Abstract
COVID-19 is the pandemic that hit the world starting December 2019. Recent studies and international statistics have shown an increased prevalence, morbidity as well as mortality of this disease in male patients compared to female patients. The aim of this brief communication is to describe the pathophysiology of this sex-discrepancy, based on the infectivity mechanism of the coronavirus including the Angiotensin-Converting Enzyme 2 (ACE2), the Type II transmembrane Serine Protease (TMPRSS2), and the androgen receptor. This could help understand the susceptibility of urological patients, especially those receiving androgen deprivation therapy for prostate cancer, and testosterone replacement therapy.
Keywords: COVID-19, Angiotensin-Converting Enzyme 2, Type II Transmembrane Serine Protease, androgen receptor
Résumé
La COVID-19 est la pandémie mondiale apparue en décembre 2019. Les études récentes et les statistiques internationales ont montré une nette prédominance de la prévalence de cette maladie ainsi que de sa morbidité et mortalité chez les hommes, comparés aux femmes. Le but de cette communication brève est d’exposer la physiopathologie de cette possible différence de sexe, en se basant sur le mécanisme de l’infectivité du virus incluant l’enzyme de conversion de l’angiotensine de type 2 (ACE2), la sérine protéase transmembranaire de type II (TMPRSS2), et le récepteur aux androgènes. Ceci pourrait justifier une étude de la susceptibilité des patients urologiques, surtout ceux recevant une déprivation androgénique pour cancer de prostate, ou une supplémentation en testostérone.
Mots clés: COVID-19, Enzyme de conversion de l’angiotensine 2, Sérine protéase transmembranaire 2, Récepteur aux androgènes
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2) is the virus responsible for the coronavirus disease of 2019 (COVID-19), declared a pandemic by the World Health Organization (WHO). International efforts are converging into depicting the pandemic's clinical course, epidemiology, prevention and treatment. Recent studies have reported that men are more affected by the disease than women, and a higher percentage of COVID-19–related morbidity, intensive care admission and mortality is found among men compared to women [1]. The basis for this discrepancy has not been established yet.
In general, differences in lifestyle and behavior exist between sexes. Females have a lower prevalence of smoking and cardiovascular diseases, which are associated with a worse prognosis for COVID-19 patients. Moreover, literature has uncovered a female predominance of anxiety disorders, especially illness and health anxiety [2], thus maybe resulting in more restrictive activities and social distancing in women.
Specific mechanisms explaining this discrepancy exist for the coronavirus family, since this sex-difference is not found in other virus families. Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) outbreaks have also shown a male predominance in disease susceptibility [3]. Male mice infected experimentally with SARS-CoV were more susceptible than female mice, and ovariectomy or estrogen receptor antagonists increased female mice mortality, thereby concluding that estrogen might have protective effect against coronavirus infection [4].
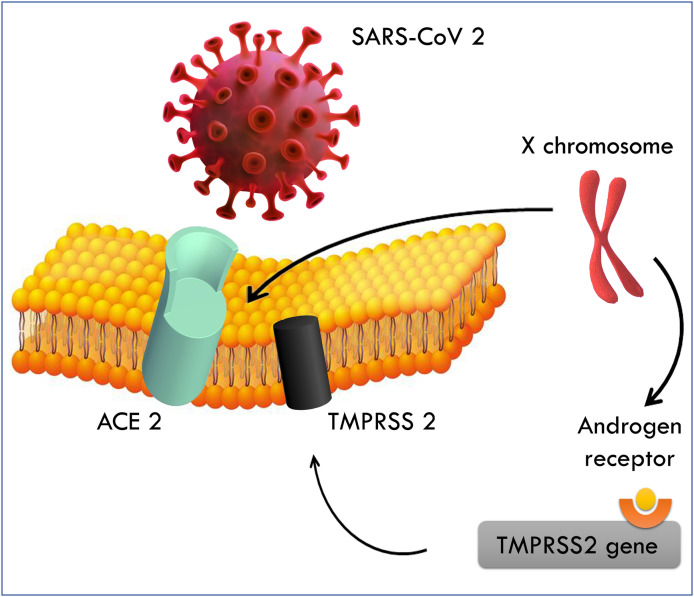
Yet, the viral pathogenicity itself could be accountable for this male-female difference in susceptibility for COVID-19. Coronavirus infectivity depends on its entry via binding of its viral spike (S) protein to Angiotensin-Converting Enzyme 2 (ACE2) receptor, and on S protein priming by the Type II Transmembrane Serine Protease (TMPRSS2) (Fig. 1 ).
Figure 1.
Pathway for SARS-CoV-2 virus infection mediated by androgen activity
First, ACE2, which is known to be a blood pressure regulator through the renin-angiotensin-aldosterone system, is a functional receptor for SARS-CoV2 allowing human cell entry and viral infectivity. ACE2 is not only found in the lungs resulting in respiratory symptoms and lung injury of the SARS-CoV2, but also in many other organs including kidneys, prostate and intestines, suggesting other possible routes of viral transmission, and testes, hence possibly requiring a long-term follow-up of the reproductive function in males. While some authors reported the absence of SARS-CoV2 in the semen of males recovering from COVID-19 [5], others have shown its presence in the semen of active and recovering patients. [6].
The virus susceptibility is affected by ACE2 expression, which could be influenced by several factors such as smoking [7] and renin-angiotensin-aldosterone system inhibitors [8]. Some authors suggest that the increased morbidity and mortality from the virus in male patients is attributed to an increased expression of ACE2 in males [9], especially that the ACE2 gene is located on the X chromosome. Others did not find a significant difference in ACE2 expression between males and females, making this hypothesis less probable [7]. More studies are needed to determine any incriminated role of ACE2 overexpression in COVID-19 susceptibility in men.
