Abstract
Purpose
Infants with autism spectrum disorder (ASD) produce fewer play actions and gestures than neurotypical infants (e.g., Mastrogiuseppe et al., 2015; Veness et al., 2012; Zwaigenbaum et al., 2005). The purpose of this study was to investigate whether different “types” of actions and gestures are more or less likely to develop atypically in ASD.
Method
We examined eight types of actions and gestures longitudinally from ages 8 to 14 months in 80 infants with a heightened risk for developing ASD by virtue of having an affected older sibling (high risk [HR]; e.g., Ozonoff et al., 2011) and 25 infants with no such familial risk (low risk). Data were collected using the MacArthur–Bates Communicative Development Inventories (Fenson et al., 1994, 1993).
Results
HR infants later diagnosed with ASD showed less growth across nearly all types of actions and gestures compared to the low-risk comparison group. Importantly, these HR infants who were later diagnosed with ASD also exhibited reduced growth in frequent deictic gestures and in actions that involve object manipulation relative to HR infants with non-ASD language delay.
Conclusions
During infancy, it is challenging for clinicians to distinguish ASD from other early communicative delays (e.g., Camarata, 2014). Our results indicate that deictic gestures, as well as actions and gestures involving object manipulation, may be useful targets of surveillance strategies for HR infants and could support early detection efforts for ASD.
During the first years of life, infants produce a variety of play actions and gestures to interact and communicate with social partners (e.g., Bates et al., 1979; Caselli et al., 2012). These actions and gestures enable preverbal infants to express desires (e.g., pointing to an out-of-reach toy), initiate shared attention (e.g., showing an object to a parent), and engage in functional and symbolic play (e.g., pretending to feed a doll). However, infants and children with autism spectrum disorder (ASD) produce many fewer actions and gestures than their neurotypical peers (see Ramos-Cabo et al., 2019, for a review). Indeed, our previous work finds substantially less growth in actions and gestures during the first 14 months among infants later diagnosed with ASD compared to neurotypical infants (Iverson et al., 2018). However, questions remain about the “types” of actions and gestures used by infants later diagnosed with ASD. Individual actions and gestures vary widely in the cognitive and motor demands they place on the child. There may be variability in the degree to which particular types of actions and gestures are affected in ASD. To address this gap in current knowledge, we further analyzed data from this sample of infants (Iverson et al., 2018) and compared the development of eight distinct types of actions and gestures among neurotypical infants and infants later diagnosed with ASD.
Because ASD is usually diagnosed after 40 months of age (D. L. Christensen et al., 2016)—long after the onset of actions and gestures in typical development—and because ASD is a relatively rare neurodevelopmental disorder (2.5% prevalence in the general population; e.g., Kogan et al., 2018; Xu et al., 2019), we examined actions and gestures in a prospective study of infants who have an older sibling with ASD. The ASD recurrence rate for these infants is estimated at 18.7% (e.g., Ozonoff et al., 2011), putting them at heightened risk (HR) of receiving an ASD diagnosis. This study involved longitudinal examination of eight types of actions and gestures between the ages of 8 and 14 months (a period when a variety of these behaviors emerge) in a sample of HR infants and a comparison group of infants with no family history of ASD (low risk [LR]). We explored whether growth trajectories for individual action and gesture types differed for HR infants later diagnosed with ASD relative to HR and LR infants who received no ASD diagnosis.
The Development of Early Actions and Gestures in Neurotypical Infants
Neurotypical infants begin to produce actions and gestures during the first year of life (e.g., Bates et al., 1979). Early actions and gestures have been assessed with a variety of instruments, including parent report (e.g., Fenson et al., 1994, 1993), video-recorded observations (e.g., Capirci et al., 2005), and experimenter-administered standardized assessments (e.g., the Early Social Communication Scales; Mundy et al., 2003). Parent report is a highly effective tool for capturing the full range of actions and gestures that infants produce. Many of these behaviors are produced infrequently (e.g., blowing kisses, pretending to use a vacuum, blowing on food to indicate that it is hot) and are easily missed in the narrow windows of time captured by observational measures. Parent report measures draw on the parents' daily experience with infants and are well suited to capture the breadth of infants' repertoires.
In particular, actions and gestures are often examined using the MacArthur–Bates Communicative Development Inventories (CDI), a parent report instrument that includes 63 distinct behaviors (Fenson et al., 1994, 1993). The CDI organizes behaviors into five categories, which follow a clear developmental progression (Caselli et al., 2012; Fenson et al., 1994). Items in the First Communicative Gestures category include deictic gestures (e.g., pointing) and culturally defined conventional gestures (e.g., shaking the head for “yes” or “no”). Infants begin to produce these behaviors between the ages of 9 and 13 months (Bates et al., 1979, 1975). At the same time, infants begin to acquire behaviors included in the Games and Routines category, which includes actions learned in the context of social interactions (e.g., call-and-response games like peekaboo).
Subsequent categories capture later emerging and more sophisticated behaviors. At the end of the first year, infants begin to produce Actions With Objects, behaviors that require knowledge of an object's function (e.g., brushing teeth with a toothbrush). Just a few months later, infants “Pretending to Be a Parent” by extending behaviors to a stuffed animal or doll (e.g., pretending to brush a doll's teeth, rather than their own teeth). The distinction is important because it provides initial evidence of symbolic understanding, as the infant is not merely repeating a ritualized action. The final category, Imitating Other Adult Actions, develops in parallel, emerging at around 14 months of age (e.g., typing at a keyboard). These actions are likely learned by observing functional actions produced by others and reproducing them independently.
The behaviors in these five categories reflect different underlying skills. For instance, those included in First Communicative Gestures are largely social bids, which serve to communicate information to a caregiver (e.g., reaching to request). Conversely, items in other categories focus on play skills that may be performed outside any social context. For instance, Actions With Objects captures infants' functional knowledge of objects, and Pretending to Be a Parent captures infants' symbolic understanding. Thus, these categories do not reflect different levels of one cohesive domain. Rather, they tap into distinct communication and play abilities.
Additionally, the motoric complexity of individual actions and gestures may also affect when in development they emerge (e.g., Sparaci & Volterra, 2017). In particular, actions and gestures that involve object manipulation are considered to be more motorically challenging than empty-handed actions and gestures (e.g., waving bye-bye). To illustrate, one item on the Actions With Objects category of the CDI asks whether infants are able to stir a spoon in a cup. At minimum, this action requires the infant to stabilize the cup with one hand while simultaneously placing the spoon correctly and stirring. This is a challenging motor sequence for an infant. In fact, actions with object manipulation tend to emerge later in development than empty-handed behaviors, regardless of which CDI category they appear in (Caselli et al., 2012). The ability to produce individual actions and gestures—in particular, those with objects—is likely influenced by infants' motoric ability, in addition to their cognitive, symbolic, and communicative skills.
The Development of Early Actions and Gestures in ASD
ASD is a neurodevelopmental disorder characterized by challenges with verbal and nonverbal communication and the presence of restricted or repetitive behaviors (American Psychiatric Association, 2013). The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) specifically includes “deficits in understanding and use of gestures” in the diagnostic criteria, which has prompted researchers to investigate the ontogeny of atypical gesture use. The literature is replete with findings indicating that gesture production is atypical in very young children with ASD by the second year (e.g., Mitchell et al., 2006; Rozga et al., 2011; Watson et al., 2013; Zwaigenbaum et al., 2005). From 12 months of age, infants with ASD gesture less frequently than neurotypical infants (e.g., Choi et al., 2019; Mitchell et al., 2006; Wetherby et al., 2004; Zwaigenbaum et al., 2005), coordinate gestures with other communicative behaviors (e.g., vocalizations) less frequently (Choi et al., 2019; Heymann et al., 2018; Parladé & Iverson, 2015; Sowden et al., 2013), and use gestures to establish social interactions less frequently (Rozga et al., 2011; Watson et al., 2013; Wetherby & Prutting, 1984).
Additionally, patterns of play actions differ in infants with ASD compared to neurotypical peers (e.g. Campbell et al., 2016; L. Christensen et al., 2010; Landa et al., 2007; Pierce, 2009; Mulligan & White, 2012; Wilson et al., 2017). Toddlers with ASD spend more time exploring object properties, perform fewer functional play actions (e.g., stirring a spoon in a bowl), and perform fewer symbolic play actions (e.g., pretending to feed teddy bear) than both neurotypical infants and infants with non-ASD developmental delays (e.g., Boucher, 1999; L. Christensen et al., 2010; Williams, 2003).
In light of the well-documented differences in both gestures and play actions, researchers have used the CDI to measure the developmental time course by which these behaviors emerge in ASD (Iverson et al., 2018; Mastrogiuseppe et al., 2015; Mitchell et al., 2006; Veness et al., 2012). However, this work has focused on the “overall sizes” of infants' repertoires, rather than on specific “types” of actions and gestures. Understanding how various types of actions and gestures develop in ASD may be important. Our previous work indicates that infants later diagnosed with ASD exhibit reduced growth in the development of actions and gestures compared to neurotypical infants. Importantly, however, group differences were amplified for later emerging actions and gestures (a category that included Actions With Objects, Pretending to Be a Parent, and Imitating Adult Actions) compared to earlier emerging actions and gestures (which included First Communicative Gestures and Games and Routines). Moreover, recent work suggests that deictic gestures (i.e., pointing, showing, reaching to request, showing objects to others) are affected to a greater extent than other conventional gestures in toddlers with ASD (Manwaring et al., 2019).
Together, this evidence supports the notion that different types of actions and gestures may be differentially affected in ASD. This makes intuitive sense, as the individual behaviors that make up this category vary in significant ways. Some behaviors are necessarily social (e.g., handing an object to a parent); others may be performed in isolation (e.g., throwing a ball). Some require symbolic knowledge of an object (e.g., pretending to feed a doll with a spoon). For others, it is difficult to differentiate the symbolic understanding from “the thing itself” (e.g., drinking from a cup). Moreover, actions and gestures vary in their motoric complexity. Because actions that involve object manipulation are more motorically difficult, they may be particularly impacted in ASD. There are well-documented motor difficulties among infants and toddlers with ASD (see West, 2019, for meta-analytic review). Fine motor disruptions could constrain early actions and gestures—especially those with objects (e.g., see Iverson et al., 2018).
