Highlights
-
•
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer death in the world.
-
•
Approximately, 0.8% of patients will present with skin lesion as the first sign of a silent internal malignancy as skin metastasis without visceral metastasis is rare in CRC.
-
•
A comprehensive literature review (including clinical features of patients, management, and outcome) covering all reported cases of cutaneous metastasis secondary to rectal cancer was included for better understanding of the disease.
Keywords: Rectal adenocarcinoma, Colorectal cutaneous metastases, Groin skin metastasis, Cutaneous cancer, Cutaneous nodule
Abstract
Background
Colorectal cancer is ranked third among the most commonly diagnosed malignancies and fourth among the leading causes of cancer death in the world. However, only a few case reports are found in the literature regarding skin metastasis originating from rectal cancer, which usually shows widespread disease and poor prognosis. Approximately, 0.8% of the patients will have skin lesion as the first indication of a silent internal malignancy, which is rare.
Case report
We report a complicated case of a 45-year-old male patient who referred to our highly specialized governmental hospital for diversion loop colostomy as well as biopsies of rectal and inguinal skin areas followed by palliative radiation therapy to the pelvis. Histopathological exam of rectal biopsies revealed moderately differentiated rectal adenocarcinoma, while the skin of the right inguinal area showed metastatic cutaneous rectal adenocarcinoma. Unfortunately, palliative radiation therapy was not started as the patient passed away secondary to respiratory failure which ended by cardiopulmonary arrest.
Conclusion
A patient who is having new or evolving skin lesions with an oncology history should be well investigated as cutaneous metastasis is a strong possibility.
1. Introduction
The concerns regarding increased colorectal cancer (CRC) incidence and mortality are increased in many medium-to-high human development index (HDI) regions including Eastern Europe, Asia, and South America compared to the highest indexed HDI regions including USA, Australia, New Zealand, and several Western European countries [1]. Currently, CRC is ranked third among the most commonly diagnosed malignancies and fourth among the leading causes of cancer death in the world. By 2030, the estimate increase in the burden of CRC is about 60% denoting more than 2 million newly diagnosed patients and 1.1 million cancer-caused deaths [2]. Metastatic skin cancer occurs from internal malignancy, which is considered extremely rare, representing only 0.001% of all skin biopsies performed [3].
Skin cancer related to CRC accounts only for about 6.5% of all biopsied lesions, most often metastasize to liver and lung. Approximately, 0.8% of patients will have skin lesion as the first indication of a silent internal malignancy which is rare compared to metastasis that occurs a few years after the detection or resection of the primary tumor (usually developing within the first 3 years of follow-up). Spared skin metastasis without visceral metastasis is rare in CRC [[3], [4], [5], [6]]. This project has been reported in line with the SCARE criteria [46].
We are reporting a complicated case of locally advanced low rectal cancer with extensive metastasis to inguinal and perineal skin and distant metastasis to multiple organs in a middle-aged Saudi male patient who was treated as a palliative case. A comprehensive literature review (including clinical features of patients, management, and outcome) covering all reported cases of secondary to rectal cancer was included for better understanding of the disease.
2. Case report
A 45-year-old male patient, known to have white matter leukodystrophy and generalized spasticity of unknown etiology which started at the age of 14, was progressive and kept bedridden for the past 7 years for which a Baclofen pump was inserted. Also, he had mental disability. The patient was diagnosed with locally advanced low rectal cancer with distant metastasis to multiple organs including perineal and inguinal skin, lung, external iliac, colon, and inguinal lymph nodes metastasis. In 2018, about 1 month prior to referral to King Faisal Specialist Hospital and Research Center, the patient was following with Neurology regarding his condition, where he was found to have lower GI bleeding and surgery was involved. He underwent investigation for lower GI bleeding including colonoscopy, and he was found to have rectal mass and could not pass the scope above it as well as the skin lesion which was biopsied. The case was discussed in the multidisciplinary tumor board and planned for diversion loop colostomy as well as rectal biopsies and inguinal area skin biopsy followed by palliative radiation therapy to the pelvis. Computed Tomography (CT) scan of the abdomen and pelvis (Fig. 1, Fig. 2, Fig. 3) demonstrated a circumferential enhancing wall thickening involving the whole rectum with ill-defined hypodense area seen 7–9 o’clock with possible involvement of the anal canal associated with diffuse edema and fat stranding of the mesorectum. There were multiple necrotic lymph nodes noted in the mesorectum and bilateral internal iliac region, the largest one in the right internal ilium measuring 2 cm. There were multiple necrotic lymph nodes seen on the right external iliac (measuring 1.6 cm) and bilateral inguinal area, the largest one on the left side measuring 3.4 × 3.3 cm. There were bilateral symmetrical hilar necrotic lymph nodes measuring on the right side 2.5 × 2 cm and on the left side 3.2 × 1.6 cm. At the perivascular space, they measured 1 cm, being at least T3 N2.
