Abstract
Purpose
Auditory deprivation has downstream effects on the development of language and executive functioning (EF) in prelingually deaf children with cochlear implants (CIs), but little is known about the very early development of EF during preschool ages in children with CIs. This study investigated the longitudinal development of EF and spoken language skills in samples of children with normal hearing (NH; N = 40) or CIs (N = 41) during preschool ages.
Method
Participants were enrolled in the study between ages 3 and 6 years and evaluated annually up to the age of 7 years. Mixed-effects models were used to evaluate and predict growth of spoken language and EF skills over time.
Results
Children with CIs scored lower than NH peers on language measures but improved significantly over time. On performance-based neurocognitive measures of controlled attention, inhibition, and working memory, children with CIs scored more poorly than the sample of NH peers but comparable to norms, whereas on a parent report behavior checklist, children with CIs scored more poorly than both NH peers and norms on inhibition and working memory. Children with CIs had poorer EF than the sample of NH peers in most domains even after accounting for language effects, and language predicted only the verbal working memory domain of EF. In contrast, EF skills consistently predicted language skills at subsequent visits.
Conclusions
Findings demonstrate that, despite significant improvement over time, some domains of EF (particularly parent-reported EF) and language skills in children with CIs lag behind those of children with NH during preschool ages. Language delays do not fully explain differences in EF development between children with CIs and NH peers during preschool ages, but EF skills predict subsequent language development in children with CIs.
Cochlear implantation in childhood results in significant improvement in spoken language skills, particularly when implantation occurs at very young ages (Geers, Nicholas, & Sedey, 2003; Niparko et al., 2010). Longitudinal studies of early-implanted children document dramatic spoken language improvement during preschool ages after cochlear implantation, which exceeds the trajectory of language development prior to implantation (Niparko et al., 2010). Many early-implanted children score in or near average ranges for language skills by ages 8–9 years (Geers, Nicholas, & Sedey, 2003).
However, despite significant advances in cochlear implant (CI) technology and clinical care, enormous variability is found in spoken language outcomes following implantation, with many deaf children achieving near-normal spoken language functioning in quiet environments, whereas others experience suboptimal spoken language outcomes (Eisenberg et al., 2007; Ganek et al., 2012; Niparko et al., 2010; Semenov et al., 2012; Tobey et al., 2013). Explaining and addressing this variability in outcomes is one of the primary unresolved research and clinical issues in the field of cochlear implantation of prelingually deaf children (Eisenberg et al., 2007; Pisoni et al., 2018). Some demographic, family, and audiologic characteristics have been associated with better speech and language outcomes in prelingually deaf, early-implanted children, allowing for advances in interventions to promote improved spoken language development. These characteristics include earlier age at implantation, better pre-implant unaided pure-tone average (PTA) thresholds, shorter duration of deafness, use of auditory–oral communication strategies, a fully active electrode array, use of updated/modern processing strategies, a smaller family size, a higher family socioeconomic status, higher parent education, and greater maternal sensitivity in parent–child interaction (Geers, 2002, 2006; Geers, Brenner, & Davidson, 2003; Geers & Nicholas, 2013; Geers, Nicholas, & Sedey, 2003; Geers & Sedey, 2011; Quittner et al., 2013; Ruffin et al., 2013). However, considerable variability in individual outcomes is found even after accounting for those predictor characteristics (Pisoni et al., 2010). Further advances in explaining individual differences in spoken language outcomes after early cochlear implantation offer the potential for development of novel interventions to improve outcomes.
A growing body of evidence suggests that deficits in exposure to auditory and language experiences have direct and indirect effects on other domains of brain-based information (neurocognitive) processing in children and adolescents with CIs (Kronenberger & Pisoni, 2018). The brain is a dynamic, self-organizing system that develops based on reciprocal experiences and activity between neural processing and stimulation from the environment (Fischer & van Geert, 2014; Goldenberg & Galván, 2015; Peltzer-Karpf, 2012; Stiles et al., 2015; van Geert, 2009), including auditory input, language exposure, and the psychological and sociocultural factors affected by auditory and language experiences (Kronenberger & Pisoni, in press). The development of neurocognitive abilities including language, memory, nonverbal reasoning, and executive functioning (EF) reflects the underlying growth of brain systems that are dependent on sensory experience, neural activity, and related stimulation as well as learning experiences within the family, educational, and clinical systems. Therefore, limitations or alterations in early auditory experience provided to the developing brain may have effects on neurocognitive functioning that extend well beyond proximal spoken language skills (Kral et al., 2016).
Early auditory experience and activity may be particularly critical for the development of the neurocognitive domain of EF (Conway & Pisoni, 2008; Conway et al., 2009; Gathercole & Baddeley, 1993). Although many theories and models of EF have been proposed, all of them share in common the definition of EF as a set of active, effortful cognitive control and supervisory oversight processes needed to engage in planned, purposeful, goal-directed behavior, typically associated with the functioning of the prefrontal cortex and related brain regions (Barkley, 2012; Diamond, 2013). Hence, executive functions are involved in conscious, effortful concentration and control of thinking and behavior, particularly when demands are made that exceed overlearned/automatic behavior or that tax mental resources (Diamond, 2013). Three domains of cognitive functioning have received support as core components of EF: inhibition (delaying thinking or behavior in order to make an effortful, conscious action), working memory (holding information in immediate memory when other concurrent mental demands are present), and mental flexibility (ability to shift mental or behavioral set in response to changing demands; Miyake et al., 2000). Additional components of EF have been identified such as controlled attention (active control of the contents and direction of cognitive awareness and processing), self-monitoring (active awareness of one's appearance, behavior, and progress toward a goal), organization (arrangement of items in space and time based on meaningful characteristics), and goal direction (resisting distraction and staying on-task; Barkley, 2012). Although there is disagreement about whether these latter domains of EF are higher order domains dependent on the three core EF components or whether they are separate functions, all of these EF domains are necessary to evaluate, monitor, develop, and carry out plans and reach goals. Consequently, deficits in these domains are associated with delays or disorders of EF, such as attention-deficit/hyperactivity disorder (Barkley, 1997).
EF develops at very young ages and shows progressive improvement throughout the school-age years. Working memory, inhibition, and shifting have been shown to develop in children as young as 2–3 years old, with steady improvement throughout preschool ages (Espy et al., 2001). EF development is affected by multiple factors, including genetics, family environment, social learning, education, language development, and behavioral contingencies in the environment (Barkley, 2012; Kronenberger & Pisoni, in press).
Auditory experience may also affect EF in several different ways. For example, auditory stimulation provides temporal patterns to the developing brain, which have been shown to be important for developing sequential processing abilities such as pattern detection, serial memory, and sustained attention (Conway et al., 2009). Auditory experience and activities also give valuable practice with selective attention (to target sounds), resisting distraction (from extraneous sounds), and working memory (storage of auditory information in the face of competing cognitive demands; Kronenberger & Pisoni, 2018), all of which are core components of EF. Additionally, auditory experience underlies the development of spoken language, which offers tools to support EF, such as encoding and representing information in working memory, holding goal-related information in mind, and using self-talk to inhibit and regulate behavior (Alderson-Day & Fernyhough, 2015; Doebel et al., 2018).
Because of deficits in auditory experience and language exposure, prelingually deaf children with CIs show considerable variability and are at risk for delays in EF. Deficits in auditory–verbal working memory, the EF subdomain required for concurrent auditory–verbal memory storage and information-processing activities, have been well documented in numerous studies of CI users (Figueras et al., 2008; Kronenberger, Colson, et al., 2014; Kronenberger, Pisoni, Henning, & Colson, 2013). Children with CIs have also shown delays in the EF domains of inhibition (Figueras et al., 2008; Kronenberger, Pisoni, Henning, & Colson, 2013) and controlled attention (concentration) under time pressure (collectively referred to as “inhibition–concentration–speed”; Kronenberger, Colson, et al., 2014), even on visual tasks with minimal language mediation (Kronenberger, Pisoni, Henning, & Colson, 2013).
Language and EF are reciprocally, bidirectionally related, particularly early in childhood (Barkley, 2012; Singer & Bashir, 1999; Ylvisaker & DeBonis, 2000). Controlled attention, working memory, and planning, all of which are critical components of EF, are also used to acquire and process language (Kronenberger & Pisoni, 2018; Rönnberg et al., 2008). EF also regulates the effort that is allocated to listening, and more efficient allocation of this effort contributes to more effective listening skills and language processing experiences, particularly in challenging conditions such as processing degraded/coarsely coded input from a CI (Pichora-Fuller et al., 2016). Language, in turn, supports EF by serving as a tool for representing goal-related information in memory and for representing plans for appropriately regulated, goal-directed behavior (Alderson-Day & Fernyhough, 2015; Doebel et al., 2018).
Consistent with a reciprocal–bidirectional model of language and EF development in prelingually deaf, early implanted children, numerous studies have demonstrated close links between EF and language outcomes in samples of CI users. Auditory–verbal working memory is associated with speech perception (Cleary et al., 2000; Nittrouer et al., 2013), vocabulary (Cleary et al., 2000; Geers et al., 2013; Nittrouer et al., 2013; Wass et al., 2008), word learning (Willstedt-Svensson et al., 2004), and verbal communication skills (Ibertsson et al., 2009; Lyxell et al., 2008) in CI users. Measures of inhibition and controlled attention are also related to speech-language outcomes in samples of CI users (Horn et al., 2004), particularly when time demands are present (Kronenberger, Colson, et al., 2014).
The Ease of Language Understanding theory (Rönnberg et al., 2013) and the Framework for Understanding Effortful Listening (Pichora-Fuller et al., 2016) propose that CI users have to allocate additional effortful resources to listening and language processing because of degraded/coarsely coded stimulation from the CI, resulting in a greater dependence on EF during language-related tasks. As a result, the association between EF and spoken language skills is stronger in CI users than in peers with normal hearing (NH; Kronenberger, Colson, et al., 2014). Correlations of EF measures of auditory–verbal working memory and inhibition–concentration–speed with speech-language skills in CI users exceed those of NH control samples (Kronenberger, Colson, et al., 2014; Kronenberger, Pisoni, Harris, et al., 2013; Nittrouer et al., 2013; Pisoni et al., 2011). Additionally, depletion of working memory resources results in poorer real-time language processing in CI users but has less impact on NH peers, indicating that working memory plays a greater role in language functioning in CI users (Kronenberger et al., 2018).
Existing research in samples of prelingually deaf, early-implanted children has demonstrated consistent growth in spoken language skills after implantation, from preschool ages through adolescence (Niparko et al., 2010). However, almost all of the research on EF has been cross-sectional and limited to school-age and older samples. Furthermore, no longitudinal research has investigated EF development during the preschool years, when language growth and adjustment to the CI are greatest. The dearth of longitudinal EF studies has limited our understanding of EF growth and the reciprocal associations between EF and language following implantation.
Longitudinal studies using digit span tests have demonstrated early deficits in verbal working memory starting at very early school ages, which persist through adolescence (Harris et al., 2013), although no research has investigated longitudinal changes in other EF subdomains. Furthermore, early vocabulary (assessed about 1 year on average after implantation) predicted verbal working memory and controlled fluency–speed scores 11 years later (Castellanos et al., 2016). Conversely, verbal working memory was associated with later vocabulary, language, and reading scores in school-age children (Kronenberger, Pisoni, Harris, et al., 2013; Pisoni et al., 2011). Hence, some longitudinal analyses have shown bidirectional predictive relationships between EF and language during school ages, but little is currently known about the longitudinal development of EF skills in CI users during preschool ages.
Cross-sectional studies of EF skills in preschool CI users indicate that EF delays in domains including working memory, controlled attention, and inhibition are found in children as young as 3 years old (Beer et al., 2014; Kronenberger, Beer, et al., 2014). Thus, investigation of the early development of EF skills in CI users over time during preschool ages is necessary for understanding and explaining how EF and language develop in young CI users. Furthermore, this knowledge may suggest novel neurocognitive interventions to promote EF and spoken language development at young ages when risks for delays in these domains first emerge (Kronenberger & Pisoni, 2016).
In this study, we investigated the longitudinal development of language and EF skills during preschool ages in prelingually deaf, early-implanted CI users compared to NH peers. Four primary research questions were addressed: (a) How does language development during preschool ages compare in CI users and NH peers? Based on prior findings (Niparko et al., 2010), we expected that language development in CI users would lag behind NH peers but would improve over time at a pace at least as rapid as that of NH peers. (b) How do EF subdomains develop during preschool ages in CI users compared to NH peers? Based on research demonstrating delays in EF domains in school-age CI users, we expected lower EF scores in the domains of verbal working memory and inhibition–concentration–speed in preschool CI users compared to NH peers, improving at a pace similar to that of NH peers. (c) What factors (demographic, language, nonverbal ability) predict EF development in CI users compared to NH peers during preschool ages? Prior work with older CI users and NH peers has demonstrated that language supports EF skills (Barkley, 2012; Kronenberger & Pisoni, in press; Singer & Bashir, 1999), but very little is known about language and other predictors of EF in preschool-aged CI users. We expected that language and nonverbal ability scores would predict EF ability and development, over and above variance accounted for by demographics. (d) Does EF predict subsequent language development during preschool years in CI users compared to NH peers? Consistent with a bidirectional model of language and EF influences, we expected that EF skills at younger ages would predict subsequent language development. The prospective longitudinal study design allows for investigation of trajectories of change in EF and language skills as well as evaluation of predictive relationships over time.