An investigation of the development of different types of actions and gestures will enhance our understanding of early communicative processes in ASD, knowledge that can be leveraged to distinguish ASD from other early communicative delays in toddlers and very young children. Making this distinction presents a number of challenges for clinicians (e.g., Camarata, 2014), but the ability to do so is critically important—particularly for HR infants, who experience elevated rates of non-ASD language delay (e.g., Marrus et al., 2018; Yirmiya et al., 2007). A similar approach has been useful in understanding language development in ASD. For example, a recent study by Bruyneel et al. (2019) found that examining specific components of language ability—phonology, grammar, semantics, and pragmatics—was effective in characterizing the language delays of HR infants above and beyond more holistic measures of language. We adopted this approach to the development of early actions and gestures in light of previously reviewed evidence indicating that (a) individual behaviors in this category reflect different underlying abilities and (b) actions and gestures develop differently among infants with ASD compared to neurotypical infants and infants with non-ASD communicative delays.
This Study
This study extended prior work (Iverson et al., 2018) by examining whether the shape of developmental change in early actions and gestures differed for three groups of HR infants who varied in developmental outcomes at the age of 3 years: those who later received an ASD diagnosis (HR-ASD), those who exhibited delayed language but “no” ASD diagnosis (language delay; HR-LD), and those who appeared to be developing typically (no diagnosis; HR-ND). The primary aim was to assess whether growth patterns in particular types of actions and gestures differed for the HR-ASD and HR-LD groups. We modeled growth using item-level data from the Actions and Gestures section of the CDI, which was completed by parents each month from infant ages 8 to 14 months. For purposes of comparison, data from a group of infants with no family history of ASD (LR) were also included.
Method
Participants
This study included two cohorts of infants followed in two separate longitudinal studies. The first consisted of 80 infants (40 girls) with an older full biological sibling with a diagnosis of autistic disorder (AD) under DSM-IV-TR criteria (American Psychiatric Association, 2000), confirmed via administration of the Autism Diagnostic Observation Schedule–Generic (Lord et al., 2000). Participants were recruited through a university autism research program, local agencies serving families of autistic individuals, and word of mouth. The second cohort included 25 LR infants (15 girls) with no first- or second-degree relatives with ASD. LR infants were recruited through publicly available birth records and word of mouth. All HR and LR infants were from monolingual English-speaking households and were born full term from uncomplicated pregnancies.
Demographics of the two cohorts were generally similar; there were no significant group differences in participants' sex, ethnicity, or parent education. Consistent with prior studies of HR infants, mothers and fathers of HR infants were significantly older than those of LR infants. Demographic data for the sample and group comparisons are presented in Table 1.
Table 1.
Demographic characteristics of low-risk (LR) and high-risk (HR) samples.
| Characteristic | LR (n = 25) |
HR (n = 80) |
||
|---|---|---|---|---|
| Sex | ||||
| Female (%) | 10 | (40) | 40 | (50) |
| Male (%) | 15 | (60) | 40 | (50) |
| Racial or ethnic minority (%) | 2 | (8) | 10 | (12.5) |
| M age for mothers (SD)* | 31.92 | (4.95) | 34.19 | (4.17) |
| M age for fathers (SD)* | 33.16 | (4.47) | 36.31 | (4.77) |
| Mean parent education a (SD) | 1.38 | (0.51) | 1.19 | (0.50) |
Parent education based on averaging education scores for mothers and fathers: 0 = high school, 1 = some college or college degree, 2 = graduate or professional school.
HR and LR groups significantly differ (p < .05).
Procedure
HR and LR infants were visited in their homes, though observation schedules differed across studies. HR infants were visited monthly from 5 to 14 months of age, with follow-up visits at 18, 24, and 36 months of age. LR infants were seen every 2 weeks from 2 to 19 months, and there were no follow-up visits in the toddler years. The current study utilized data from the 8-, 9-, 10-, 11-, 12-, 13-, and 14-month time points for both LR and HR infants (i.e., we did not include data from half-month time points for any LR infant). The general procedure at all visits involved videotaping infants for approximately 45 min as they engaged in unstructured and semistructured play with a primary caregiver.
Dependent Measures
At each visit between the ages of 8 and 14 months, primary caregivers completed the CDI: Words and Gestures form (CDI-I; Fenson et al., 1993). The CDI-I form is a parent report measure of receptive and productive vocabulary and early communicative actions and gestures. It has excellent internal consistency (rs = .95–.96), test–retest reliability (rs = .80–.90), and concurrent validity with experimenter-administered measures (r = .72; Bates et al., 1988; Fenson et al., 1994). Moreover, it is sensitive to language delays in infancy (Dale et al., 1989; Fenson et al., 1994, 1993; Heilmann et al., 2005; Miller et al., 1995; Thal et al., 1999). This study focused on data from the Actions and Gestures portion of the CDI-I. Using items from this measure, we created 10 dependent variables: four focused on gestures, four focused on play actions, and two that compared behaviors with and without object manipulation.
Gestures
The CDI measures 12 infant gestures, which we divided into two categories: Deictic Gestures and Nondeictic Gestures (see Table 2 for additional detail about items). Parents indicate whether their infant produces each gesture “often,” “sometimes,” or “not yet.” Final data included four dependent variables. First, we computed infants' repertoires of Deictic Gestures by summing the number of deictic gestures produced (i.e., an infant would receive equal credit for a gesture regardless of whether it was produced “often” or “sometimes”). Second, we calculated infant repertoires of Frequent Deictic Gestures by summing the number of deictic gestures that infants produced “often.” This process was repeated to calculate each infants' repertoire of Nondeictic Gestures and their repertoire of Frequent Nondeictic Gestures.
Table 2.
Definitions and information about the eight categories of actions and gestures.
| Action and gesture variables | Definition | No. items |
|---|---|---|
| Deictic Gestures | Ritualized requesting, showing objects to a parent, giving objects to a parent, pointing with an index finger. Here, we summed behaviors regardless of whether caregivers indicated they were produced “often” or “sometimes.” | 4 |
| Frequent Deictic Gestures | Behaviors were the same as in Deictic Gestures. However, here we summed only behaviors that caregivers reported were produced “often.” | 4 |
| Nondeictic Gestures | Early-appearing communicative gestures, e.g., nodding the head “yes,” shaking the head “no,” waving bye-bye. Here, we sum behaviors regardless of whether caregivers indicated they were produced “often” or “sometimes.” | 8 |
| Frequent Nondeictic Gestures | Behaviors were the same as in Nondeictic Gestures. However, here we summed only behaviors caregivers reported were produced “often.” | 8 |
| Games and Routines | Social games (e.g., playing peekaboo, “so big,” singing, dancing). | 6 |
| Actions With Objects | Culturally defined actions performed on associated objects, e.g., holding a comb or brush to the hair, eating with a spoon or fork. | 17 |
| Pretending to Be a Parent | Actions performed on a stuffed animal or doll, e.g., brushing the hair of a doll or stuffed animal (as opposed to their own hair), feeding a doll with a spoon. | 13 |
| Imitating Other Adult Actions | Actions with objects traditionally carried out by caregivers, e.g., pretending to vacuum, pretending to type at a computer. | 15 |
| Actions and Gestures With Object Manipulation a | Of the 63 total items, 43 items are behaviors that involve holding and manipulating objects (e.g., holding a plane and making it fly, brushing teeth with a toothbrush). We calculated the proportion of the 43 items with object manipulation that parents endorsed as being produced by their infant. | 43 |
| Empty-Handed Actions and Gestures a | Of the 63 total items, 20 items are behaviors that do not involve object manipulation (e.g., dancing, nodding the head to indicate “yes”). We calculated the proportion of the 20 empty-handed items that parents endorsed as being produced by their infant. | 20 |
Proportions were computed for Actions and Gestures With Object Manipulation and Empty-Handed Actions and Gestures in order to permit direct comparison between these variables, considering the total numbers of items in each category differed.
Actions
The CDI includes 51 items that measure infants' play actions, which were divided into four categories: Games and Routines, Actions With Objects, Pretending to Be a Parent, and Imitating Other Adult Actions. For each item, parents selected “yes” or “no” to indicate whether or not their infant has begun to produce a given action. We summed the number of behaviors that infants performed within each category (e.g., to calculate the total number of Actions With Objects that the infant had begun to produce). Detailed information about the content of these categories is provided in Table 2.
Actions and Gestures With Versus Without Object Manipulation
Finally, we created two variables to examine behaviors that require object manipulation (and therefore are more motorically complex) versus those that do not. Of the 63 items measuring all actions and gestures, 43 involved object manipulation. For each infant, we calculated the proportion of these 43 behaviors that parents endorsed as being produced by their infant (Actions and Gestures With Object Manipulation). The 20 remaining items described behaviors that did not require object manipulation (e.g., blowing to indicate something is hot). We calculated the proportion of these 20 items that parents endorsed as being performed by their infant (Empty-Handed Actions and Gestures). Proportions allowed for direct comparison between Actions and Gestures With Object Manipulation and Empty-Handed Actions and Gestures (see Table 2 for additional information).
Outcome Measures and Classification
For HR infants only, additional measures were collected at 18, 24, and 36 months of age for purposes of outcome classification. The Mullen Scales of Early Learning (MSEL; Mullen, 1995) was administered when HR infants were 18, 24, and 36 months old. Additionally, at 18 months old, caregivers completed either the CDI-I (described above) or the CDI: Words and Sentences form (CDI-II), a 680-item vocabulary checklist in which caregivers are asked to report which words their infant says. The CDI-II also includes questions regarding the morphology and syntax of the infants' language. The form parents received depended on the infants' language ability. If the child had very few words (as indicated by the caregiver), the caregiver completed the CDI-I. If the infant was producing words frequently or combining words, the caregiver completed the CDI-II. When HR infants were 24 months old, caregivers completed the CDI-II. At 36 months old, caregivers completed the CDI: Words Produced (CDI-III), a 100-item checklist in which caregivers are asked to report on words their infant says, their grammatical complexity, and the semantics and pragmatics of speech. Finally, at 36 months old, HR infants were administered the Autism Diagnostic Observation Scale–Revised (Lord et al., 2000) by a research-reliable clinician naïve to previously collected data and study hypotheses.
HR infants were classified as HR-ASD if they met or exceeded the algorithm cutoffs for ASD or AD on scores on the Autism Diagnostic Observation Scale–Revised “and” they received a clinical best estimate diagnosis of AD or Pervasive Developmental Disorder–Not Otherwise Specified using DSM-IV-TR criteria (these evaluations took place before the publication of the DSM-5). Using these criteria, 11 HR infants (four girls) were diagnosed with ASD 1 (HR-ASD).
