Highlight
-
•
This is the first study comparing tocilizumab to standard of care in severe COVID-19
-
•
Tocilizumab in severe COVID-19 patients did influence 28-day clinical outcomes
-
•
Tocilizumab safety was satisfactory except for ICU-admitted patients
Keywords: Tocilizumab, COVID-19, Coronavirus, Safety, Efficacy, Interleukin-6, Italy
Abstract
Background
Tocilizumab (TCZ), a humanized monoclonal antibody targeting the interleukin-6 (IL-6) receptor, has been proposed for the treatment of COVID-19 patients; however, limited data are available on the safety and efficacy.
Methods
We performed a retrospective study on severe COVID-19 patients with hyper-inflammatory features admitted outside intensive care units (ICUs). Patients treated with intravenous TCZ in addition to standard of care were compared to patients treated with standard of care alone. Safety and efficacy were assessed over a 28-day follow-up.
Results
65 patients were included. Among them, 32 were treated with TCZ. At baseline, all patients were on high-flow supplemental oxygen and most (78% of TCZ patients and 61% of standard treatment patients) were on non-invasive ventilation. During the 28-day follow-up, 69% of TCZ patients experienced a clinical improvement compared to 61% of standard treatment patients (p = 0.61). Mortality was 15% in the tocilizumab group and 33% in standard treatment group (p = 0.15). In TCZ group, at multivariate analysis, older age was a predictor of death, whereas higher baseline PaO2:FiO2 was a predictor of clinical improvement at day 28. The rate of infection and pulmonary thrombosis was similar between the two groups.
Conclusions
At day 28, clinical improvement and mortality were not statistically different between tocilizumab and standard treatment patients in our cohort. Bacterial or fungal infections were recorded in 13% of tocilizumab patients and in 12% of standard treatment patients. Confirmation of efficacy and safety will require ongoing controlled trials.
1. Introduction
Starting from December 2019, the World has faced a global pandemic of a novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. As of May 2nd, 2020, the pandemic has affected more than 3.400.000 people worldwide [2]. The Lombardy region in Italy has become the epicentre of the European COVID-19 outbreak, and an exponential surge in COVID-19 patients posed a critical burden on the National Health System [3,4]. To date, no pharmacologic therapy has been approved for the treatment of COVID-19.
Tocilizumab is a humanized monoclonal antibody which selectively targets the interleukin-6 (IL-6) receptor. It is currently approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, and giant cell arteritis [5]. Recently, tocilizumab has become one of the therapeutic options for the management of cytokine release syndrome (CRS), a life-threatening complication of chimeric antigen receptor (CAR)- T cell therapy [6]. CRS is the consequence of uncontrolled immune activation with release of pro-inflammatory cytokines and chemokines (e.g., IL-1β, IL-6, IL-18, and monocyte chemoattractant protein-10) [7]. Since a proportion of hospitalized patients with respiratory failure due to COVID-19 develop clinical and laboratory features reminiscent of CRS (including high fever, intense fatigue and myalgia, and elevated serum inflammatory markers C-reactive protein, ferritin, and IL-6) [8,9], it was hypothesized that timely inhibition of inflammation with tocilizumab could be clinically effective for this population [10]. So far, the experience with tocilizumab in COVID-19 patients is limited [11], [12], [13], [14]. Despite preliminary encouraging results, studies suffered from the lack of a standardized therapeutic scheme, a short post-treatment follow-up, and the absence of a comparison group. Here, we compare the outcomes at 28 days of a large cohort of patients with severe COVID-19 pneumonia treated with tocilizumab in addition to standard management, with those of concomitantly hospitalized patients who received standard management only.
2. Methods
2.1. Patients and setting
Patients hospitalized for COVID-19 at San Raffaele Hospital, Milan, Italy are recruited in an Institutional observational protocol (COVID-BioB Study, Ethical Committee approval no. 34/int/2020, ClinicalTrials.gov NCT04318366). All patients gave written informed consent to data collection and to compassionate use of tocilizumab.
