Summary
Super-spreading events in an outbreak can change the nature of an epidemic. Therefore, it is useful for public health teams to determine whether an ongoing outbreak has any contribution from such events, which may be amenable to interventions. We estimated the basic reproductive number (R0) and the dispersion factor (k) from empirical data on clusters of epidemiologically linked coronavirus disease 2019 (COVID-19) cases in Hong Kong, Japan and Singapore. This allowed us to infer the presence or absence of super-spreading events during the early phase of these outbreaks. The relatively large values of k implied that large cluster sizes, compatible with super-spreading, were unlikely.
Keywords: Super-spreading, Transmission clusters, COVID-19, Hong Kong, Japan, Singapore
Introduction
The spread of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), from Wuhan to other Asian regions is ongoing. As of March 3rd, 2020, the number of laboratory-confirmed COVID-19 cases in Hong Kong (HK), Japan (JP) and Singapore (SG) were 101, 284 and 110, whereas the numbers of deaths were 2, 6 and 0, respectively. Human-to-human transmission clusters have been observed, including 16 cases originating from a Buddhist worship hall (HK); 28 cases linked to a couple returning from Hawaii (JP), and 31 cases related to a church gathering (SG). Here, we explore whether super-spreading is involved in the early phase of the COVID-19 outbreaks in these populations.
Generally, super-spreaders are individuals who generate a more-than-expected number of secondary cases. Super-spreading events (SSEs), where an unexpectedly large number of cases are generated from a single gathering, have been reported with other coronavirus outbreaks, including severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome [1,2]. Therefore, it is worth investigating whether any of the recent COVID-19 outbreaks may also have involved SSEs.
The present study applied a previously published method to empirical data on clusters of epidemiologically linked COVID-19 cases [3]. With estimates of the basic reproductive number (R 0), which is the number of secondary cases generated by a typical index case in a wholly susceptible population, and the dispersion factor (k), which is a measure of variability in empirical cluster sizes, the study explored the presence of SSEs in outbreaks of COVID-19 in HK, JP and SG.
Methods
Using publicly available data on laboratory-confirmed cases of COVID-19 (up to March 3rd, 2020) for HK, JP and SG, from the Centre for Health Protection (HK), Ministry of Health, Labour and Welfare (JP) and Ministry of Health (SG), respectively, an empirical distribution of secondary case transmission cluster sizes was obtained from each population. In this context, a cluster was defined where each case within it could be epidemiologically linked to the others. Such epidemiological links included temporal and geographical groupings that involve one or more index cases and secondary transmissions. A single index case was considered as a cluster of size one.
Cases with unclear epidemiological links were excluded from the analysis. These included: (i) imported cases intercepted at borders with subsequent quarantine or medical surveillance, for example, cases evacuated from affected areas by government-chartered flights (SG, JP); and (ii) cases identified from the international conveyance who were then isolated (HK, JP). Cases that appeared to be re-infected upon hospital discharge (one case in JP) were only counted once.
A previously published method was applied to the extracted data (Appendix A) to estimate R 0 and k, and determine whether SSEs had been involved in recent COVID-19 outbreaks in HK, JP and SG hitherto [3]. For k < 1, it represents a greater degree of dispersion, hence more variability in transmission cluster sizes, such that SSEs may be more likely.
Results
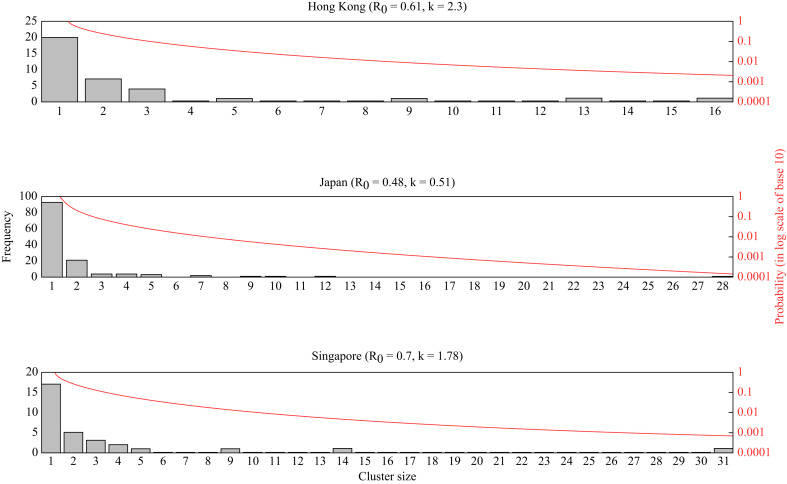
In total, 89 (HK), 251 (JP) and 103 (SG) cases were included in the analysis, consisting of 35 (HK), 131 (JP), and 31 (SG) clusters of secondary infections. The mean cluster sizes were 2.54 (HK), 1.92 (JP) and 3.32 (SG); the maximum were 16 (HK), 28 (JP) and 31 (SG). In all populations, the minimum cluster size was one.
