Abstract
Purpose
Previous studies of neural processing of rhyme discrimination in 7- to 8-year-old children who stutter (CWS) distinguished children who had recovered, children who had persisted, and children who did not stutter (CWNS; Mohan & Weber, 2015). Here, we investigate neural processing mediating rhyme discrimination for early acquired real words in younger CWS and CWNS (4;1–6;0 years;months), when rhyming abilities are newly emerging, to examine possible relationships to eventual recovery (CWS-eRec) and persistence in stuttering (CWS-ePer).
Method
Children performed a rhyme discrimination task while their event-related brain potentials were recorded. CWNS, CWS-eRec, and CWS-ePer had similar speech and language abilities. Inclusionary criteria incorporated at least 70% accuracy for rhyme discrimination. Analyses focused on the mean amplitude of the N400 component elicited by rhyming and nonrhyming words in anterior and posterior regions of interest.
Results
CWNS, CWS-eRec, and CWS-ePer displayed a classic event-related potential rhyme effect for rhyme discrimination characterized by larger amplitude, posteriorly distributed N400s elicited by nonrhyming targets compared to rhyming targets. CWNS displayed a more robust anterior rhyme effect compared to the CWS groups with a larger amplitude N400 anteriorly for the rhyming targets. This effect was more consistent across individual CWNS than CWS.
Conclusions
The groups of CWNS, CWS-eRec, and CWS-ePer, who had all developed rhyming discrimination abilities, exhibited similar underlying neural processes mediating phonological processing of early acquired words for the classic central-parietal rhyme effect. However, individual variability of the anterior rhyme effect suggested differences in specific aspects of phonological processing for some CWS-eRec and CWS-ePer compared to CWNS.
Identifying factors that may predict recovery and persistence is a major goal of research in stuttering. Researchers estimate that 5%–8% of preschool children begin to stutter and that 80% of these children eventually recover (Yairi & Ambrose, 1999, 2013). Efficient allocation of limited treatment resources depends on the ability to better predict the trajectory of stuttering outcomes in young children who stutter (CWS). Factors such as gender, family history of stuttering, age of stuttering onset, and time since onset are recommended for assessing risk of persistence (Walsh et al., 2018; Yairi & Ambrose, 2005; Yairi & Seery, 2015). In addition, the multifactorial dynamic pathways theory of stuttering describes how the development of motor, linguistic, and emotional abilities may influence the trajectory of stuttering in preschool CWS (Smith & Weber, 2017). These contributing factors are rapidly developing during the preschool years when stuttering begins. Furthermore, the neurodevelopmental processes mediating these abilities are not independent of one another. They are thought to interact throughout the onset and trajectory of stuttering to either support or inhibit speech motor development, which ultimately results in either typically fluent speech or the disfluencies identified as stuttering. Interactions between the speech motor system and linguistic and psychosocial factors may be especially important for understanding stuttering recovery and persistence (Smith & Weber, 2017). One linguistic factor associated with stuttering persistence is phonological development (Paden et al., 1999; Spencer & Weber-Fox, 2014). Using a rhyme discrimination task for early acquired real words, the current study aims to deepen our understanding of the neurodevelopment of phonological processing in CWS and how this factor may be related to eventual recovery and persistence in stuttering.
Phonological Development in CWS
Developmental acquisition of phonology occurs rapidly through experience with speech perception and production (Rvachew & Brosseau-Lapré, 2018). Children construct phonological representations, which contain an acoustic–phonetic representation for how a word is perceived, and an articulatory–phonetic representation for how a word is produced (Edwards et al., 2004; Rvachew & Brosseau-Lapré, 2018; Walley, 1993). Speech production abilities (articulation accuracy) and phonological awareness skills (knowledge of sublexical units) are based on development and refinement of these phonological representations (Edwards et al., 2011; Metsala, 1997; Rvachew & Brosseau-Lapré, 2018; Shiller et al., 2010; Walley, 1993).
Speech Production Abilities in CWS
The acoustic–phonetic component of the phonological representation is thought to be a model or target for a child's developing speech production abilities and formulation of the articulatory–phonetic representation (Edwards et al., 2011; Shiller et al., 2010). Accurate speech production, as measured by percentage of whole words correct, increases from approximately 30% at age of 2 years to 88% at 5 years of age (Rvachew & Brosseau-Lapré, 2018, pp. 164–169). Ninety-five percent of stuttering onsets occur by 4 years of age (Yairi & Ambrose, 2013), which coincides with this rapid increase in speech production accuracy (Smith & Weber, 2017). Furthermore, speech production abilities in CWS have been linked to stuttering recovery and persistence. For example, Paden et al. (1999) found that, as a group, preschool CWS who eventually persisted (ePer) scored lower than CWS who eventually recovered (eRec) on the Assessment of Phonological Processes–Revised. This gap in speech production accuracy eventually closed as participants were followed for 2 years (Paden et al., 2002). This finding highlights that factors of stuttering recovery and persistence may depend on a child's age at assessment (Paden et al., 2002; Walsh et al., 2018). In another study of speech production, Spencer and Weber-Fox (2014) found that lower scores of articulatory abilities measured by the Bankson-Bernthal Test of Phonology–Consonant Inventory (Bankson & Bernthal, 1990) and poorer performance on a nonword repetition task (Dollaghan & Campbell, 1998) differentiated groups of CWS-ePer from CWS-eRec. Findings from Paden et al. and Spencer and Weber-Fox highlight speech production abilities as a potential factor contributing to the trajectory of stuttering outcome in young CWS.
Phonological Awareness in CWS
Phonological awareness is thought to develop through the refinement and reorganization of phonological representations within the growing lexicon (Metsala, 1997; Walley, 1993). This development proceeds from awareness of larger units, such as onsets and rimes, to smaller units, such as phonemes (Anthony & Francis, 2005; Carroll et al., 2003). This organization around sublexical units allows for efficient processing of words that sound similar (e.g., minimal pairs) within a growing lexicon (Metsala, 1997; Walley, 1993). CWS may be delayed in the refinement of phonological representations as sublexical units, which influences their phonological awareness abilities compared to children who do not stutter (CWNS; Byrd et al., 2007; Gerwin et al., 2019; Pelczarski & Yaruss, 2014). Byrd et al. (2007) measured speech reaction times for picture naming after CWS and CWNS were primed with a neutral prime (tone), the first phoneme of the word (incremental prime), or the whole word except the initial phoneme (holistic prime). Three-year-old CWNS responded more quickly to the holistic prime, whereas 5-year-old CWNS responded more quickly to the incremental prime. The authors explained this change as evidence for a developmental shift from processing and representing words as wholes to processing and representing them as smaller components, such as phonemes. Interestingly, CWS at ages 3 and 5 years responded more quickly to the holistic prime. This preference for the holistic prime suggests that CWS may be delayed in the refinement and processing of phonological representations as sublexical units (Byrd et al., 2007).
Group differences have also been noted for phonological awareness tasks; however, these differences tend to be subtle and subclinical. For example, although both CWNS and CWS ages 5–6 years scored within normal limits on standardized subtests targeting phonological awareness skills, on certain tasks, the CWS group scored significantly lower than the CWNS group (Pelczarski & Yaruss, 2014). Specifically, CWS scored lower than CWNS on blending and elision tasks, which required the manipulation of phonological units ranging in size from whole words to individual phonemes. The groups performed similarly on a receptive task that involved selecting words with the same initial or final phoneme from provided options. This result highlighted that detecting group effects in phonological awareness may depend on selecting tasks with sufficient complexity for the linguistic development of participants (Pelczarski & Yaruss, 2014).
Subclinical group differences were also found in a recent study of rhyme discrimination and production related to stuttering recovery and persistence in CWS ages 4–5 years (Gerwin et al., 2019). Rhyming abilities develop in a similar time frame as stuttering onset, starting as young as 2–3 years of age and increasing rapidly between 4 and 5 years of age (Carroll et al., 2003; Lonigan et al., 1998; Maclean et al., 1987). Although CWNS, CWS-eRec, and CWS-ePer with typical speech sound development demonstrated similar accuracy on both a rhyme discrimination and a rhyme production task, the strategies used to produce rhyming words differentiated CWS-ePer and CWNS. Children were asked to produce a word that rhymed with 10 common, early acquired words such as “can,” “brother,” and “shower.” Accurate rhyme production could be achieved with a real-word strategy (e.g., can, pan) or a nonword strategy (e.g., can, slan). CWS-ePer produced nonword rhymes at approximately twice the rate of CWNS. Reliance on the nonword strategy in CWS-ePer was hypothesized to reflect differences in refinement and flexibility of phonological representations and/or ease of phonological access to the lexicon based on the stimulus word. In other words, CWS-ePer may not as readily separate and manipulate words into onsets and rimes or as readily activate networks of words in the lexicon based on common sublexical units (Gerwin et al., 2019).
Taken together, the findings in these studies suggest subtle differences in the way phonological representations are organized, accessed, or processed by CWS compared to CWNS (Byrd et al., 2007; Gerwin et al., 2019; Pelczarski & Yaruss, 2014). Importantly, these differences, as found by Gerwin and colleagues, may be greater for the CWS-ePer, thus potentially providing an additional clinical tool for assessing the likelihood of persistence in stuttering. However, previous findings also highlight that differences between CWNS and CWS (Pelczarski & Yaruss, 2014) or CWS-eRec and CWS-ePer (Gerwin et al., 2019) depend on task complexity including factors such as the size of the sublexical unit being assessed or whether the task provides response options (receptive) or requires independent response generation (expressive). The current study extends investigations of phonological awareness in young CWS by examining the underlying neural activity mediating a real-word rhyme discrimination task.
Event-Related Potentials and Phonological Processing
Electroencephalography (EEG) is used to measure neural activity that is volume conducted through the scalp (Luck, 2014). Event-related potentials (ERPs) result from EEG that has been time-locked to the onset of a stimulus and averaged across multiple trials in the same condition. ERP methodology is particularly useful for investigating the time course of cognitive processes. The phonological processes underlying rhyme discrimination tasks have been investigated using ERPs in both children and adults (Coch et al., 2002, 2005; Grossi et al., 2001). These rhyme tasks involve presenting word pairs including a prime followed by a rhyming or nonrhyming target word. Rhyme discrimination tasks are well suited to ERP investigations of phonological processing because they do not involve overt speech production, which can add artifact to the continuous EEG recording due to muscle activity from the articulators (Luck, 2014). Specific ERP components, namely, the contingent negative variation (CNV) and N400 rhyme effects, have been elicited in both visual and auditory rhyme tasks.
