. doi: 10.1159/000206753. [DOI] [PubMed] [Google Scholar]
- Quintin J. Fungal mediated innate immune memory, what have we learned? Semin. Cell Dev. Biol. 2019;89:71–77. doi: 10.1016/j.semcdb.2018.05.023. [DOI] [PubMed] [Google Scholar]
- Quintin J., Saeed S., Martens J.H.A., Giamarellos-Bourboulis E.J., Ifrim D.C., Logie C., Jacobs L., Jansen T., Kullberg B.J., Wijmenga C. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–232. doi: 10.1016/j.chom.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed S., Quintin J., Kerstens H.H., Rao N.A., Aghajanirefah A., Matarese F., Cheng S.C., Ratter J., Berentsen K., van der Ent M.A. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slight S.R., Khader S.A. Chemokines shape the immune responses to tuberculosis. Cytokine Growth Factor Rev. 2013;24:105–113. doi: 10.1016/j.cytogfr.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara I., Yamada H., Hua S., Mizuno S. Role of interleukin (IL)-1 type 1 receptor in mycobacterial infection. Microbiol. Immunol. 2001;45:743–750. doi: 10.1111/j.1348-0421.2001.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Tameris M.D., Hatherill M., Landry B.S., Scriba T.J., Snowden M.A., Lockhart S., Shea J.E., McClain J.B., Hussey G.D., Hanekom W.A. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thye T., Nejentsev S., Intemann C.D., Browne E.N., Chinbuah M.A., Gyapong J., Osei I., Owusu-Dabo E., Zeitels L.R., Herb F. MCP-1 promoter variant -362C associated with protection from pulmonary tuberculosis in Ghana, West Africa. Hum. Mol. Genet. 2009;18:381–388. doi: 10.1093/hmg/ddn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J.W., Barza M., Wolff S.M., Dinarello C.A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc. Natl. Acad. Sci. USA. 1988;85:1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrall A.J., Netea M.G., Alisjahbana B., Hill P.C., van Crevel R. Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology. 2014;141:506–513. doi: 10.1111/imm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetvicka V. Glucan-immunostimulant, adjuvant, potential drug. World J. Clin. Oncol. 2011;2:115–119. doi: 10.5306/wjco.v2.i2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2018. Global Tuberculosis Report 2018, World Health Organization. License: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/handle/10665/274453. [Google Scholar]
- Yamada H., Mizumo S., Horai R., Iwakura Y., Sugawara I. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab. Invest. 2000;80:759–767. doi: 10.1038/labinvest.3780079. [DOI] [PubMed] [Google Scholar]
- Yao Y., Jeyanathan M., Haddadi S., Barra N.G., Vaseghi-Shanjani M., Damjanovic D., Lai R., Afkhami S., Chen Y., Dvorkin-Gheva A. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell. 2018;175:1634–1650.e17. doi: 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genes induced by Mtb exposure on day 6 in RPMI- or β-glucan-treated human monocytes with a fold change of at least 1,5 and p < 0.05.
Data Availability Statement
Raw data files of the RNA-sequencing analysis on β-glucan-trained/control monocytes exposed to Mtb/RPMI have been deposited in the NCBI Gene Expression Omnibus under accession number GEO: GSE141656.