HR infants were classified as demonstrating language delay (HR-LD) if they “did not” receive an ASD diagnosis “and” met one or both of the following criteria (e.g., Iverson et al., 2018; Parladé & Iverson, 2015):
standardized scores on the CDI-II and/or CDI-III at or below the 10th percentile at “more than one time point” between 18 and 36 months of age and
standardized scores on the CDI-III at or below the 10th percentile and standardized scores on the Receptive and/or Expressive subscale of the MSEL equal to or greater than 1.5 SDs below the mean at 36 months of age.
These criteria were developed for the purpose of identifying children who demonstrated a pattern of delayed language development (not to provide clinical diagnosis). They have been used previously to identify language delay in both community and HR samples (e.g., Gershkoff-Stowe et al., 1997; Heilmann et al., 2005; Ozonoff et al., 2010; Parladé & Iverson, 2015; Robertson & Weismer, 1999; Weismer & Evans, 2002). Using these criteria, 24 infants (12 boys) were classified as HR-LD. By definition, all HR-LD infants exhibited delays in expressive language (i.e., had scores below the 10th percentile on the CDI-III at least once). In addition, 18 of 24 HR-LD infants also exhibited delays in receptive language (as measured by the MSEL). Delays in other domains were also observed among HR-LD infants. At 36 months of age, eight HR-LD infants had low scores (i.e., 1.5 SDs below the mean) on the MSEL Fine Motor subscale, two infants displayed low scores on the Visual Reception subscale, and one infant displayed low scores on both subscales.
The remaining 45 HR infants (21 boys) were classified as having no delay or diagnosis (HR-ND). Table 3 provides additional information on each of these HR outcome groups by presenting descriptive data from the MSEL, including the Visual Reception, Fine Motor, Receptive Language, and Expressive Language subscale scores at 36 months of age. LR infants were not followed during the toddler years, and the MSEL was not administered to them. There were never any developmental concerns expressed by parents or researchers by the final 19-month visit, and no infants received any early intervention services.
Table 3.
Means and standard deviations for Mullen Scales of Early Learning (MSEL) subscale t scores for high-risk (HR) outcome groups at 36 months of age.
| Group | MSEL subscale scores |
|||
|---|---|---|---|---|
| Visual Reception | Fine Motor | Receptive Language | Expressive Language | |
| HR-ND (n = 45) |
58.36 (12.13) | 48.89 (12.08) | 53.69 (8.44) | 57.81 (8.54) |
| HR-LD (n = 24) |
51.75 (14.32) | 42.13 (12.68) | 43.67 (8.23) | 47.79 (9.86) |
| HR-ASD (n = 11) |
31.71 (15.02) | 26.00 (7.72) | 28.25 (11.42) | 32.33 (11.95) |
Note. HR-ND = HR infants with no diagnosis; HR-LD = HR infants with language delay; HR-ASD = HR infants with autism spectrum disorder.
Analytic Approach
The central aim of this study was to model growth trajectories of categories of actions and gestures in relation to HR infants' 36-month outcome classification (HR-ND, HR-LD, HR-ASD), using the LR infants as a reference group. We utilized hierarchical linear modeling (HLM), which is optimally suited for data with a nested hierarchical structure (Bryk & Raudenbush, 1992). HLM models assessed variation in the dependent variables described above at two levels: Level 1 assessed within-subject variation across time points, nested within individual infants, and Level 2 assessed between-subjects variation, with individual infants nested within outcome groups. Additionally, HLM accommodates unequally spaced or missing data (Huttenlocher et al., 1991; Singer, 1998; Willett et al., 1998). For our sample, 632 of 735 (86%) observations were complete. Table 4 presents information on the numbers of complete observations across outcome groups and time points. Data were analyzed using Version 7 of HLM for Windows (Raudenbush et al., 2011).
Table 4.
The number and percentage of complete observations for each outcome group across time points.
| Group | Age in months |
||||||
|---|---|---|---|---|---|---|---|
| 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| LR | 25 (100%) | 16 (64%) | 16 (64%) | 18 (72%) | 16 (64%) | 18 (82%) | 18 (82%) |
| HR-ND | 40 (89%) | 42 (93%) | 43 (95%) | 42 (93%) | 44 (98%) | 45 (100%) | 43 (95%) |
| HR-LD | 19 (79%) | 21 (88%) | 20 (83%) | 22 (92%) | 22 (92%) | 23 (96%) | 22 (92%) |
| HR-ASD | 7 (64%) | 8 (72%) | 11 (100%) | 10 (91%) | 11 (100%) | 11 (100%) | 10 (91%) |
Note. LR = low-risk infants; HR-ND = HR infants with no diagnosis; HR-LD = HR infants with language delay; HR-ASD = HR infants with autism spectrum disorder.
For each variable, we began by running an unconditional linear model—that is, with AGE (in months) at Level 1 and no other predictors. Second, we ran an unconditional quadratic model (i.e., with predictors AGE and AGE2 only). The quadratic model was selected for the final model if “both” of the following criteria were met:
The quadratic term (AGE2) was significant in the unconditional model.
A chi-square deviance test indicated that the quadratic model explained significantly more variance than the linear model.
If one of these criteria was not met, the linear model was retained for parsimony.
Final Linear Models
For linear models, Level 1 estimated individual linear growth from 8 to 14 months of age as a function of AGE. We chose to center the data at the initial age point (8 months) to provide information about infants' repertoires of actions and gestures at the time of entry. The equation for Level 1 is as follows:
| (1) |
Here, the intercept (π0i) represents an infant i's score at 8 months of age. The term π1i represents the linear slope—the rate and direction of change across the period—for infant i.
For Level 2, time-invariant variables (i.e., variables that remain constant over the period, e.g., sex) were included as predictors of the intercept and linear slope. This included a dummy variable for each HR outcome classification group (HR-ND, HR-LD, and HR-ASD); the LR infants served as a reference group. In addition, a dummy variable for Sex was included as a control. The final Level 2 equations for the prediction model were as follows:
| (2) |
| (3) |
Here, coefficients (the β terms) represent the deviation of each HR group from the LR reference group. For instance, β00 represents the LR group's score at the intercept, and β02 represents the deviation of the HR-ND group from the LR group.
Final Quadratic Models
For quadratic models, Level 1 estimated individual growth from 8 to 14 months of age as a function of AGE and AGE2. Again, we centered the data at 8 months of age. The equation for Level 1 is as follows:
| (4) |
The intercept (π0i) represents an infant i's score at 8 months of age. In this quadratic model, the term π1i represents the instantaneous linear slope for infant i—this term indicates the rate and the direction of change “at the intercept.” The term π2i represents the quadratic growth—acceleration or deceleration over time—for infant i.
Again, Level 2 included dummy variables for each outcome group (HR-ND, HR-LD, and HR-ASD) and Sex as predictors of the intercept, instantaneous linear slope, and quadratic slope from Level 1. The equations for Level 2 are as follows:
| (5) |
| (6) |
| (7) |
These models allow us to determine whether each HR group differed significantly from the LR group, but they do not permit comparisons between the HR subgroups. For this reason, we supplemented each primary model with planned follow-up analyses wherein we rotated the reference group in order to conduct comparisons between HR groups.
Results
This study compared growth trajectories for different action and gesture types for three outcome groups of HR infants (HR-ND, HR-LD, and HR-ASD) to those of a comparison group of LR infants. This approach enabled us to measure the shape of change for each group and the extent to which trajectories for HR groups diverged from those of infants with no family history of ASD. Primary model estimates for each of the final models can be found in Tables 5, 6, and 7. Results for each of these categories will be discussed in turn.
Table 5.
Final model estimates for trajectories of gesture types with outcome group and sex.
| Variable | Deictic Gestures |
Frequent Deictic Gestures |
Nondeictic Gestures |
Frequent Nondeictic Gestures |
||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | Coefficient | SE | Coefficient | SE | |
| Intercept, β00 | 1.302*** | 0.211 | 0.024 | 0.133 | 1.394*** | 0.199 | 0.485*** | 0.133 |
| Sex, β01 | 0.470 | 0.228 | 0.144 | 0.125 | 0.072 | 0.192 | 0.001 | 0.133 |
| HR-ND, β02 | −0.215 | 0.275 | −0.126 | 0.161 | −0.189 | 0.244 | −0.198 | 0.175 |
| HR-LD, β03 | −0.602 | 0.322 | −0.227 | 0.187 | −0.852** | 0.284 | −0.501** | 0.171 |
| HR-ASD, β04 | −0.440 | 0.471 | 0.495* | 0.244 | −0.733* | 0.335 | −0.360 | 0.195 |
| Instantaneous linear slope, β10 | 0.928*** | 0.116 | 0.666*** | 0.057 | 0.723*** | 0.074 | 0.480*** | 0.071 |
| Sex, β11 | −0.082 | 0.141 | −0.027 | 0.049 | 0.002 | 0.060 | −0.011 | 0.055 |
| HR-ND, β12 | −0.285 | 0.163 | −0.169* | 0.067 | −0.163† | 0.084 | −0.115 | 0.079 |
| HR-LD, β13 | −0.443* | 0.187 | −0.344*** | 0.076 | −0.157 | 0.100 | −0.146 | 0.094 |
| HR-ASD, β14 | −0.242 | 0.258 | −0.450*** | 0.095 | −0.185 | 0.118 | −0.115 | 0.106 |
| Quadratic slope, β20 | −0.084*** | 0.017 | — | — | — | — | — | — |
| Sex, β21 | 0.006 | 0.021 | ||||||
| HR-ND, β22 | 0.055* | 0.024 | ||||||
| HR-LD, β23 | 0.084** | 0.028 | ||||||
| HR-ASD, β24 | 0.031 | 0.031 | ||||||
Note. Em dashes indicate that a linear model was used (and thus, there was no quadratic term). SE = standard error; HR-ND = high risk with no diagnosis; HR-LD = high risk with language delay; HR-ASD = high risk with autism spectrum disorder.
p < .05.
p < .01.
p < .001.
p < .10.
Table 6.