Method
Participants
Participants for this study were 41 CI users and 40 NH peers between ages 3 and 6 years at the first study visit. Inclusion criteria for the CI sample were as follows: (a) onset of severe-to-profound hearing loss prior to the age of 3 years, (b) cochlear implantation at the age of 3 years or younger, (c) use of CI for at least 6 months, (d) enrollment in an aural rehabilitative program and/or educational setting that encouraged the development of speaking and listening skills, and (e) use of modern multichannel CIs. Inclusion criteria for all participants with NH included (a) NH and language development as assessed by parent report at the time of enrollment (including never having been enrolled in any program for hearing loss and never having used a sensory aid or assistive device for listening) and (b) PTA within normal range as assessed by a hearing screening of each ear individually at 20 dB HL for 500, 1000, 2000, and 4000 Hz using a GSI 61 Clinical Audiometer and Telephonics TDH 50P Headphones in an IAC Acoustics 404A soundbooth. Inclusion criteria for both the CI and NH samples were (a) age of 3–6 years at the time of the first visit, (b) absence of any history of neurological or developmental conditions that required chronic management by a physician or special accommodations in the school or at home (other than those associated with hearing loss in the CI sample), and (c) home environment in which English was the primary language spoken. With the exception of the hearing screening, all inclusion and exclusion criteria were assessed using a combination of questionnaires and interviews with trained research personnel who were licensed speech-language pathologists.
CI participants were recruited from populations currently being seen for clinical services at a large university hospital-based CI clinic or who responded to advertisements posted in the community, including offices and organizations serving families of children with hearing loss. Children with NH were recruited through advertisements posted in the same hospital and community settings that were used for recruitment of children with CIs.
Sample characteristics are presented in Table 1. The CI sample averaged 4.2 years of age at the first visit (n = 25 three-year-olds, 8 four-year-olds, 6 five-year-olds, and 2 six-year-olds), and the NH sample had a similar distribution (n = 24 three-year-olds, 13 four-year-olds, 1 five-year-olds, and 2 six-year-olds). All but one of the CI participants had onset of hearing loss at birth (n = 1 had hearing loss at the age of 2 months), and the majority (n = 25) were implanted between 1 and 2 years of age (n = 5 prior to 1 year of age and n = 11 between 2 and 3 years of age). Eighteen of the children had used their CIs for 2–3 years, with 14 having used CIs for under 2 years and nine having used their CIs for over 3 years. Four children in the CI sample communicated using simultaneous communication strategies, whereas the remainder used no formal sign language, with listening accompanied by occasional lipreading used for communication (Geers & Brenner, 2003). Etiology of hearing loss was auditory neuropathy (n = 3), meningitis (n = 1), Mondini malformation (n = 2), genetic (e.g., Waardenburg syndrome; n = 8), and unknown or familial (n = 27).
Table 1.
Sample characteristics at Year 1 (first annual evaluation).
| Characteristic | CI | NH | t |
|---|---|---|---|
| Demographics and hearing history, Year 1 | |||
| Age (years) | 4.2 (1.0) | 4.0 (0.9) | 0.63 |
| Age at implantation (months) | 19.6 (7.6) | NA | NA |
| Duration of CI use (years) | 2.5 (1.1) | NA | NA |
| Preimplant residual hearing a | 98.3 (13.9) [36] | NA | NA |
| Income level b | 7.0 (3.0) [37] | 7.6 (2.0) | 0.99 |
| Income categories (low–medium–high) c | 17/5/15 | 12/17/11 | |
| Sex (female/male) | 20/21 | 19/21 | |
| Race (White/Black) d | 38/7 | 36/5 | |
| Neurocognitive and language measures, Year 1 | |||
| DAS-II Picture Similarities T-score e | 53.3 (9.1) [39] | 60.4 (12.4) | 2.90** |
| Leiter-R Attention Sustained scaled score | 9.6 (2.8) [39] | 12.1 (2.3) [39] | 4.36*** |
| Leiter-R Forward Memory scaled score | 10.5 (3.3) [30] | 12.1 (2.5) [36] | 2.30* |
| WISC-III Digit Span Forward raw score | 3.6 (1.6) [26] | 4.0 (1.8) | 0.97 |
| PPVT-4 standard score | 84.3 (20.7) [40] | 118.5 (10.3) | 9.37*** |
| PLS-4 Total standard score | 80.9 (23.9) [38] | 119.2 (12.5) | 8.92*** |
| Executive functioning behavior checklist measures, Year 1 | |||
| BRIEF Inhibit T-Score | 58.2 (14.6) [39] | 49.8 (9.3) [39] | 3.06** |
| BRIEF Shift T-Score | 50.3 (9.0) [39] | 48.5 (8.8) [39] | 0.90 |
| BRIEF Working Memory T-Score | 59.1 (13.7) [39] | 51.8 (12.0) [39] | 2.50* |
Note. N = 41 for CI sample and 40 for NH sample, unless otherwise indicated by N in brackets. Groups did not differ on sex (Fisher's exact test, p = 1.0) or race (Fisher's exact test, p = .77) but did differ on income categories, χ2(2) = 7.9, p = .02. t tests compare CI and NH samples (df = 79 for the full sample). Underlined values are significantly different from the test normative mean score at p < .05. CI = cochlear implant; NH = normal hearing; NA = not applicable; DAS-II = Differential Ability Scales–Second Edition; Leiter-R = Leiter International Performance Scale–Revised; WISC-III = Wechsler Intelligence Scale for Children–Third Edition; PPVT-4 = Peabody Picture Vocabulary Test–Fourth Edition; PLS-4 = Preschool Language Scale–Fourth Edition; BRIEF = Behavior Rating Inventory of Executive Function.
Preimplant unaided pure-tone average for 500, 1000, and 2000 Hz in dB HL.
Income is on a 1 (under $5,500) to 10 ($95,000 and over) scale (Kronenberger, Pisoni, Henning, & Colson, 2013).
Income categories are low (under $50,000), medium ($50,000–$94,999), and high ($95,000+).
Numbers add to more than study N because some participants reported more than one race.
A measure of nonverbal ability.
p < .05.
p < .01.
p < .001.
Annual household income was assessed using a self-report scale of 1 (< $5,000) to 10 (≥ 95,000), with values of 3, 5, and 7 corresponding to income values of $10,000–$15,999, $25,000–$34,999, and $50,000–$64,999, respectively. Because income was unevenly distributed across the 1–10 range, income values were divided by tertiles across the combined samples, with 37.7% (n = 29) of the combined CI and NH samples reporting income of under $50,000 (ratings of 1–6), 28.6% (n = 22) reporting income of $50,000–$94,999 (ratings of 7–9), and 33.8% (n = 26) reporting income of $95,000 and higher (rating of 10; n = 4 families chose not to report income). Although the groups did not differ in income ratings on the 1–10 scale, t(75) = 0.99, p > .10, the groups did differ on the three income categories, χ2(2) = 7.92, p < .02, reflecting larger numbers in the CI sample in the $95,000+ range and larger numbers in the NH sample in the $50,000–$94,999 range. Subsequent analyses treated the three income levels as nominal variables. The CI and NH samples did not differ in age, t(79) = 0.63, p = .53; sex (Fisher's exact p = 1.0); or race (White vs. Black or multiple races; Fisher's exact p = .77; see Table 1).
Procedure
Data were collected as part of a multiyear longitudinal study of neurocognitive and speech-language development of young children with CIs and NH peers during a 6-year period, during which enrollment of new participants occurred during the first 5 years; all eligible participants who volunteered for the study during that time were entered (rolling enrollment), resulting in a total of 41 participants in the CI sample and 40 participants in the NH sample. Neurocognitive and speech-language tests were administered during one or two annual evaluation sessions (in rare cases, a third session was available, depending on child fatigue, motivation, or engagement) by age, between ages 3 and 7 years. Evaluation sessions during the same annual testing were typically conducted in the same week (82% of the CI sample and 85% of the NH sample) or in the same month (92% of the CI sample and 96% of the NH sample) and could occur no more than 6 months apart. As a result, one to five annual evaluations, each consisting of one to two sessions in the same 6-month period, were obtained for each participant, depending on the child's age at enrollment and the time period of the study. Numbers of participants completing annual evaluations were as follows: 10 CI and three NH, five annual evaluations; nine CI and 11 NH, four annual evaluations; eight CI and nine NH, three evaluations; eight CI and 10 NH, two evaluations; and six CI and seven NH, one evaluation. Therefore, totals of 132 annual evaluations and 113 annual evaluations were performed for the CI and NH samples, respectively. Average time between annual evaluations was 1.0 years (SD = 0.2, range: 0.5–1.9 years). The CI and NH samples did not differ in number of sessions per annual evaluation, t(215) = 0.99, p = .33, or in time between annual evaluations, t(143) = 1.57, p = .12.
All study procedures were reviewed and approved by the university institutional review board, and parents were consented (with assent by children as appropriate) prior to initiation of study procedures. Children were evaluated by licensed speech-language pathologists with extensive experience evaluating individuals with hearing loss and CIs. Participants were paid $20 per hour plus travel expenses.
Measures
A battery of individually administered, performance-based, neurocognitive ability tests was selected to provide a broad assessment of nonverbal ability, language, and EF appropriate for children aged 3–7 years with hearing loss. With the exception of the digit span task (explained below), all measures were components of well-established, normed test batteries that have been validated across the age range of the study and that have been used with children with hearing loss. All tests were accompanied by verbal directions, visual demonstrations, and practice to establish that children fully understood the task prior to administration. Performance-based tests were administered to evaluate two core areas of EF at risk in children with CIs: working memory and inhibition–concentration–speed (Kronenberger, Beer, et al., 2014; Kronenberger, Pisoni, Henning, & Colson, 2013). Parents also completed a questionnaire of child EF behaviors in order to provide additional information about EF outcomes in everyday behaviors, consistent with research demonstrating that individually administered, performance-based tests of EF show relatively modest overlap (correlations in the range of 0.30) with behavior checklist measures of EF (Barkley, 2012).
Demographics and Hearing History
Measures of demographics and hearing history included chronological age at each time (year) of evaluation, sex, time between evaluations, age at the time of cochlear implantation, duration of CI use, age of onset of deafness, preimplant residual hearing (PTA for 500, 1000, and 2000 Hz in dB HL), communication mode (coded on a 1 [sign emphasis] to 6 [auditory verbal] scale for which scores of 1–3 reflect simultaneous communication strategies and 4–6 reflect oral communication strategies [Geers & Brenner, 2003]), and income level. Because 90% (37/41) of participants in the CI sample had a communication mode score of 5 at the first annual evaluation (defined on the communication mode scale as “Auditory Oral: … no formal sign language is used; … child both watches and listens to talker”) and 100% of the sample had a communication mode of 5 by the third annual evaluation, the communication mode variable was excluded from further analysis.
Nonverbal Intelligence
The Picture Similarities subtest of the Differential Ability Scales–Second Edition (DAS-II; Elliott, 2007) was administered to assess nonverbal/fluid ability. For this subtest, participants match an object (depicted as a picture on a card) with a conceptually similar object from a row of four pictures (e.g., match a picture of a table with a picture of a chair, because both are furniture). Although many (albeit not all) of the pictures on the Picture Similarities subtest can be verbally labeled, Picture Similarities requires identification of a concept linking two pictures; this concept does not need to be verbally named by the child and is not directly represented by the names of the objects. Nevertheless, verbal labeling may be used to assist performance on some Picture Similarities items, indicating a modest verbally mediated component to the Picture Similarities score. Despite this, Picture Similarities loads on a nonverbal/nonverbal reasoning factor on the DAS-II that is distinct from (but associated with) DAS-II verbal subtests, and it shares less than 20% of its variance with specific DAS-II verbal subtests (e.g., r with Naming Vocabulary = .42 in the normative sample; Elliott, 2007). Thus, Picture Similarities scores are reliable and valid measures of nonverbal reasoning in children 2.5–8 years of age (Elliott, 2007). Raw scores are converted to T-scores based on age norms from a nationally representative sample.
Language
Participants completed two measures of language: the Peabody Picture Vocabulary Test–Fourth Edition (PPVT-4; Dunn & Dunn, 2007) and the Preschool Language Scale–Fourth Edition (PLS-4; Zimmerman et al., 2002). The PPVT-4 is a measure of single-word receptive vocabulary for ages 2.5–90 years that requires the child to select a picture from among a set of four pictures that corresponds to a word spoken by the examiner. The PLS-4 is a broad measure of developmental language skills that assesses language comprehension and expression in children aged 0–6;11 (years;months; for children aged 7 years and older, 6;11 norms were used to obtain standard scores). The PPVT-4 and PLS-4 have been used in the past to measure language development in preschool-aged children with CIs (Nicholas & Geers, 2013; Wang et al., 2017) and are well-established, validated, norm-based measures with excellent psychometrics. In this study, norm-based PPVT-4 standard scores were used to assess vocabulary, and PLS-4 Total Language standard scores were used to assess global language development.