CNV
The CNV is a late, slow wave that occurs during tasks involving anticipation between a warning and target stimulus (Brunia et al., 2012). In rhyme discrimination tasks, the CNV is elicited by the prime word prior to the presentation of the target and is thought to reflect working memory, covert rehearsal, or allocation of cognitive resources (Coch et al., 2002, 2005; Grossi et al., 2001; Rugg, 1984). The CNV has been elicited using rhyme discrimination in children as young as 6 years old (Coch et al., 2005).
Posterior Rhyme Effect
The N400 is a negative component that peaks approximately 400 ms after presentation of a stimulus word and is thought to index ease and integration of lexical retrieval (Kutas & Federmeier, 2011; Kutas & Hillyard, 1984). This component is elicited for both prime and target words in rhyme discrimination tasks. The N400 rhyme effect occurs when nonrhyming target words elicit an N400 component with larger mean amplitude compared to rhyming target words (Coch et al., 2002, 2005; Grossi et al., 2001; Praamstra & Stegeman, 1993; Rugg, 1984). Considered within the family of N400, the rhyme effect is thought to reflect the processes underlying comparison of phonological representations of the prime and target words (Praamstra & Stegeman, 1993; Rugg, 1984). This effect has a maximal distribution over central, parietal, and occipital electrode sites (Coch et al., 2002, 2005); therefore, we refer to it as the posterior rhyme effect.
Developmentally, the posterior rhyme effect, as indexed by the N400, has been reported in children as young as 3–5 years old (Andersson et al., 2018). This effect is considered adultlike by approximately 6–7 years of age, as no significant differences in amplitude, distribution, or latency were seen across ages 6–21 years (Coch et al., 2002, 2005). However, despite its early development, the N400 elicited by rhyme processing can be influenced by increasing task complexity. Weber-Fox et al. (2003) demonstrated that, when orthographic interference was added to a visual rhyme task (thrown/own vs. cake/own vs. gown/own vs. cone/own), children 9–10 years of age show less efficient processing in the left hemisphere compared to young adults. They exhibited longer peak latencies and less differentiated processing across the conditions when compared to the young adults (Weber-Fox et al., 2003).
Anterior Rhyme Effect
The anterior rhyme effect is elicited by the target words over frontal and anterior temporal sites, with rhyming targets eliciting a larger N400 compared to nonrhyming targets (Coch et al., 2002, 2005). The anterior rhyme effect is influenced by the lexicality of stimuli such that, when real-word stimuli were used, the effect was left lateralized; however, with nonwords, it was bilateral (Coch et al., 2002, 2005; Mohan & Weber, 2015). The anterior rhyme effect is hypothesized to index facilitation of processing for rhyming targets compared to nonrhyming targets based on the phonological information contained in the prime word (Mohan & Weber, 2015).
Phonological Processing in Children and Adults Who Stutter
Using rhyme discrimination tasks to investigate phonological processing, the CNV, posterior rhyme effect, and anterior rhyme effect have been elicited in both children and adults who stutter (Mohan & Weber, 2015; Weber-Fox et al., 2004, 2008). A visual rhyme discrimination task that included orthographic interference was found to differentiate children and adults who stutter from their typically fluent peers. Adults who stutter (AWS) exhibited delayed reaction times relative to adults who do not stutter (AWNS) for rhyme judgments of prime–target word pairs that were orthographically similar but did not rhyme (e.g., gown, own; Weber-Fox et al., 2004). This suggested that, as a group, the AWS were more susceptible to phonological processing breakdowns when increased cognitive load was imposed by misleading orthographic information. Although the amplitude of the rhyme effect (nonrhyme ERP amplitude minus rhyme ERP amplitude) was similar for AWS and AWNS, the hemispheric distribution of the effect distinguished the groups. The AWS showed a larger amplitude rhyme effect over the right hemisphere compared to the left hemisphere, whereas the rhyme effect was similar across hemispheres in the AWNS (Weber-Fox et al., 2004). No differences were noted in the CNV elicited by the primes between AWS and AWNS (Weber-Fox et al., 2004, 2008).With the same visual paradigm in children ages 9–13 years, the N400 in CWNS peaked earlier for electrode sites over the left hemisphere compared to the right, whereas in CWS, the N400 peak latency was similar over both hemispheres (Weber-Fox et al., 2008). In addition, the CNV elicited by the primes was less mature in CWS compared to CWNS. Together, these studies indicate subtle neural differences underlying visual rhyme processing in CWS and AWS compared to their typically fluent peers. Specifically, the groups who stuttered may have recruited the right hemisphere for compensatory processing. Furthermore, given that the pattern of results was not identical in CWS and AWS, the dynamics of development revealed characteristics related to stuttering that were not constant across the life span.
A more recent ERP study of rhyme processing examined whether neural processes of CWS who were persisting in their stuttering (CWS-per) at ages 7–8 years could be distinguished from those of CWS who had recovered (CWS-rec) by that age (Mohan & Weber, 2015). In that study, the children were required to make rhyme judgments based on the auditory presentation of nonwords (e.g., feap–neap vs. bry–pag). Although all groups demonstrated near-ceiling rhyme judgment accuracy, the ERP waveforms distinguished CWS-per from CWS-rec. Furthermore, the ERPs of both groups of CWS differed from those of the CWNS group. All three groups showed a classic rhyme effect over central parietal sites with a larger N400 mean amplitude elicited by nonrhyming targets compared to rhyming targets. In addition, groups showed similar mean amplitudes of the N400 elicited by the primes indicating similar processes of lexical access of prime words across groups. However, the groups differed in the anterior rhyme effect where there was an earlier onset of the N400 for rhyming targets compared to nonrhyming targets over anterior lateral sites. The ERPs of the CWNS showed a bilateral anterior rhyme effect while CWS-per lacked the effect all together. CWS-rec showed the anterior rhyme effect; however, it was lateralized over the right hemisphere. The authors also assessed individual data from the CWS-per group and noted that the majority of participants did not show a typical anterior rhyme effect. Absence of anterior onset rhyme effect in CWS-per was thought to reflect decreased saliency of the phonological representation of the primes and therefore decreased facilitation for processing the rhyme targets (Mohan & Weber, 2015). Atypical laterality and absence of this anterior rhyme effect is consistent with observed differences in the structure and connectivity of the left inferior frontal gyrus (IFG) in CWS. The gray matter volume and white matter connections of the left IFG were found to be decreased in CWS compared to CWNS (Chang et al., 2008, 2015; Chang & Zhu, 2013).
Current Study
The current study was designed to build on and extend investigations of the neural processes underlying phonological awareness in CWS. The primary aim was to examine whether phonological processes indexed by ERPs may differentiate eventual stuttering recovery and persistence in younger, preschool CWNS, CWS-eRec, and CWS-ePer ages 4;1–6;0 (years;months). Compared to previous studies of rhyme processing, these children were closer to the onset of stuttering and within the age range of rapid development of rhyme abilities. We used early-developing real-word stimuli because nonword rhyme discrimination is challenging for children this age (Wagensveld et al., 2013, 2012).
The current study utilizes stuttering outcome data to retrospectively examine the neural processes underlying developing phonological awareness for real-word rhyming in preschool-age CWNS, CWS-eRec, and CWS-ePer. We expected that this rhyme task would elicit the anterior and posterior rhyme effects, which have been observed in typically developing children as young as 3–5 years of age (Andersson et al., 2018). Furthermore, because we used real-word stimuli, we expected that the anterior rhyme effect would be left lateralized, particularly in the CWNS (Coch et al., 2002, 2005). However, the current study is the first to measure neural activity related to rhyming using real-word stimuli in this age range; thus, it is unknown when lateralization of the anterior rhyme effect elicited by real words develops.
Given the findings of Mohan and Weber (2015) in 7- to 8-year-old children for nonword rhyme discrimination, we predicted that the CNV and N400 elicited by the prime words would be similar across groups. Differences between CWS and CWNS for the CNV have only been noted in an older age population using a visual rhyming task with orthographic interference (Weber-Fox et al., 2008). Based on the study of Mohan and Weber, we also expected that the posterior rhyme effect elicited by target words would be similar across groups but that the amplitude or distribution of the anterior rhyme effect would distinguish CWNS, CWS-eRec, and CWS-ePer. Finally, stuttering is a heterogeneous disorder, and previous studies of CWS report on the individual variability within groups for ERP measures (Kaganovich et al., 2010; Kreidler et al., 2017; Usler & Weber-Fox, 2015). Therefore, we examined characteristics of the individual data to improve our understanding of how rhyme processing is developing in each group.
Method
Participants
Participants were part of a longitudinal study of behavioral, motor, emotional, and language factors as they relate to developmental stuttering and eventual recovery and persistence. All procedures included in the project were approved by the institutional review board at Purdue University, and informed consent was obtained for each participant. The current study examined neural activity elicited for phonological processing of rhyme judgments in 29 children aged 4;1–6;0. Eleven participants were CWNS, and 18 were CWS. Of the 18 CWS, nine were CWS-eRec and nine were CWS-ePer.
All children were native English speakers and passed a hearing screening at 250, 500, 1000, 2000, 4000, and 6000 Hz presented at 20 dB HL. They had normal or corrected-to-normal vision and no history of neurological or emotional disorders per parent report. Participants also did not demonstrate social interaction impairments or activity restrictions as measured by the Childhood Autism Rating Scale (Schopler et al., 1988). All participants displayed nonverbal intelligence within normal limits as measured by the Primary Test of Nonverbal Intelligence (Ehler & McGhee, 2008).
Formation of CWNS, CWS-eRec, and CWS-ePer Groups
A participant was diagnosed as stuttering in their first year of the longitudinal study if they met the following criteria based on Yairi and Ambrose (1999): (a) The child's parent(s) and the project speech-language pathologist perceived the child as stuttering, (b) stuttering was rated as a 2 or greater on an 8-point scale (0 = no stuttering, 7 = severe stuttering), and (c) the child produced at least three stuttering-like dysfluencies (SLDs) per 100 syllables in a spontaneous speech sample. The Test of Childhood Stuttering (TOCS; Gilliam et al., 2009) was also administered to quantify stuttering. In four cases, a score within the stuttering range on the TOCS was used in lieu of three SLDs per 100 syllables to diagnose a child as stuttering.