Final model estimates for trajectories of action types with outcome group and sex.
| Variable | Games and Routines |
Actions With Objects |
Pretending to Be Parent |
Imitating Other Actions |
||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | Coefficient | SE | Coefficient | SE | |
| Intercept, β00 | 1.486** | 0.263 | 2.824*** | 0.750 | 0 | 4.282*** | 1.196 | |
| Sex, β01 | −0.646* | 0.290 | −1.806* | 0.752 | 0 | −0.504 | 0.819 | |
| HR-ND, β02 | 0.032 | 0.349 | −0.708 | 0.965 | 0 | −1.853 | 1.332 | |
| HR-LD, β03 | −0.824* | 0.356 | −2.388** | 0.847 | 0 | −1.840 | 1.294 | |
| HR-ASD, β04 | −0.466 | 0.537 | −2.357* | 0.951 | 0 | −4.337* | 1.699 | |
| Instantaneous linear slope, β10 | 0.616*** | 0.098 | −0.008 | 0.490 | 0.660*** | 0.193 | −0.570 | 0.405 |
| Sex, β11 | 0.152 | 0.083 | −0.291 | 0.262 | −0.002 | 0.137 | −0.360 | 0.236 |
| HR-ND, β12 | −0.122 | 0.117 | 0.616 | 0.518 | −0.001 | 0.220 | 0.943* | 0.439 |
| HR-LD, β13 | −0.025 | 0.118 | 0.716 | 0.537 | −0.393 | 0.212 | 0.384 | 0.430 |
| HR-ASD, β14 | −0.151 | 0.128 | 0.648 | 0.594 | −0.561** | 0.203 | 0.922 | 0.579 |
| Quadratic slope, β20 | — | — | 0.242*** | 0.060 | — | — | 0.199 | 0.058 |
| Sex, β21 | 0.127** | 0.041 | 0.064 | 0.042 | ||||
| HR-ND, β22 | −0.125 | 0.068 | −0.139* | 0.067 | ||||
| HR-LD, β23 | −0.143* | 0.067 | −0.050 | 0.066 | ||||
| HR-ASD, β24 | −0.208** | 0.076 | −0.186* | 0.087 | ||||
Note. Em dashes indicate that a linear model was used (and thus, there was no quadratic term). SE = standard error; HR-ND = high risk with no diagnosis; HR-LD = high risk with language delay; HR-ASD = high risk with autism spectrum disorder.
p < .05.
p < .01.
p < .001.
Table 7.
Final model estimates of the proportions of Actions and Gestures With Object Manipulation and Empty-Handed Actions and Gestures, including outcome group and sex.
| Variable | Proportion of Actions and Gestures With Object Manipulation |
Proportion of Empty-Handed Actions and Gestures |
||
|---|---|---|---|---|
| Coefficient | SE | Coefficient | SE | |
| Intercept, β00 | 0.145*** | 0.044 | 0.172*** | 0.022 |
| Sex, β01 | −0.081 | 0.042 | −0.053* | 0.026 |
| HR-ND, β02 | −0.019 | 0.056 | −0.011 | 0.031 |
| HR-LD, β03 | −0.119* | 0.047 | −0.107*** | 0.031 |
| HR-ASD, β04 | −0.130* | 0.051 | −0.059 | 0.039 |
| Instantaneous linear slope, β10 | 0.001 | 0.026 | 0.091*** | 0.008 |
| Sex, β11 | −0.021 | 0.013 | 0.0126 | 0.007 |
| HR-ND, β12 | 0.028 | 0.027 | −0.018 | 0.010 |
| HR-LD, β13 | 0.016 | 0.028 | −0.015 | 0.011 |
| HR-ASD, β14 | 0.033 | 0.032 | −0.03** | 0.011 |
| Quadratic slope, β10 | 0.009** | 0.003 | — | — |
| Sex, β11 | 0.006** | 0.002 | ||
| HR-ND, β12 | −0.004 | 0.004 | ||
| HR-LD, β13 | −0.002 | 0.003 | ||
| HR-ASD, β14 | −0.009* | 0.004 | ||
Note. Em dashes indicate that a linear model was used (and thus, there was no quadratic term). SE = standard error; HR-ND = high risk with no diagnosis; HR-LD = high risk with language delay; HR-ASD = high risk with autism spectrum disorder.
p < .05.
p < .01.
p < .001.
Gestures
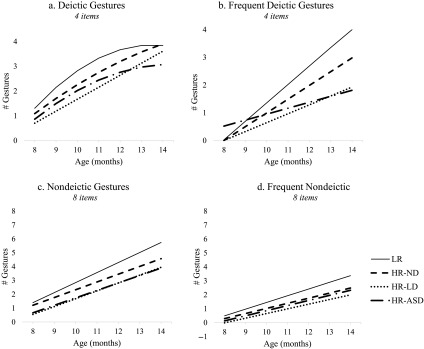
Deictic Gestures
Estimated trajectories for Deictic Gestures are shown in Figure 1a. LR infants displayed positive growth with a gradual deceleration over time. At 8 months of age, LR infants produced about 1.31 of the 4 total Deictic Gestures on average. By 14 months of age, this increased to 3.84—they were essentially at ceiling. The HR-ND and HR-LD groups also displayed positive growth but showed less deceleration over time than LR infants (ps = .025 and .004, respectively). However, both HR-ND and HR-LD groups had also reached ceiling by 14 months of age, producing approximately 3.91 and 3.61 Deictic Gestures, respectively. HR-ASD infants displayed a similar pattern as LR infants and did not differ on any parameter. Additionally, follow-up analyses revealed that HR-ASD and HR-LD groups did not differ from one another on any parameter.
Figure 1.
Estimated growth trajectories for Gesture categories by outcome group from 8 to 14 months of age. LR = low risk; HR-ND = high risk with no diagnosis; HR-LD = high risk with language delay; HR-ASD = high risk with autism spectrum disorder.
Frequent Deictic Gestures
Estimated trajectories for Frequent Deictic Gestures—those gestures reported by parents as being produced often by their infants—are shown in Figure 1b. LR infants displayed positive growth over time. At 8 months of age, LR infants produced almost none deictic gestures frequently, only 0.02 gestures on average. However, they showed dramatic growth over time and, by 14 months of age, produced all four deictic gestures frequently.
Every HR group differed from the LR reference group, and they also differed from one another. The HR-ND infants exhibited less growth than LR infants (p = .014), and by 14 months of age, they produced 2.98 deictic gestures frequently. The HR-LD infants experienced even less growth over time, diverging from both the LR group, p < .001, and the HR-ND group, p = .009. At 14 months of age, HR-LD infants produced only 1.92 of deictic gestures frequently.
By contrast, the HR-ASD infants produced “more” of deictic gestures frequently at 8 months of age than LR, HR-ND, and HR-LD infants (ps = .045, .007, and .004, respectively). But the HR-ASD infants showed the slowest growth rate of any group, significantly differing from the LR and HR-ND groups (all ps < .001). Functionally, this meant that HR-ASD and HR-LD infants were comparable to one another by 14 months of age (respectively producing 1.81 and 1.92 of deictic gestures frequently).
Nondeictic Gestures
Estimated trajectories for Nondeictic Gestures are shown in Figure 1c. LR infants displayed positive linear growth over time. At 8 months of age, LR infants produced 1.39 of the 8 total Nondeictic Gestures on average. This increased to 5.73 by 14 months of age. HR-ND infants displayed a similar pattern as LR infants and did not differ significantly on any model parameter.
Compared to LR infants, HR-LD and HR-ASD groups started out with fewer Nondeictic Gestures at 8 months of age, on average producing only 0.54 and 0.66 of the eight items, respectively (ps = .003 and .031, respectively). Over time, HR-LD and ASD infants increased at a similar rate as LR infants, and therefore, the initial gap was maintained over time. Follow-up analyses revealed that the HR-ASD and HR-LD infants did not differ from one another on any parameter.
Frequent Nondeictic Gestures
Estimated trajectories for Frequent Nondeictic Gestures (i.e., only nondeictic gestures that infants produced often) are shown in Figure 1d. LR infants displayed positive growth over time. At 8 months of age, LR infants produced very few nondeictic gestures frequently (0.48 gestures on average). By 14 months of age, they produced 3.37 of the 8 total Frequent Nondeictic Gestures. HR-ND infants displayed a similar pattern to LR infants and did not differ significantly on any model parameter.
The HR-LD infants produced fewer nondeictic gestures frequently, relative to the LR group at 8 months of age. In fact, none of the HR-LD infants produced a single nondeictic gesture frequently at 8 months of age. However, over time, HR-LD infants increased at a similar rate as LR infants, and therefore, the initial gap was maintained. The HR-ASD infants did not differ from the LR infants or any other HR group on any model parameter.
Actions
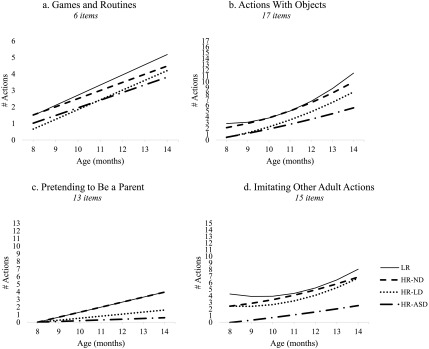
Games and Routines
Estimated trajectories for Games and Routines are shown in Figure 2a, showing that the LR infants displayed positive linear growth. At 8 months of age, LR infants produced 1.49 of the 6 total Games and Routines on average. This increased to 5.18 by 14 months of age. The HR-ND and HR-ASD groups displayed a similar pattern to LR infants and did not differ on any parameter. In contrast, compared to LR infants, HR-LD infants produced fewer Games and Routines at 8 months of age (only 0.66 of the 6 total), p = .023. Because they increased at a rate similar to LR infants, the initial gap was maintained over time. Follow-up analyses revealed that the HR-ASD and HR-LD infants did not differ from one another on any parameter.
Figure 2.
Estimated growth trajectories for Action categories by outcome group from 8 to 14 months of age. LR = low risk; HR-ND = high risk with no diagnosis; HR-LD = high risk with language delay; HR-ASD = high risk with autism spectrum disorder.
Actions With Objects
Estimated trajectories for Actions With Objects are shown in Figure 2b. LR infants displayed initially flat growth and accelerated over time. At 8 months of age, LR infants were reported to produce 2.82 of the 17 total Actions With Objects on average and increased to 11.49 by 14 months of age. HR-ND infants displayed a similar pattern to LR infants and did not differ on any parameter. Relative to LR infants, HR-LD and HR-ASD infants produced fewer Actions With Objects at 8 months of age (0.44 and 0.47 of the 17 total, respectively), ps = .006 and .015, respectively. They also displayed reduced acceleration compared to LR infants (ps = .035 and .008, respectively), and accordingly, their trajectories diverged from that of the LR infants over time. By 14 months of age, HR-LD infants were reported to produce 8.25 of the Actions With Objects on average, and HR-ASD infants were producing 5.52 (less than half of the LR infants' repertoire). Follow-up analyses revealed that HR-LD and HR-ASD infants did not differ significantly from one another on any parameter.
