Abstract
We report the first two cases of Coronavirus Disease 2019 (COVID-19) who were receiving intensive care including favipiravir, and were clinically diagnosed with neuroleptic malignant syndrome (NMS) to focus attention on NMS in COVID-19 management. Case 1: A 46-year-old-man with acute respiratory distress syndrome (ARDS) caused by COVID-19 infection was being administered favipiravir. Fentanyl, propofol, and rocuronium were also given. On day 3, midazolam administration was initiated for deep sedation. On day 5, his high body temperature increased to 41.2 °C, creatine kinase level elevated, and he developed tachycardia, tachypnea, altered consciousness, and diaphoresis. NMS was suspected, and supportive therapy was initiated. High-grade fever persisted for 4 days and subsided on day 9. Case 2: A 44-year-old-man with ARDS caused by COVID-19 infection was being treated with favipiravir. On day 5, risperidone was started for delirium. On day 7, his body temperature suddenly increased to 40.8 °C, his CK level elevated, and he developed tachycardia, tachypnea, altered consciousness, and diaphoresis. NMS diagnosis was confirmed, and both, favipiravir and risperidone were discontinued on day 8. On the same day, his CK levels decreased, and his body temperature normalized on day 9.
Patients with COVID-19 infection frequently require deep sedation and develop delirium; therefore, more attention should be paid to the development of NMS in patients who are being administered such causative agents. The mechanism underlying the occurrence of NMS in COVID-19 patients treated with favipiravir remains unknown. Therefore, careful consideration of NMS development is necessary in the management of COVID-19 patients.
Keywords: COVID-19, Neuroleptic malignant syndrome, Fever
1. Introduction
The mortality of patients with Coronavirus Disease 2019 (COVID-19) who were receiving mechanical ventilation in Japan is considerably lower than that in the United States and European countries, possibly because of the intensive care provide in Japan that included the use of favipiravir to battle the exponential rise in the number of infections [[1], [2], [3]]. Favipiravir has been investigated for its efficacy against influenza [4], and has been widely used for COVID-19 patients who were receiving mechanical ventilation in observational studies in Japan.
Patients with acute respiratory distress syndrome (ARDS) associated with COVID-19 require deep sedation, neuromuscular blockade, and steroid administration in some instances [5], and family visits are sometimes restricted owing to the high-risk of infection spread involved in contact with COVID-19 patients [6]. Thus, patients with COVID-19 require sedatives and frequently develop delirium that is treated with the administration of major tranquilizers, such as risperidone. In such clinical situations, we treated two COVID-19 cases in patients who had high body temperature with increased serum creatine kinase (CK) during intensive care that included the administration of favipiravir.
We report the first two cases of COVID-19 that were clinically diagnosed with neuroleptic malignant syndrome (NMS) to focus attention on NMS in COVID-19 management.
2. Case presentation
2.1. Case 1
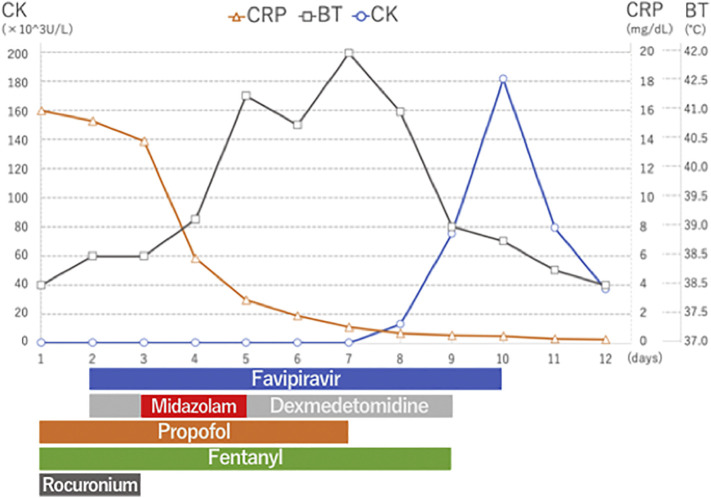
A 46-year-old-man with ARDS caused by COVID-19 infection was receiving mechanical ventilation and was being administered favipiravir (1800 mg ∗ 2 on the first day followed by 800 mg ∗ 2/day for the subsequent 9 days), mPSL (1 g/day for 3 days), and antibiotics ceftriaxone (CTRX) 2 g/day and azithromycin (AZM) 500 mg/day for 3 days. Fentanyl, propofol, and rocuronium were also given. On day 3, midazolam administration was initiated for deep sedation; however, it was discontinued on day 5 because of light sedation after oxygenation improved. On day 5, his high body temperature increased to 41.2 °C, creatine kinase level elevated, and he developed tachycardia, tachypnea, altered consciousness, and diaphoresis. NMS was suspected, and supportive therapy was initiated. His breathing stabilized, and extubation was performed on day 8. High-grade fever persisted for 4 days and subsided on day 9. Blood test performed on day 9 showed severely increased CK level (181,650 U/L) and acute kidney injury (Cre 2.6 mg/dL). Thereafter, the CK level decreased gradually, and his condition normalized. Supplemental oxygen was weaned off, and he was admitted to the general ward for rehabilitation therapy (see Fig. 1 ).
Fig. 1.
Hospital course in case 1.
CRP; c-reactive protein, BT; body temperature, CK; creatinine kinase.