A follow-up online survey was used to determine stuttering outcome status. The average length of time from ERP recording to follow-up survey response was 4 years (range: 1.5–6 years). The probability of recovery after 4 years of stuttering is low and has been estimated at 5% (Yairi & Ambrose, 2005). On the survey, parents responded to questions about the child's fluency and rated their child's speech on an 8-point scale to indicate severity. In combination with responses describing the child's fluency, a rating of 0 or 1 was considered within normal limits and likely recovered. A rating of 2 or higher accompanied by the parent's description of stuttering indicated that the parent considered the child's stuttering to be persisting. A follow-up survey was not completed for one CWS. In this case, stuttering status was determined from this child's fourth and final year of participation in the longitudinal study. A child was considered recovered in the final year of participation if they no longer met the initial diagnostic criteria based on the study of Yairi and Ambrose (1999) in their entirety; otherwise, a child was considered persisting. Of the 18 CWS, nine were considered eRec and nine ePer. Table 1 provides a description of each CWS who participated in the current study, including stuttering outcome, gender, age at ERP session, and parent report of treatment received prior to the first year of participation.
Table 1.
Characteristics of each child who stutters.
| Participant | Gender | Age at ERP session (years;months) | Follow-up duration (years) | Stuttering outcome | Therapy received prior to first year |
|---|---|---|---|---|---|
| CWS 1 | M | 5;6 | 6 | Persisting | N |
| CWS 2 | M | 5;11 | 6 | Persisting | S, L |
| CWS 3 | M | 5;4 | 4 | Persisting | N |
| CWS 4 | M | 5;2 | 5 | Persisting | N |
| CWS 5 | M | 5;2 | 5.5 | Persisting | N |
| CWS 6 | M | 4;5 | 4 | Persisting | N |
| CWS 7 | F | 5;0 | 4 | Persisting | S |
| CWS 8 | F | 5;1 | 3.5 | Persisting | S |
| CWS 9 | M | 4;1 | 1.5 | Persisting | N |
| CWS 10 | M | 6;0 | 4.5 | Recovered | N |
| CWS 11 | M | 5;7 | 4 | Recovered | A |
| CWS 12 | M | 5;0 | 4 | Recovered | N |
| CWS 13 | M | 5;6 | 3 | Recovered | N |
| CWS 14 | M | 5;11 | 4 | Recovered | S, A |
| CWS 15 | M | 5;0 | 5 | Recovered | N |
| CWS 16 | F | 4;8 | 3 | Recovered | N |
| CWS 17 | F | 4;1 | 4 | Recovered | N |
| CWS 18 | M | 4;2 | 1.5 | Recovered | N |
Note. ERP = event-related potential; CWS = child who stutters; M = male; Persisting = child's stuttering continued at time of follow-up; N = none; S = parent report of stuttering therapy prior to first year of participation; L = parent report of language therapy prior to first year of participation; Recovered = child no longer stuttered at the time of follow-up; A = parent report of articulation therapy prior to first year of participation; F = female.
CWNS, CWS-eRec, and CWS-ePer groups were matched for age, F(2, 26) = 0.21, p = .81; nonverbal intelligence, F(2, 26) = 0.31, p = .74; and mother's level of education, F(2, 26) = 0.14, p = .87. Mother's level of education estimated socioeconomic status using the Hollingshead Education Scale (Hollingshead, 1975). This scale provided a rating of education level from a rating of 1 = less than seventh grade through 7 = graduate professional training (graduate degree) (Hollingshead, 1975). Each of the group means for maternal level of education was approximately 6, which indicates “standard college or university graduation.” There were two left-handed participants, one CWNS and one CWS-eRec. All other participants were right-handed. TOCS scores for CWNS and each of the CWS groups at their initial testing differed significantly, confirming group categorization based on fluency: CWNS and CWS-eRec, F(1, 18) = 19.43, p < .01; CWNS and CWS-ePer, F(1, 18) = 66.11, p < .01. CWS-eRec and CWS-ePer produced a similar number of SLDs per 100 syllables, F(1, 16) = 0.60, p = .45; however, these groups demonstrated differences on the TOCS index score, F(1, 16) = 4.40, p = .05, indicating greater stuttering severity for the CWS-ePer group at initial testing. This is consistent with previous research that has suggested that, at 4–5 years of age, greater stuttering severity may help predict eventual persistence in stuttering (Walsh et al., 2018). Group characteristics are summarized for the CWNS, CWS-eRec, and CWS-ePer groups in Table 2.
Table 2.
Characteristics of the CWNS, CWS-eRec, and CWS-ePer groups.
| Group | n (female) | Handedness Right (left) |
Age in months M (SE) |
MLE M (SE) |
PTONI M (SE) |
TOCS M (SE) |
|---|---|---|---|---|---|---|
| CWNS | 11 (3) | 10 (1) | 59.18 (2.27) | 6.09 (0.28) | 113.91 (2.31) | 99.45 (3.39) |
| CWS-eRec | 9 (2) | 8 (1) | 61.22 (2.82) | 5.89 (0.39) | 110.89 (3.19) | 74.22 (4.80) |
| CWS-ePer | 9 (2) | 9 (0) | 60.89 (2.19) | 6.11 (0.31) | 114.67 (4.90) | 62.56 (2.81) |
Note. SE = standard error; MLE = mother's level of education as measured by the Hollingshead Education Scale (Hollingshead, 1975); PTONI = standard score on the Primary Test of Nonverbal Intelligence (Ehler & McGhee, 2008); TOCS = standard score on the Test of Childhood Stuttering (Gilliam et al., 2009); CWNS = children who do not stutter; CWS-eRec = children who stutter and eventually recovered; CWS-ePer = children who stutter and eventually persisted.
Assessment Battery
The Bankson-Bernthal Test of Phonology–Consonant Inventory was administered to assess each participant's speech production abilities (Bankson & Bernthal, 1990). The Structured Photographic Expressive Language Test–Third Edition assessed the participants' expressive language abilities (Dawson et al., 2003), and the Clinical Evaluation of Language Fundamentals Preschool–Second Edition Receptive Language index (Wiig et al., 2004) assessed the participants' receptive language. CWNS, CWS-eRec, and CWS-ePer groups performed similarly on these speech and language assessments, F(2, 26) < 1.72, p > .20. Group means and standard errors on the speech and language assessment battery are presented in Table 3.
Table 3.
Assessment battery means for all groups.
| Group | BBTOP-CI M (SE) |
SPELT-3 M (SE) |
CELF-P2 M (SE) |
PAT rhyme discrimination M (SE) |
PAT rhyme production M (SE) |
ERP rhyme task M (SE) |
|---|---|---|---|---|---|---|
| CWNS | 108.36 (2.64) | 108.64 (3.13) | 106.45 (3.64) | 91.82 (3.25) | 71.82 (6.00) | 89.89 (2.72) |
| CWS-eRec | 106.33 (3.84) | 103.33 (3.18) | 106.22 (2.69) | 94.44 (5.56) | 83.33 (6.45) | 91.39 (3.02) |
| CWS-ePer | 99.67 (4.46) | 101.78 (4.73) | 96.11 (6.40) | 93.33 (2.89) | 82.22 (7.78) | 87.07 (3.20) |
Note. BBTOP-CI = standard score on the Bankson-Bernthal Test of Phonology–Consonant Inventory (Bankson & Bernthal, 1990); SE = standard error; SPELT-3 = standard score on the Structured Photographic Expressive Language Test–Third Edition (Dawson et al., 2005); CELF-P2 = standard score on the Clinical Evaluation of Language Fundamentals Preschool–Second Edition Receptive Language Subtest (Wiig et al., 2004); PAT = percent correct for each task of the Phonological Awareness Test Rhyming subtest (Robertson & Salter, 2007); ERP Rhyme Task = percent correct on event-related potential rhyme task; CWNS = children who do not stutter; CWS-eRec = children who stutter and eventually recovered; CWS-ePer = children who stutter and eventually persisted.
Inclusionary criteria for the current study included rhyming ability to ensure that the participants had developed the phonological awareness abilities necessary for performing the rhyme discrimination task. All participants included in the current study obtained a score of at least 7 of 10 on either the rhyme discrimination or rhyme production task of the Phonological Awareness Test (PAT) Rhyming subtest (Robertson & Salter, 2007). In addition, they performed with a minimum of 70% rhyme judgment accuracy on the ERP rhyme discrimination task (described below in Procedure section). Accuracy scores above chance on at least two measures ensured that participants had developed rhyming abilities. As a preliminary study of the neural activity underlying rhyme discrimination in CWS in this age range, these inclusionary criteria were necessary to compare results with previous studies of children who achieved high rhyme accuracy (Coch et al., 2002, 2005; Mohan & Weber, 2015).
ERP Stimuli
The stimuli for the ERP study included 160 naturally spoken real words. All words were produced by a female native English speaker. All but two words were early developing and familiar words taken from the MacArthur–Bates Communicative Development Inventories (Fenson & Paul, 2007). Forty of these words were also used in the studies of Coch et al. (2002) and Grossi et al. (2001). Stimuli were divided into two lists of 80 pairs each. Each list contained 40 rhyming pairs (e.g., comb–home) and 40 nonrhyming pairs (e.g., blow–grass). The lists were counterbalanced such that the target words of each list were the same; however, on one list, a target was part of a rhyming pair (e.g., comb–home), and on the second list, it was part of a nonrhyming pair (e.g., sheep–home). The average durations of the primes and the targets were 660 ms (SD = 85 ms, range: 460–920 ms) and 650 ms (SD = 89 ms, range: 480–910 ms), respectively.
Procedure
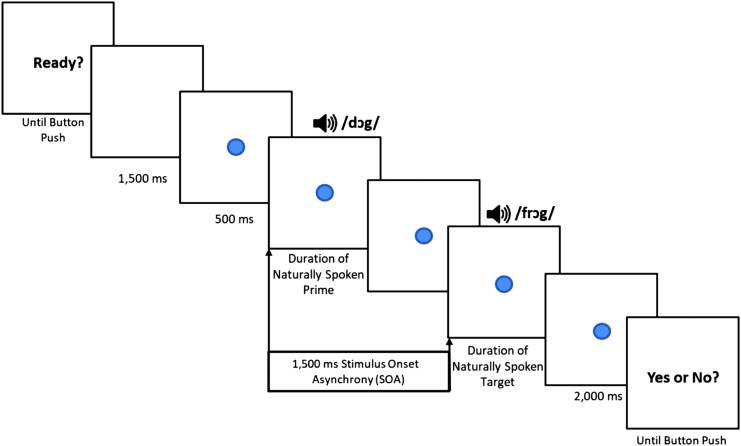
In a sound-attenuating booth, participants sat at a distance of 164 cm from a 47.5-cm monitor with a central speaker above it. A colored circle appeared on the display screen 500 ms prior to each auditory word pair and served as a fixation point through each trial. The prime–target pairs were presented with a 1,500-ms stimulus onset asynchrony at 70–75 dB SPL. The circle remained on the screen as the auditory prime and target words were presented until 2,000 ms after the target word offset. The total presentation time of the circle on the screen was 4,500–5,000 ms, depending on the duration of the target word in a given trial. Figure 1 summarizes the progression of the stimulus presentation. To add variety for interest, the appearance of the fixation circles varied randomly across trials in color (red, orange, yellow, green, blue, indigo, and violet) and size (1.5–4.75 cm). The visual angles of the circles were between 0.52° and 1.66° vertically and horizontally.