Pretending to Be a Parent
Estimated trajectories for Pretending to Be a Parent are shown in Figure 2c. Note that almost none of the infants produced these actions at 8 months of age (only six of the 105 infants), and the intercept was not significantly different from zero, p = .15. For this reason, we fixed the 8-month intercept to zero and estimated growth terms. LR infants displayed positive linear growth over time, increasing by approximately 0.66 actions each month. By the 14-month visit, they produced 3.96 of the 13 total Pretending to Be a Parent actions. The HR-ND group displayed a similar pattern to LR infants and did not differ on any parameter.
HR-LD and HR-ASD infants increased at a significantly slower rate than LR infants (ps = .07 and .01, respectively), with nearly flat growth over time. Even at the final 14-month time point, HR-ASD infants produced very few Pretending to Be a Parent actions: Seven HR-ASD infants never produced any, and the other four produced only one to two Pretending to Be a Parent actions. Follow-up analyses revealed that HR-LD and HR-ASD infants did not differ significantly on any parameter.
Imitating Other Adult Actions
Estimated trajectories for Imitating Other Adult Actions are shown in Figure 2d. LR infants displayed initially flat growth and accelerated over time. At 8 months of age, LR infants produced 4.28 of the 15 total Imitating Other Adult Actions on average and increased to 8.04 by 14 months of age. The HR-LD group displayed a similar pattern to LR infants and did not differ on any parameter. Conversely, HR-ND infants showed greater initial growth than LR infants at 8 months of age, p = .03, but exhibited less acceleration over time, p = .04. By 14 months of age, HR-ND infants produced 6.84 Imitating Other Adult Actions.
The HR-ASD infants differed significantly from LR infants in Imitating Other Adult Actions at 8 months of age: Indeed, no HR-ASD infants were reported to produce any of these actions at this age, p = .012. Moreover, they displayed less acceleration than LR infants, p = .03. Therefore, HR-ASD infants not only started with fewer actions, but their trajectory progressively diverged from that of the LR group over time. By 14 months of age, HR-ASD infants had only 2.53 of the Imitating Other Adult Actions in their repertoires. Follow-up analyses revealed that HR-LD and HR-ASD infants did not differ significantly on any parameter.
Actions and Gestures With Versus Without Object Manipulation
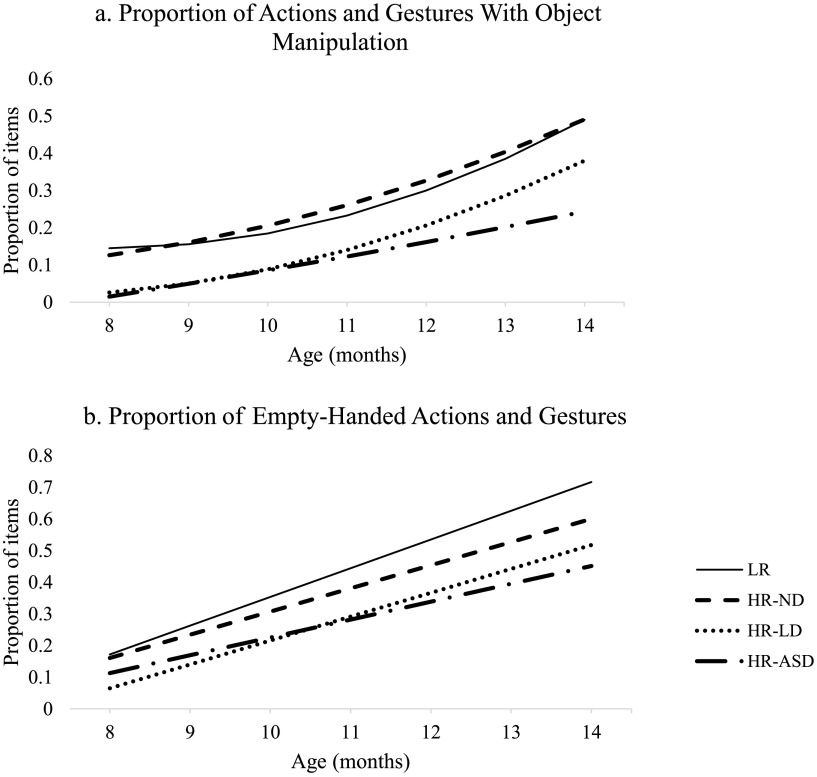
Actions and Gestures With Object Manipulation
Next, we examined all items from the previous six categories that involved object manipulation (43 total). We report these data as a proportion (the number of actions and gestures with objects endorsed by the parent divided by the total number of actions and gestures with objects) to allow direct comparison with Empty-Handed Actions and Gestures (20 items).
Estimated trajectories for Actions and Gestures With Object Manipulation are presented in Figure 3a. LR infants displayed initially flat growth and accelerated over time. At 8 months of age, LR infants produced approximately 15% of the Actions and Gestures With Object Manipulation on average. This increased substantially to about 49% by 14 months of age. HR-ND infants showed a similar pattern and did not differ from LR infants on any model parameter. In contrast, HR-LD and HR-ASD infants produced smaller proportions of the Actions and Gestures With Object Manipulation at 8 months of age (2.6% and 1.5%, respectively), ps = .012. Although the growth rate for HR-LD infants was similar to that of the LR infants, HR-ASD infants displayed less acceleration over time, p = .04. Therefore, the gap in Actions and Gestures With Object Manipulation remained stable over time for HR-LD infants but widened for HR-ASD infants. Follow-up analyses revealed greater acceleration among the HR-LD infants compared to HR-ASD infants, p = .033. Thus, by 14 months of age, HR-LD infants produced approximately 36% of the Actions and Gestures With Object Manipulation, while HR-ASD infants produced only about 21% on average.
Figure 3.
Estimated growth trajectories for gestures and actions with and without objects, depicted for each outcome group from 8 to 14 months of age. LR = low risk; HR-ND = high risk with no diagnosis; HR-LD = high risk with language delay; HR-ASD = high risk with autism spectrum disorder.
Empty-Handed Actions and Gestures
Estimated trajectories of Empty-Handed Actions and Gestures are displayed in Figure 3b. LR infants displayed positive linear growth over time. At 8 months of age, LR infants produced approximately 17% of the Empty-Handed Actions and Gestures on average, increasing to about 72% by 14 months of age. The HR-ND group displayed a similar pattern to LR infants and did not differ on any parameter. In contrast, HR-LD infants produced a smaller proportion of the Empty-Handed Actions and Gestures at 8 months of age (only 6.5%) compared to LR infants, p < .001. However, they increased at a similar rate to LR infants, and so the initial gap was maintained over time. By 14 months of age, HR-LD infants produced about 52% of the Empty-Handed Actions and Gestures.
A different pattern was evident for HR-ASD infants. At 8 months of age, HR-ASD infants produced a similar proportion of the Empty-Handed Actions and Gestures to that of the LR infants (about 11%) but displayed slower growth, diverging from LR infants over time, p = .003. The HR-LD and HR-ASD infants did not differ significantly on any parameter.
Discussion
This study examined various types of actions and gestures in three groups of HR infants (HR-ASD, HR-LD, and HR-ND) and a comparison sample of LR infants. Both HR-ASD and HR-LD infants consistently showed atypical growth patterns when compared to LR infants. However, they also differed in nuanced ways from one another. First, HR-ASD infants showed slower growth in their acquisition of frequently used deictic gestures relative to HR-LD infants. Second, compared to the HR-LD group, HR-ASD infants showed attenuated growth in actions and gestures that involve object manipulation. We discuss these findings below and describe their implications for early identification and intervention efforts for HR infants.
The Development of Gestures in HR Infants
Among infants' earliest forms of communication are deictic gestures (e.g., pointing, ritualized requests) and nondeictic conventional gestures like waving goodbye (e.g., Bates et al., 1979). Typically developing infants (LR and HR-ND) made extensive gains in both types of gestures, more than tripling the sizes of their gesture repertoires from 8 to 14 months of age. Consistent with past research, the HR-LD and HR-ASD infants also showed increases, but their gains were more modest than those of their peers (e.g., Ramos-Cabo et al., 2019).
Past work finds that deictic gestures may be affected differently for infants with ASD compared to infants with non-ASD language delay (see Manwaring et al., 2018, for review). Our data partially support this finding, and suggest that the “frequency” of deictic gestures distinguishes the two groups. There were no differences in the rate at which HR-ASD and HR-LD infants acquired deictic gestures overall. Instead, HR-ASD infants showed flatter growth in their acquisition of “frequently used” deictic gestures compared to HR-LD infants. Notably, there was no indication that nondeictic gestures were acquired at different rates for HR-ASD and HR-LD infants.
This raises the question of why deictic gestures are affected differently for HR-ASD and HR-LD infants but nondeictic gestures are not. Deictic gestures require infants to divide and alternate their attention between an object and a social partner (e.g., by pointing to or showing a toy). This kind of coordinated social attention occurs less frequently among infants with ASD and their caregivers (e.g., see Bruinsma et al., 2004, for a review). Conventional gestures (e.g., waving goodbye, nodding,), however, typically involve only interaction with a social partner, rather than requiring coordinated attention to objects “and” social partners.
The Development of Play Actions in HR Infants
We also analyzed the development of four types of play actions. With regard to games and routines (i.e., highly structured behaviors like playing peekaboo or “so big”), these were the only action type for which HR-ASD infants kept pace with LR infants. It is possible that the highly ritualized aspect of these behaviors may support their development in ASD (i.e., the call-and-response nature of these behaviors may promote their production). For all other types of actions, HR-ASD and HR-LD infants showed slower growth than their LR peers, but never differed significantly from one another.
Although the pretending actions (e.g., pretending to feed a doll with a spoon) did not differ for HR-ASD and HR-LD infants, it is possible that differences might emerge later in development. Pretending actions are particularly advanced because they require not only knowledge of functional actions but also the extension of those actions to others. These behaviors were just emerging during the 8- to 14-month study period, even among the LR and HR-ND infants (who produced less than a third of the items by 14 months). Previous work suggests that young children with ASD perform these pretend behaviors less often than neurotypical children in the second and third years (e.g., Campbell et al., 2018; Charman et al., 1997; Jarrold, 2003; Sigman & Ungerer, 1984).