EF: Individually Administered, Performance-Based Tests
The working memory component of EF was evaluated with the Forward Memory subtest of the Leiter International Performance Scale–Revised (Leiter-R; Roid & Miller, 1997) and the Digit Span Forward subtest of the Wechsler Intelligence Scale for Children–Third Edition (WISC-III; Wechsler, 1991). The Forward Memory subtest assesses visual short-term/working memory with no audibility demands. For this subtest, the examiner points to a sequence of pictures of common objects on an easel page, which the subject must then reproduce by pointing to the same pictures in the same sequence. The sequence length begins with one item and increases as the child answers items correctly. Scores for the Forward Memory subtest in this study were scaled scores (M = 10, SD = 3) by age, based on a large nationally representative sample of normal-hearing, typically developing children and young adults.
The Digit Span Forward subtest measures auditory–verbal working memory capacity. For this subtest, the examiner presents a series of digits using live voice at a rate of one item per second, and the child then repeats the sequence in spoken format in the same order. Sequences begin with two digits and increase by one digit every two items until the child fails both items at the same sequence length. The Digit Span Forward subtest is a well-validated measure of auditory–verbal short-term/working memory capacity for children aged 6 years and older in the WISC-III (Wechsler, 1991), but similar measures have been found to be reliable and valid for children as young as 2.5 years old (Elliott, 2007). In adolescents and adults, digit span forward tests require little effort other than maintenance of the digit sequence in immediate memory; as a result, digit span forward at older ages places limited demands on the active executive control processes required for working memory (Engle et al., 1999). However, in very young children and children with hearing loss, digit span forward tests place significant additional demands on speech perception, management of underspecified phonological representations of words in short-term memory, and sustained attention/effort, concurrently with the primary task of maintenance of the digit sequence in immediate memory. Thus, digit span forward tasks in preschool children and/or children with hearing loss are valid measures of verbal working memory (Kronenberger & Pisoni, 2018; Pisoni et al., 2011). In the current study, this WISC-III subtest was used for children as young as 3 years of age in order to maintain consistency with prior research in the lab; the Digit Span Backward subtest (which requires reproduction of digits in backward order) was not used because of the young ages of the samples. Scores used for Digit Span Forward were the total number of sequences reproduced correctly by the child.
The inhibition–concentration–speed component of EF was assessed using the Attention Sustained Total subtest scaled score of the Leiter-R (Roid & Miller, 1997). This subtest is a timed measure of concentration and speed that requires participants to cancel (cross out) target pictures within a larger array of target and distractor pictures. Raw scores are the number of target pictures canceled (correct answers) minus the number of distractor pictures canceled (errors) within the time limit. Raw scores are converted to age-based scaled scores.
EF: Behavior Checklist
Parents completed the Behavior Rating Inventory of Executive Function–Preschool Version for ages 3–5 years (BRIEF-P; Gioia et al., 2003) or the Behavior Rating Inventory of Executive Function (BRIEF) for ages 6–7 years (Gioia et al., 2000) as a behavior checklist measure of EF in daily behaviors shown by the child, as rated by the parent. The BRIEF is the most widely used questionnaire measure of EF and has been shown to have strong psychometric properties, including excellent validity as a measure of EF in everyday behavior (Roth et al., 2015, 2014). BRIEF scores are associated with other measures of EF, and samples of children with poor EF (especially attention-deficit/hyperactivity disorder) have been shown to score more poorly on BRIEF subscales than nonreferred populations (Gioia et al., 2000; Roth et al., 2014). BRIEF and BRIEF-P raw subscale scores are converted to T-scores using an age-based normative sample, such that higher scores indicate poorer EF. For this study, three BRIEF/BRIEF-P subscale T-scores, namely, Inhibit (example item: “Interrupts others”), Shift (“Tries the same approach to a problem over and over even when it does not work”), and Working Memory (“Has trouble remembering things, even for a few minutes”), were chosen for analysis because these subscales match the three core subdomains of EF identified by Miyake et al. (2000). These subscales have been extensively validated as measures of their respective constructs and are widely used in research and clinical settings (Roth et al., 2014).
Of 241 BRIEF scales completed by parents during the course of the study, 210 were completed by mothers, seven by grandmothers, 23 by fathers, and one by a female guardian. For 231 of the 241 BRIEF scales completed in the study, the same parent filled out the BRIEF for the same participant at every annual evaluation, indicating very strong consistency in BRIEF rating across annual evaluations (over 95% of annual evaluations).
Data Analyses
Descriptive statistics are reported for all participants at their first study visit, along with information about number of annual evaluations attended (reported earlier; see Table 1). Because the CI and NH samples differed on nonverbal ability and socioeconomic status, those variables were tested and controlled as terms in mixed-method models (see below). In order to test the first two hypotheses (language and EF development over time in CI and NH samples), mixed-effects models were used to investigate patterns and differences in development of nonverbal ability, language, and EF skills over time. Specifically, CI and NH samples were compared for intercept (score at the age of 3 years from a mixed model) and slope (change in score by year between 3 and 7 years in the model) scores on the measures of nonverbal ability, language, and EF.
In order to test the third hypothesis (prediction of EF outcomes), mixed-effects models were used to evaluate the predictive association between measures of demographics, nonverbal ability, and language at earlier annual evaluations with EF measures at subsequent annual evaluations. First, hearing group (CI vs. NH), demographics (sex, income, age), and nonverbal ability scores at each annual evaluation were entered into equations predicting EF scores at the next annual evaluation, for all annual evaluations taking place between ages 3 and 7 years (Model 1). Demographics were entered in order to control for potential confounds and because EF and language skills may be influenced by developmental level (age) and by environmental stimulation and advantages associated with socioeconomic status (Barkley, 2012; Blair & Raver, 2014). Next, language (PPVT-4 and PLS-4) scores were tested for entry into the equations separately for hearing group and were retained only if they produced statistically significant terms for one or both hearing groups (forward entry method, p < .05) and if the Language × Hearing Group interaction was statistically significant. Separate terms were used for language scores by hearing group based on prior research demonstrating different EF–language associations for children with CIs and NH peers (Kronenberger, Colson, et al., 2014). Finally, if the Language × Hearing Group interaction variables were not statistically significant predictors of EF scores, a single term for each language measure across the combined CI and NH samples was tested for entry into the equation (forward entry method, p < .05) in order to evaluate whether a main effect of language predicting EF was found across both groups (Model 2).
In order to test the fourth hypothesis (prediction of language outcomes from EF), mixed-effects models were used to predict scores on the language variables (PPVT-4 and PLS-4) at each annual evaluation using demographic, hearing group, nonverbal ability, and EF variables from the prior annual evaluation. First, hearing group (CI vs. NH), demographics (sex, income, age), and nonverbal ability scores at each annual evaluation were entered into equations predicting language scores at the next annual evaluation (Model 1). Next, EF (Attention Sustained, Forward Memory, Digits Forward, BRIEF Inhibit, BRIEF Shift, and BRIEF Working Memory) scores were tested for entry into the equations separately for hearing group and were retained only if they produced statistically significant terms for one or both hearing groups (forward entry method, p < .05) and if the EF × Hearing Group interaction was statistically significant. Finally, if the EF × Hearing Group terms were not statistically significant predictors of language scores, a single term for each EF measure across the combined CI and NH samples was tested for entry into the equation (forward entry method, p < .05) in order to evaluate whether a main effect of EF predicting language was found across both groups (Model 2).
Results
Descriptive Statistics
Demographic characteristics of the samples (described earlier) are shown in Table 1, along with descriptive statistics for the nonverbal ability, EF, and language variables. At the first annual evaluation (Year 1), no differences were found between the hearing groups in age or sex, but the groups differed by income category. Although the CI and NH samples each scored above the normative sample mean of 50 on the DAS-II Picture Similarities subtest at their first evaluation, the NH sample scored significantly higher than the CI sample on that measure of nonverbal ability (see Table 1). No significant differences were found between the groups for digit span forward or parent-reported shift behavior at the first annual evaluation.
Compared to NH peers, the CI sample showed significantly poorer EF performance on the Leiter-R Attention Sustained, Leiter-R Forward Memory, BRIEF Inhibit, and BRIEF Working memory subtests in Year 1 (see Table 1). The NH sample scored significantly higher than the normative mean for the Leiter-R Attention Sustained and Forward Memory subtests at Year 1, while the CI sample scored higher than the norm mean (indicating more EF problems) for the BRIEF Inhibit and Working Memory subscales. Language scores for the CI sample were lower than those for the NH sample and lower than test norms for PPVT-4 and PLS-4 at Year 1. The NH sample scored above the norm mean on the PPVT-4 and PLS-4 in Year 1 (see Table 1).
In order to evaluate the extent to which group differences in language and EF at Year 1 were a result of nonverbal ability, analyses of covariance were conducted comparing language and EF scores in the NH and CI samples while statistically controlling for DAS-II Picture Similarities scores. With the exception of Leiter-R Forward Memory, F(1, 63) = 3.67, p < .06, all results were similar to the t tests comparing hearing groups (a table with all analysis of covariance results is available from the authors).
Language Development
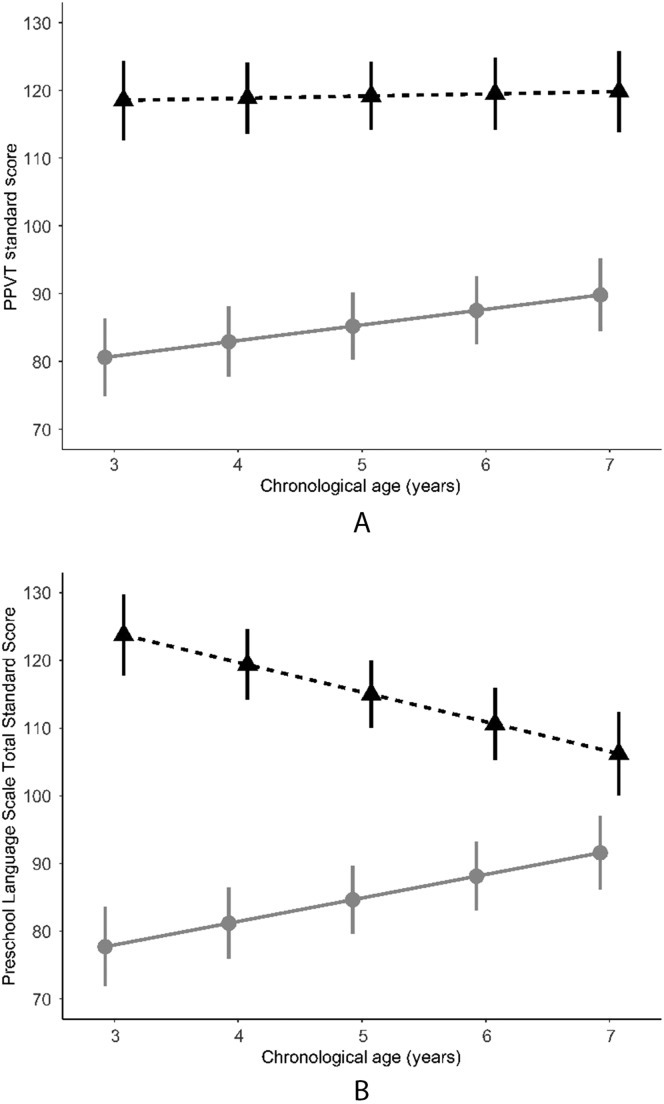
Results of mixed-effects models of language development between ages 3 and 7 years demonstrated significant differences between the two groups at baseline (CI sample PPVT-4 and PLS-4 scores < NH sample PPVT-4 and PLS-4 scores; see Table 2 and Figure 1). Furthermore, the CI sample showed significant improvement (slope) in PPVT-4 and PLS-4 scores over time, and their improvement exceeded the rate of change for the NH sample for the PLS-4. Mean PPVT-4 and PLS-4 scores for the CI sample rose from approximately 80 at the age of 3 years to approximately 90 by the age of 7 years. NH sample scores were well above average for PPVT-4 and PLS-4 at baseline, in the 118–124 range, and their PPVT-4 scores did not change over time. The NH sample showed a decline in PLS-4 scores over time, reflecting ceiling effects for the PLS-4 at later preschool ages as opposed to an actual decline in performance (see Discussion section; see Table 2 and Figure 1).
Table 2.
Mixed-effects models of language development.
| Scale | CI | NH | Difference | t |
|---|---|---|---|---|
| PPVT-4 standard score | ||||
| Intercept | 80.6 (74.9, 86.3) | 118.5 (112.6, 124.4) | 37.9 (29.7, 46.1) | 9.16*** |
| Slope | 2.3 (1.1, 3.5) | 0.3 (−1.3, 1.9) | −2.0 (−4.0, 0.0) | 1.94 a |
| PLS-4 Total standard score | ||||
| Intercept | 77.7 (71.8, 83.6) | 123.8 (117.8, 129.7) | 46.1 (37.7, 54.4) | 10.87*** |
| Slope | 3.5 (2.1, 4.8) | −4.4 (−6.1, −2.6) | −7.9 (−10.1, −5.7) | 7.04*** |
Note. Values in parentheses are 95% confidence interval. Intercept is for the baseline age of 3 years. Slope is the change in standard score points per age-year. CI = cochlear implant; NH = normal hearing; PPVT-4 = Peabody Picture Vocabulary Test–Fourth Edition; PLS-4 = Preschool Language Scale–Fourth Edition.
p < .10.
p < .05.
p < .01.
p < .001.