2.2. Case 2
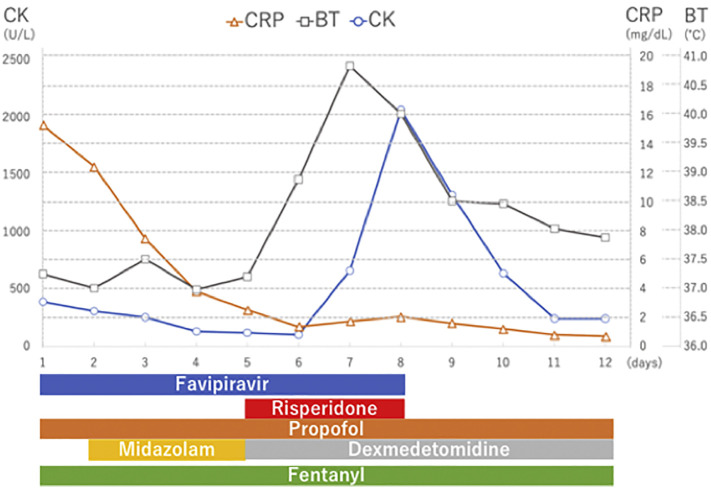
A 44-year-old-man with ARDS caused by COVID-19 infection was receiving mechanical ventilation and was being treated with favipiravir (1800 mg ∗ 2 on the first day, followed by 800 mg ∗ 2/day for 8 days), mPSL (100 mg/day for 3 days), and antibiotics [tazobactam/piperacillin (TAZ/PIPC) and azithromycin (AZM)]. On day 5, risperidone was started for delirium. On day 7 (10 day after the development of high-grade fever in case 1), his body temperature suddenly increased to 40.8 °C, his CK level elevated, and he developed tachycardia, tachypnea, altered consciousness, and diaphoresis. NMS diagnosis was confirmed, and both, favipiravir and risperidone were discontinued on day 8. On the same day, his CK levels decreased, and his body temperature normalized on day 9. On day 13, tracheostomy was performed, and mechanical ventilation was weaned off on day 22, and his condition was stabilized (see Fig. 2 ).
Fig. 2.
Hospital course in case 2.
CRP; c-reactive protein, BT; body temperature, CK; creatinine kinase.
3. Discussion
Two patients with COVID-19 infection who were receiving mechanical ventilation fulfilled Levenson's criteria for the diagnosis of NMS [7]. Causative agents, such as midazolam and fentanyl were discontinued in Case 1. Following the experience of case 1, risperidone was discontinued quickly in case 2, thus preventing the worsening of the condition.
Several cases of NMS caused by midazolam use have been reported in Japan [8], and risperidone-induced NMS has been commonly reported [9]. However, the short duration of midazolam use did not allow us to exclude it as the possible cause of NMS. Risperidone was administered in case 2 for delirium control and was discontinued earlier in case 2, on the basis of the experience of case 1 in terms of NMS. Patients with COVID-19 infection frequently require deep sedation and develop delirium; therefore, more attention should be paid to the development of NMS in patients who are being administered such causative agents.
With regard to the association of favipiravir administration (favipiravir is a potent and selective RNA-dependent RNA polymerase inhibitor) and NMS development, it is considered that favipiravir could cause rhabdomyolysis because patients with influenza treated with favipiravir exhibited increased CK levels [10]. However, the direct association of favipiravir with NMS remains unknown. In the current two cases, favipiravir was discontinued on day 10 in case 1 and on day 8 in case 2. Consequently, fever subsided in both the cases in conjunction with other therapies. The number of COVID-19 positive patients being treated with favipiravir is increasing not only in Japan, but also across the world, including countries like China and the United States [11,12]; therefore, further research on the association between favipiravir use and NMS development in COVID-19 patients is warranted.
COVID-19 infection could cause rhabdomyolysis as a late complication [13]. In the current two cases, the serum CRP levels that reflected inflammation remained low with the rise in body temperature; therefore, the possibility of COVID-19-induced rhabdomyolysis concomitant with increased inflammation is considered less likely.
4. Conclusion
The mechanism underlying the occurrence of NMS in COVID-19 patients who are receiving intensive care and are being treated with favipiravir remains unknown. Therefore, careful consideration of NMS development is necessary in the management of COVID-19 patients.
Funding
None.
References
- 1.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020 doi: 10.1001/jama.2020.677. Published online April 22, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diseases hJAfI Case presentations of COVID-19. http://www.kansensho.or.jp/modules/topics/index.php?content_id=31 at.
- 4.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209 doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhazzani W., Moller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving Sepsis campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosa R.G., Falavigna M., da Silva D.B., Sganzerla D., Santos M.M.S., Kochhann R. Effect of flexible family visitation on delirium among patients in the intensive care unit: the ICU visits randomized clinical trial. JAMA. 2019;322:216–228. doi: 10.1001/jama.2019.8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levenson J.L. Neuroleptic malignant syndrome. Am J Psychiatry. 1985;142:1137–1145. doi: 10.1176/ajp.142.10.1137. [DOI] [PubMed] [Google Scholar]
- 8.N M A case of neuroleptic malignant syndrome associated with midazolam administration. Chiryou. 2009;91:1996–1999. [Google Scholar]
- 9.Bajjoka I., Patel T., O'Sullivan T. Risperidone-induced neuroleptic malignant syndrome. Ann Emerg Med. 1997;30:698–700. doi: 10.1016/s0196-0644(97)70091-x. [DOI] [PubMed] [Google Scholar]
- 10.Evaluation and licensing division PaFSBMoH, labour and welfareAvigan Tablet. 2014;200 mg. [Google Scholar]
- 11.Study of the use of Favipiravir in hospitalized subjects with COVID-19. https://clinicaltrials.gov/ct2/show/NCT04358549?term=Favipiravir&cond=COVID&draw=2&rank=4 at.
- 12.Favipiravir combined with Tocilizumab in the treatment of Corona Virus Disease 2019. https://clinicaltrials.gov/ct2/show/NCT04310228?term=Favipiravir&cond=COVID&draw=2&rank=8 at.
- 13.Jin M., Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]