Figure 1.
Progression of the auditory and visual stimuli during the event-related potential rhyme discrimination task.
The experimenter gave the following instructions to each participant, “Now you will listen to some words. Sometimes the words will rhyme, like ‘dog’ and ‘frog.’ Sometimes the words won't rhyme, like ‘bee’ and ‘shoe.’ Listen to the pairs of words and try to tell if they rhyme or if they don't rhyme. It is important that you sit very still. While you are listening to the words, look at the circle on the screen in front of you. Every once in a while, you will see a picture pop up. This is the time when you can move! Every time you see a picture, you can take one Lego block and stack it. You will see 10 pictures total. How high can you build your tower? When your tower is done, you will get a surprise! We will start now. Remember to listen carefully to the words that rhyme and don't rhyme. Are you ready?”
After hearing the stimuli for each trial, the participant told the experimenter whether they thought the words rhymed or not by saying “yes” or “no.” The experimenter then entered the participant's response with a key press on the response pad so that the child's answer was recorded and associated with each trial. When a picture (e.g., airplanes, balloons, bubblegum) appeared on the screen, the child took a break to participate in a rewarding activity (Legos, Connect Four, fishing puzzle, etc.).
ERP Recording
Children were fitted with an elastic cap with 32 channels embedded in it with scalp positions that correspond to the International 10–10; system including lateral (F7/F8, FC5/FC6, T7/T8, CP5/CP6, P7/P8), medial (FP1/FP2, AF3/AF4, FC1/FC2, F3/F4, C3/C4, CP1/CP2, P3/P4, PO3/PO4, O1/O2), and midline sites (FZ, CZ, PZ, OZ; Jurcak et al., 2007). The continuous electroencephalogram was recorded using the Biosemi ActiveTwo system. Reference electrodes were placed on the participants' right and left mastoids. Additionally, electrodes were placed on the outer canthi of the right and left eyes to measure horizontal eye movements (horizontal electrooculogram) as well as on the inferior and superior orbital ridges to measure vertical eye movements (vertical electrooculogram).
ERP Analyses
ERPs were time-locked to the onset of the prime and target words. The continuous electroencephalogram was analyzed using EEGLAB (Delorme & Makeig, 2004) and ERPLAB (Lopez-Calderon & Luck, 2014), which are MATLAB toolboxes (MathWorks). The EEG was down-sampled at a rate of 256 Hz and band-pass filtered from 0.1 to 30 Hz. Two trained raters used independent component analysis, a statistical tool included in EEGLAB, to identify and remove eye artifacts, including blinks and movements. If the raters disagreed on which components to remove, a third trained rater worked to form consensus.
The continuous EEG data were epoched from 200 ms before the onset of the prime or target to 2,000 ms after the stimulus onset. Epochs were baseline corrected from −200 ms to stimulus onset (0 ms). Automatic artifact rejection algorithms were run with a 200-ms window moving in 50-ms increments. Epochs were also inspected manually and removed if they contained a remaining artifact such as drift that had not already been eliminated. Each participant had at least 21 artifact-free trials remaining in the rhyme and nonrhyme conditions. Finally, the artifact-free epochs were averaged to produce an ERP waveform at each electrode site for each condition (rhyme and nonrhyme) for each participant. The ERP waveforms of all participants within a group were averaged at each electrode site to produce grand-averaged ERPs. The grand-averaged ERPs for each participant group contained all artifact-free trials, regardless of accuracy. Averages of all artifact-free trials for each participant, rather than correct trials only, were used for analysis to ensure an adequate number of trials in each condition and the most representative ERPs for each participant. The mean number of trials for each condition and group was between 29 and 32 trials. The groups had a similar number of trials in the rhyme condition, F(2, 26) = 0.14, p = .87, and the nonrhyme condition, F(2, 26) = 1.23, p = .31. In addition, groups obtained a similar number of correct trials in each condition of the ERP rhyme task: rhyme condition, F(2, 26) = 0.37, p = .70; nonrhyme condition, F(2, 26) = 2.79, p = .08.
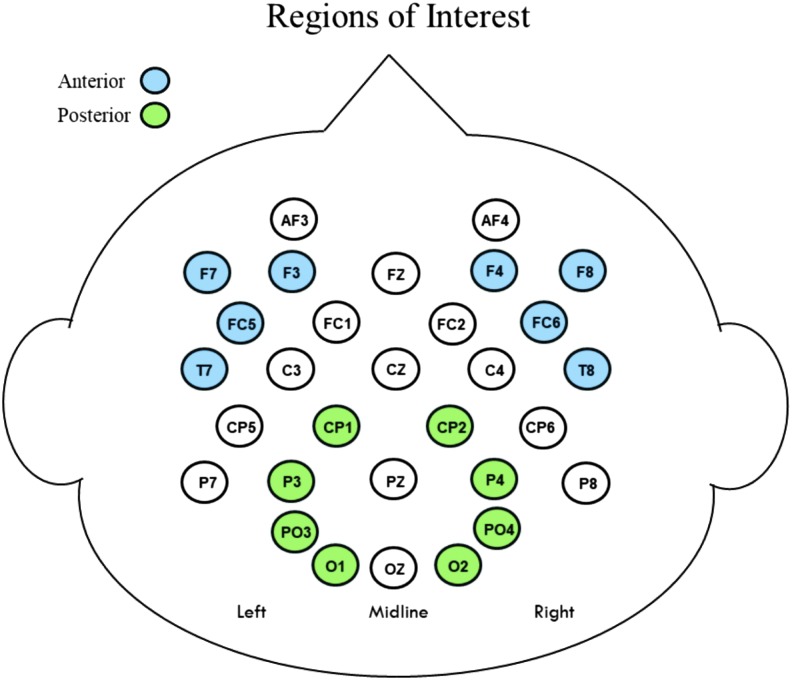
Analyses focused on measuring the mean amplitudes of the ERP components elicited by the prime and target words in each individual's waveforms. Regions of interest (ROIs) and time windows for mean amplitude measurement were chosen based on the distributions of effects in previous studies (Andersson et al., 2018; Coch et al., 2002, 2005; Mohan & Weber, 2015) in conjunction with visual inspection of the waveforms elicited in the current study, which were consistent with earlier findings for this type of task in young children. The CNV elicited by the prime words and the anterior N400s elicited by rhyming and nonrhyming targets were measured over lateral anterior and temporal sites. The posterior N400s elicited by the primes and rhyming and nonrhyming target words were measured over midlateral central parietal and occipital sites. Figure 2 illustrates the specific electrode locations included in these anterior and posterior ROIs.The mean amplitude of the CNV elicited by the primes was measured in a temporal window of 900–1,300 ms, and the N400s elicited by both prime and target words were measured in a temporal window of 300–500 ms.
Figure 2.
Electrode distribution on the head. Separate analyses were performed for the anterior and posterior regions of interest.
Statistical Analyses
Statistical analyses were performed separately for the ERPs elicited by the prime and target words. For the N400 elicited by primes, a repeated-measures analysis of variance (ANOVA) compared the mean amplitude in the posterior ROI between groups (CWNS vs. CWS-eRec vs. CWS-ePer) and within factors of hemisphere (left, right) and parietal-occipital scalp distribution (CP1/2, P3/4, PO3/4, O1/2). A repeated-measures ANOVA compared mean amplitude of the CNV in the anterior ROI between groups (CWNS vs. CWS-eRec vs. CWS-ePer) and within factors of hemisphere (left, right) and frontal-temporal scalp distribution (F7/8, F3/4, FC5/5, T7/8).
For ERPs elicited by the targets, one repeated-measures ANOVA compared N400 mean amplitude in the posterior ROI between groups (CWNS vs. CWS-eRec vs. CWS-ePer) and within factors of condition (rhyme, nonrhyme), hemisphere (left, right), and parietal-occipital scalp distribution (CP1/2, P3/4, PO3/4, O1/2). A separate repeated-measures ANOVA assessed the mean amplitude of the N400 in the anterior ROI between groups (CWNS vs. CWS-eRec vs. CWS-ePer) and within factors of condition (rhyme, nonrhyme), hemisphere (left, right), and frontal-temporal scalp distribution (F7/8, F3/4, FC5/5, T7/8). In addition to assess the effect of condition in each group independently, planned repeated-measures ANOVAs were used to evaluate the anterior and posterior rhyme effects within each of the groups. Results from midline electrodes were consistent with the lateral and medial results and are not reported. The Huynh–Feldt adjusted p values were utilized for repeated measures (Picton et al., 2000). Significance was set at p values of < .05. Effect sizes (ηp 2) are reported for significant effects.
To aid in interpretation of condition and/or group effects, individual patterns of N400 mean amplitude in the rhyme and nonrhyme conditions were also explored in the anterior and posterior ROIs. We measured a nominal variable with only two options (effect present, effect absent) by counting the number of children in each group who demonstrated the expected anterior and posterior effects. The exact test of goodness of fit was run to determine whether the proportion of CWS-eRec and CWS-ePer with and without the anterior or posterior rhyme effect was consistent with theoretical expectations, which we estimated from the CWNS group. Four exact tests were run to include assessment of each rhyme effect (anterior, posterior) in each group (CWS-eRec, CWS-ePer). The method of small p values was applied due to expected proportions other than 50:50, and two-tailed p values were reported (McDonald, 2014).
Results
Rhyme Task Accuracy
Children who were included in the current study demonstrated at least 70% accuracy on either the rhyme discrimination or rhyme production of the PAT and on the ERP rhyme discrimination task to ensure they had developed rhyming abilities. There were no group differences on the PAT rhyme discrimination task, F(2, 26) = 0.11, p = .89; PAT rhyme production task, F(2, 26) = 0.95, p = .40; or the ERP rhyme task, F(2, 26) = 0.51, p = .61. Group means and standard errors on the rhyming tasks are presented in Table 3.
ERPs Elicited by Prime Words
N400
There was no main effect of group, F(1, 26) = 0.97, p = .39, nor were there any significant interactions with group (ps > .12). These results indicated that the mean amplitude of the N400s elicited by the primes was similar across CWNS, CWS-eRec, and CWS-ePer.
CNV
There was no main effect of group, F(1, 26) = 0.90, p = .42, nor were there any interactions with group (ps > .41). These results indicated that the mean amplitude of the CNVs elicited by the primes was similar across CWNS, CWS-eRec, and CWS-ePer.