Figure 1.
Language (Peabody Picture Vocabulary Test–Fourth Edition [PPVT; Panel A] and Preschool Language Scale–Fourth Edition [Panel B] standard scores) development by chronological age (in years) in samples of children with cochlear implants (gray circles) or normal hearing (black triangles). Values are mean ± 1 SE.
Neurocognitive Development
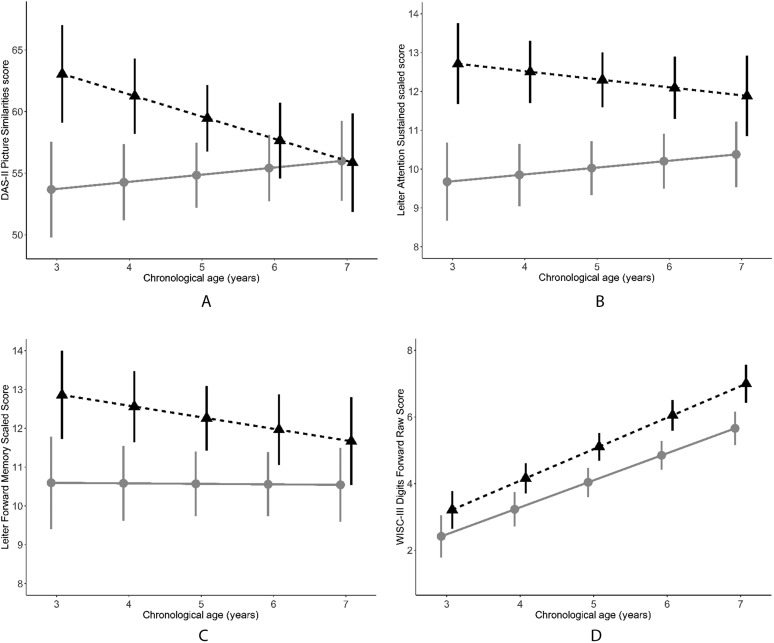
Results of mixed-effects models of development of nonverbal ability (DAS-II Picture Similarities) between ages 3 and 7 years demonstrated significant differences between the groups at baseline (CI < NH; see Table 3 and Figure 2). However, the NH sample showed a significantly declining slope in nonverbal ability scores over time, whereas the CI sample showed no significant change in scores, resulting in overlapping distributions by the age of 6 years (see Table 3 and Figure 2).
Table 3.
Mixed-effects models of nonverbal ability and executive functioning development.
| Scale | CI | NH | Difference | t |
|---|---|---|---|---|
| DAS-II Picture Similarities T-score | ||||
| Intercept | 53.7 (49.8, 57.6) | 63.1 (59.1, 67.0) | 9.4 (3.8, 14.9) | 3.33*** |
| Slope | 0.6 (−0.6, 1.8) | −1.8 (−3.3, −0.3) | −2.4 (−4.3, −0.5) | 2.45* |
| Leiter-R Attention Sustained scaled score | ||||
| Intercept | 9.7 (8.7, 10.7) | 12.7 (11.7, 13.8) | 3.0 (1.6, 4.5) | 4.14*** |
| Slope | 0.2 (−0.1, 0.5) | −0.2 (−0.6, 0.2) | −0.4 (−0.9, 0.1) | 1.53 |
| Leiter-R Forward Memory scaled score | ||||
| Intercept | 10.6 (9.4, 11.8) | 12.9 (11.7, 14) | 2.3 (0.6, 3.9) | 2.71** |
| Slope | 0.0 (−0.4, 0.3) | −0.3 (−0.7, 0.1) | −0.3 (−0.8, 0.2) | 1.08 |
| WISC-III Digit Span Forward raw score | ||||
| Intercept | 2.4 (1.8, 3.1) | 3.2 (2.6, 3.8) | 0.8 (−0.1, 1.6) | 1.85 a |
| Slope | 0.8 (0.6, 1.0) | 0.9 (0.7, 1.1) | 0.1 (−0.1, 0.4) | 0.99 |
| BRIEF Inhibit T-score | ||||
| Intercept | 58.8 (55.0, 62.6) | 50.0 (46.0, 53.9) | −8.8 (−14.3, −3.4) | 3.18** |
| Slope | −1.2 (−2.1, −0.2) | −0.4 (−1.7, 0.9) | 0.8 (−0.8, 2.4) | 0.95 |
| BRIEF Shift T-score | ||||
| Intercept | 48.9 (45.7, 52.1) | 50.7 (47.5, 54.0) | 1.8 (−2.7, 6.4) | 0.80 |
| Slope | 0.7 (−0.3, 1.6) | −1.7 (−2.9, −0.5) | −2.4 (−3.9, −0.9) | 3.08** |
| BRIEF Working Memory T-score | ||||
| Intercept | 59.7 (55.7, 63.7) | 51.7 (47.5, 55.9) | −8.0 (−13.8, −2.2) | 2.71** |
| Slope | −1.3 (−2.4, −0.2) | −1.1 (−2.5, 0.4) | 0.3 (−1.5, 2.1) | 0.28 |
Note. Values in parentheses are 95% confidence interval. Intercept is for the age of 3 years. Slope is the change in points per age-year. CI = cochlear implant; NH = normal hearing; DAS-II = Differential Ability Scales–Second Edition (T-scores); Leiter-R = International Performance Scale–Revised (scaled scores); WISC-III = Wechsler Intelligence Scale for Children–Third Edition (raw scores); BRIEF = Behavior Rating Inventory of Executive Function (T-scores).
p < .10.
p < .05.
p < .01.
p < .001.
Figure 2.
Nonverbal ability (Differential Ability Scales–Second Edition [DAS-II] Picture Similarities T-scores; Panel A) and executive functioning (Leiter-R Attention Sustained [Panel B] and Forward Memory [Panel C] scaled scores, and Wechsler Intelligence Scale for Children–Third Edition Digit Span Forward raw scores [Panel D]) development by chronological age (in years) in samples of children with cochlear implants (gray circles) or normal hearing (black triangles). Values are mean ± 1 SE.
On individually administered, performance-based tests of EF, the CI sample scored significantly lower than the NH sample on the Leiter-R Attention Sustained and Leiter-R Forward Memory subtests at the baseline age of 3 years (see Table 3 and Figure 2). This difference was due to the above-average scores on the Leiter-R subscales obtained by the NH sample, with scaled scores almost 1 SD above the mean at baseline; in contrast, the CI sample scored approximately at the normative mean at baseline. Results comparing the hearing groups on Digit Span Forward scores at baseline did not reach statistical significance (p = .066; see Table 3 and Figure 2). The samples did not differ significantly in change (slope) of scores on individually administered, performance-based tests of EF over the period of the study. Neither sample showed a significant increase or decrease on Leiter-R subscale scores, indicating development of EF at an average rate. Both groups showed improvement in Digit Span Forward raw scores, indicating growth in verbal short-term memory with age.
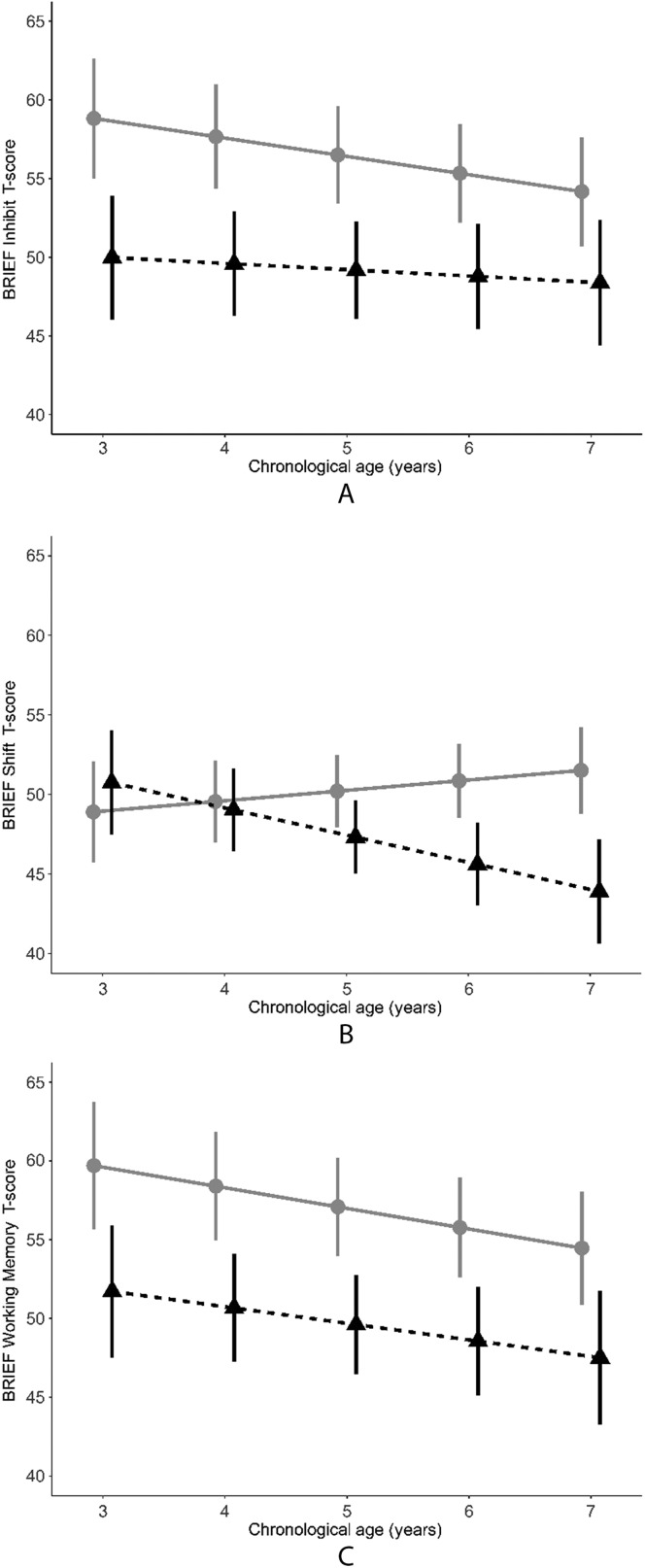
For parent-completed questionnaire (BRIEF) measures of EF, the CI sample scored significantly worse (higher) than the NH sample on the Inhibit and Working Memory subscales at baseline. The CI sample scored well above the normative mean of 50, whereas the NH sample mean BRIEF scores were not significantly different from the normative mean. No baseline difference between groups was found on the Shift subscale (see Table 3 and Figure 3). The CI sample showed significant improvement (in the form of a declining slope) on Inhibit and Working Memory subscale scores over time, whereas the NH sample showed no change on those subscales over time; however, this rate of change was not significantly different between the samples. For the Shift subscale, the NH sample showed significant improvement (declining slope) over time, but the CI sample did not; this difference in slope was statistically significant between the samples (see Table 3 and Figure 3).
Figure 3.
Parent-rated executive functioning (Behavior Rating Inventory of Executive Function [BRIEF] Inhibit [Panel A], Shift [Panel B], and Working Memory [Panel C] T-scores) development by chronological age (in years) in samples of children with cochlear implants (gray circles) or normal hearing (black triangles). Values are mean ± 1 SE.
Prediction of EF Development
NH and higher family income at earlier annual evaluations significantly predicted Leiter-R Attention Sustained and Digit Span Forward scores at later annual evaluations, while older age also predicted higher Digit Span Forward scores at later annual evaluations (see Model 1 in Table 4). No other language or demographic variable significantly predicted later Attention Sustained scores (see Model 2 in Table 4). Higher PPVT-4 scores in the combined hearing groups (main effect) predicted stronger Digit Span Forward scores at later annual evaluations (see Model 2 in Table 4). Furthermore, entry of PPVT-4 scores into the model predicting Digit Span Forward scores resulted in the substantial attenuation of terms for hearing group and income, such that neither variable predicted Digit Span Forward after the entry of PPVT-4 (see Model 2 in Table 4). For Leiter-R Forward Memory, only age at earlier annual evaluations predicted scores at subsequent annual evaluations, with younger age predicting higher Leiter-R Forward Memory scores (see Models 1 and 2 in Table 4).
Table 4.