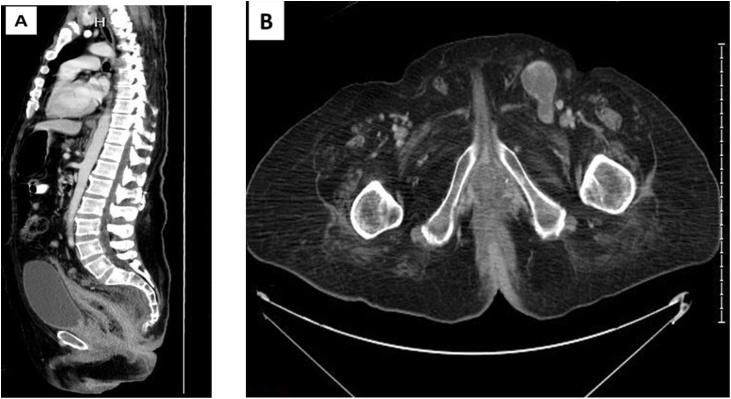
Fig. 1.
Sagittal and axial (B) reformatting for abdomen and pelvis CT scan show diffuse wall thickening of the colon with haziness of the meso-rectal fat planes and multiple regional enlarged lymph nodes. The thickening is extending into the anus. There are also multiple enlarged lymph nodes in inguinal areas.
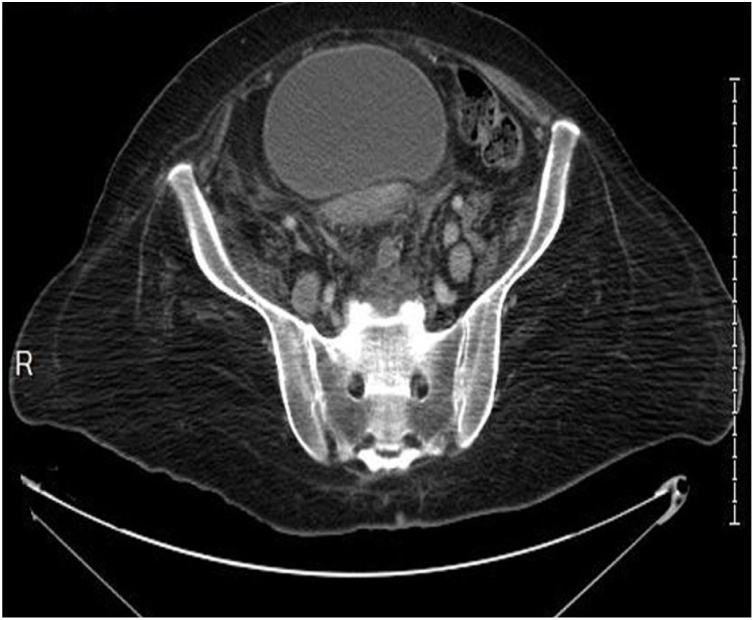
Fig. 2.
Axial CT scan of the pelvis shows multiple enlarged lymph nodes seen along the iliac vessels.
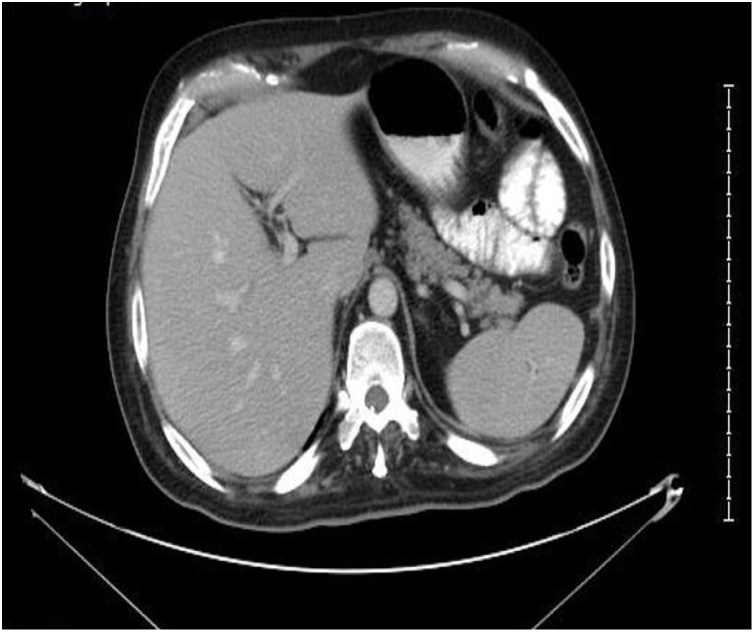
Fig. 3.