2.2. Eligibility criteria
Eligibility criteria for tocilizumab administration were: a diagnosis of COVID-19 confirmed upon reverse-transcriptase Polymerase Chain Reaction (RT-PCR) positivity for SARS-CoV-2 on nasopharyngeal swab; hyper-inflammation defined as elevation in either C-reactive protein (CRP, ≥ 100 mg/L, normal values <6 mg/L) or ferritin (≥ 900 ng/mL, normal value <400 ng/mL), in the presence of increased lactate dehydrogenase (LDH, > 220 U/L); severe respiratory involvement defined by typical radiological findings at chest X-ray and/or computed tomography (CT) scan, in the presence of an oxygen saturation (SaO2) ≤92% while breathing ambient air or a ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2) (PaO2:FiO2) ≤300 mmHg [15]. Exclusion criteria were: evidence of concomitant bacterial infection, history of diverticular disease, neutropenia < 1500 109 cells/L, concomitant use of other immunosuppressive biologic drugs, baseline elevation of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels > 5-fold the upper limit of the normal range. No concomitant corticosteroid therapy was allowed.
2.3. Treatment
All patients received the same background treatment, following an Institutional protocol for standard of care: hydroxychloroquine 400 mg daily, lopinavir/ritonavir 400/100 mg twice daily, ceftriaxone 2 gr for 6 days, azithromycin 500 mg daily until a negative report of urine antigen for L. pneumophila, anti-coagulation prophylaxis with enoxaparin 4000 UI subcutaneously once a day.
Tocilizumab was administrated intravenously at a dose of 400 mg. A second dose of 400 mg of tocilizumab was given after 24 hours in case of respiratory worsening (defined as need to increase FiO2, to start Non-invasive Ventilation (NIV), or to start mechanical ventilation) after the first tocilizumab infusion. Due to a shortage of tocilizumab in Italy, only patients who were admitted between March 13th and March 19th, 2020 were treated with tocilizumab. Patients admitted to hospital before or after the time period of tocilizumab availability who retrospectively fulfilled eligibility criteria for tocilizumab treatment were used as a comparison group. These patients received the same Institutional standard treatment outside ICU, did not receive anti-inflammatory drugs or glucocorticoids, and were not enrolled in other clinical trials.
2.4. Study population
The following baseline features were analysed: age, sex, duration of symptoms, comorbidities (smoking history, chronic kidney disease, arterial hypertension, cancer, type 2 diabetes mellitus, coronary artery disease and chronic obstructive pulmonary disease), baseline clinical status (PaO2:FiO2 ratio, need for supplementary oxygen therapy, need for NIV, body temperature), and serum inflammatory markers (CRP, ferritin, LDH).
2.5. Outcomes
Patients’ clinical status was assessed daily using a six-category ordinal scale, similar to previous published studies of COVID-19 [16], [17]. The categories were defined as follows: 1) patient discharged, 2) hospitalization not requiring supplemental oxygen, 3) hospitalization requiring supplemental low-flow oxygen (FiO2 < 40%), 4) hospitalization requiring high-flow supplemental oxygen (FiO2 ≥ 40%) and/or NIV, 5) hospitalization requiring invasive mechanical ventilation or Extra-Corporeal Membrane Oxygenation, 6) death. Overall survival and the proportion of patients with clinical improvement, defined as live discharge from hospital or decrease of at least 2 points from baseline on the six-category ordinal scale at 28 days, were assessed. Predictors of mortality and clinical improvement were analysed in tocilizumab patients.
2.6. Monitoring of adverse events
The occurrence of adverse events was recorded on a daily basis, with a focus on: bacterial or fungal infections, elevation of AST or ALT level > 3x the upper limit of normal range, neutropenia < 1500 109 cells/L, pulmonary thrombosis, and pneumothorax.
2.7. Statistical analysis
Data were analysed using SPSS version 22.0 (SPSS, Chicago, IL-USA). Continuous variables are reported as medians and interquartile ranges. Categorical variables are reported as numbers and percentages. Wilcoxon rank-sum tests were applied to continuous variables and two-tailed Fisher's exact tests were used for categorical variables. Survival analysis was performed with the Kaplan-Meier approach, log-rank test was used to compare survival curves. Survival and clinical and laboratory features at baseline were analysed using proportional hazard Cox regression models. Results of the Cox regression model are presented as hazard ratio (HR) with 95% confidence interval. P-values <0.05 were considered statistically significant.