Models predicting executive functioning development: individually administered measures.
| Model | Leiter-R Attention Sustained |
Leiter-R Forward Memory |
Digit Span Forward |
|||
|---|---|---|---|---|---|---|
| Estimate | p | Estimate | p | Estimate | p | |
| Model 1 | ||||||
| Hearing group | −1.37 (−2.59, −0.16) | .03 | −1.34 (−2.70, 0.03) | .06 | −0.99 (−1.67, −0.32) | .004 |
| Age | −0.32 (−0.69, 0.05) | .09 | −0.40 (−0.78, −0.02) | .04 | 0.62 (0.44, 0.81) | .001 |
| Sex | 0.52 (−0.62, 1.67) | .37 | −0.41 (−1.70, 0.88) | .53 | −0.30 (−0.93, 0.34) | .36 |
| Income $50–$95K a | 1.47 (0.00, 2.94) | .05 | 1.12 (−0.50, 2.74) | .17 | 0.90 (0.10, 1.70) | .03 |
| Income $95K+ a | 1.32 (0.01, 2.63) | .05 | 1.28 (−0.20, 2.75) | .09 | 0.42 (−0.32, 1.15) | .26 |
| Nonverbal ability | 0.02 (−0.02, 0.06) | .28 | 0.03 (−0.01, 0.07) | .15 | 0.00 (−0.02, 0.01) | .65 |
| Model 2 b | ||||||
| Hearing group | −1.37 (−2.59, −0.16) | .03 | −1.34 (−2.70, 0.03) | .06 | −0.31 (−1.17, 0.56) | .48 |
| Age | −0.32 (−0.69, 0.05) | .09 | −0.40 (−0.78, −0.02) | .04 | 0.57 (0.38, 0.76) | .001 |
| Sex | 0.52 (−0.62, 1.67) | .37 | −0.41 (−1.70, 0.88) | .53 | −0.21 (−0.81, 0.39) | .48 |
| Income $50–$95K a | 1.47 (0.00, 2.94) | .05 | 1.12 (−0.50, 2.74) | .17 | 0.71 (−0.06, 1.47) | .08 |
| Income $95K+ a | 1.32 (0.01, 2.63) | .05 | 1.28 (−0.20, 2.75) | .09 | 0.07 (−0.67, 0.81) | .84 |
| Nonverbal ability | 0.02 (−0.02, 0.06) | .28 | 0.03 (−0.01, 0.07) | .15 | −0.01 (−0.03, 0.01) | .50 |
| PPVT-4 | — | — | — | — | 0.02 (0.00, 0.04) | .03 |
Note. Hearing group coded as cochlear implant = 1 and normal hearing = 0. Sex coded as male = 0 and female = 1. Estimates are unstandardized regression coefficients. Values in parentheses are 95% confidence interval. Values in bold are p < .05. Leiter-R = Leiter International Performance Scale–Revised; Nonverbal ability = Differential Ability Scales–Second Edition, Picture Similarities subtest T-score; PPVT-4 = Peabody Picture Vocabulary Test–Fourth Edition, standard score.
Compared to the reference group of $0–$50K.
Neither PPVT-4 nor Preschool Language Scale–Fourth Edition met entry criteria of p < .05 for entry into the equation predicting Leiter-R Attention Sustained or Leiter-R Forward Memory.
NH at earlier annual evaluations significantly predicted lower (better) BRIEF Inhibit, Shift, and Working Memory scores at later annual evaluations (see Model 1 in Table 5). For BRIEF Shift, higher income also significantly predicted lower (better) scores. No other language or demographic variable significantly predicted BRIEF scores at later annual evaluations (see Model 2 in Table 5).
Table 5.
Models predicting executive functioning development: questionnaire measures.
| Model | BRIEF Inhibit |
BRIEF Shift |
BRIEF Working Memory |
|||
|---|---|---|---|---|---|---|
| Estimate | p | Estimate | p | Estimate | p | |
| Model 1 | ||||||
| Hearing group | 5.57 (0.47, 10.68) | 0.04 | 4.62 (0.88, 8.36) | 0.02 | 6.46 (1.49, 11.43) | 0.02 |
| Age | −0.88 (−2.01, 0.24) | 0.12 | −0.38 (−1.60, 0.84) | 0.54 | −0.56 (−1.84, 0.72) | 0.39 |
| Sex | −0.76 (−5.65, 4.12) | 0.76 | 3.03 (−0.50, 6.56) | 0.10 | 0.68 (−4.05, 5.41) | 0.77 |
| Income $50–$95K a | −0.46 (−6.30, 5.37) | 0.87 | −3.92 (−8.45, 0.61) | 0.09 | −1.72 (−7.56, 4.12) | 0.56 |
| Income $95K+ a | −3.32 (−8.84, 2.21) | 0.23 | −7.44 (−11.48, −3.40) | 0.001 | −3.71 (−9.10, 1.67) | 0.17 |
| Nonverbal ability | 0.00 (−0.12, 0.12) | 0.97 | 0.01 (−0.12, 0.13) | 0.90 | −0.09 (−0.22, 0.05) | 0.21 |
| Model 2 b | — | — | — | — | — | — |
Note. Hearing group coded as cochlear implant = 1 and normal hearing = 0. Sex coded as male = 0 and female = 1. Estimates are unstandardized regression coefficients. Values in parentheses are 95% confidence interval. Values in bold are p < .05. BRIEF = Behavior Rating Inventory of Executive Function T-score; Nonverbal ability = Differential Ability Scales–Second Edition, Picture Similarities subtest T-score.
Compared to the reference group of $0–$50K.
Neither Peabody Picture Vocabulary Test–Fourth Edition nor Preschool Language Scale–Fourth Edition met entry criteria of p < .05 for entry into the equation predicting any of the three BRIEF measures; therefore, Model 2 is the same as Model 1.
Prediction of Language Development
NH and higher family income at earlier annual evaluations significantly predicted higher PPVT-4 and PLS-4 scores at later annual evaluations (see Model 1 in Table 6), and higher nonverbal ability significantly predicted PPVT-4 scores at later annual evaluations (see Model 1 in Table 6). Lower (better) scores on BRIEF Shift predicted higher PPVT-4 scores at later annual evaluations in the CI sample only (p = .008 for the interaction effect of Shift × Hearing Group predicting PPVT-4). Hearing group, income, and nonverbal ability remained significant in the model predicting PPVT-4 with BRIEF Shift scores included, and older age also significantly predicted higher PPVT-4 scores in that model (see Model 2 in Table 6).
Table 6.
Models predicting language development.
| Model | PPVT-4 standard score |
PLS-4 Total standard score |
||
|---|---|---|---|---|
| Estimate | p | Estimate | p | |
| Model 1 | ||||
| Hearing group | −31.74 (−39.91, −23.57) | .001 | −22.04 (−29.28, −14.81) | .001 |
| Age | 1.32 (−0.05, 2.69) | .06 | −0.04 (−1.99, 1.91) | .97 |
| Sex | −0.19 (−8.11, 7.73) | .96 | 1.18 (−5.70, 8.07) | .73 |
| Income $50–$95K a | 6.54 (−2.22, 15.29) | .14 | 5.60 (−3.01, 14.21) | .20 |
| Income $95K+ a | 15.14 (6.43, 23.85) | .001 | 9.84 (1.93, 17.75) | .02 |
| Nonverbal ability | 0.17 (0.02, 0.32) | .03 | 0.19 (−0.01, 0.39) | .06 |
| Model 2 | ||||
| Hearing group | −31.65 (−39.65, −23.65) | .001 | −17.72 (−23.76, −11.68) | .001 |
| Age | 1.53 (0.18, 2.88) | .03 | −3.25 (−5.81, −0.69) | .02 |
| Sex | −0.27 (−8.04, 7.50) | .94 | 1.18 (−4.03, 6.39) | .65 |
| Income $50–$95K a | 6.05 (−2.58, 14.68) | .17 | −0.02 (−6.83, 6.78) | .99 |
| Income $95K+ a | 13.80 (5.17, 22.43) | .002 | 5.78 (−0.39, 11.96) | .07 |
| Nonverbal ability | 0.15 (0.00, 0.30) | .05 | 0.17 (−0.03, 0.37) | .09 |
| BRIEF Shift (NH) | 0.27 (−0.08, 0.61) | .13 | — | — |
| BRIEF Shift (CI) | −0.30 (−0.55, −0.06) | .02 | — | — |
| Leiter-R Forward Memory | — | — | 1.13 (0.24, 2.01) | .02 |
| Digit Span Forward (NH) | — | — | 1.19 (−1.03, 3.41) | .29 |
| Digit Span Forward (CI) | — | — | 5.27 (3.06, 7.48) | .001 |
Note. Hearing group coded as CI (cochlear implant) = 1 and NH (normal hearing) = 0. Sex coded as male = 0 and female = 1. Estimates are unstandardized regression coefficients. Values in parentheses are 95% confidence interval. Values in bold are p < .05. PPVT-4 = Peabody Picture Vocabulary Test–Fourth Edition; PLS-4 = Preschool Language Scale–Fourth Edition; Nonverbal ability = Differential Ability Scales–Second Edition, Picture Similarities subtest T-score; BRIEF = Behavior Rating Inventory of Executive Function; Leiter-R = Leiter International Performance Scale–Revised.
Compared to the reference group of $0–$50K.
For the PLS-4, higher scores on the Leiter-R Forward Memory subtest in the combined groups (main effect) and higher Digit Span Forward scores in the CI group only (interaction effect; p = .008) predicted better language outcomes. Hearing group remained significant in the model predicting PLS-4 with Leiter-R Forward Memory and Digit Span Forward scores included. Furthermore, younger age significantly predicted higher PLS-4 scores in the latter model (see Model 2 in Table 6).
Discussion
The purpose of this study was to investigate and explain the longitudinal development of spoken language and EF skills during preschool ages (3–7 years) in prelingually deaf, early-implanted CI users compared to NH peers. Four hypotheses were tested: (a) Children with CIs will lag behind NH peers in language development but will improve over time at a pace at least as fast as that of NH peers, (b) children with CIs will score lower than NH peers on performance- and questionnaire-based measures of EF and will improve in EF over time at a pace similar to that of NH peers, (c) language and nonverbal ability will predict subsequent EF abilities over time during preschool ages, and (d) EF will predict subsequent language skills over time during preschool ages. Although a few studies have investigated the longitudinal development of spoken language skills in preschool CI users, this is the first study to investigate the longitudinal development of multiple EF abilities in preschool-aged CI users beginning at very young ages (3 years) and to report the predictive association between EF and language skills across time in preschool-aged children with CIs.
The first hypothesis was that children with CIs would lag behind NH peers in language development but would improve at a pace equal to or faster than that of NH peers. Study results were consistent with this hypothesis. Children with CIs showed lower scores on measures of vocabulary (PPVT-4) and global language (PLS-4) than NH peers at baseline as well as in subsequent years but showed statistically significant improvement over time in PPVT-4 and PLS-4 scores (95% confidence interval for slope is entirely greater than 0; see Table 2). Their growth rate for PLS-4 scores was significantly greater than that of NH peers and resulted in mean PLS-4 and PPVT-4 scores at the age of 7 years that were within the broad average range for age (greater than 1 SD below the normative mean). Because PPVT-4 and PLS-4 standard scores are norm based by age, higher scores over time indicate not only growth in absolute abilities but also improvement in skills relative to the normal developmental trajectory of same-aged peers. These findings are consistent with prior studies demonstrating rapid development of spoken language skills following implantation (Niparko et al., 2010), with many children with CIs falling in the broad average range (1 SD below the normative mean or higher) by early school ages (Geers, Nicholas, & Sedey, 2003; Geers & Sedey, 2011). Hence, the present results demonstrate a cumulative benefit of cochlear implantation for the development of spoken language skills, with a developmental trajectory greater than that of norms and same-aged peers during the preschool years.
Children in the NH sample, by contrast, showed consistency over time in PPVT-4 standard scores (reflecting age-appropriate development) but a decline in PLS-4 standard scores over time. No change in PPVT-4 norm-based scores over time indicates improvement in raw scores (absolute growth in vocabulary) at the same rate as same-aged peers, which would be expected for an NH sample. The decline in PLS-4 standard scores in the NH sample is an artifact of ceiling effects for high performers beginning at 5 years of age. Specifically, at the age of 4;0, perfect performance on Auditory Comprehension and Expressive Communication subtests of the PLS-4 corresponds to standard scores of 136 and 143, respectively. However, by the age of 6;6, the highest possible scores on those subtests are 109 and 118, respectively, and missing only 1 point on each results in scores of 94 and 105, respectively. Thus, for high performers scoring above 120 at the age of 3 years, the PLS-4 ceiling effect produces a false trend of declining scores between the ages of 3 and 6 years. As a result, the PLS-4 trend line of the NH sample over ages 3–7 years should not be interpreted as valid. On the other hand, PLS-4 scores of the CI sample are well within the floor and ceiling, and therefore, the slope of increasing PLS-4 scores is valid for the CI sample.