CT scan upper abdomen with IV contrast shows no abdominal organs metastases.
Magnetic Resonance Imaging (MRI) for local staging was contraindicated as the patient was with an implanted pump. CT Chest (Fig. 4) demonstrated multiple bilateral tiny pulmonary nodules, the largest one measuring 4 mm in the right upper lobe. Upon examination, the patient was bedridden with poor functional status. Glasgow coma score (GCS) was 15/15, having generalized spasticity. Perineal examination revealed multiple exophytic masses in the scrotal skin, inguinal folds, and perineum and gluteal folds, which were firm to hard in consistency with a few being ulcerated especially over scrotum with little oozing of the serosanguinous fluid. There was no mechanical obstruction of orifices (anal or urethral). The patient was in the general surgical ward to continue postoperative care and management. Histopathological exam of rectal biopsies revealed moderately differentiated rectal adenocarcinoma, while the skin of the right inguinal area showed metastatic cutaneous rectal adenocarcinoma (Fig. 5a, b, c). Unfortunately, later, the patient developed respiratory failure secondary to aspiration pneumonia which ended by cardiopulmonary arrest and death.
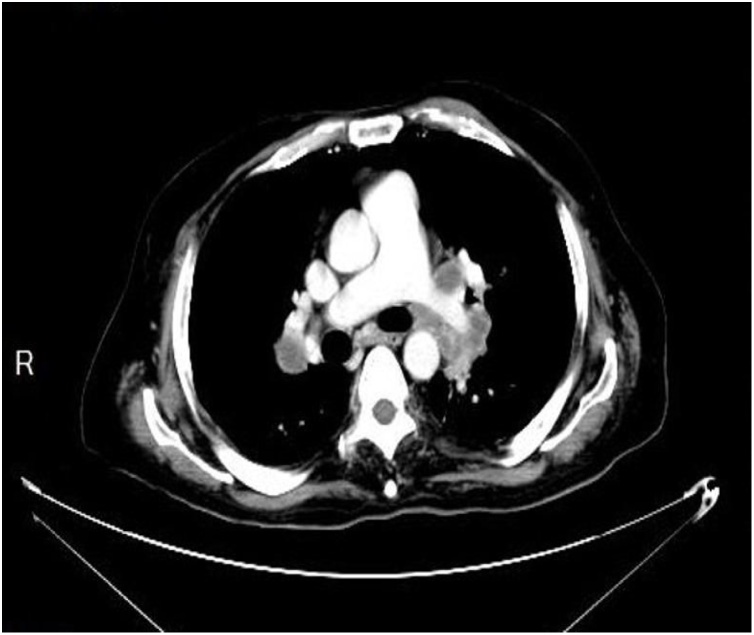
Fig. 4.
CT scan of the chest with IV contrast shows bilateral hilar and mediastinal enlarged lymph nodes.
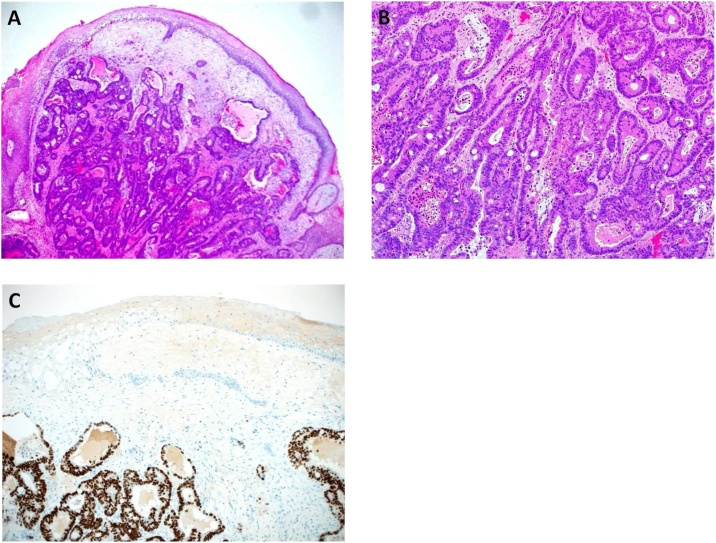
Fig. 5.
a) The tumor involved the dermis and subcutaneous tissue [hematoxylin-eosin (H&E), original magnification ×40]. b) The tumor consisted of complex and single neoplastic glandular structures with intervening desmoplastic stroma [H&E, original magnification ×100]. c) Immunohistochemical staining for CDX2 shows diffuse and strong nuclear staining in the neoplastic cells [original magnification ×100].