3. Results
3.1. Baseline patients’ characteristics
A total of 65 severe COVID-19 pneumonia patients were included in the study. 32 were treated with tocilizumab as per our study protocol, whereas 33 received only the Institutional standard of care. Baseline characteristics of both cohorts are summarized in Table 1 . Median age was similar in tocilizumab and standard of treatment group: 65 (53 – 75) compared to 60 (55 – 75.5) years respectively (p = 0.52). The vast majority of patients were male (91% in tocilizumab and 82% in standard of treatment group, p = 0.47). At baseline, most patients were on NIV (78% in tocilizumab and 61% in standard of treatment group, p = 0.18), whereas all remaining patients were on high-flow supplemental oxygen.
Table 1.
Baseline clinical and laboratory features of COVID-19 patients treated with tocilizumab compared with patients treated with standard treatment.
| Patients features | Tocilizumab (32 patients) | Standard treatment (33 patients) | p-value |
|---|---|---|---|
| Age (years), median (IQR) | 64 (53 – 75) | 60 (55 – 75.5) | 0.52 |
| Sex (male), n (%) | 29 (91) | 27 (82) | 0.47 |
| Duration of symptoms (days), median (IQR) | 11 (8 – 14) | 9 (8 – 10) | 0.14 |
| PaO2:FiO2 ratio, median (IQR) | 107 (82 – 181) | 124 (91 – 172) | 0.40 |
| NIV, n (%) | 25 (78) | 20 (61) | 0.18 |
| FiO2, median (IQR) | 60 (60 – 80) | 80 (50 – 80) | 0.96 |
| Body temperature (°C), median (IQR) | 37.6 (37.0 – 38.3) | 38.2 (37.3 – 38.6) | 0.06 |
| CRP (mg/L), median (IQR) | 156 (100 – 208) | 169 (98 – 226) | 0.63 |
| LDH (U/L), median (IQR) | 469 (362 – 548) | 479 (394 – 591) | 0.26 |
| Ferritin (ng/mL), median (IQR) | 1400 (1027 – 2777) | 1448 (793 – 4131) | 0.88 |
| Comorbidities, n (%) | 20 (62) | 20 (61) | |
| Smoking | 0 | 2 (6) | 0.49 |
| CKD | 3 (9) | 5 (15) | 0.71 |
| Arterial hypertension | 12 (37) | 16 (48) | 0.32 |
| COPD | 1 (3) | 2 (6) | 0.61 |
| Cancer | 2 (6) | 1 (3) | 0.61 |
| T2DM | 4 (12) | 6 (18) | 0.51 |
| CAD | 4 (12) | 6 (18) | 0.51 |
NIV = non-invasive ventilation. CRP = C-reactive protein. LDH = lactate dehydrogenase. CKD = chronic kidney disease. COPD = chronic obstructive pulmonary disease. T2DM = type-2 diabetes mellitus. CAD = coronary artery disease.
3.2. Tocilizumab treatment
Patients included in the tocilizumab group received a first intravenous dose of 400 mg of tocilizumab. In 9 (28%) patients a second intravenous dose of 400 mg of tocilizumab was administered due to progressive respiratory worsening. Seven of these patients (78%) where on NIV at baseline. One patient could not be treated with a second dose of tocilizumab because he died within the first 12 hours after the first tocilizumab infusion.