The NH sample also showed a slight decline in nonverbal ability scores over time (DAS-II Picture Similarities T-score), despite robust coverage of the DAS-II norms at very low and very high levels of functioning. However, the 95% confidence interval for this decline included values close to 0 (−0.3, where a value of 0 would reflect no change; see Table 3), and the average decline per year in the NH sample was under 2 T-score points (see Table 3). Thus, this change may reflect a modest effect of regression to the mean or the greater value of an enriched home environment at very young ages when not all children are universally exposed to the educational environments of preschool and kindergarten. In contrast to the NH sample showing a decline in nonverbal ability scores during the period of the study, the CI sample showed a nonsignificant increase in nonverbal ability scores that was statistically significantly greater than that of the NH sample, such that by 5–6 years of age, the nonverbal ability scores of the groups were overlapping and not significantly different. Thus, while the groups differed in nonverbal ability at baseline, they did not differ in nonverbal ability for most of the age ranges in the study. It is possible that the significantly greater improvement in nonverbal ability scores in the CI sample relative to the NH sample was a downstream result of improved language skills (acting to enhance nonverbal problem solving or to improve performance on verbally mediated stimuli on the Picture Similarities subtest) and/or enriched auditory and language experiences after implantation. Further investigation of the course of and influences on nonverbal development in young children after cochlear implantation is recommended.
The second hypothesis of this study was that children with CIs would score lower than NH peers on measures of EF and would improve in EF over time at a pace similar to that of NH peers. This hypothesis was supported for most of the EF measures. Significant hearing group differences (CI poorer than NH) were found for Leiter-R Attention Sustained, Leiter-R Forward Memory, BRIEF Inhibit, and BRIEF Working Memory at baseline. Furthermore, the rate of change in scores over time did not differ between the CI and NH samples for five out of the six EF measures.
For the performance-based, individually administered Leiter-R Attention Sustained and Forward Memory tests, we found above-average performance by the NH group and average performance by the CI group compared to norms. Thus, the CI group did not show below-average performance on the Leiter-R EF measures relative to norms, but only relative to the NH control sample. However, comparison to scale norms may not be appropriate for the samples in this study, both of which had higher-than-average nonverbal ability and family income (mean income for both samples was in the $65,000–$74,999 range [see Table 1], compared to State of Indiana median income of $52,182 at www.census.gov). Furthermore, BRIEF Inhibit and Working Memory scores for the CI sample were significantly higher than the normative mean (indicating EF delays), whereas BRIEF scores for the NH sample did not differ from the normative mean. Thus, although the CI sample scored close to the normative mean on the Leiter-R EF measures, sample characteristics and BRIEF results suggest that EF delays were present, on average, in the CI sample.
The difference in Digit Span Forward raw scores between samples was of moderate magnitude but did not reach statistical significance across the age range of the study (p = .066; see Table 3). Unlike the other performance-based EF measures, the Digit Span test used in this study was not specifically designed for 3- to 5-year-olds; as a result, below the age of 6 years, the WISC-III version of forward digit span may not have been sufficiently sensitive. Counting skills emerge in the 3-year-old age range, and some 3-year-olds cannot count to 9 (Mullen, 1995), even though repetition of a span of two or three numerals forward is not uncommon in 3-year-olds (Elliott, 2007; Mullen, 1995). Furthermore, measurement of EF in general, and working memory in particular, is challenging in preschool samples (Espy et al., 2006).
Significant differences in Digit Span Forward scores were evident between the CI and NH samples in this study by the age of 5 years and persisted thereafter (see Figure 2), and in analyses controlling for demographic variables, the groups differed significantly in Digit Span Forward scores across the duration of the study (see Table 4). Hence, study results in general show differences in Digit Span Forward between samples. It is not surprising that the Digit Span Forward test was challenging for the preschool-aged participants in this study, because as a measure of working memory in that age range, it taxes not only rote immediate verbal memory but also concurrent cognitive processes of controlled attention/effort and (in children with hearing loss) rapid/automatic phonological coding used in speech perception (Kronenberger & Pisoni, 2018; Pisoni et al., 2011). We used the WISC-III Digit Span Forward test in this study to maintain congruence with our other projects, but future research should use a more age-appropriate test such as the DAS-II Recall of Digits Forward.
Finally, although BRIEF Shift scores did not differ between groups at baseline, a pattern of improving scores in the NH sample over time, coupled with a pattern of worsening scores in the CI sample (see Figure 3), resulted in significant differences between groups by the age of 7 years. Like Digit Span, variability in and mastery of flexibility and shifting may develop later in the preschool years such that differences are less detectable at younger ages.
Both groups showed no statistically significant change over time in the individually administered, performance-based EF subtests that yield normed scores (Leiter-R Attention Sustained and Forward Memory), indicating development at the same rate as age-normed peers. Both groups improved markedly in Digit Span Forward scores, consistent with the use of raw scores for Digit Span; hence, this improvement in absolute (raw score) performance does not suggest improvement relative to norms (which are not available for Digit Span Forward at preschool ages). Relative to each other, the NH and CI samples did not show differences in rate of change on any of the individually administered, performance-based EF tests. In contrast, the CI sample showed significant improvement in BRIEF Inhibit and Working Memory scores over time (indicated by negative values for the entire range of the 95% confidence interval), whereas the NH sample showed no statistically significant change in scores on those BRIEF subtests. However, change in BRIEF Inhibit and Working Memory scores was not statistically significantly different for the NH and CI samples. Additional investigation will be needed to better understand this pattern of change over time. For BRIEF Shift, CI users showed no change over time, while NH peers showed significant improvement, resulting in a statistically significant difference between the samples in BRIEF Shift change over time.
Thus, children with CIs showed delays in most domains of EF relative to NH peers as early as 3 years of age. This finding is consistent with other research showing very early differences between deaf children and NH peers in domains of cognitive information processing such as attention maintenance, visual habituation, and object exploration as early as infancy and even prior to receiving CIs (Fagan, 2019; Monroy et al., 2019). In addition, study findings indicate that EF delays in children with CIs start early, do not worsen with time, and in fact may improve for some domains with development. Because EF changes in children with CIs mirror or exceed the development of EF in normative peers, having a CI does not contribute to or worsen EF delays; rather, for some EF domains, EF development after implantation was accelerated compared to norms.
The third hypothesis of this study was that spoken language and nonverbal ability would both predict subsequent EF abilities over time. Language and nonverbal ability skills are related but distinct core constituents of global cognitive ability or intelligence (“g”; Kaufman & Lichtenberger, 2005). As a result, not surprisingly, Picture Similarities scores were associated with subsequent PPVT scores in the combined samples (see Table 6), and post hoc tests showed significant correlations (p < .05) of .38 between Picture Similarities scores and PPVT scores and .40 between Picture Similarities scores and PLS scores in the combined samples at Year 1. Hence, it was expected that the language and nonverbal ability measures, while modestly correlated (sharing less than 20% of variance), would each independently predict EF abilities.
However, in models including terms for hearing group, demographics, and nonverbal ability, this hypothesis was supported only for PPVT-4 predicting Digit Span Forward scores at subsequent annual evaluations. On the other hand, for the other five EF variables measured in this study, namely, Leiter-R Attention Sustained, Leiter-R Forward Memory, and BRIEF Inhibit, Shift, and Working Memory, language did not significantly predict EF outcome scores in models that included hearing group, demographics, and nonverbal ability. For four of those five EF measures, NH (vs. CI) significantly predicted better EF outcomes, and for the fifth EF variable (Leiter-R Forward Memory), hearing group approached significance (p < .06; see Table 4) in predicting subsequent EF. Nonverbal ability did not predict any of the EF variables in the models.
These findings indicate that language may be more highly predictive of future EF skills in domains that involve verbal, spoken language measured by individually administered, performance-based EF tests such as Digit Span Forward, in contrast to visual performance-based EF tests (Leiter-R) or EF parent report questionnaires reflecting behavior in the everyday environment (BRIEF). It is notable that the language (PPVT-4) and EF (Digit Span) tests that showed a significant predictive relationship involved the same administration methodology (both are performance-based tests individually administered in a clinic/lab setting) and the same verbal subdomain (both use spoken language). It is possible that method bias (shared variance arising from the methodology of administration; e.g., being a good “test-taker”) accounts for some of this association, but these findings are also consistent with a large body of other converging evidence demonstrating that language facilitates working memory development, particularly in CI users (Kronenberger & Pisoni, 2018, in press).
In contrast, the lack of a predictive association between language test scores and behavior checklist measures of EF, while unexpected, may reflect fundamental differences in the kinds of behaviors and abilities assessed by those measures. Specifically, parent-rating scales reflect EF behaviors in the complex, day-to-day, real-world environment, while individually administered language tests reflect language abilities without the context of application and functioning in the daily environment. It may be that a functional measure of language in daily life would more closely relate to behavior checklist measures of EF.
The lack of predictors of questionnaire-measured EF outcomes indicates the need for better knowledge about the validity and interpretation of different methods for measuring EF. It is well established that performance-based and questionnaire-based measures of EF correlate only modestly (but significantly), showing that they each measure different components of EF (Barkley, 2012). In the absence of perfect agreement between different methods of measuring a construct, a multimethod–multitrait approach to assessment is recommended (Holmbeck et al., 2002), evaluating outcomes using multiple assessment methods, as was done in the current study. Future research to explain EF outcomes should adopt a biopsychosocial systems approach (Kronenberger & Pisoni, in press), examining not only individually administered language tests but also measures of hearing quality, functional everyday language, family, and educational environments (Holt et al., 2013; Quittner et al., 2013).
Contrary to our initial expectations, nonverbal ability was not a significant predictor of EF outcomes in this study. This finding suggests that nonverbal ability and EF may not be as closely associated in preschool-aged children as we expected. Alternatively, the lack of a significant association between nonverbal ability and EF may be a result of less stable or valid results for nonverbal ability obtained at younger ages, or nonverbal ability may have been related to EF but not over and above the variance accounted for by hearing group and income measures. Furthermore, both samples in this study had above-average mean nonverbal ability scores; in samples with a wider range of nonverbal ability, including more participants in below-average ranges, nonverbal ability may relate with EF. Another possibility is that this study used only one measure of nonverbal ability (DAS-II Picture Similarities) that relied heavily on concept formation and categorization skills and that may have allowed for some verbal mediation. Other types of nonverbal reasoning, such as analogic reasoning or sequential reasoning (Hammill et al., 2009), might relate better to EF outcomes. Further investigation of the role of nonverbal ability in early EF development of children with CIs is recommended.
It is also notable that hearing group significantly predicted EF for all questionnaire measures (BRIEF), for one of the three performance-based EF measures (Leiter-R Attention Sustained), and nearly for a second performance-based EF measure (Leiter-R Forward Memory), even when language scores were tested for entry into Model 2. These findings indicate that language does not fully explain EF differences between NH and CI samples of preschool children during early development, consistent with theories positing multiple influences on EF outcomes in children with hearing loss, including not only language but also early auditory experience, family environment, and educational interventions (Kronenberger & Pisoni, in press).
Our fourth hypothesis was that EF would predict subsequent language skills over time during preschool ages. For PPVT-4, BRIEF Shift scores in the CI sample (but not in the NH sample) predicted vocabulary scores at the next annual evaluation. The BRIEF Shift scale measures mental flexibility in problem solving and the ability to transition easily from one component of a problem/situation to another. Thus, children with CIs who had more flexibility, openness, and tolerance for transitions had better vocabulary at later annual evaluations, perhaps as a result of openness and exposure to new vocabulary and learning experiences. This finding is consistent with recent findings showing that children with CIs who had less controlling families also had stronger receptive vocabularies (Holt et al., 2013).
EF domains reflecting short-term/working memory significantly predicted PLS-4 scores at subsequent annual evaluations, over and above the contributions of hearing group and demographic variables. Furthermore, Digit Span Forward was more predictive of PLS-4 scores in the CI sample than in the NH sample. The EF domain of working memory may be particularly influential in language outcomes of children with CIs, based on predictions from the Ease of Language Understanding (Rönnberg et al., 2013) and Framework for Understanding Effortful Listening (Pichora-Fuller et al., 2016) theories that posit that children with CIs must engage more directed, focused mental effort during language processing tasks than children with NH as a result of underspecified, coarsely coded internal representations of language in long-term memory.
Findings regarding the predictive relationship of EF for future language skills are consistent with contemporary models positing that EF influences language development and language functioning, both for basic vocabulary and for global language skills (Kronenberger & Pisoni, 2018). For children with CIs, EF appears to play a greater role in language functioning than for NH peers, as demonstrated by significant interaction effects in the mixed-effects models. This finding replicates earlier effects found in cross-sectional studies (Kronenberger, Colson, et al., 2014) and experimental studies (Kronenberger et al., 2018) showing that EF is particularly important in language processing of CI users. Although flexibility/shifting and verbal working memory showed the strongest predictive relationships with language outcomes, it is possible that relationships between other EF variables and language outcomes were statistically significant but did not add to the predictive power of shifting/flexibility and working memory. Additional work with larger samples is recommended to investigate associations of other domains of EF and language outcomes.
Summary, Limitations, and Future Research
This is the first longitudinal study of EF development in preschool-aged children with CIs as young as 3 years of age and the first longitudinal study investigating the predictive associations between EF and language outcomes in preschool-aged children with CIs. The results of this study demonstrate that children with CIs are at risk for EF delays beginning at very early preschool ages and that EF in preschoolers with CIs develops at an average or faster rate compared to NH peers. Language predicted verbal working memory in both CI and NH samples but was not predictive of parent-reported EF behaviors in daily life or of performance-based EF measures presented in the visual modality. Some domains of EF (flexibility/shifting and verbal working memory) were predictive of future language skills, although predominantly in the CI sample alone. Thus, some reciprocal predictive associations between EF and language skills were found for children with CIs during preschool ages, but these associations were not present for all measures and did not entirely account for future language or EF outcomes. This pattern of results is consistent with a complex system of reciprocal biopsychosocial influences (Kronenberger & Pisoni, in press) operating to determine language and EF outcomes in preschool CI users.