A notable finding was that imitating adult actions (e.g., pretending to vacuum or typing at a keyboard) was an area of relative strength for HR-LD infants. Their growth trajectory was essentially indistinguishable from that of the LR group. The HR-ASD group displayed a flatter trajectory than their LR peers (although they did not differ from HR-LD infants). This difference likely persists into childhood, considering well-documented evidence that children with ASD demonstrate less imitation than do neurotypical children (e.g., Campbell et al., 2018; Charman et al., 1997; Jarrold, 2003; Sigman & Ungerer, 1984).
Actions and Gestures With Versus Without Object Manipulation
Across all types of play actions and gestures, individual behaviors varied as to whether they involved object manipulation. Some require complex action sequences with objects (e.g., sweeping with a broom, eating with a spoon), while others are independent of objects (e.g., blowing to indicate something is hot). This difference may be important. Researchers have proposed that actions and gestures with objects are more motorically difficult—and so require greater fine motor skill—than empty-handed actions and gestures (e.g., Caselli et al., 2012). Because infants with ASD exhibit early-appearing disruptions in fine motor skills compared to neurotypical peers (e.g., Iverson et al., 2019), actions and gestures with objects may be especially challenging for these infants (see Sparaci et al., 2018).
Our data were consistent with this perspective. Although HR-ASD infants showed attenuated growth in actions and gestures with “and” without objects, this attenuation was more pronounced for those with objects. Importantly, actions and gestures with object manipulation significantly distinguished the HR-ASD and HR-LD infants. All actions are inherently motor behaviors, but they vary widely in their motoric complexity. Past work often attributes delayed gesture and play development in ASD to reduced social engagement or communicative intent. It is possible that delayed development of play actions and gestures may be compounded, at least in part, by weaknesses in fine motor skill (see Gernsbacher et al., 2008, for further discussion of this idea).
Developmental Cascades
The different developmental patterns of actions and gestures among HR groups may have cascading effects in other domains. In particular, there is an established developmental link between gestures, play actions, and language learning (e.g., Iverson et al., 1994; LeBarton et al., 2015; Roemer et al., 2019; Sparaci et al., 2018; Volterra et al., 1979). Infant actions and gestures frequently elicit verbal responses from caregivers (Bornstein & Tamis-LeMonda, 1989). In fact, some estimates suggest that caregivers respond verbally to 85%–96% of infant gestures (e.g., Choi et al., 2019; Leezenbaum et al., 2014), and 50%–70% of actions with objects (e.g., Bornstein et al., 2008). The timing of these responses is advantageous for word learning (see also West & Iverson, 2017). During these actions, the infants' attention is focused on an object when the label is presented (as an illustration, an infant may point to a teddy bear and the caregiver responds, “do you want your bear?”). This timely language input scaffolds word learning by temporally aligning the word or phrase with the infants' attention (e.g., Carpenter et al., 1998; Tomasello & Farrar, 1986).
Caregivers of HR and LR infants are equally responsive to actions and gestures (e.g., Leezenbaum et al., 2014). However, because HR-LD and HR-ASD infants produce actions and gestures less frequently—and they have smaller repertoires of some of these behaviors—their caregivers have fewer opportunities to respond, so the base rates of verbal responses are lower (Choi et al., 2019; Leezenbaum et al., 2014). Receiving fewer verbal responses may limit opportunities for word learning, potentially affecting language development among HR-ASD and HR-LD infants. Differences in infants' actions and gestures may also be relevant for caregiver input and responses. Our data revealed that growth in repertoires of some types of actions and gestures, such as those that involve object manipulation, was slowed for HR-LD and HR-ASD relative to LR infants. These behaviors may also differ in the extent to which they elicit language input. That is, caregivers may be more likely to respond to some actions than to others, and they may respond to different actions in different ways (e.g., gestures with objects may be more likely to elicit object labels than games and routines such as peekaboo or pat-a-cake). More research is needed to examine the nature and content of caregiver responses to different types of actions and gestures and whether these may vary for HR-LD and HR-ASD infants, whose repertoires expand more slowly.
Limitations and Future Directions
The study has notable strengths, including a prospective longitudinal design with frequent observations, but there are several limitations. First, as typical of studies with HR infants, the subgroup of infants who later developed ASD was relatively small. This may have limited our ability to detect more subtle differences between the HR-LD and HR-ASD groups. Second, these data were collected as part of a larger study focused on development in the first year of life. Infants continue to acquire actions and gestures well into the second year, and it is possible that developmental trends among HR infants would continue to shift further into development. Future studies should confirm findings with larger samples of HR-ASD infants and investigate whether and how these patterns may change into the second and third years.
Third, as previously discussed, the CDI has some limitations. It is a parent report measure and thus relies on the accuracy of parents' recall and reporting, which may differ across CDI categories. Some categories—like gestures—are used specifically to communicate. Parents may be more accurate reporters of communicative items compared to play skills, which infants could perform outside social contexts (e.g., typing on a computer). Furthermore, many of these skills are explicitly taught by parents (e.g., playing pat-a-cake) and may be strongly influenced by parental scaffolding. It is important that future studies validate these findings with other behavioral measures.
Additionally, the CDI provides limited information about the frequency of behaviors. Only the items related to gestures inquire about frequency, and even then, there is little specificity because selections are limited to “often,” “sometimes,” or “never.” The CDI also does not provide information about the context in which these behaviors are produced or their functions. Thus, for example, two infants may be credited with pointing, but they could differ substantially in the functions of their pointing gestures. One infant may point only to request out-of-reach objects, while the other points to initiate shared attention with a parent. These infants would receive equal credit for pointing despite the fact that their gestures clearly serve different communicative functions, and indeed, there is ample work utilizing naturalistic observations, laboratory tasks, and standardized assessments showing that infants with ASD gesture less frequently and under different circumstances and produce fewer play actions than neurotypical peers (e.g., LeBarton & Iverson, 2017; Parladé & Iverson, 2015; Rozga et al., 2011; Sowden et al., 2013; Watson et al., 2013; Wetherby et al., 2004). Future studies should bridge these approaches to assess whether the frequency and context of production varies for particular gesture types.
Clinical Implications
These findings have several potential clinical implications. First, they suggest that some types of actions and gestures could be used to distinguish infants with ASD from non-ASD language delay. In particular, actions and gestures with object manipulation develop differently for HR-LD and HR-ASD infants. Detection of differences between infants with ASD versus language delay may require frequent observation schedules (e.g., Iverson et al., 2018; West et al., 2019). It is encouraging that differences were evident using the CDI, a parent report checklist of early communicative behaviors that is inexpensive and easy to administer and score and, therefore, could be administered at multiple, frequent time points.
Additionally, frequent surveillance of different types of actions and gestures may inform intervention strategies, both for children with ASD and those with general communicative delays. Although group-level data do not translate directly to treatment decisions on the individual level, frequent surveillance of toddlers' production and repertoires of actions and gestures can provide invaluable information about strengths and weaknesses to target in treatment. For example, play actions and gestures are regularly assessed using a curriculum checklist to inform treatment in the Early Start Denver Model (Rogers & Dawson, 2010), which has substantial positive effects on receptive and expressive language development (e.g., Dawson et al., 2010). In combination with other studies demonstrating a predictable sequence of gestures and gesture–word combinations in toddlers with ASD (e.g., Talbott et al., 2018), the present findings may inform treatment providers in efforts to provide a context for individualized treatment. Furthermore, assessing individual actions and gestures that a child has produced (but may not consistently produce in a treatment session) can continue to inform communicative strengths to build on for both children with ASD and language delays.
Acknowledgments
This study was funded by Autism Speaks and the National Institutes of Health (R01 HD41607 and R01 HD54979, awarded to Jana M. Iverson), with additional support from Grants HD35469 and HD055748, awarded to N. J. Minshew. During the preparation of this article, J. N. received support through a T32 training grant from the National Institute of Mental Health (T32MH018269). Additionally, K. W. received support through an F32 training grant from the National Institutes of Health (F32DC017903).
Funding Statement
This study was funded by Autism Speaks and the National Institutes of Health (R01 HD41607 and R01 HD54979, awarded to Jana M. Iverson), with additional support from Grants HD35469 and HD055748, awarded to N. J. Minshew. During the preparation of this article, J. N. received support through a T32 training grant from the National Institute of Mental Health (T32MH018269). Additionally, K. W. received support through an F32 training grant from the National Institutes of Health (F32DC017903).
Footnote
One of the 11 HR-ASD infants received an ASD diagnosis at 24 months as part of a separate study (see Campbell et al., 2015, for study details) but withdrew prior to 36 months. We ran analyses with and without this infant included, and the pattern of results was unchanged. We therefore retained this participant in the HR-ASD group.