3.3. Survival and clinical outcome
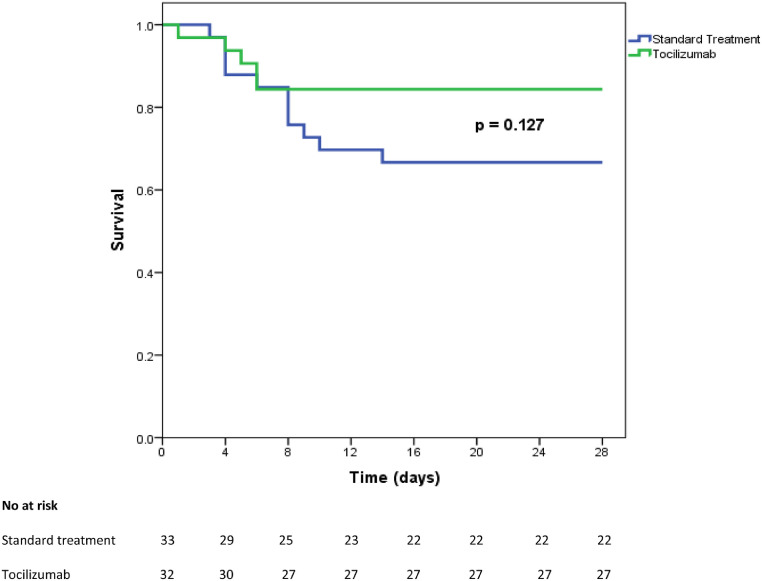
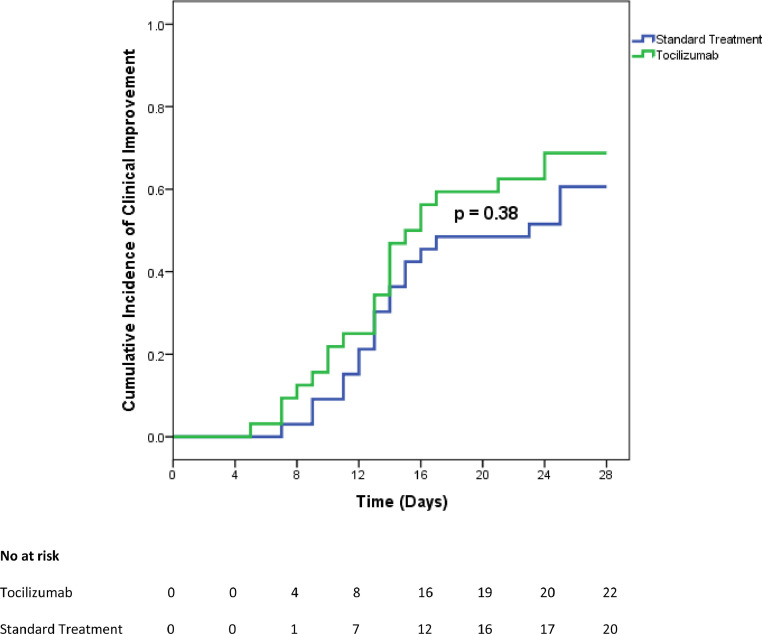
By day 28, 5 (16%) patients in the tocilizumab group compared to 11 (33%) patients in the standard of treatment group died (p = 0.150). Survival curves are presented in Fig. 1 . Median time to death was 5 (4 - 6) days after first tocilizumab infusion. Four (13%) patients in the tocilizumab group and two (6%) patients in the standard treatment group required mechanical ventilation, p = 0.43. Tocilizumab-treated patients were admitted to ICU and intubated within the first 24 hours after the first tocilizumab infusion, received a second dose while in the ICU, and were extubated and discharged from the ICU by day 13, after a median time of 8.5 (5 – 11) days. The two patients in the standard treatment group were admitted to the ICU at day 2 and 3 of follow up; one of these patients died within 24 after ICU admission while the other was discharged after 9 days. Twenty (63%) patients in the tocilizumab group compared to 16 (49%) patients in the standard treatment group were discharged from the hospital (p = 0.32), with a similar median time to discharge: 13.5 (10 – 16.7) for tocilizumab versus 14 (12 – 15.5) days for standard of treatment patients (p = 0.99). Outcomes at day 28 are summarized in Table 2 . Clinical improvement was reached in 22 (69%) patients of the tocilizumab group and in 20 patients (61%) of the standard treatment group, p = 0.61. Incidence of clinical improvement curves in tocilizumab and standard treatment groups are presented in Fig. 2 .
Fig. 1.
Overall survival curves from baseline to day 28 in tocilizumab and standard treatment severe COVID-19 patients.
Table 2.
Clinical status of patients at baseline and at day 28 according to the six-category ordinal scale in tocilizumab and standard treatment patients.
| Clinical status | Baseline TCZ | Baseline ST | Day 28 TCZ | Day 28 ST |
|---|---|---|---|---|
| 1. Discharged from hospital, n (%) | 0 | 0 | 20 (63) | 16 (48) |
| 2. Hospitalization, not requiring supplemental O2, n (%) | 0 | 0 | 2 (6) | 2 (6) |
| 3. Hospitalization, requiring supplemental low-flow O2, n (%) | 0 | 0 | 2 (6) | 2 (6) |
| 4. Hospitalization, requiring NIV and/or high-flow supplemental O2, n (%) | 32 (100) | 33 (100) | 3 (9) | 1 (3) |
| 5. Hospitalization, requiring invasive mechanical ventilation or ECMO, n (%) | 0 | 0 | 0 | 1 (3) |
| 6. Death, n (%) | 0 | 0 | 5 (16) | 11 (33) |
NIV = non-invasive ventilation. ECMO = extracorporeal membrane oxygenation. TCZ = tocilizumab. ST = standard treatment
Fig. 2.