ERPs Elicited by Target Words
Posterior N400
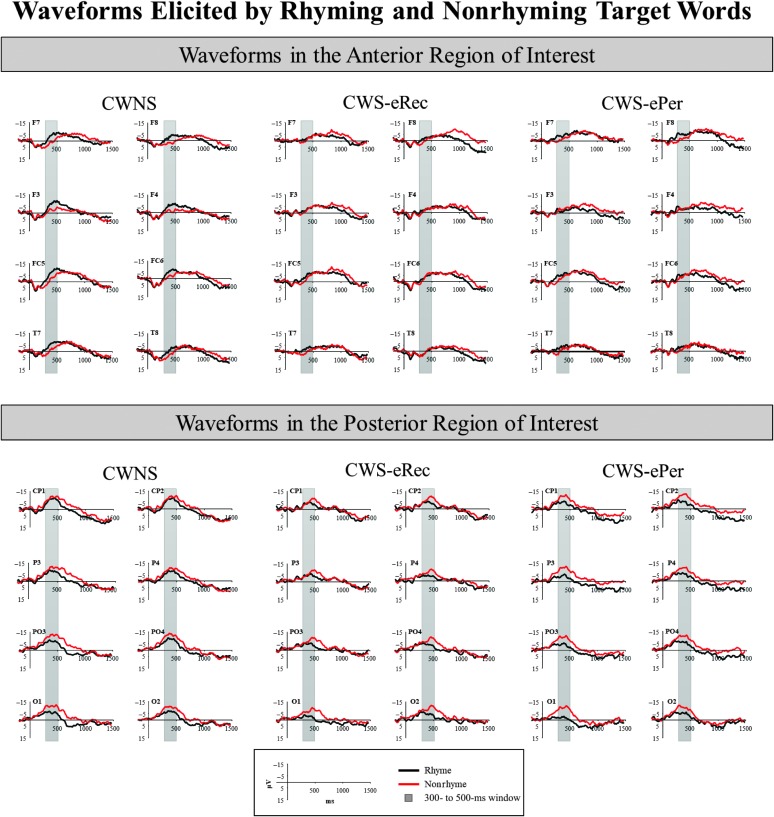
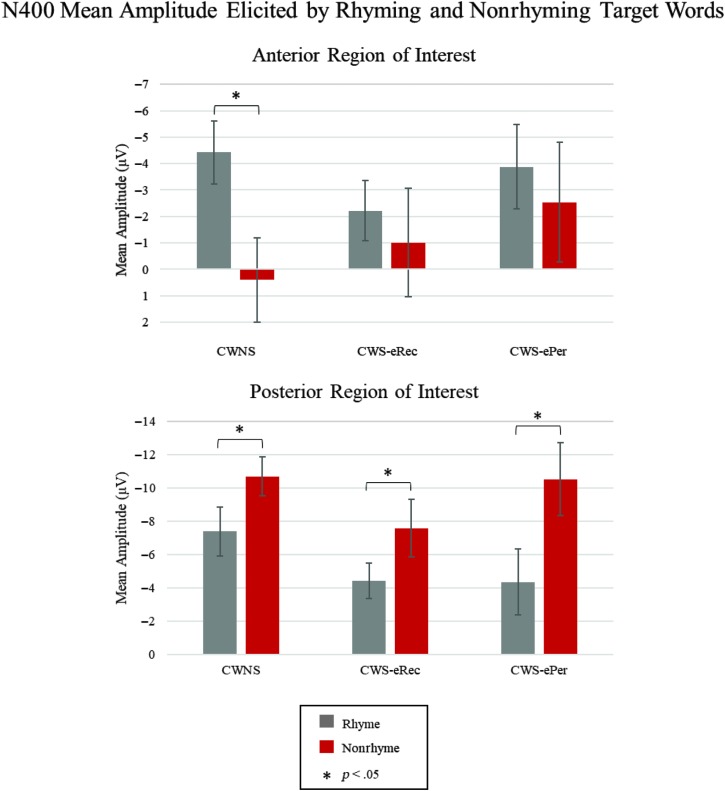
The average waveforms elicited by the rhyming and nonrhyming targets in the posterior ROI are illustrated in the lower plot of Figure 3 for each group. There was a main effect of condition, F(1, 26) = 23.22, p < .01, ηp 2 = .47, indicating a classic rhyme effect characterized by a larger amplitude N400 for nonrhyming targets relative to rhyming targets was elicited in this ROI. There were no Condition × Group interactions (ps > .28). The repeated-measures ANOVA run for each group separately revealed a main effect of condition in each group, CWNS, F(1, 10) = 9.85, p = .01, ηp 2 = .50; CWS-eRec, F(1, 8) = 6.16, p = .04, ηp 2 = .44; and CWS-ePer, F(1, 8) = 8.25, p = .02, ηp 2 = .51. As displayed in Figure 4, all groups demonstrated the posterior rhyme effect.
Figure 3.
Grand-averaged waveforms elicited by rhyming (black) and nonrhyming (red) targets in the anterior (upper plot) and posterior (lower plot) regions of interest. The 300- to 500-ms time window used for mean amplitude measurement is depicted in gray. CWNS = children who do not stutter; CWS-eRec = children who stutter and eventually recovered; CWS-ePer = children who stutter and eventually persisted.
Figure 4.
N400 mean amplitude by condition and group in the anterior (upper plot) and posterior (lower plot) regions of interest. CWNS = children who do not stutter; CWS-eRec = children who stutter and eventually recovered; CWS-ePer = children who stutter and eventually persisted.
Anterior N400
The average waveforms elicited by rhyming and nonrhyming targets in the anterior ROI are illustrated in the upper plot of Figure 3 for each group. There was a main effect of condition in this region, F(1, 26) = 9.30, p < .01, ηp 2 = .26, indicating a larger amplitude N400 for rhyming targets compared to nonrhyming targets, characteristic of the anterior rhyme effect. This effect was observed at each of the ROI electrode sites for the CWNS in Figure 3. The difference between the rhyme and nonrhyme conditions was not as apparent in the grand-averaged waveforms of the CWS-eRec and CWS-ePer groups (see Figure 3). Condition × Group interactions did not reach significance (ps > .12); however, repeated-measures ANOVA run for each group separately revealed a main effect of condition only for the CWNS group, F(1, 10) = 18.25, p < .01, ηp 2 = .65; CWS-eRec, F(1, 8) = 0.54, p = .49; and CWS-ePer, F(1, 8) = 0.87, p = .38. As illustrated in the upper plot of Figure 4, only the CWNS group demonstrated the anterior rhyme effect. In addition, this effect in CWNS did not involve interactions with hemispheric laterality (ps > .74), indicating a bilateral anterior rhyme effect elicited by real words at this young age.
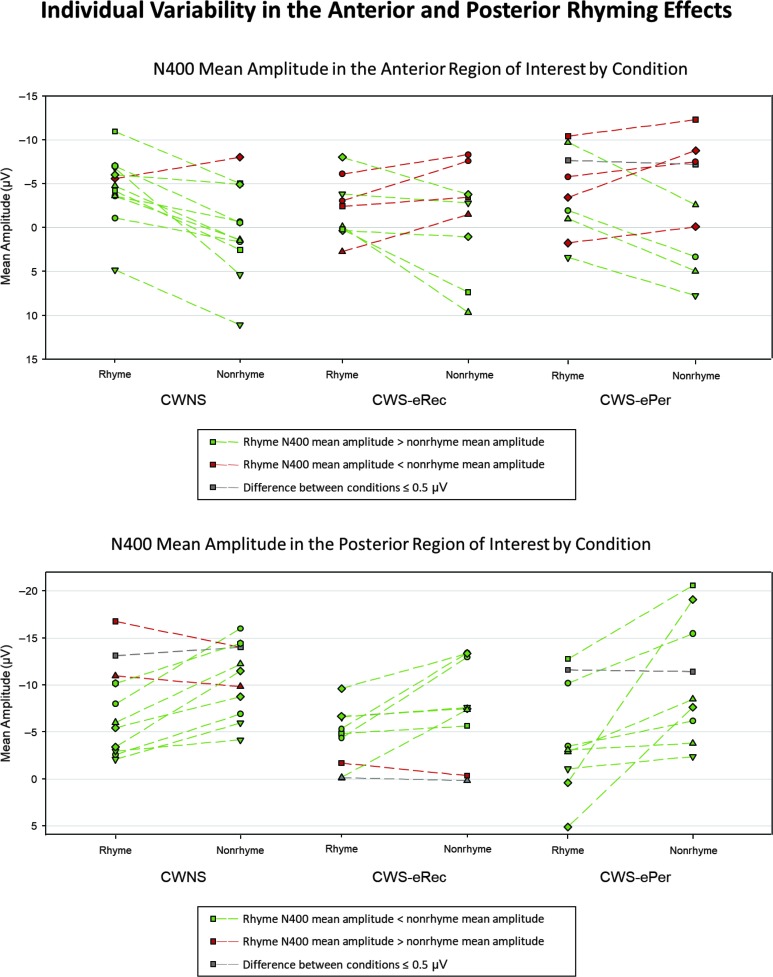
Individual Variability for N400 Rhyme Effects
Individual participant patterns of N400 mean amplitudes elicited in the rhyme and nonrhyme conditions were explored to determine how many children in each group showed the anterior and posterior rhyme effects. Figure 5 illustrates the N400 mean amplitude by condition for each participant in each group in the posterior (lower plot) and anterior (upper plot) ROIs. In the posterior ROI, the majority of participants in each group demonstrated ERPs consistent with the rhyme effect, that is, a larger mean amplitude was elicited by nonrhyming targets compared to rhyming targets. The posterior rhyme effect was shown by eight participants in the CWNS group (73%), seven participants in the CWS-eRec group (78%), and eight participants in the CWS-ePer group (89%). For the exact test of goodness of fit (McDonald, 2014), we set the expected proportion (effect present/effect absent) to 70:30 based on the CWNS data. Significant differences from the expected proportion were not noted for the posterior rhyme effect in the CWS-eRec (p = 1.0) or CWS-ePer (p = .30) groups. In other words, the number of CWS-eRec and CWS-ePer demonstrating the posterior rhyme effect was consistent with the expectation set by the proportion in the CWNS group.
Figure 5.
N400 mean amplitude by condition for each participant in each group in the anterior (upper plot) and posterior (lower plot) regions of interest. CWNS = children who do not stutter; CWS-eRec = children who stutter and eventually recovered; CWS-ePer = children who stutter and eventually persisted.