These findings provide guidance for potential novel approaches to assessment and intervention for very young children with CIs. Specifically, findings indicate that delays in EF can be measured as young as the age of 3 years and that children with CIs may develop an elevated risk of EF delays at very early preschool ages. As a result, assessment and intervention to address EF delays in at-risk children with CIs should occur during preschool ages if possible. Improvement of EF skills with environmental and behavioral interventions at preschool ages has been demonstrated (Blair & Raver, 2014; Diamond & Lee, 2011) and should be implemented as needed for children with CIs who show EF delays. Furthermore, study results demonstrate a predictive link between EF development and language skills in children with CIs, suggesting a potential value for integrating EF interventions into speech-language interventions for children with CIs, although one caveat of this study is the small sample size. Future research should use larger samples and should investigate the efficacy of early, integrated interventions involving both EF and language skills in preschool children with CIs.
Study results should be interpreted with several limitations in mind. First, the study involved rolling entry and annual assessment of participants over a 6-year period, resulting in unequal numbers of annual evaluations and different ages at the time of entry. Second, the sample sizes, while large compared to the existing literature on preschool children with CIs, allowed for detection of medium or greater effect sizes; small effect sizes may have been present and would not have been detected as significant because of sample size. Relatedly, nonsignificant results that approached statistical significance should be interpreted with caution, and considerable variability was seen in both the CI and NH samples. Third, differences in nonverbal ability between the CI and NH samples were found at baseline, although nonverbal ability differences were not present after the age of 5–6 years. Nevertheless, given the well above-average nonverbal ability and language scores in the NH group, it is likely that NH volunteers for the study were high functioning, particularly for language ability. In order to address this issue, we included nonverbal ability as a term in all predictive mixed models. Fourth, the groups differed in a categorical measure that divided family income into three categories, with the CI sample showing more participants in the high category and the NH sample showing more participants in the middle category. Both samples had higher income than the median for the state in which the study was conducted, consistent with nonverbal ability results suggesting that samples were from more advantaged backgrounds. As a result, income was also controlled in all analyses predicting outcomes. Finally, PLS-4 results in the NH sample should be interpreted with caution because of ceiling effects at the ages of 5–6 years and because of a lack of 7-year-old norms (6;11 norms were used for 7-year-olds). In contrast, the PLS-4 was a good measure of language development for the CI sample because it has a low floor and is developmentally appropriate for very young children with language delays.
An important component of study methodology is the method and scope of measurement of EF. Measurement of EF in very young children is challenging because of limited understanding, motivation, and stamina. Classic EF tests often are too difficult for young children to understand, requiring the use of a smaller set of tests that may have floor effects or limited validity (Espy et al., 1999). Behavior checklist measures, on the other hand, are affected not only by the target construct but also by rater characteristics including insight, bias, attitude, understanding of questions, and awareness of child behavior. Thus, measurement of EF in very young children has numerous challenges that should be addressed and understood in interpretation of results. The recommended method for addressing the complexity of EF measurement, a multimethod–multitrait approach, was adopted in this study in order to provide the most valid and comprehensive assessment of EF in this preschool population.
Given the limitations of study methodology, it is important to view the present results in the context of existing research and as the catalyst for future validating research. For example, given study limitations, the lack of predictive value of early language for future EF found in the mixed-effects models in this study should be regarded with caution, as the sample size was small and other EF measures may be tested in the future. Future research should investigate additional predictors of language and EF outcomes in children with CIs, including family, education, early language exposure, and audiological factors. For example, length and intensity of exposure to sign language prior to implantation may relate to later outcomes. Given that EF–language associations are different for children with CIs and children with NH, within-group analyses specifically focusing on explanation of individual differences in CI samples, independent of factors explaining EF in NH populations, are recommended. Variability in EF and language outcomes within CI samples demonstrates that, while a proportion of CI users are at risk for suboptimal outcomes, many pediatric CI users display average or better outcomes. Investigating resilience in children with CIs who show average or better development of language and EF skills offers the potential for understanding factors contributing to positive outcomes and for suggesting novel interventions to assist children at risk for suboptimal outcomes.
Acknowledgments
This work was supported by the National Institute on Deafness and Other Communication Disorders (R01DC015257 to William G. Kronenberger and David B. Pisoni and R01DC009581 to David B. Pisoni). The authors thank Shirley Henning, Allison Ditmars, Bethany Colson, and Caitlin Montgomery for their help with this project.
Funding Statement
This work was supported by the National Institute on Deafness and Other Communication Disorders (R01DC015257 to William G. Kronenberger and David B. Pisoni and R01DC009581 to David B. Pisoni).
References
- Alderson-Day B., & Fernyhough C. (2015). Inner speech: Development, cognitive functions, phenomenology, and neurobiology. Psychological Bulletin, 141(5), 931–965. https://doi.org/10.1037/bul0000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley R. A. (1997). ADHD and the nature of self-control. Guilford Press. [Google Scholar]
- Barkley R. A. (2012). Executive functions: What they are, how they work, and why they evolved. Guilford Press. [Google Scholar]
- Beer J., Kronenberger W. G., Castellanos I., Colson B. G., Henning S. C., & Pisoni D. B. (2014). Executive functioning skills in preschool-age children with cochlear implants. Journal of Speech, Language, and Hearing Research, 57(4), 1521–1534. https://doi.org/10.1044/2014_JSLHR-H-13-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C., & Raver C. C. (2014). Closing the achievement gap through modification of neurocognitive and neuroendocrine function: Results from a cluster randomized controlled trial of an integrative approach to the education of children in kindergarten. PLOS ONE, 9(11), e112393 https://doi.org/10.1371/journal.pone.0112393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos I., Pisoni D. B., Kronenberger W. G., & Beer J. (2016). Early expressive language skills predict long-term neurocognitive outcomes in cochlear implant users: Evidence from the MacArthur–Bates CDI. American Journal of Speech-Language Pathology, 25(3), 381–392. https://doi.org/10.1044/2016_AJSLP-15-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M., Pisoni D. B., & Kirk K. I. (2000). Working memory spans as predictors of spoken word recognition and receptive vocabulary in children with cochlear implants. The Volta Review, 102(4), 259–280. [PMC free article] [PubMed] [Google Scholar]
- Conway C. M., & Pisoni D. B. (2008). Neurocognitive basis of implicit learning of sequential structure and its relation to language processing. Annals of the New York Academy of Sciences, 1145(1), 113–131. https://doi.org/10.1196/annals.1416.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway C. M., Pisoni D. B., & Kronenberger W. G. (2009). The importance of sound for cognitive sequencing abilities: The auditory scaffolding hypothesis. Current Directions in Psychological Science, 18(5), 275–279. https://doi.org/10.1111/j.1467-8721.2009.01651.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A. (2013). Executive functions. Annual Review of Psychology, 64, 135–168. https://doi.org/10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A., & Lee K. (2011). Interventions shown to aid executive function development in children 4 to 12 years old. Science, 333(6045), 959–964. https://doi.org/10.1126/science.1204529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebel S., Dickerson J. P., Hoover J. D., & Munakata Y. (2018). Using language to get ready: Familiar labels help children engage proactive control. Journal of Experimental Child Psychology, 166, 147–159. https://doi.org/10.1016/j.jecp.2017.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L. M., & Dunn D. M. (2007). Peabody Picture Vocabulary Test–Fourth Edition (PPVT-4). Pearson Assessments. [Google Scholar]
- Eisenberg L. S., Widen J. E., Yoshinaga-Itano C., Norton S., Thal D., Niparko J. K., & Vohr B. (2007). Current state of knowledge: Implications for developmental research—Key issues. Ear and Hearing, 28(6), 773–777. https://doi.org/10.1097/aud.0b013e318157f06c [DOI] [PubMed] [Google Scholar]
- Elliott C. D. (2007). Differential Ability Scales (2nd ed.). The Psychological Corporation. [Google Scholar]
- Engle R. W., Tuholski S. W., Laughlin J. E., & Conway A. R. A. (1999). Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General, 128(3), 309–331. https://doi.org/10.1037/0096-3445.128.3.309 [DOI] [PubMed] [Google Scholar]
- Espy K. A., Bull R., Martin J., & Stroup W. (2006). Measuring the development of executive control with the shape school. Psychological Assessment, 18(4), 373–381. https://doi.org/10.1037/1040-3590.18.4.373 [DOI] [PubMed] [Google Scholar]
- Espy K. A., Kaufmann P. M., Glisky M. L., & McDiarmid M. D. (2001). New procedures to assess executive functions in preschool children. The Clinical Neuropsychologist, 15(1), 46–58. https://doi.org/10.1076/clin.15.1.46.1908 [DOI] [PubMed] [Google Scholar]
- Espy K. A., Kaufmann P. M., McDiarmid M. D., & Glisky M. L. (1999). Executive functioning in preschool children: Performance on A-not-B and other delayed response format tasks. Brain and Cognition, 41(2), 178–199. https://doi.org/10.1006/brcg.1999.1117 [DOI] [PubMed] [Google Scholar]
- Fagan M. K. (2019). Exploring in silence: Hearing and deaf infants explore objects differently before cochlear implantation. Infancy, 24(3), 338–355. https://doi.org/10.1111/infa.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueras B., Edwards L., & Langdon D. (2008). Executive function and language in deaf children. Journal of Deaf Studies and Deaf Education, 13(3), 362–377. https://doi.org/10.1093/deafed/enm067 [DOI] [PubMed] [Google Scholar]
- Fischer K. W., & van Geert P. (2014). Dynamic development of brain and behavior. In Molenaar P. C. M., Lerner R. M., & Newell K. M. (Eds.), Handbook of developmental systems theory and methodology (pp. 287–315). Guilford Press. [Google Scholar]
- Ganek H., McConkey Robbins A., & Niparko J. K. (2012). Language outcomes after cochlear implantation. Otolaryngologic Clinics of North America, 45(1), 173–185. https://doi.org/10.1016/j.otc.2011.08.024 [DOI] [PubMed] [Google Scholar]
- Gathercole S. E., & Baddeley A. D. (1993). Working memory and language. Erlbaum. [Google Scholar]
- Geers A. E. (2002). Factors affecting the development of speech, language, and literacy in children with early cochlear implantation. Language, Speech, and Hearing Services in Schools, 33(3), 172–183. https://doi.org/10.1044/0161-1461(2002/015) [DOI] [PubMed] [Google Scholar]
- Geers A. E. (2006). Factors influencing spoken language outcomes in children following early cochlear implantation. Advances in Oto-Rhino-Laryngology, 64, 50–65. https://doi.org/10.1159/000094644 [DOI] [PubMed] [Google Scholar]
- Geers A. E., & Brenner C. (2003). Background and educational characteristics of prelingually deaf children implanted by five years of age. Ear and Hearing, 24(Suppl. 1), 2S–14S. https://doi.org/10.1097/01.aud.0000051685.19171.bd [DOI] [PubMed] [Google Scholar]
- Geers A. E., Brenner C., & Davidson L. (2003). Factors associated with development of speech perception skills in children implanted by age five. Ear and Hearing, 24(1), 24S–35S. https://doi.org/10.1097/01.aud.0000051687.99218.0f [DOI] [PubMed] [Google Scholar]
- Geers A. E., & Nicholas J. G. (2013). Enduring advantages of early cochlear implantation for spoken language development. Journal of Speech, Language, and Hearing Research, 56(2), 643–655. https://doi.org/10.1044/1092-4388(2012/11-0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers A. E., Nicholas J. G., & Sedey A. L. (2003). Language skills of children with early cochlear implantation. Ear and Hearing, 24(1), 46S–58S. https://doi.org/10.1097/01.aud.0000051689.57380.1b [DOI] [PubMed] [Google Scholar]