Cumulative incidence of clinical improvement from baseline to day 28 in tocilizumab and standard treatment patients.
3.4. Safety and adverse events
Serious adverse events were recorded in 8 (25%) patients from the tocilizumab group and in 9 (27%) patients from the standard treatment group. Bacteremia occurred in four patients (13%) from the tocilizumab group and in 4 patients (12%) from the standard treatment group (p = 0.99). Infections in the tocilizumab group were observed while in the ICU in 3 patients (1 at day 8 and 2 at day 9 after tocilizumab treatment) and in the Medicine Ward in 1 patient (at day 13 after tocilizumab treatment). Notably, one of the ICU-admitted patients also experienced candidemia (day 18) and invasive pulmonary aspergillosis (day 22). Three of these patients were eventually discharged, whereas the patient with fungal infections was still hospitalized at day 28. The rate of infection was lower in patients treated with a single dose (9%) compared to patients treated with two doses of tocilizumab (33%), p=0.06. In the standard treatment group, one patient died, one was diagnosed with infection while in ICU and at day 28 was still intubated and on antibiotic therapy, and the two remaining patients were still admitted to Hospital. The rate of pulmonary thrombosis documented at contrast-enhanced thorax CT scan was also similar between the two groups: 2 (6%) patients in the tocilizumab group compared to 3 (9%) patients in the standard treatment group (p = 0.99). One patient per group developed a pneumothorax while on NIV. Transitory increase in serum AST or ALT was observed in 5 (15%) tocilizumab patients and in 6 (18%) standard treatment patients (p = 0.99). In the tocilizumab group the highest serum level of ALT was reached at a median time of 11 (9 – 13) days. A transitory neutropenia was observed in 5 (16%) tocilizumab patients and no standard treatment patient (p = 0.024). In no patient the neutrophil count dropped < 1.000 109 cells/L. No infection developed in patients with transient neutropenia. No infusion-related adverse reaction was recorded.
3.5. Predictors of survival and clinical improvement in tocilizumab-treated patients
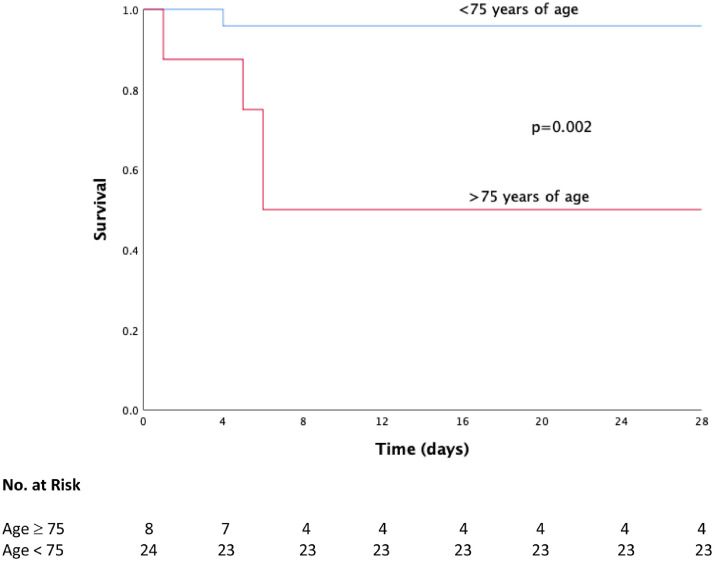
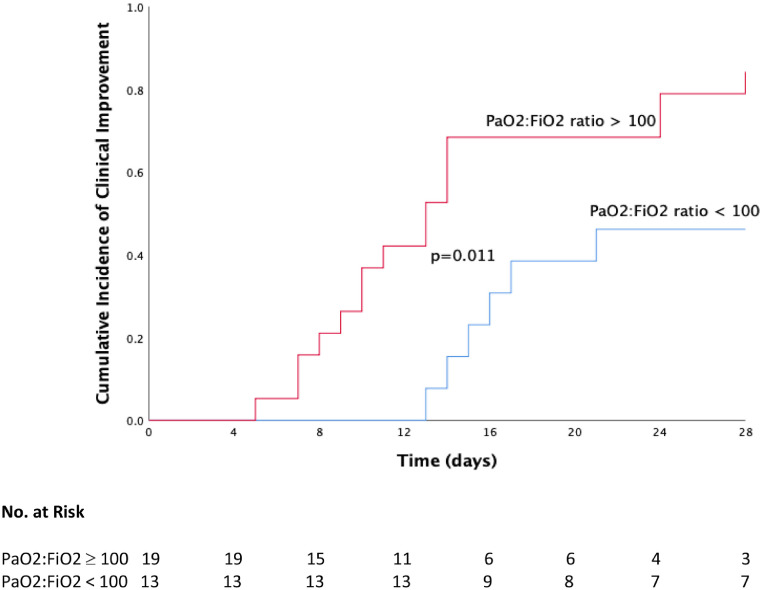
Univariate analyses for overall survival and clinical improvement of tocilizumab patients are presented in Tables 3 and 4 . At multivariate analysis, patients with age <75 years had a higher survival (HR 1.46, 1.03 – 2.08, p=0.03, see Fig. 3 ), whereas patients with baseline PaO2:FiO2 ratio ≥100 showed a slightly higher incidence of clinical improvement at day 28 (HR 1.01, 1.00 – 1.02, p=0.006, see Fig. 4 ).