Patterns in the anterior ROI were not as consistent across individuals, particularly for the CWS groups. The anterior rhyme effect, a larger mean amplitude elicited by the rhyming targets compared to nonrhyming targets, was demonstrated by 10 CWNS (91%), but only five CWS-eRec (56%) and only four CWS-ePer (45%). In addition, in each of the CWS groups, four participants (45%) showed the opposite pattern expected for the anterior rhyme effect. For the exact test of goodness of fit (McDonald, 2014), we set the expected proportion (effect present/effect absent) to 90:10 based on the CWNS data. Significant differences from the expected proportion were noted for the anterior rhyme effect in both the CWS-eRec (p < .01) and CWS-ePer (p < .01) groups. This finding indicates that, for CWS-eRec and CWS-ePer, the number of children demonstrating the anterior rhyme effect is not consistent with the proportion of typically developing peers demonstrating the rhyme effect.
Discussion
The current study used a real-word rhyme discrimination task to investigate the neural underpinnings of phonological processing in CWNS, CWS-eRec, and CWS-ePer ages 4;1–6;0. Compared to previous studies using rhyme discrimination, these children were closer to the onset of stuttering and within the age range of rapid development of rhyme abilities. We assessed whether phonological processing at this young age may predict eventual recovery or persistence in stuttering. In addition, we investigated how patterns of individual data from each CWS group compared to the CWNS group. All children included in the study demonstrated rhyme discrimination abilities for real words, and the groups did not differ in their task accuracy; however, differences were noted in certain ERP components. This finding suggests that successful rhyme discrimination was achieved with different underlying processing. The CNV and N400 ERP components elicited by the prime words did not distinguish the groups, indicating similar lexical access and working memory or rehearsal processing elicited by presentation of the primes. ERPs elicited by rhyming and nonrhyming targets were characterized by the classic posterior rhyme effect, a larger mean amplitude elicited by nonrhyming targets compared to rhyming targets, over central-parietal and occipital sites. The anterior rhyme effect, a larger mean amplitude elicited by rhyming targets compared to nonrhyming targets, over frontal and temporal sites was also elicited. This effect was consistent across individuals in the CWNS group but was present in only half of the CWS-eRec and CWS-ePer participants. Results indicate that specific aspects of phonological processing for early acquired real words may differ for some CWS at ages 4;1–6;0, but these aspects do not predict eventual recovery or persistence at this age. However, differences in the consistency of the presence of an anterior rhyme effect suggested that facilitation of processing for rhyming targets compared to nonrhyming targets based on the phonological information contained in the prime word may still be emerging for a greater percentage of CWS compared to their typically fluent peers.
Overall, the current findings add to our understanding of the underlying processes for phonological awareness in CWS. Greater variability in some aspects of the ERPs for CWS, both CWS-eRec and CWS-ePer, compared to CWNS is consistent with behavioral studies, indicating that phonological awareness may be delayed in some preschool-age CWS (Byrd et al., 2007; Gerwin et al., 2019; Pelczarski & Yaruss, 2014). Furthermore, the current study is the first to examine ERPs elicited by rhyming real words in children as young as 4 years of age. Prior studies in older children have shown that the anterior rhyme effect for real-word stimuli is lateralized over anterior regions of the left hemisphere (Coch et al., 2002). In the current study, the distribution of the anterior rhyme effect was bilateral. These findings suggest that the laterality of the anterior rhyme effect is still developing at this young age when rhyming abilities are emerging.
ERPs Indexing Phonological Processing Related to Stuttering Outcome
The anterior and posterior ERP rhyme effects elicited by early acquired real words were similar for young CWS-eRec and CWS-ePer who were closer to the onset of stuttering and within the time window of rapid development of rhyming abilities. These results contrast with findings from Mohan and Weber (2015), which differentiated 7- to 8-year-old CWNS, CWS-rec, and CWS-per. In that study, which used a nonword rhyme discrimination task, the CWS-per did not demonstrate the anterior rhyme effect, while CWS-rec showed a right-lateralized effect and CWNS showed a bilateral distribution. The differences in findings for the anterior rhyme effect across these studies in CWS highlight the need to investigate factors related to stuttering recovery and persistence through the onset and trajectory of stuttering (Smith & Weber, 2017). Because motor, linguistic, and emotional factors are undergoing rapid development near stuttering onset, identifying predictors of recovery and persistence is dependent on identifying specific temporal windows for comparison of developmental abilities (Smith & Weber, 2017). As noted earlier, differences between CWNS and CWS, and CWNS, CWS-eRec, and CWS-ePer in phonological processing tend to be subtle and subclinical, and detecting these differences may be dependent on the phonological task having sufficient complexity to challenge participants at their current stage of development (Gerwin et al., 2019; Pelczarski & Yaruss, 2014). First, we consider the results of the current study in light of research examining brain structures and functions in CWS and CWNS that are known to be involved in mediating phonological processing. Second, we consider the role of semantic information provided by our rhyme discrimination task that utilized early acquired real-word stimuli, as a facilitator of phonological processing in CWS who eventually recover and those who persist.
Variability of the Anterior Rhyme Effect in CWS Is Consistent With Delays in Maturation in the Left IFG
Variability of the anterior rhyme effect in individual CWS may indicate that this component is still developing, both in CWS-eRec and CWS-ePer. While the majority of CWNS in the current study exhibited the anterior rhyme effect, only half of each CWS group demonstrated the effect. This delayed emergence of the anterior rhyme effect in some CWS may be related to differences between CWS and CWNS in the structure and connectivity of left hemisphere language regions of the brain (Chang et al., 2008, 2015; Chang & Zhu, 2013), including the left IFG, which has been strongly associated with phonological and semantic processing (Liakakis et al., 2011). In neuroimaging studies using rhyme discrimination tasks, the IFG was activated in children and adults in conjunction with more posterior brain areas such as the superior temporal gyrus, fusiform gyrus, and supramarginal gyrus (Cone et al., 2008; Debska et al., 2016; Hurschler et al., 2015; Macsweeney et al., 2009; Zhuang et al., 2016). In addition, increasing cortical thickness of the left IFG, measured longitudinally, was associated with the development of phonological processing skills, such as phoneme elision, in typically developing children ages 5–11 years (Lu et al., 2007). Although the neuroimaging studies in CWS did not use rhyme discrimination to investigate task-related brain activation, differences were noted between CWS and CWNS groups in the structure and connectivity of brain regions shown to be involved in rhyme discrimination, specifically the left IFG (Chang et al., 2008, 2015; Chang & Zhu, 2013). Furthermore, findings from a recent study suggested that stuttering persistence in CWS is associated with decreased growth rate of white matter tracts connecting the left IFG to areas such as the right IFG and supplementary motor areas (Chow & Chang, 2017). Taken together, greater variability in the development of specific aspects of phonological processing, as indexed by the anterior rhyme effect in some young CWS, may be consistent with an underlying delay in the maturation of the left IFG reported for CWS compared to CWNS in this age range.
Semantic Content as a Facilitator of Rhyme Processing in CWS
Earlier findings in 7- to 8-year-old CWS for a nonword rhyme discrimination task indicated the children who were persisting in stuttering did not demonstrate the typical anterior rhyme effect (Mohan & Weber, 2015). A right-lateralized anterior rhyme effect in CWS-rec by this age revealed that they continued to process the nonword rhyme stimuli differently compared to their typically fluent peers (Mohan & Weber, 2015). Given that the children in the current study were younger, we may have predicted similar or greater differences in the anterior rhyme effect for the CWS compared to the CWNS. However, because real-word pairs were used for rhyme discrimination in the current study, it was not known whether the ERPs would distinguish the groups given that real-word rhyme discrimination is less complex compared to nonword rhyme discrimination for young children (Wagensveld et al., 2013, 2012). In the current study of young CWS, the proportion of individual CWS-eRec and CWS-ePer showing the anterior rhyme effect was significantly different from that of CWNS. Approximately half of CWS-ePer and CWS-eRec displayed a typical anterior rhyme effect in their ERPs, whereas in the study of Mohan and Weber (2015), the majority of the persisting group did not. This finding suggests that the semantic information contained in the real-word prime facilitated processing of rhyming compared to nonrhyming target words for some CWS in the current study, including both CWS-eRec and CWS-ePer.
The anterior rhyme effect reflects the differentiation of neural activation for processing rhyming versus nonrhyming targets. It is important to note that the same target words were used in the rhyme and nonrhyme conditions. Therefore, the differences in ERPs for processing the same stimulus words in each condition must reflect the phonological relationship between the prime and target word. In other words, the differences in neural activity were specifically related to rhyme processing. Given the close relationship between phonological development and growth of the lexicon (Edwards et al., 2004, 2011; Metsala, 1997), real-word primes, with their familiarity and semantic information, may facilitate access to phonological representations and networks of phonologically related words within the lexicon. In rhyming pairs, these networks may contain and therefore activate the target word (Gray et al., 2012).
However, when nonword stimuli are used for rhyme discrimination, successful task completion relies solely on analysis of the phonological representation, specifically the acoustic–phonetic representation abstracted from the stimuli by each child (Wagensveld et al., 2012). This process may be one reason rhyme discrimination with nonwords is more challenging than real words for young children (Wagensveld et al., 2013, 2012). In addition, there is some evidence to suggest that CWS have deficits in central speech sound discrimination (Jansson-Verkasalo et al., 2014) and integration of auditory speech information into internal representations of speech sounds (Beal et al., 2011). Therefore, in the current study, some young CWS, both CWS-eRec and CWS-ePer, may have benefitted from the semantic content of the real words to access the lexicon, which resulted in the anterior rhyme effect. In contrast, we hypothesize that the majority of CWS who persisted at 7–8 years of age did not demonstrate the anterior rhyme effect because nonwords did not provide additional semantic support for accessing phonological representations.
The role of semantic content in facilitating language processing for CWS who are persisting is not limited to rhyme processing. Usler and Weber-Fox (2015) compared processing of syntactic violations in English sentences and Jabberwocky (nonword) sentences in CWNS, CWS-rec, and CWS-per ages 6–7 years. In the English sentences, all groups showed the expected ERP component, a P600, elicited by a phrase structure syntactic violation (e.g., “He wants to play with those his toys.”). However, ERPs elicited by phrase structure syntactic violations in Jabberwocky sentences (e.g., “Ho digbay to tangwon those his bowz.”), which are made up of nonwords while maintaining English syntax with closed-class words, distinguished the groups. While CWNS and CWS-rec exhibit a similar P600 compared to English sentences, CWS-per exhibited an earlier developing N400-like response. This N400-like ERP elicited by syntactic violations in Jabberwocky sentences compared to the P600 elicited by violations in English sentences indicated that semantic context in English sentences supported the syntactic processing in CWS-per. However, without the support of semantic context, CWS-per differed from their CWS-rec peers revealing a less mature syntactic processing strategy (Usler & Weber-Fox, 2015). Thus, converging ERP evidence suggests that, depending on age and the task complexity, CWS, in particular those who persist, may benefit from semantic contextual support for language processing (Mohan & Weber, 2015; Usler & Weber-Fox, 2015).