References
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). https://doi.org/10.1176/appi.books.9780890425596 [Google Scholar]
- Bates E., Benigni L., Bretherton I., Camaioni L., & Volterra V. (1979). The emergence of symbols: Cognition and communication in infancy. Academic Press. [Google Scholar]
- Bates E., Bretherton I., & Snyder L. (1988). From first words to grammar. Cambridge University Press. [Google Scholar]
- Bates E., Camaioni L., & Volterra V. (1975). The acquisition of performatives prior to speech. Merrill-Palmer Quarterly, 21(3), 205–226. [Google Scholar]
- Bornstein M. H., & Tamis-LeMonda C. S. (1989). Maternal responsiveness and cognitivedevelopment in children. New Directions for Child and Adolescent Development, 1989(43), 49–61. https://doi.org/10.1002/cd.23219894306 [DOI] [PubMed] [Google Scholar]
- Bornstein M. H., Tamis-LeMonda C. S., Hahn C.-S., & Haynes O. M. (2008). Maternalresponsiveness to young children at three ages: Longitudinal analysis of a multidimensional, modular, and specific parenting construct. Developmental Psychology, 44(3), 867–874. https://doi.org/10.1037/0012-1649.44.3.867 [DOI] [PubMed] [Google Scholar]
- Boucher J. (1999). Editorial: Interventions with children with autism—Methods based on play. Child Language Teaching and Therapy, 15(1), 1–5. https://doi.org/10.1177/026565909901500101 [Google Scholar]
- Bruinsma Y., Koegel R. L., & Koegel L. K. (2004). Joint attention and children with autism: A review of the literature. Mental Retardation and Developmental Disabilities ResearchReviews, 10(3), 169–175. https://doi.org/10.1002/mrdd.20036 [DOI] [PubMed] [Google Scholar]
- Bruyneel E., Demurie E., Zink I., Warreyn P., & Roeyers H. (2019). Exploring receptive and expressive language components at the age of 36 months in siblings at risk for autism spectrum disorder. Research in Autism Spectrum Disorders, 66, 101419 https://doi.org/10.1016/j.rasd.2019.101419 [Google Scholar]
- Bryk A. S., & Raudenbush S. W. (1992). Hierarchical linear models: Applications and data analysis methods. Sage. [Google Scholar]
- Camarata S. (2014). Early identification and early intervention in autism spectrum disorders: Accurate and effective? International Journal of Speech-Language Pathology, 16(1), 1–10. https://doi.org/10.3109/17549507.2013.858773 [DOI] [PubMed] [Google Scholar]
- Campbell S. B., Leezenbaum N. B., Mahoney A. S., Moore E. L., & Brownell C. E. (2016). Pretend play and social engagement in toddlers at high and low genetic risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 46(7), 2305–2316. https://doi.org/10.1007/s10803-016-2764-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. B., Leezenbaum N. B., Schmidt E. N., Day T. N., & Brownell C. A. (2015). Concern for another's distress in toddlers at high and low genetic risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(11), 3594–3605. https://doi.org/10.1007/s10803-015-2505-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. B., Mahoney A. S., Northrup J., Moore E. L., Leezenbaum N. B., & Brownell C. A. (2018). Developmental changes in pretend play from 22 to 34 months in younger siblings of children with autism spectrum disorder. Journal of Abnormal Child Psychology, 46(3), 639–654. https://doi.org/10.1007/s10802-017-0324-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capirci O., Contaldo A., Caselli M. C., & Volterra V. (2005). From action to language through gesture: A longitudinal perspective. Gesture, 5(1–2), 155–177. https://doi.org/10.1075/gest.5.1-2.12cap [Google Scholar]
- Carpenter M., Nagell K., Tomasello M., Butterworth G., & Moore C. (1998). Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development, 63(4), i–174. https://doi.org/10.2307/1166214 [PubMed] [Google Scholar]
- Caselli M. C., Rinaldi P., Stefanini S., & Volterra V. (2012). Early action and gesture “vocabulary” and its relation with word comprehension and production. Child Development, 83(2), 526–542. https://doi.org/10.1111/j.1467-8624.2011.01727.x [DOI] [PubMed] [Google Scholar]
- Charman T., Swettenham J., Baron-Cohen S., Cox A., Baird G., & Drew A. (1997). Infants with autism: An investigation of empathy, pretend play, joint attention, and imitation. Developmental Psychology, 33(5), 781–789. https://doi.org/10.1037/0012-1649.33.5.781 [DOI] [PubMed] [Google Scholar]
- Choi B., Shah P., Rowe M. L., Nelson C. A., & Tager-Flusberg H. (2019). Gesture development, caregiver responsiveness, and language and diagnostic outcomes in infants at high and low risk for autism. Journal of Autism and Developmental Disorders. Epub ahead of print. https://doi.org/10.1007/s10803-019-03980-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen D. L., Bilder D. A., Zahorodny W., Pettygrove S., Durkin M. S., Fitzgerald R. T., Kurzius-Spencer M., Baio J., & Yeargin-Allsopp M. (2016). Prevalence and characteristics of autism spectrum disorder among 4-year-old children in the autism and developmental disabilities monitoring network. Journal of Developmental & Behavioral Pediatrics, 37(1), 1–8. https://doi.org/10.1097/DBP.0000000000000235 [DOI] [PubMed] [Google Scholar]
- Christensen L., Hutman T., Rozga A., Young G. S., Ozonoff S., Rogers S. J., Baker B., & Sigman M. (2010). Play and developmental outcomes in infant siblings of children with autism. Journal of Autism and Developmental Disorders, 40(8), 946–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale P. S., Bates E., Reznick J. S., & Morisset C. (1989). The validity of parent report instrument of child language at twenty months. Journal of Child Language, 16(2), 239–249. https://doi.org/10.1017/S0305000900010394 [DOI] [PubMed] [Google Scholar]
- Dawson G., Rogers S., Munson J., Smith M., Winter J., Greenson J., Donaldson A., & Varley J. (2010). Randomized, controlled trial of an intervention for toddlers with autism: The early start denver model. Pediatrics, 125(1), e17–e23. https://doi.org/10.1542/peds.2009-0958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L., Dale P. S., Reznick J. S., Bates E., Thal D. J., & Pethick S. J. (1994). Variability in early communicative development. Monographs of the Society for Research in Child Development, 59(5), 1–173. https://doi.org/10.2307/1166093 [PubMed] [Google Scholar]
- Fenson L., Dale P., Reznick J. S., Thal D., Bates E., Hartung J., Pethick S. J., & Reilly J. (1993). The MacArthur Communicative Development Inventories: User's guide and technical manual. Singular. [Google Scholar]
- Gernsbacher M. A., Sauer E. A., Geye H. M., Schweigert E. K., & Hill Goldsmith H. (2008). Infant and toddler oral- and manual-motor skills predict later speech fluency in autism. The Journal of Child Psychology and Psychiatry, 49(1), 43–50. https://doi.org/10.1111/j.1469-7610.2007.01820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershkoff-Stowe L., Thal D. J., Smith L. B., & Namy L. L. (1997). Categorization and its developmental relation to early language. Child Development, 68(5), 843–859. https://doi.org/10.1111/j.1467-8624.1997.tb01966.x [DOI] [PubMed] [Google Scholar]
- Heilmann J., Weismer S. E., Evans J., & Hollar C. (2005). Utility of MacArthur–Bates Communicative Development Inventories in identifying language abilities of late-talking and typically developing toddlers. American Journal of Speech-Language Pathology, 14(1), 40–51. https://doi.org/10.1044/1058-0360(2005/006) [DOI] [PubMed] [Google Scholar]
- Heymann P., Northrup J. B., West K. L., Parladé M. V., Leezenbaum N. B., & Iverson J. M. (2018). Coordination is key: Joint attention and vocalisation in infant siblings of children with autism spectrum disorder. International Journal of Language & Communication Disorders, 53(5), 1007–1020. https://doi.org/10.1111/1460-6984.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher J., Haight W., Bryk A., Seltzer M., & Lyons T. (1991). Early vocabulary growth: Relation to language input and gender. Developmental Psychology, 27(2), 236–248. https://doi.org/10.1037/0012-1649.27.2.236 [Google Scholar]
- Iverson J. M., Capirci O., & Caselli M. C. (1994). From communication to language in two modalities. Cognitive Development, 9(1), 23–43. https://doi.org/10.1016/0885-2014(94)90018-3 [Google Scholar]
- Iverson J. M., Northrup J. B., Leezenbaum N. B., Koterba E. A., Parladé M. V., & West K. L. (2018). Early communicative and language development in infant siblings of children with autism. Journal of Autism and Developmental Disorders, 48(1), 55–71. https://doi.org/10.1007/s10803-017-3297-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson J. M., Shic F., Wall C. A., Chawarska K., Curtin S., Estes A., Gardner J. M., Hutman T., Landa R. J., Levin A. R., Libertus K., Messinger D. S., Nelson C. A., Ozonoff S., Sacrey L.-A. R., Sheperd K., Stone W. L., Tager-Flusberg H. B., Wolff J. J., … Young G. S. (2019). Early motor abilities in infants at heightened versus low risk for ASD: A Baby Siblings Research Consortium (BSRC) study. Journal of Abnormal Psychology, 128(1), 69–80. https://doi.org/10.1037/abn0000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrold C. (2003). A review of research into pretend play in autism. Autism, 7(4), 379–390. https://doi.org/10.1177/1362361303007004004 [DOI] [PubMed] [Google Scholar]
- Kogan M. D., Vladutiu C. J., Schieve L. A., Ghandour R. M., Blumberg S. J., Zablotsky B., Perrin J. M., Shattuck P., Kuhlthau K. A., Hardwood R. L., & Lu M. C. (2018). The prevalence of parent-reported autism spectrum disorder among U.S. children. Pediatrics, 142(6), e20174161 https://doi.org/10.1542/peds.2017-4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa R. J., Holman K. C., & Garrett-Mayer E. (2007). Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry, 64(7), 853–864. https://doi.org/10.1001/archpsyc.64.7.853 [DOI] [PubMed] [Google Scholar]
- LeBarton E. S., Goldin-Meadow S., & Raudenbush S. (2015). Experimentally induced increases in early gesture lead to increases in spoken vocabulary. Journal of Cognition and Development, 16(2), 199–220. https://doi.org/10.1080/15248372.2013.858041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBarton E. S., & Iverson J. M. (2017). Gesture's role in learning interactions. In Church R. B., Alibali M. W., & Kelly S. D. (Eds.), Why gesture?: How the hands function in speaking, thinking and communicating (pp. 331–351). John Benjamins; https://doi.org/10.1075/gs.7.16leb [Google Scholar]
- Leezenbaum N. B., Campbell S. B., Butler D., & Iverson J. M. (2014). Maternal verbal responses to communication of infants at low and heightened risk of autism. Autism, 18(6), 694–703. https://doi.org/10.1177/1362361313491327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E. H. Jr., Leventhal B. L., DiLavore P. C., Pickles A., & Rutter M. (2000). The Autism Diagnostic Observation Schedule–Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30(3), 205–223. https://doi.org/10.1023/A:1005592401947 [PubMed] [Google Scholar]