Table 3.
Baseline univariate predictors of survival in tocilizumab patients.
| Survived (27 patients) | Dead (5 patients) | p-value | |
|---|---|---|---|
| Age (years), median (IQR) | 61 (53 – 73) | 77 (74 – 81) | 0.003 |
| Sex (male), n (%) | 25 (93) | 4 (80) | 0.41 |
| PaO2:FiO2, median (IQR) | 118 (86 – 181) | 69 (65 – 94) | 0.034 |
| Body temperature (°C), median (IQR) | 37.5 (38.9 – 38.2) | 38 (37.5 – 38) | 0.85 |
| CRP (mg/L), median (IQR) | 139 (88.5 – 204) | 185 (146 – 185) | 0.46 |
| LDH (U/L), median (IQR) | 439 (357 – 546) | 443 (438 – 456) | 0.94 |
| NIV, n (%) | 20 (74) | 5 (100) | 0.56 |
| Duration of symptoms (days), median (IQR) | 11 (7 – 14) | 12 (9 – 15) | 0.36 |
| CKD, n (%) | 3 (9) | 0 | 0.99 |
| Arterial hypertesion, n (%) | 11 (34) | 1 (20) | 0.63 |
| COPD, n (%) | 0 | 1 (20) | 0.16 |
| Cancer, n (%) | 2 (7) | 0 | 0.99 |
| T2DM, n (%) | 3 (11) | 1 (20) | 0.51 |
| CAD, n (%) | 2 (7) | 2 (40) | 0.10 |
| Re-treatment, n (%) | 8 (30) | 1 (20) | 0.99 |
NIV = non-invasive ventilation. CRP = C-reactive protein. LDH = lactate dehydrogenase. CKD = chronic kidney disease. COPD = chronic obstructive pulmonary disease. T2DM = type-2 diabetes mellitus. CAD = coronary artery disease.
Table 4.
Baseline univariate predictors of clinical improvement in tocilizumab patients.
| Improved (22 patients) | Not improved (10 patients) | p-value | |
|---|---|---|---|
| Age (years), median (IQR) | 59,5 (51 – 72) | 74 (69.5 – 78) | 0.009 |
| Sex (male), n (%) | 22 (100) | 7 (70) | 0.024 |
| PaO2:FiO2, median (IQR) | 137 (90 – 185) | 86 (68 – 103) | 0.008 |
| Body temperature (°C), median (IQR) | 37.6 (36.5 – 38.3) | 37.5 (37.4 – 38.1) | 0.77 |
| CRP (mg/L), median (IQR) | 128 (69 – 187) | 186 (137 – 2702) | 0.038 |
| LDH (U/L), median (IQR) | 429 (354 – 552) | 446 (426 – 564) | 0.54 |
| NIV, n (%) | 16 (73) | 9 (41) | 0.39 |
| Duration of symptoms (days), median (IQR) | 11 (7 – 14) | 11.5 (10 – 14) | 0.28 |
| CKD, n (%) | 2 (9) | 1 (10) | 0.99 |
| Arterial hypertension, n (%) | 7 (32) | 5 (50) | 0.44 |
| COPD, n (%) | 0 | 1 (10) | 0.31 |
| Cancer, n (%) | 1 (4) | 1 (10) | 0.53 |
| T2DM, n (%) | 3 (14) | 1 (10) | 0.99 |
| CAD, n (%) | 2 (9) | 2 (20) | 0.57 |
| Re-treatment, n (%) | 5 (23) | 4 (40) | 0.41 |
NIV = non-invasive ventilation. CRP = C-reactive protein. LDH = lactate dehydrogenase. CKD = chronic kidney disease. COPD = chronic obstructive pulmonary disease. T2DM = type-2 diabetes mellitus. CAD = coronary artery disease.