Second, the role of TMPRSS2 is to activate the spike protein and facilitate viral entry. Notably, the TMPRSS2 gene has been reported in the pathophysiology of prostate cancer, when fused with the oncogenic transcription factor ERG. ERG is normally regulated by androgens, and the gene fusion juxtaposes the androgen receptor elements of these two genes; ERG gene is therefore controlled by androgen receptor signaling [10].
Since SARS-CoV and SARS-CoV-2 have a similar molecular structure, their viral pathogenesis is likely similar. Interestingly, TMPRSS2 gene expression is promoted through androgen receptor only (since no other regulatory elements have been reported to date), and increases upon exposure to androgens [11]. Several androgen receptor elements (ARE) are located upstream of the transcription start site and the first intron of TMPRSS2 gene [12].
This upregulation of TMPRSS2 by androgens could explain the increased susceptibility to COVID-19 in men. This pathophysiology could also explain the less symptomatic disease in children who have low expression of the androgen receptor.
However, a recent study has shown that the constitutive TMPRSS2 expression in lung tissue does not differ between men and women [13], and low levels of androgens in women could be enough to sustain this TMPRSS2 expression in lungs. Song et al. have reported a small percentage of TMPRSS2 and ACE2 co-expression in prostate, and a higher co-expression in pneumocytes in males compared to females [14].
Given that TMPRSS2 levels are influenced by androgens not only in the prostate but also in the lung, Montopoli et al. have shown recently that a significantly lower risk of COVID-19 was found in patients with prostate cancer receiving androgen deprivation therapy (ADT) compared to those who did not receive ADT [15]. Therefore, ADT could give partial protection from SARS-CoV2 infections, while taking into account that cancer itself is a negative prognosticator in COVID-19 [15]. More studies are needed to confirm these findings.
Studying prevalence of COVID-19 and its morbidity and mortality, as well as TMPRSS2 protein expression in lung tissue of patients with prostate cancer receiving ADT, females with polycystic ovary syndrome (a baseline increased androgen status), patients with congenital adrenal hyperplasia (21-hydroxylase deficiency), and patients on testosterone therapy, might lead to a better understanding of this mechanism. TMPRSS2 inhibitors are now being tested on SARS-CoV2 (ClinicalTrials.gov, NCT04321096).
All these assumptions could help us depict underlying explanations for the difference found for morbidity and mortality of the novel COVID-19 pandemic between male and female patients. Understanding this discrepancy could help depict the disease pathophysiology in order to develop promising targeted therapies or vaccines. Future studies must further assess the specific susceptibility of urological patients, especially prostate cancer patients receiving androgen deprivation therapy, and patients receiving testosterone replacement therapy.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet Lond Engl. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahrami F., Yousefi N. Females are more anxious than males: a metacognitive perspective. Iran J Psychiatry Behav Sci. 2011;5(2):83–90. [PMC free article] [PubMed] [Google Scholar]
- 3.Karlberg J., Chong D.S.Y., Lai W.Y.Y. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. 2004;159(3):229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol Baltim Md. 1950 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song C., Wang Y., Li W., Hu B., Chen G., Xia P. Detection of 2019 novel coronavirus in semen and testicular biopsy specimen of COVID-19 patients. medRxiv. 2020 2020.03.31.20042333. [Google Scholar]
- 6.Li D., Jin M., Bao P., Zhao W., Zhang S. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3(5):e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith J.C., Sheltzer J.M. Cigarette smoke triggers the expansion of a subpopulation of respiratory epithelial cells that express the SARS-CoV-2 receptor ACE2. bioRxiv. 2020 2020.03.28.013672. [Google Scholar]
- 8.Sama I.E., Ravera A., Santema B.T., van Goor H., Ter Maaten J.M., Cleland J.G.F. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin J.-M., Bai P., He W., Wu F., Liu X.-F., Han D.-M. Gender differences in patients with COVID-19: focus on severity and mortality. Infectious Diseases (except HIV/AIDS) 2020 doi: 10.1101/2020.02.23.20026864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas J.M., True L., Hawley S., Matsumura M., Morrissey C., Vessella R. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. J Pathol. 2008;215(2):118–125. doi: 10.1002/path.2330. [DOI] [PubMed] [Google Scholar]
- 11.Lin B., Ferguson C., White J.T., Wang S., Vessella R., True L.D. Prostate-localized and Androgen-regulated Expression of the Membrane-bound Serine Protease TMPRSS2. Cancer Res. 1999;59(17):4180–4184. [PubMed] [Google Scholar]
- 12.Shen L.W., Mao H.J., Wu Y.L., Tanaka Y., Zhang W. TMPRSS2: a potential target for treatment of influenza virus and coronavirus infections. Biochimie. 2017;142:1–10. doi: 10.1016/j.biochi.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stopsack K.H., Mucci L.A., Antonarakis E.S., Nelson P.S., Kantoff P.W. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song H., Seddighzadeh B., Cooperberg M.R., Huang F.W. Expression of ACE2, the SARS-CoV-2 Receptor, and TMPRSS2 in Prostate Epithelial Cells. Eur Urol. 2020 doi: 10.1016/j.eururo.2020.04.065. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7200365/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (n=4532) Ann Oncol Off J Eur Soc Med Oncol. 2020 doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]