In contrast to the anterior rhyme effect, the classic posterior rhyme effect, thought to reflect comparison of the phonological representations of the prime and target word (Rugg, 1984), was found to be robust in all the groups in the current study. This is consistent with findings of the posterior rhyme effect in typically developing children as young as 3 years of age for nonwords (Andersson et al., 2018) and children as young as 6 years of age for real words (Coch et al., 2002), as well as the findings in CWS-rec and CWS who had persisted from Mohan and Weber (2015).
Limitations and Future Directions
As an initial study of phonological processing mediating rhyme discrimination, the current study included only children with high rhyme discrimination accuracy for early acquired real words. Although limiting the sample size, this criterion allowed for comparisons of ERPs across groups of CWS related to eventual recovery and persistence that demonstrated similar speech, language, and rhyming abilities. Children with deficits in speech sound production, namely, speech sound disorder, are at risk for deficits in phonological awareness (Anthony et al., 2011; Rvachew & Grawburg, 2006). In future studies, the neural underpinnings of children with low rhyme accuracy may be investigated to examine whether phonological processing in children who are just developing rhyming abilities may distinguish eventual recovery and persistence. Future studies may also include CWS with concomitant speech sound disorder. Including children with varying degrees of phonological awareness and concomitant disorders may provide a more representative sample of the CWS population to broaden our understanding of the underlying neurodevelopment of phonological processing and identify other possible indicators of eventual recovery and persistence.
Conclusions
The anterior and posterior rhyme effects were elicited in the ERPs of young CWNS, CWS-eRec, and CWS-ePer who completed a real-word rhyme discrimination task with at least 70% accuracy. Although these effects did not distinguish stuttering recovery and persistence, the individual variability in the presence of the anterior rhyme effect in the CWS groups compared to CWNS may reflect underlying differences in structure and connectivity of brain areas such as the left IFG, which has been associated with phonological processing (Chang et al., 2008, 2015; Chang & Zhu, 2013; Liakakis et al., 2011; Lu et al., 2007). In addition, when compared with previous work using nonword tasks, the results of the current study point to the role of semantic information as a facilitator of language processing in CWS (Mohan & Weber, 2015; Usler & Weber-Fox, 2015). Specifically, these findings suggest that the semantic content of our real-word rhyme discrimination task likely supported phonological access to the lexicon for some individual CWS, both CWS-eRec and CWS-ePer. However, approximately half of the CWS exhibited altered neural activity over anterior regions for rhyme processing, which was similar to the patterns of older CWS completing a more complex nonword rhyme processing task.
Acknowledgments
This research was funded by grants from the National Institute on Deafness and Other Communication Disorders (DC000559 to Smith and Weber co-PIs and 4T32DC000030-25 to PI Laurence Leonard). We would like to thank the members of the Neural Systems for Language Processing Lab, Jennifer Schumaker, Carlie Lepore, and Rhiana Ragheb, for their assistance in data collection and processing. We would also like to thank Amanda Hampton Wray and Ranjini Mohan for their helpful comments on this study.
Funding Statement
This research was funded by grants from the National Institute on Deafness and Other Communication Disorders (DC000559 to Smith and Weber co-PIs and 4T32DC000030-25 to PI Laurence Leonard).
References
- Andersson A., Sanders L. D., Coch D., Karns C. M., & Neville H. J. (2018). Anterior and posterior erp rhyming effects in 3- to 5-year-old children. Developmental Cognitive Neuroscience, 30, 178–190. https://doi.org/10.1016/j.dcn.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony J. L., Aghara R. G., Dunkelberger M. J., Anthony T. I., Williams J. M., & Zhang Z. (2011). What factors place children with speech sound disorders at risk for reading problems? American Journal of Speech-Language Pathology, 20(2), 146–160. https://doi.org/10.1044/1058-0360(2011/10-0053) [DOI] [PubMed] [Google Scholar]
- Anthony J. L., & Francis D. J. (2005). Development of phonological awareness. Current Directions in Psychological Science, 14(5), 255–259. https://doi.org/10.1111/j.0963-7214.2005.00376.x [Google Scholar]
- Bankson N. W., & Bernthal J. E. (1990). Bankson–Bernthal Test of Phonology. Pro-Ed. [Google Scholar]
- Beal D. S., Quraan M. A., Cheyne D. O., Taylor M. J., Gracco V. L., & De Nil L. F. (2011). Speech-induced suppression of evoked auditory fields in children who stutter. NeuroImage, 54(4), 2994–3003. https://doi.org/10.1016/j.neuroimage.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunia C. H. M., van Boxtel G. J. M., & Böcker K. B. E. (2012). Negative slow waves as indices of anticipation: The Bereitschaftspotential, the contingent negative variation, and the stimulus-preceding negativity. In Luck S. J. & Kappenman E. S. (Eds.), The Oxford handbook of event-related potential components (pp. 189–207). Oxford University Press; https://doi.org/10.1093/oxfordhb/9780195374148.013.0108 [Google Scholar]
- Byrd C. T., Conture E. G., & Ohde R. N. (2007). Phonological priming in young children who stutter: Holistic versus incremental processing. American Journal of Speech-Language Pathology, 16(1), 43–53. https://doi.org/10.1044/1058-0360(2007/006) [DOI] [PubMed] [Google Scholar]
- Carroll J. M., Snowling M. J., Stevenson J., & Hulme C. (2003). The development of phonological awareness in preschool children. Developmental Psychology, 39(5), 913–923. https://doi.org/10.1037/0012-1649.39.5.913 [DOI] [PubMed] [Google Scholar]
- Chang S.-E., Erickson K. I., Ambrose N. G., Hasegawa-Johnson M. A., & Ludlow C. L. (2008). Brain anatomy differences in childhood stuttering. NeuroImage, 39(3), 1333–1344. https://doi.org/10.1016/j.neuroimage.2007.09.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.-E., & Zhu D. C. (2013). Neural network connectivity differences in children who stutter. Brain: A Journal of Neurology, 136(Pt. 12), 3709–3726. https://doi.org/10.1093/brain/awt275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.-E., Zhu D. C., Choo A. L., & Angstadt M. (2015). White matter neuroanatomical differences in young children who stutter. Brain, 138(3), 694–711. https://doi.org/10.1093/brain/awu400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow H. M., & Chang S.-E. (2017). White matter developmental trajectories associated with persistence and recovery of childhood stuttering. Human Brain Mapping, 38(7), 3345–3359. https://doi.org/10.1002/hbm.23590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch D., Grossi G., Coffey-Corina S., Holcomb P. J., & Neville H. J. (2002). A developmental investigation of ERP auditory rhyming effects. Developmental Science, 5(4), 467–489. https://doi.org/10.1111/1467-7687.00241 [Google Scholar]
- Coch D., Grossi G., Skendzel W., & Neville H. (2005). ERP nonword rhyming effects in children and adults. Journal of Cognitive Neuroscience, 17(1), 168–182. https://doi.org/10.1162/0898929052880020 [DOI] [PubMed] [Google Scholar]
- Cone N. E., Burman D. D., Bitan T., Bolger D. J., & Booth J. R. (2008). Developmental changes in brain regions involved in phonological and orthographic processing during spoken language processing. NeuroImage, 41(2), 623–635. https://doi.org/10.1016/j.neuroimage.2008.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J., Stout C., & Eyer J. (2003). Structured Photographic Expressive Language Test–Third Edition (SPELT-3). Janelle Publications. [Google Scholar]
- Debska A., Łuniewska M., Chyl K., Banaszkiewicz A., Zelechowska A., Wypych M., Marchewka A., Pugh K. R., & Jednoróg K. (2016). Neural basis of phonological awareness in beginning readers with familial risk of dyslexia—Results from shallow orthography. NeuroImage, 132, 406–416. https://doi.org/10.1016/j.neuroimage.2016.02.063 [DOI] [PubMed] [Google Scholar]
- Delorme A., & Makeig S. (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Dollaghan C., & Campbell T. (1998). Nonword repetition and child language impairment. Journal of Speech, Language, and Hearing Research, 41(5), 1136–1146. https://doi.org/10.1044/jslhr.4105.1136 [DOI] [PubMed] [Google Scholar]
- Edwards J., Beckman M. E., & Munson B. (2004). The interaction between vocabulary size and phonotactic probability effects on children's production accuracy and fluency in nonword repetition. Journal of Speech, Language, and Hearing Research, 47(2), 421–436. https://doi.org/10.1044/1092-4388(2004/034) [DOI] [PubMed] [Google Scholar]
- Edwards J., Munson B., & Beckman M. E. (2011). Lexicon–phonology relationships and dynamics of early language development—A commentary on Stoel-Gammon's relationships between lexical and phonological development in young children. Journal of Child Language, 38(1), 35–40. https://doi.org/10.1017/S0305000910000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehler D. J., & McGhee R. L. (2008). PTONI: Primary Test of Nonverbal Intelligence. Pro-Ed. [Google Scholar]
- Fenson L., & Paul H. (2007). MacArthur–Bates Communicative Development Inventories. Brookes; https://doi.org/10.1037/t11538-000 [Google Scholar]
- Gerwin K., Brosseau-Lapré F., Brown B., Christ S., & Weber C. (2019). Rhyme production strategies distinguish stuttering recovery and persistence. Journal of Speech, Language, and Hearing Research, 62(9), 3302–3319. https://doi.org/10.1044/2019_JSLHR-S-18-0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam R. B., Logan K. J., & Pearson N. A. (2009). Test of Childhood Stuttering (TOCS). Pro-Ed. [Google Scholar]
- Gray S., Reiser M., & Brinkley S. (2012). Effect of onset and rhyme primes in preschoolers with typical development and specific language impairment. Journal of Speech, Language, and Hearing Research, 55(1), 32–44. https://doi.org/10.1044/1092-4388(2011/10-0203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi G., Coch D., Coffey-Corina S., Holcomb P. J., & Neville H. J. (2001). Phonological processing in visual rhyming: A developmental ERP study. Journal of Cognitive Neuroscience, 13(5), 610–625. https://doi.org/10.1162/089892901750363190 [DOI] [PubMed] [Google Scholar]