Fig. 3.
Overall survival curves from baseline to day 28 in tocilizumab patients according to age.
Fig. 4.
Cumulative incidence of clinical improvement from baseline to day 28 in tocilizumab patients according to baseline PaO2:FiO2 ratio.
4. Discussion
This study describes the long-term real-life effectiveness and safety of tocilizumab in severe COVID-19 patients in an Italian tertiary hospital compared to patients receiving standard management. At 28 days, no statistically significant differences in clinical outcomes between the two groups were observed. The 28-day mortality (15%) was considerable also in tocilizumab patients, yet similar between the two groups and to another Italian series [12]. However, the majority of patients in both groups experienced a clinical improvement and was eventually discharged from the hospital.
IL-6 is currently considered to play a central role in the systemic inflammatory status of severe COVID-19 patients [18], [19], [20] and its blockade is currently investigated in two International randomized controlled trials and in several uncontrolled International clinical trials [21]. The case series published so far [11], [12], [13], [14] have all obtained encouraging results, still the absence of a comparative group and of a homogeneous population are major limitation for the interpretation of the results.
The treatment protocol we used consisted of a pre-defined dose of 400 mg of tocilizumab, followed by a second infusion in case of respiratory worsening after 24 hours. To assess whether repeated tocilizumab administrations were associated with different clinical outcomes, we compared re-treated patients with those treated with a single tocilizumab infusion; however, no statistically significant difference emerged. In the multivariate analysis performed in tocilizumab patients, only older age was associated with reduced survival, whereas a higher baseline PaO2:FiO2 ratio was associated with clinical improvement. These findings are in keeping with the previously published predictors of mortality in critically-ill COVID-19 patients [22,23]. Notably, the duration of symptoms prior to tocilizumab therapy was not associated with improved outcome or survival in this cohort of patients.
The main concern regarding tocilizumab therapy is the occurrence of severe infections [24]. In this study, four patients (13%) experienced bacterial infections after tocilizumab treatment; of these, three patients were in the ICU. As all ICU-admitted patients also received a second dose of tocilizumab, it is hard to discriminate whether ICU-level care (i.e. vascular catheters, invasive ventilation) or a higher tocilizumab dose could have played a role in the increased rate of infection observed in this population. Overall, the rate of infection was comparable in the standard treatment group, with 4 patients also experiencing blood-stream infections. In the tocilizumab group, three out of four patients with infectious adverse events were eventually discharged from hospital. Nonetheless, the occurrence of pulmonary aspergillosis and candidemia in one patient treated with high-dose tocilizumab and still hospitalized at the last time point raises a concern on opportunistic fungal infections in ICU patients treated with IL-6 blockers. Also the rate of pulmonary thrombosis, a recently recognized complication of severe COVID-19 [25] was not different between the two groups.
Four patients (13%) were admitted to ICU within the first 12 hours after the first tocilizumab infusion. All of them were eventually discharged from ICU after a median time of 8.5 days. The good prognosis of these tocilizumab-treated patients admitted to ICU is encouraging, as the mortality in ICU-admitted patients with COVID-19 is high, recently published study on COVID-19 ICU patients in Lombardy, Italy, reported a mortality rate of 23% [26], [27], [28].
Our study has some limitations. The retrospective design and the small size of the cohort preclude clear conclusions on the efficacy of tocilizumab in severe COVID-19 patients. Strengths include a clear and consistent treatment scheme, the homogeneity of the population studied, a 28-day follow-up, the absence of concomitant corticosteroid therapy, and the presence of a comparison group.
5. Conclusions
In our study, we did not observe clear improvements in patients receiving tocilizumab compared to standard management. Infectious adverse events require careful monitoring to evaluate long-term risks. The results of ongoing randomized placebo-controlled trials are eagerly awaited to establish the role of IL-6 blockade in severe COVID-19 patients, and whether tocilizumab therapy might be safely and effectively used for treating COVID-19.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowlegments
We wish to thank all the patients who participated in this study and their families. We are indebted to the healthcare personnel and the management of IRCCS San Raffaele Scientific Institute for making this study possible. We dedicate this work to the memory of health care workers who have given their lives in the care of patients with COVID-19.