By fitting negative binomial models to these empirical cluster size distributions, the maximum likelihood estimates (MLEs) of R 0 were determined, and found to be statistically smaller than one in all three populations (HK: R 0 = 0.61 (90% confidence interval (CI): 0.47–0.78); JP: R 0 = 0.48 (0.39–0.59); SG: R 0 = 0.70 (0.55–0.89)) (Figure 1 ). Similarly, our estimates of k did not statistically deviate from one, though we note that the upper bounds of some values for k are infinite (HK: k = 2.30 (90% CI: 0.39–∞); JP: k = 0.51 (0.26–1.42); SG: k = 1.78 (0.36–∞)).
Figure 1.
Empirical distribution of transmission cluster size and the best-fitted curve from negative binomial models.
We also investigated the relationship among R 0, k and the probabilities of observing a cluster with size being at least the empirically maximum cluster size as of March 3rd, 2020 (Supplementary Figure S1). p n was denoted as the probability of observing a cluster p of at least size n. For a fixed value of k, p 16 (HK), p 28 (JP) and p 31 (SG) all increased with R 0. When R 0 is small (say 0.3), a higher variation in the number of secondary cases (that is, a smaller k) increases the chance of observing a large transmission cluster (suggestive of a SSE). This effect was reversed when R0 > 0.9. These two effects were consistent across the three populations.
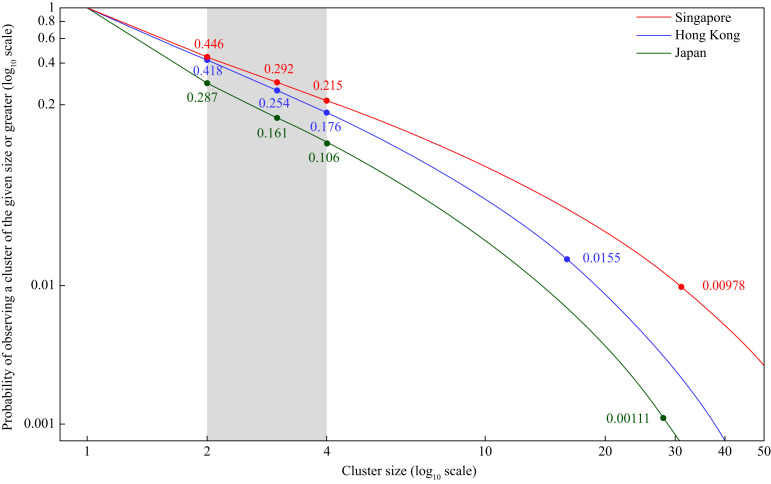
With MLEs of R 0 and k, p n was computed (Figure 2 ). The probability of observing a cluster of size ≥4 (p 4) ranged from 0.106 to 0.215. The estimates for p 16 (HK), p 28 (JP) and p 31 (SG) were 0.0155, 0.00111 and 0.00978, respectively (Figure 2).
Figure 2.
Probability of observing a cluster of a given size or greater, assuming the maximum likelihood estimates of R0 and k.
Furthermore, with an arbitrary introduction of 50 infections, the probabilities that at least one SSE will occur with a cluster size of ≥30 are: 0.114 (HK), 0.0411 (JP) and 0.412 (SG) (Supplementary Figure S2). By increasing the number of new infections to 250, this resulted in a substantial increase in the chance of observing at least one cluster with size ≥30: 0.455 (HK) 0.189 (JP) and 0.929 (SG) (Supplementary Figure S2). By keeping the number of new infections unchanged and increasing the minimum cluster size from 30 to 120, the chances of observing one such SSE were smaller than 0.015 in all three populations (Supplementary Figure S2).
Discussion
SSEs can change the nature of an epidemic, so it is useful for public health teams to determine whether an ongoing outbreak has any contribution from them, which may be amenable to interventions. To make this assessment, we applied a previously published method to publicly available data (the empirical transmission cluster sizes) of laboratory-confirmed COVID-19 cases in HK, JP and SG to estimate epidemiological parameters (R 0, k) [3]. This assessment improves our understanding of the dispersion of clustered COVID-19 cases, but it does rely on a certain level of detail when recording new cases and linking them to existing clusters.