- Geers A. E., Pisoni D. B., & Brenner C. (2013). Complex working memory span in cochlear implanted and normal hearing teenagers. Otology & Neurotology, 34(3), 396–401. https://doi.org/10.1097/MAO.0b013e318277a0cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers A. E., & Sedey A. L. (2011). Language and verbal reasoning skills in adolescents with 10 or more years of cochlear implant experience. Ear and Hearing, 32(1), 39S–48S. https://doi.org/10.1097/AUD.0b013e3181fa41dc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia G. A., Espy K. A., & Isquith P. K. (2003). Behavior Rating Inventory of Executive Function–Preschool Version. Psychological Assessment Resources. [Google Scholar]
- Gioia G. A., Isquith P. K., Guy S. C., & Kenworthy L. (2000). BRIEF: Behavior Rating Inventory of Executive Function professional manual. Psychological Assessment Resources. [Google Scholar]
- Goldenberg D., & Galván A. (2015). The use of functional and effective connectivity techniques to understand the developing brain. Developmental Cognitive Neuroscience, 12, 155–164. https://doi.org/10.1016/j.dcn.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammill D. D., Pearson N. A., & Wiederholt J. L. (2009). Comprehensive test of nonverbal intelligence (2nd ed.). Pro-Ed. [Google Scholar]
- Harris M. S., Kronenberger W. G., Gao S., Hoen H. M., Miyamoto R. T., & Pisoni D. B. (2013). Verbal short-term memory development and spoken language outcomes in deaf children with cochlear implants. Ear and Hearing, 34(2), 179–192. https://doi.org/10.1097/AUD.0b013e318269ce50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmbeck G. N., Li S. T., Schurman J. V., Friedman D., & Coakley R. M. (2002). Collecting and managing multisource and multimethod data in studies of pediatric populations. Journal of Pediatric Psychology, 27(1), 5–18. https://doi.org/10.1093/jpepsy/27.1.5 [DOI] [PubMed] [Google Scholar]
- Holt R. F., Beer J., Kronenberger W. G., & Pisoni D. B. (2013). Developmental effects of family environment on outcomes in pediatric cochlear implant recipients. Otology & Neurotology, 34(3), 388–395. https://doi.org/10.1097/MAO.0b013e318277a0af [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn D. L., Davis R. A. O., Pisoni D. B., & Miyamoto R. T. (2004). Visual attention, behavioral inhibition and speech/language outcomes in deaf children with cochlear implants. International Congress Series/Excerpta Medica, 1273, 332–335. https://doi.org/10.1016/j.ics.2004.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibertsson T., Hansson K., Asker-Àrnason L., Sahlén B., & Mäki-Torkko E. (2009). Speech recognition, working memory and conversation in children with cochlear implants. Deafness & Education International, 11(3), 132–151. https://doi.org/10.1002/dei.261 [Google Scholar]
- Kaufman A. S., & Lichtenberger E. O. (2005). Assessing adolescent and adult intelligence (3rd ed.). Wiley. [Google Scholar]
- Kral A., Kronenberger W. G., Pisoni D. B., & O'Donoghue G. M. (2016). Neurocognitive factors in sensory restoration of early deafness: A connectome model. The Lancet Neurology, 15(6), 610–621. https://doi.org/10.1016/S1474-4422(16)00034-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger W. G., Beer J., Castellanos I., Pisoni D. B., & Miyamoto R. T. (2014). Neurocognitive risk in children with cochlear implants. JAMA Otolaryngology—Head & Neck Surgery, 140(7), 608–615. https://doi.org/10.1001/jamaoto.2014.757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger W. G., Colson B. G., Henning S. C., & Pisoni D. B. (2014). Executive functioning and speech-language skills following long-term use of cochlear implants. Journal of Deaf Studies and Deaf Education, 19(4), 456–470. https://doi.org/10.1093/deafed/enu011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger W. G., Henning S. C., Ditmars A. M., & Pisoni D. B. (2018). Language processing fluency and verbal working memory in prelingually deaf long-term cochlear implant users: A pilot study. Cochlear Implants International, 19(6), 312–323. https://doi.org/10.1080/14670100.2018.1493970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger W. G., & Pisoni D. B. (2016). Working memory training in deaf children with cochlear implants. In Young N. M. & Kirk K. I. (Eds.), Pediatric cochlear implantation: Learning and the brain (pp. 275–292). Springer. [Google Scholar]
- Kronenberger W. G., & Pisoni D. B. (2018). Neurocognitive functioning in deaf children with cochlear implants. In Knoors H. & Marschark M. (Eds.), Evidence-based practices in deaf education. Oxford. [Google Scholar]
- Kronenberger W. G., & Pisoni D. B. (in press). Why are children with cochlear implants at risk for executive functioning delays: Language only or something more. In Marschark M. & Knoors H. (Eds.), Oxford handbook of deaf studies in learning and cognition. Oxford. [Google Scholar]
- Kronenberger W. G., Pisoni D. B., Harris M. S., Hoen H. M., Xu H., & Miyamoto R. T. (2013). Profiles of verbal working memory growth predict speech and language development in children with cochlear implants. Journal of Speech, Language, and Hearing Research, 56(3), 805–825. https://doi.org/10.1044/1092-4388(2012/11-0356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberger W. G., Pisoni D. B., Henning S. C., & Colson B. G. (2013). Executive functioning skills in long-term users of cochlear implants: A case control study. Journal of Pediatric Psychology, 38(8), 902–914. https://doi.org/10.1093/jpepsy/jst034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyxell B., Sahlén B., Wass M., Ibertsson T., Larsby B., Hällgren M., & Mäki-Torkko E. (2008). Cognitive development in children with cochlear implants: Relations to reading and communication. International Journal of Audiology, 47(Suppl. 2), S47–S52. https://doi.org/10.1080/14992020802307370 [DOI] [PubMed] [Google Scholar]
- Miyake A., Friedman N. P., Emerson M. J., Witzki A. H., Howerter A., & Wager T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. https://doi.org/10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- Monroy C., Shafto C., Castellanos I., Bergeson T., & Houston D. (2019). Visual habituation in deaf and hearing infants. PLOS ONE, 14(2), e0209265 https://doi.org/10.1371/journal.pone.0209265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. M. (1995). Mullen Scales of Early Learning: AGS Edition. NCS Pearson. [Google Scholar]
- Nicholas J. G., & Geers A. E. (2013). Spoken language benefits of extending cochlear implant candidacy below 12 months of age. Otology & Neurotology, 34(3), 532–538. https://doi.org/10.1097/MAO.0b013e318281e215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niparko J. K., Tobey E. A., Thal D. J., Eisenberg L. S., Wang N.-Y., Quittner A. L., Fink N. E., & CDaCI Investigative Team. (2010). Spoken language development in children following cochlear implantation. JAMA, 303(15), 1498–1506. https://doi.org/10.1001/jama.2010.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittrouer S., Caldwell-Tarr A., & Lowenstein J. H. (2013). Working memory in children with cochlear implants: Problems are in storage, not processing. International Journal of Pediatric Otorhinolaryngology, 77(11), 1886–1898. https://doi.org/10.1016/j.ijporl.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer-Karpf A. (2012). The dynamic matching of neural and cognitive growth cycles. Nonlinear Dynamics, Psychology, and Life Sciences, 16(1), 61–78. [PubMed] [Google Scholar]
- Pichora-Fuller M. K., Kramer S. E., Eckert M. A., Edwards B., Hornsby B. W. Y., Humes L. E., Lemke U., Lunner T., Matthen M., Mackersie C. L., Naylor G., Phillips N. A., Richter M., Rudner M., Sommers M. S., Tremblay K. L., & Wingfield A. (2016). Hearing impairment and cognitive energy: The framework for understanding effortful listening (FUEL). Ear and Hearing, 37(Suppl. 1), 5S–27S. https://doi.org/10.1097/AUD.0000000000000312 [DOI] [PubMed] [Google Scholar]
- Pisoni D. B., Conway C. M., Kronenberger W., Henning S., & Anaya E. (2010). Executive function, cognitive control, and sequence learning in deaf children with cochlear implants. In Marshark M. & Spencer P. E. (Eds.), The Oxford handbook of deaf studies, language, and education (Vol. 2, pp. 439–457). Oxford University Press; https://doi.org/10.1093/oxfordhb/9780195390032.013.0029 [Google Scholar]
- Pisoni D. B., Kronenberger W. G., Harris M. S., & Moberly A. C. (2018). Three challenges for future research on cochlear implants. World Journal of Otorhinolaryngology—Head and Neck Surgery, 3(4), 240–254. https://doi.org/10.1016/j.wjorl.2017.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoni D. B., Kronenberger W. G., Roman A. S., & Geers A. E. (2011). Measures of digit span and verbal rehearsal speed in deaf children after more than 10 years of cochlear implantation. Ear and Hearing, 32(Suppl. 1), 60S–74S. https://doi.org/10.1097/AUD.0b013e3181ffd58e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittner A. L., Cruz I., Barker D. H., Tobey E., Eisenberg L. S., Niparko J. K., & Childhood Development after Cochlear Implantation Investigative Team. (2013). Effects of maternal sensitivity and cognitive and linguistic stimulation on cochlear implant users' language development over four years. The Journal of Pediatrics, 162(2), 343–348.e343. https://doi.org/10.1016/j.jpeds.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid G. H., & Miller L. J. (1997). Leiter International Performance Scale–Revised. Stoelting Company. [Google Scholar]
- Rönnberg J., Lunner T., Zekveld A., Sörqvist P., Danielsson H., Lyxell B., Dahlström O., Signoret C., Stenfelt S., Pichora-Fuller M. K., & Rudner M. (2013). The Ease of Language Understanding (ELU) model: Theoretical, empirical, and clinical advances. Frontiers in Systems Neuroscience, 7(31). https://doi.org/10.3389/fnsys.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg J., Rudner M., Foo C., & Lunner T. (2008). Cognition counts: A working memory system for ease of language understanding (ELU). International Journal of Audiology, 47(Suppl. 2), S99–S105. https://doi.org/10.1080/14992020802301167 [DOI] [PubMed] [Google Scholar]
- Roth R. M., Erdodi L. A., McCulloch L. J., & Isquith P. K. (2015). Much ado about norming: The Behavior Rating Inventory of Executive Function. Child Neuropsychology, 21(2), 225–233. https://doi.org/10.1080/09297049.2014.897318 [DOI] [PubMed] [Google Scholar]
- Roth R. M., Isquith P. K., & Gioia G. A. (2014). Assessment of executive functioning using the Behavior Rating Inventory of Executive Function (BRIEF). In Goldstein S. & Naglieri J. A. (Eds.), Handbook of executive functioning (pp. 301–331). Springer Science + Business Media; https://doi.org/10.1007/978-1-4614-8106-5_18 [Google Scholar]
- Ruffin C. V., Kronenberger W. G., Colson B. G., Henning S. C., & Pisoni D. B. (2013). Long-term speech and language outcomes in prelingually deaf children, adolescents and young adults who received cochlear implants in childhood. Audiology and Neuro-Otology, 18(5), 289–296. https://doi.org/10.1159/000353405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov Y. R., Martinez-Monedero R., & Niparko J. K. (2012). Cochlear implants: Clinical and societal outcomes. Otolaryngologic Clinics of North America, 45(5), 959–981. https://doi.org/10.1016/j.otc.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Singer B. D., & Bashir A. S. (1999). What are executive functions and self-regulation and what do they have to do with language-learning disorders. Language, Speech, and Hearing Services in Schools, 30(3), 265–273. https://doi.org/10.1044/0161-1461.3003.265 [DOI] [PubMed] [Google Scholar]
- Stiles J., Brown T. T., Haist F., & Jernigan T. L. (2015). Brain and cognitive development. In Liben L. S., Müller U., & Lerner R. M. (Eds.), Handbook of child psychology and developmental science, Vol. 2: Cognitive processes (7th ed., pp. 9–62). Wiley & Sons. [Google Scholar]
- Tobey E. A., Thal D., Niparko J. K., Eisenberg L. S., Quittner A. L., Wang N.-Y., & The CDaCI Investigative Team. (2013). Influence of implantation age on school-age language performance in pediatric cochlear implant users. International Journal of Audiology, 52(4), 219–229. https://doi.org/10.3109/14992027.2012.759666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geert P. (2009). Nonlinear complex dynamical systems in developmental psychology. In Guastello S. J., Koopmans M., & Pincus D. (Eds.), Chaos and complexity in psychology: The theory of nonlinear dynamical systems (pp. 242–281). Cambridge University Press. [Google Scholar]
- Wang Y., Bergeson T. R., & Houston D. M. (2017). Infant-directed speech enhances attention to speech in deaf infants with cochlear implants. Journal of Speech, Language, and Hearing Research, 60(11), 3321–3333. https://doi.org/10.1044/2017_JSLHR-H-17-0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass M., Ibertsson T., Lyxell B., Sahlén B., Hällgren M., Larsby B., & Mäki-Torkko E. (2008). Cognitive and linguistic skills in Swedish children with cochlear implants—Measures of accuracy and latency as indicators of development. Scandinavian Journal of Psychology, 49(6), 559–576. https://doi.org/10.1111/j.1467-9450.2008.00680.x [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1991). Wechsler Intelligence Scale for Children–Third Edition. The Psychological Corporation. [Google Scholar]
- Willstedt-Svensson U., Löfqvist A., Almqvist B., & Sahlén B. (2004). Is age at implant the only factor that counts? The influence of working memory on lexical and grammatical development in children with cochlear implants. International Journal of Audiology, 43(9), 506–515. https://doi.org/10.1080/14992020400050065 [DOI] [PubMed] [Google Scholar]
- Ylvisaker M., & DeBonis D. (2000). Executive function impairment in adolescence: TBI and ADHD. Topics in Language Disorders, 20(2), 29–57. https://doi.org/10.1097/00011363-200020020-00005 [Google Scholar]
- Zimmerman I. L., Steiner V. G., & Pond R. E. (2002). Preschool Language Scale–Fourth Edition. The Psychological Corporation. [Google Scholar]