TOCI-RAF Study Group members: Piera Angelillo, Andrea Assanelli, Elena Baldissera, Nicola Boffini, Stefania Calvisi, Corrado Campochiaro, Diana Canetti, Adriana Cariddi, Antonella Castagna, Giulio Cavalli, Fabio Ciceri, Lorenzo Dagna, Francesco De Cobelli, Giacomo De Luca, Emanuel Della Torre, Nicola Farina, Maria Fazio, Giovanni Landoni, Gaia Mancuso, Alessandro Marinosci, Giacomo Monti, Chiara Oltolini, Marco Ripa, Patrizia Rovere-Querini, Annalisa Ruggeri, Silvia Sartorelli, Paolo Scarpellini, Marzia Spessot, Alessandro Tomelleri, Moreno Tresoldi, Alberto Zangrillo.
Affilations of TOCI-RAF Study Group: Vita-Salute San Raffaele University, Via Olgettina 60, 20132 Milan, Italy.
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;0(0) doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. April 2020 doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zangrillo A, Beretta L, Silvani P. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020 Apr 1 doi: 10.51893/2020.2.pov1. Online ahead of print. PMID: 32227819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur S, Bansal Y, Kumar R, Bansal G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorganic Med Chem. 2020;28(5) doi: 10.1016/j.bmc.2020.115327. [DOI] [PubMed] [Google Scholar]
- 6.Le RQ, Li L, Yuan W. FDA Approval Summary: Tocilizumab for Treatment of Chimeric Antigen Receptor T Cell‐Induced Severe or Life‐Threatening Cytokine Release Syndrome. Oncologist. 2018;23(8):943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DW, Gardner R, Porter DL. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;1 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. April 2020 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. April 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sciascia S, Aprà F, Baffa A. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin Exp Rheumatol. 2020 May 1 [PubMed] [Google Scholar]
- 13.Xu X, Han M, Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020 Apr 29 doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Giambenedetto S, Ciccullo A, Borghetti A. Off-label Use of Tocilizumab in Patients with SARS-CoV-2 Infection. J Med Virol. 2020 Apr 16 doi: 10.1002/jmv.25897. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome advances in diagnosis and treatment. JAMA - J Am Med Assoc. 2018;319(7):698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 16.Cao B, Wang Y, Wen D. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. March 2020 doi: 10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO | Coronavirus disease (COVID-2019) R&D. https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus/en/. Accessed April 19, 2020.
- 18.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China [published online ahead of print, 2020 Mar 12]. Clin Infect Dis. 2020;ciaa248. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed]
- 20.de Simone G, Mancusi C. COVID-19: Timing is Important. Eur J Intern Med. 2020 Apr 13. pii: S0953-6205(20)30133-3. doi: 10.1016/j.ejim.2020.04.019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 21.U.S. National Library of Medicine, ClinicalTrials.gov(www.clinicaltrials.gov) accessed on 06 May 2020.
- 22.Y Shu H, Xia J, Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins-Filho PR, Tavares CSS, Santos VS.Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. Eur J Intern Med. 2020 Apr 23. pii: S0953-6205(20)30165-5. doi: 10.1016/j.ejim.2020.04.043. [DOI] [PMC free article] [PubMed]
- 24.Pawar A, Desai RJ, Solomon DH. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: A multidatabase cohort study. Ann Rheum Dis. 2019;78(4):456–464. doi: 10.1136/annrheumdis-2018-214367. [DOI] [PubMed] [Google Scholar]
- 25.Ciceri F, Beretta L, Scandroglio AM. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020 Apr 15 doi: 10.51893/2020.2.pov2. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasselli G, Pesenti A, Cecconi M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast during an Emergency Response. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 27.Zangrillo A, Beretta L, Scandroglio AM. Characteristics, Treatment, Outcomes, and Cause of Death of Invasively Ventilated Patients with COVID-19 ARDS in Milan, Italy. Critical Care Resuscitation. 2020 doi: 10.1016/S1441-2772(23)00387-3. published online ahead of print, 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalli G, De Luca G, Campochiaro C. Interleukin 1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheum. 2020 doi: 10.1016/S2665-9913(20)30127-2. published online ahead of print, 2020 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]