Our results suggest that SARS-CoV-2 transmission in HK, JP and SG was not over-dispersed as of March 3rd, 2020 (with relatively large values of k), so there was no strong evidence for the presence of SSEs. Values of R 0 may impact on the degree of correlation between p n and k, making the prediction of the presence or absence of SSEs more variable. For R 0, our estimates (0.48–0.70) were much lower than those in China (2.20–2.68) [4,5]. The observed smaller R 0 in HK, JP and SG may be due to the early enactment of social distancing interventions or the high level of adoption of personal protective measures [6,7]. However, for given values of R 0, k and the final cluster size, increasing numbers of new infections increase the chance of observing clusters of epidemiologically linked cases (Supplementary Figure S2). We should therefore be cautious about the emergence of SSEs due to the increasing number of cases as COVID-19 progresses.
The results of this study highlight the importance of social distancing to combat COVID-19. With the probability of observing secondary case clusters of size ≥4 ranging from 0.106 to 0.215 and with a large proportion of households with size <4 (HK: 71%; JP: 60%; SG: 60%), there are transmission chains that extend beyond households into the community (Figure 2). Governments have enacted different levels of non-pharmaceutical interventions (NPIs). HK, SG and JP have avoided crowding (such as mandatory cancellation of mass gatherings), closed schools, conducted contact tracing, and quarantined seemingly healthy individuals exposed to COVID-19 [6]. Both HK and SG enacted social distancing as soon as cases were first reported in the regions, whereas JP initiated these measures when the number of local cases reached 163 (excluding the international conveyance) in late February 2020 [6]. With possible transmission in the community, stay-at-home order becomes a core social distancing measure, which confines SARS-CoV-2 within households at the expense of increased risk of infection among household members, particularly in the dense migrant dormitory in SG. Therefore, setting-wide wearing of face masks is recommended for cramped residential areas [8]. Besides the community, nosocomial outbreaks are possible as foretold by the past experience in SARS and featured by the containment delay after hospitalization experienced by COVID-19 cases [1,9]. Therefore, heightened measures in isolating suspected inpatients should be enforced in hospitals.
Combinations of interventions were effective in mitigating past pandemics, and they have been widely adopted to combat COVID-19 even though the relative contributions of some individual measures are obscure [10]. Therefore, determining the optimal set of non-pharmaceutical interventions remains a challenge. The resurgence of COVID-19 cases following the lifting of NPIs may prompt the emergence of SSEs, triggering a cascade of infections that is difficult to interrupt. To this end, further research is warranted to unravel predictors for SSEs, such as older age as observed in other diseases [1].
Acknowledgement
We thank Li Ka Shing Institute of Health Sciences for technical support.
Conflict of interest statement
None declared.
Funding sources
Research Fund for the Control of Infectious Diseases, Hong Kong (Number: INF-CUHK-1); General Research Fund (Number: 14112818); Health and Medical Research Fund (Ref.: 18170312); Wellcome Trust (UK, 200861/Z/16/Z).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.05.027.
Contributor Information
K.O. Kwok, Email: kkokwok@cuhk.edu.hk.
J.W.T. Tang, Email: Julian.tang@uhl-tr.nhs.uk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shen Z., Ning F., Zhou W., He X., Lin C., Chin D.P. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10:256–260. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun B.C. Understanding and modeling the super-spreading events of the Middle East respiratory syndrome outbreak in Korea. Infect Chemother. 2016;48:147–149. doi: 10.3947/ic.2016.48.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kucharski A.J., Althaus C.L. The role of superspreading in Middle East respiratory syndrome coronavirus (MERS-CoV) transmission. Euro Surveill. 2015;20:14–18. doi: 10.2807/1560-7917.es2015.20.25.21167. [DOI] [PubMed] [Google Scholar]
- 4.Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Euro Surveill. 2020;25(4) doi: 10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu J.T., Leung K., Leung G.M. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imai N., Gaythorpe K.A.M., Abbott S., Bhatia S., Sv Elsland, Prem K. Adoption and impact of non-pharmaceutical interventions for COVID-19. Wellcome Open Res. 2020 doi: 10.12688/wellcomeopenres.15808.1. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7255913/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwok K.O., Li K.K., Chan H.H.H., Yi Y.Y., Tang A., Wei W.I. Community responses during early phase of COVID-19 epidemic, Hong Kong. Emerg Infect Dis. 2020;26(7) doi: 10.3201/eid2607.200500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng V., Wong S., Chuang V., So S., Chen J., Sridhar S. The role of community-wide wearing of face mask for control of coronavirus disease 2019 (COVID-19) epidemic due to SARS-CoV-2. J Infect. 2020;81(1):107–114. doi: 10.1016/j.jinf.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwok K.O., Wong V.W.Y., Wei W.I., Wong S.Y.S., Tang J.W. Epidemiological characteristics of the first 53 laboratory-confirmed cases of COVID-19 epidemic in Hong Kong, 13 February 2020. Euro Surveill. 2020;25(16) doi: 10.2807/1560-7917.ES.2020.25.16.2000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson N.M., Cummings D.A., Fraser C., Cajka J.C., Cooley P.C., Burke D.S. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.