- Manwaring S. S., Stevens A. L., Mowdood A., & Lackey M. (2018). A scoping review of deictic gesture use in toddlers with or at-risk for autism spectrum disorder. Autism & Developmental Language Impairments, 3, 1–27. https://doi.org/10.1177/2396941517751891 [Google Scholar]
- Manwaring S. S., Swineford L., Mead D. L., Yeh C. C., Zhang Y., & Thurm A. (2019). The gesture–language association over time in toddlers with and without language delays. Autism & Developmental Language Impairments, 4, 1–15. https://doi.org/10.1177/2396941519845545 [Google Scholar]
- Marrus N., Hall L. P., Paterson S. J., Elison J. T., Wolff J. J., Swanson M. R., Parish-Morris J., Eggebrecht A. T., Pruett J. R., Hazlett H. C., Zwaigenbaum L., Dager S., Estes A. M., Schultz R. T., Botteron K. N., Piven J., & Constantino J. N. (2018). Language delay aggregates in toddler siblings of children withautism spectrum disorder. Journal of Neurodevelopmental Disorders, 10(1), 29 https://doi.org/10.1186/s11689-018-9247-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiuseppe M., Capirci O., Cuva S., & Venuti P. (2015). Gestural communication in children with autism spectrum disorders during mother–child interaction. Autism, 19(4), 469–481. https://doi.org/10.1177/1362361314528390 [DOI] [PubMed] [Google Scholar]
- Miller J. F., Sedey A. L., & Miolo G. (1995). Validity of parent report measures of vocabulary development for children with Down syndrome. Journal of Speech and Hearing Research, 38(5), 1037–1044. https://doi.org/10.1044/jshr.3805.1037 [DOI] [PubMed] [Google Scholar]
- Mitchell S., Brian J., Zwaigenbaum L., Roberts W., Szatmari P., Smith I., & Bryson S. (2006). Early language and communication development of infants later diagnosed with autism spectrum disorder. Journal of Developmental & Behavioral Pediatrics, 27(2), S69–S78. https://doi.org/10.1097/00004703-200604002-00004 [DOI] [PubMed] [Google Scholar]
- Mullen E. M. (1995). Mullen Scales of Early Learning: AGS Edition. AGS. [Google Scholar]
- Mulligan S., & White B. P. (2012). Sensory and motor behaviors of infant siblings of children with and without autism. The American Journal of Occupational Therapy, 66(5), 556–566. https://doi.org/10.5014/ajot.2012.004077 [DOI] [PubMed] [Google Scholar]
- Mundy P., Delgado C., Block J., Venezia A., & Siebert J. (2003). Early Social Communication Scales. University of Miami. [Google Scholar]
- Ozonoff S., Iosif A., Baguio F., Cook I. C., Moore Hill M., Hutman T., Young G. S., Rogers S. J., Rozga A., Sangha S., Sigman M., & Steinfeld M. B. (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry, 49(3), 256.e1-2–266.e1-2. https://doi.org/10.1016/j.jaac.2009.11.009 [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S., Young G. S., Carter A., Messinger D., Yirmiya N., Zwaigenbaum L., Bryson S., Carver L. J., Constantino J. N., Dobkins K., Hutman T., Iverson J. M., Landa R., Rogers S. J., Sigman M., & Stone W. L. (2011). Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics, 128(3), e488–e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parladé M. V., & Iverson J. M. (2015). The development of coordinated communication in infants at heightened risk for autism spectrum disorder. Journal of Autism and Developmental Disorders, 45(7), 2218–2234. https://doi.org/10.1007/s10803-015-2391-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce H. K. (2009). Exploratory, functional, and symbolic play behaviors of toddlers with autism spectrum disorders. Unpublished dissertation. [Google Scholar]
- Ramos-Cabo S., Vulchanova M., & Vulchanov V. (2019). Gesture and language trajectories in early development: An overview from the autism spectrum disorder perspective. Frontiers in Psychology, 10, 1211 https://doi.org/10.3389/fpsyg.2019.01211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush S. W., Bryk A. S., Cheong Y. F., Congdon R., & du Toit M. (2011). HLM statistical software: Version 7 [Computer software].
- Robertson S. B., & Weismer S. E. (1999). Effects of treatment on linguistic and social skills in toddlers with delayed language development. Journal of Speech, Language, and Hearing Research, 42(5), 1234–1248. https://doi.org/10.1044/jslhr.4205.1234 [DOI] [PubMed] [Google Scholar]
- Roemer E. J., West K. L., Northrup J. B., & Iverson J. M. (2019). Word comprehension mediates the link between gesture and word production: Examining language development in infant siblings of children with autism spectrum disorder. Developmental Science, 22(3), e12767 https://doi.org/10.1111/desc.12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. J., & Dawson G. (2010). Early Start Denver Model for young children with autism: Promoting language, learning, and engagement. Guilford. [Google Scholar]
- Rozga A., Hutman T., Young G. S., Rogers S. J., Ozonoff S., Dapretto M., & Sigman M. (2011). Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother–infant interaction and nonverbal communication. Journal of Autism and Developmental Disorders, 41(3), 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M., & Ungerer J. A. (1984). Cognitive and language skills in autistic, mentally retarded, and normal children. Developmental Psychology, 20(2), 293–302. https://doi.org/10.1037/0012-1649.20.2.293 [Google Scholar]
- Singer J. D. (1998). Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics, 23(4), 323–355. https://doi.org/10.3102/10769986023004323 [Google Scholar]
- Sowden H., Clegg J., & Perkins M. (2013). The development of co-speech gesture in the communication of children with autism spectrum disorders. Clinical Linguistics & Phonetics, 27(12), 922–939. https://doi.org/10.3109/02699206.2013.818715 [DOI] [PubMed] [Google Scholar]
- Sparaci L., Northrup J. B., Capirci O., & Iverson J. M. (2018). From using tools to using language in infant siblings of children with autism. Journal of Autism and Developmental Disorders, 48(7), 2319–2334. https://doi.org/10.1007/s10803-018-3477-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparaci L., & Volterra V. (2017). Hands shaping communication: From gestures to signs. In Bertolaso M. & Stefano N. (Eds.), The hand (pp. 29–54). Springer; https://doi.org/10.1007/978-3-319-66881-9_3 [Google Scholar]
- Talbott M. R., Young G. S., Munson J., Estes A., Vismara L. A., & Rogers S. J. (2018). The developmental sequence and relations between gesture and spoken language in toddlers with autism spectrum disorder. Child Development. Epub ahead of print. https://doi.org/10.1111/cdev.13203 [DOI] [PubMed] [Google Scholar]
- Thal D. J., O'Hanlon L., Clemmons M., & Fralin L. (1999). Validity of a parent report measure of vocabulary and syntax for preschool children with language impairment. Journal of Speech, Language, and Hearing Research, 42(2), 482–496. https://doi.org/10.1044/jslhr.4202.482 [DOI] [PubMed] [Google Scholar]
- Tomasello M., & Farrar M. J. (1986). Joint attention and early language. Child Development, 57(6), 1454–1463. https://doi.org/10.2307/1130423 [PubMed] [Google Scholar]
- Veness C., Prior M., Bavin E., Eadie P., Cini E., & Reilly S. (2012). Early indicators of autism spectrum disorders at 12 and 24 months of age: A prospective, longitudinal comparative study. Autism, 16(2), 163–177. https://doi.org/10.1177/1362361311399936 [DOI] [PubMed] [Google Scholar]
- Volterra V., Bates E., Benigni L., Bretherton I., & Camaioni L. (1979). First words in language and action: A qualitative look. In Bates E. (Ed.), The emergence of symbols: Cognition and communication in infancy (pp. 141–222). Academic Press. [Google Scholar]
- Watson L. R., Crais E. R., Baranek G. T., Dykstra J. R., & Wilson K. P. (2013). Communicative gesture use in infants with and without autism: A retrospective home video study. American Journal of Speech-Language Pathology, 22(1), 25–39. https://doi.org/10.1044/1058-0360(2012/11-0145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismer S. E., & Evans J. L. (2002). The role of processing limitations in early identification of specific language impairment. Topics in Language Disorders, 22(3), 15–29. https://doi.org/10.1097/00011363-200205000-00004 [Google Scholar]
- West K. L. (2019). Infant motor development in autism spectrum disorder: A synthesis and meta-analysis. Child Development, 90(6), 2053–2070. https://doi.org/10.1111/cdev.13086 [DOI] [PubMed] [Google Scholar]
- West K. L., & Iverson J. M. (2017). Language learning is hands-on: Exploring links between infants' object manipulation and verbal input. Cognitive Development, 43, 190–200. https://doi.org/10.1016/j.cogdev.2017.05.004 [Google Scholar]
- West K. L., Leezenbaum N. B., Northrup J. B., & Iverson J. M. (2019). The relation between walking and language in infant siblings of children with autism spectrum disorder. Child Development, 90(3), e356–e372. https://doi.org/10.1111/cdev.12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby A. M., & Prutting C. A. (1984). Profiles of communicative and cognitive-social abilities in autistic children. Journal of Speech and Hearing Research, 27(3), 364–377. https://doi.org/10.1044/jshr.2703.364 [DOI] [PubMed] [Google Scholar]
- Wetherby A. M., Woods J., Allen L., Cleary J., Dickinson H., & Lord C. (2004). Early indicators of autism spectrum disorders in the second year of life. Journal of Autism and Developmental Disorders, 34(5), 473–493. https://doi.org/10.1007/s10803-004-2544-y [DOI] [PubMed] [Google Scholar]
- Willett J. B., Singer J. D., & Martin N. C. (1998). The design and analysis of longitudinal studies of development and psychopathology in context: Statistical models and methodological recommendations. Development and Psychopathology, 10(2), 395–426. https://doi.org/10.1017/S0954579498001667 [DOI] [PubMed] [Google Scholar]
- Williams E. (2003). A comparative review of early forms of object-directed play and parent–infant play in typical infants and young children with autism. Autism, 7(4), 361–374. https://doi.org/10.1177/1362361303007004003 [DOI] [PubMed] [Google Scholar]
- Wilson K. P., Carter M. W., Wiener H. L., DeRamus M. L., Bulluck J. C., Watson L. R., Crais E. R., & Baranek G. T. (2017). Object play in infants with autism spectrum disorder: A longitudinal retrospective video analysis. Autism and Developmental Language Impairments, 2 2396941517713186. https://doi.org/10.1177/2396941517713186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Strathearn L., Liu B., O'brien M., Kopelman T. G., Zhu J., Snetselaar L. G., & Bao W. (2019). Prevalence and treatment patterns of autism spectrum disorder in the United States, 2016. JAMA Pediatrics, 173(2), 153–159. https://doi.org/10.1001/jamapediatrics.2018.4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N., Gamliel I., Shaked M., & Sigman M. (2007). Cognitive and verbal abilities of 24- to 36-month-old siblings of children with autism. Journal of Autism and Developmental Disorders, 37(2), 218–229. https://doi.org/10.1007/s10803-006-0163-5 [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L., Bryson S., Rogers T., Roberts W., Brian J., & Szatmari P. (2005). Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience, 23(2–3), 143–152. https://doi.org/10.1016/j.ijdevneu.2004.05.001 [DOI] [PubMed] [Google Scholar]