- Hollingshead A. B. (1975). Four factor index of social status [Unpublished working paper]. Yale University. [Google Scholar]
- Hurschler M. A., Liem F., Oechslin M., Stämpfli P., & Meyer M. (2015). fMRI reveals lateralized pattern of brain activity modulated by the metrics of stimuli during auditory rhyme processing. Brain and Language, 147, 41–50. https://doi.org/10.1016/j.bandl.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Jansson-Verkasalo E., Eggers K., Järvenpää A., Suominen K., Van den Bergh B., De Nil L., & Kujala T. (2014). Atypical central auditory speech-sound discrimination in children who stutter as indexed by the mismatch negativity. Journal of Fluency Disorders, 41, 1–11. https://doi.org/10.1016/j.jfludis.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Jurcak V., Tsuzuki D., & Dan I. (2007). 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. NeuroImage, 34(4), 1600–1611. https://doi.org/10.1016/j.neuroimage.2006.09.024 [DOI] [PubMed] [Google Scholar]
- Kaganovich N., Wray A. H., & Weber-Fox C. (2010). Non-linguistic auditory processing and working memory update in pre-school children who stutter: An electrophysiological study. Developmental Neuropsychology, 35(6), 712–736. https://doi.org/10.1080/87565641.2010.508549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidler K., Hampton Wray A., Usler E., & Weber C. (2017). Neural indices of semantic processing in early childhood distinguish eventual stuttering persistence and recovery. Journal of Speech, Language, and Hearing Research, 60(11), 3118–3134. https://doi.org/10.1044/2017_JSLHR-S-17-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M., & Federmeier K. D. (2011). Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP). Annual Review of Psychology, 62(1), 621–647. https://doi.org/10.1146/annurev.psych.093008.131123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M., & Hillyard S. A. (1984). Brain potentials during reading reflect word expectancy and semantic association. Nature, 307(5947), 161–163. https://doi.org/10.1038/307161a0 [DOI] [PubMed] [Google Scholar]
- Liakakis G., Nickel J., & Seitz R. J. (2011). Diversity of the inferior frontal gyrus—A meta-analysis of neuroimaging studies. Behavioural Brain Research, 225(1), 341–347. https://doi.org/10.1016/j.bbr.2011.06.022 [DOI] [PubMed] [Google Scholar]
- Lonigan C. J., Burgess S. R., Anthony J. L., & Barker T. A. (1998). Development of phonological sensitivity in 2- to 5-year-old children. Journal of Educational Psychology, 90(2), 294–311. https://doi.org/10.1037/0022-0663.90.2.294 [Google Scholar]
- Lopez-Calderon J., & Luck S. J. (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8(213). https://doi.org/10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L. H., Leonard C. M., Thompson P. M., Kan E., Jolley J., Welcome S. E., Toga A. W., & Sowell E. R. (2007). Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: A longitudinal MRI analysis. Cerebral Cortex, 17(5), 1092–1099. https://doi.org/10.1093/cercor/bhl019 [DOI] [PubMed] [Google Scholar]
- Luck S. J. (2014). An introduction to the event-related potential technique (2nd ed.). MIT Press. [Google Scholar]
- Maclean M., Bryant P., & Bradley L. (1987). Rhymes, nursery rhymes and reading in early childhood. Merrill-Palmer Quarterly, 33(3), 255–281. https://www.jstor.org/stable/23086536 [Google Scholar]
- Macsweeney M., Brammer M. J., Waters D., & Goswami U. (2009). Enhanced activation of the left inferior frontal gyrus in deaf and dyslexic adults during rhyming. Brain, 132(7), 1928–1940. https://doi.org/10.1093/brain/awp129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. H. (2014). Handbook of biological statistics (3rd ed.). Sparky House Publishing. [Google Scholar]
- Metsala J. (1997). An examination of word frequency and neighborhood density in the development of spoken-word recognition. Memory & Cognition, 25(1), 47–56. https://doi.org/10.3758/BF03197284 [DOI] [PubMed] [Google Scholar]
- Mohan R., & Weber C. (2015). Neural systems mediating processing of sound units of language distinguish recovery versus persistence in stuttering. Journal of Neurodevelopmental Disorders, 7(1), 28 https://doi.org/10.1186/s11689-015-9124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden E. P., Ambrose N. G., & Yairi E. (2002). Phonological progress during the first 2 years of stuttering. Journal of Speech, Language, and Hearing Research, 45(2), 256–267. https://doi.org/10.1044/1092-4388(2002/020) [DOI] [PubMed] [Google Scholar]
- Paden E. P., Yairi E., & Ambrose N. G. (1999). Early childhood stuttering II: Initial status of phonological abilities. Journal of Speech, Language, and Hearing Research, 42(5), 1113–1124. https://doi.org/10.1044/jslhr.4205.1113 [DOI] [PubMed] [Google Scholar]
- Pelczarski K. M., & Yaruss J. S. (2014). Phonological encoding of young children who stutter. Journal of Fluency Disorders, 39, 12–14. https://doi.org/10.1016/j.jfludis.2013.10.003 [DOI] [PubMed] [Google Scholar]
- Picton T. W., Bentin S., Berg P., Donchin E., Hillyard S. A., Johnson R. Jr., Miller G. A., Ritter W., Ruchkin D. S., Rugg M. D., & Taylor M. J. (2000). Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology, 37(2), 127–152. https://doi.org/10.1111/1469-8986.3720127 [PubMed] [Google Scholar]
- Praamstra P., & Stegeman D. F. (1993). Phonological effects on the auditory N400 event-related brain potential. Cognitive Brain Research, 1(2), 73–86. https://doi.org/10.1016/0926-6410(93)90013-U [DOI] [PubMed] [Google Scholar]
- Robertson C., & Salter W. (2007). Phonological Awareness Test-2 (PAT-2). LinguiSystems. [Google Scholar]
- Rugg M. D. (1984). Event-related potentials in phonological matching tasks. Brain and Language, 23(2), 225–240. https://doi.org/10.1016/0093-934X(84)90065-8 [DOI] [PubMed] [Google Scholar]
- Rvachew S., & Brosseau-Lapré F. (2018). Developmental phonological disorders: Foundations of clinical practice (2nd ed.). Plural. [Google Scholar]
- Rvachew S., & Grawburg M. (2006). Correlates of phonological awareness in preschoolers with speech sound disorders. Journal of Speech, Language, and Hearing Research, 49(1), 74–87. https://doi.org/10.1044/1092-4388(2006/006) [DOI] [PubMed] [Google Scholar]
- Schopler E., Reichler R. J., & Renner B. R. (1988). The Childhood Autism Rating Scale (CARS) for diagnostic screening and classification of autism. Western Psychological Services. [Google Scholar]
- Shiller D., Rvachew S., & Brosseau-Lapré F. (2010). Importance of the auditory perceptual target to the achievement of speech production accuracy. Canadian Journal of Speech-Language Pathology and Audiology, 34(3), 181–192. [Google Scholar]
- Smith A., & Weber C. (2017). How stuttering develops: The multifactorial dynamic pathways theory. Journal of Speech, Language, and Hearing Research, 60(9), 2483–2505. https://doi.org/10.1044/2017_JSLHR-S-16-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C., & Weber-Fox C. (2014). Preschool speech articulation and nonword repetition abilities may help predict eventual recovery or persistence of stuttering. Journal of Fluency Disorders, 41, 32–46. https://doi.org/10.1016/j.jfludis.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usler E., & Weber-Fox C. (2015). Neurodevelopment for syntactic processing distinguishes childhood stuttering recovery versus persistence. Journal of Neurodevelopmental Disorders, 7(1), 4 https://doi.org/10.1186/1866-1955-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagensveld B., Segers E., van Alphen P., & Verhoeven L. (2013). The role of lexical representations and phonological overlap in rhyme judgments of beginning, intermediate and advanced readers. Learning and Individual Differences, 23(1), 64–71. https://doi.org/10.1016/j.lindif.2012.09.007 [Google Scholar]
- Wagensveld B., van Alphen P., Segers E., & Verhoeven L. (2012). The nature of rhyme processing in preliterate children. British Journal of Educational Psychology, 82(4), 672–689. https://doi.org/10.1111/j.2044-8279.2011.02055.x [DOI] [PubMed] [Google Scholar]
- Walley A. C. (1993). The role of vocabulary development in children's spoken word recognition and segmentation ability. Developmental Review, 13(3), 286–350. https://doi.org/10.1006/drev.1993.1015 [Google Scholar]
- Walsh B., Usler E., Bostian A., Mohan R., Gerwin K., Brown B., Weber C., & Smith A. (2018). What are predictors for persistence in childhood stuttering? Seminars in Speech and Language, 39(4), 299–312. https://doi.org/10.1055/s-0038-1667159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox C., Spencer R., Cuadrado E., & Smith A. (2003). Development of neural processes mediating rhyme judgments: Phonological and orthographic interactions. Developmental Psychobiology, 43(2), 128–145. https://doi.org/10.1002/dev.10128 [DOI] [PubMed] [Google Scholar]
- Weber-Fox C., Spencer R. M. C., Spruill J. E., & Smith A. (2004). Phonologic processing in adults who stutter: Electrophysiological and behavioral evidence. Journal of Speech, Language, and Hearing Research, 47(6), 1244–1258. https://doi.org/10.1044/1092-4388(2004/094) [DOI] [PubMed] [Google Scholar]
- Weber-Fox C., Spruill J. E., Spencer R., & Smith A. (2008). Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Developmental Science, 11(2), 321–337. https://doi.org/10.1111/j.1467-7687.2008.00678.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig E. H., Secord W. A., & Semel E. (2004). Clinical Evaluation of Language Fundamentals Preschool–Second Edition (CELF Preschool-2). The Psychological Corporation/A Harcourt Assessment Company. [Google Scholar]
- Yairi E., & Ambrose N. G. (1999). Early childhood stuttering I. Journal of Speech, Language, and Hearing Research, 42(5), 1097–1112. https://doi.org/10.1044/jslhr.4205.1097 [DOI] [PubMed] [Google Scholar]
- Yairi E., & Ambrose N. G. (2005). Early childhood stuttering for clinicians by clinicians. Pro-Ed. [Google Scholar]
- Yairi E., & Ambrose N. G. (2013). Epidemiology of stuttering: 21st century advances. Journal of Fluency Disorders, 38(2), 66–87. https://doi.org/10.1016/j.jfludis.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E., & Seery C. H. (2015). Stuttering: Foundations and clinical applications (2nd ed.). Pearson. [Google Scholar]
- Zhuang J., Johnson M. A., Madden D. J., Burke D. M., & Diaz M. T. (2016). Age-related differences in resolving semantic and phonological competition during receptive language tasks. Neuropsychologia, 93(Pt. A), 189–199. https://doi.org/10.1016/j.neuropsychologia.2016.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]