3. Discussion
CRC is the most common cancer affecting males and the third common cancer affecting females in Saudi Arabia, which accounts for 11.5% of the newly diagnosed cases in 2014 [39]. Krathen et al. conducted a meta-analysis which demonstrated the incidence of colorectal cutaneous metastasis as ranging between 3.4% and 4.0% [4,40].
Worldwide, a few case reports were reported regarding skin metastasis associated with rectal cancer, and none of them was from the Kingdom of Saudi Arabia or Arabic Gulf countries. Accordingly, we report the first case report of palliative Saudi male patient diagnosed with rectal cancer associated with extensive skin metastasis to the perineum and inguinal area.
Grossly, the specimen consisted of a single, dome-shape piece of skin with the underlying soft tissue, measuring 0.8 × 0.6 × 0.3 cm. The skin was intact and showed no evidence of ulceration. Sections from the tumor were fixed in 10% buffered formalin, paraffin-embedded, sectioned at 5 μm, mounted on coated glass-slides, and stained by routine hematoxylin and eosin stain. A CDX2 immunohistochemical (IHC) stain (clone EPR2764Y, Ventana) with proper positive control study was performed following the manufacturer’s guidelines, using an automated platform (Ventana Benchmark XT, Tucson, AZ, USA), and heat antigen retrieval by ultracell condition solution PH 8.4. An ultra-view universal DAB detection kit was used for reaction visualization.
Histopathological examination showed polypoid, moderately differentiated adenocarcinoma, which involved the dermis and subcutaneous soft tissue (Fig. 5a). The tumor consisted of complex and single glandular structures with intervening desmoplastic stroma. The neoplastic cells exhibited a moderate degree of pleomorphism and had round, hyperchromatic and vesicular nuclei, visible nucleoli, moderate amount of cytoplasm, and poorly-defined cell membranes (Fig. 5b). Mitoses, single cell necrosis, and central, comedo-type necrosis were seen. The neoplastic cells exhibited diffuse and strong nuclear positivity for CDX2 (Fig. 5c). The overlying epidermis showed mild hyperkeratosis with no dysplasia or melanocytic atypia. The pathological findings showed compatability with colorectal metastatic adenocarcinoma.
Skin metastasis usually developed during the first three years postoperatively or the diagnosis of rectal cancer which may be the first sign or symptom of asymptomatic, unsuspected occult malignancy associated with poor prognosis, a failure of ongoing management or recurrence. Multiple theories of the possible rout of skin metastasis were introduced. These included direct extension, lymphatics, and implantation at the time of biopsy or operative procedure, hematogenous spread, and spread along the embryonic ligaments [4,28].
CRC metastases to the skin are well-differentiated, often mucin-secreting adenocarcinomas. They are typically presented with a rapidly growing painless flesh-colored dermal or subcutaneous nodule or as a mass with occasional ulceration. Cutaneous metastasis is mostly on the postoperative scars. It is also found, however, on the extremities, head, neck, and penis skin. It commonly denotes widespread disease and poor prognosis with an estimated median survival of 18–20 months after the occurrence of skin metastases [[3], [4], [5]].
An extensive literature review was conducted which demonstrated a limited number of reported cases demonstrated as the following: Reingold investigated 2300 cases of internal malignancies through autopsy studies and found only one case of skin metastasis secondary to rectal cancer out of 32 cases of cutaneous metastases [4,41]. At Hershey Medical Center in Pennsylvania, two wide-scale retrospective studies were conducted by Lookingbill et al. investigating the cutaneous metastases of internal malignancies as they had four and eighteen cases, respectively, but the result did not clearly show whether the metastasis is secondary to colon or rectum cancers [28,42].
Up to date, only 43 cases of cutaneous metastases secondary to rectal cancer were reported, as 28 cases were found by Dehal et al. as listed in Table 1, in addition to 15 cases found by Hakami et al. as listed in Table 2. The patients had a mean age of 59 years (range 29–84), with male predominance as 27 were men; the majority of them presented with skin nodules and had adenocarcinoma as the underlying histology. High-risk features such as mucinous (n = 8), signet ring cell (n = 4), and poor differentiation (n = 7) appeared in 44.18% of the patients. In our case, histopathological exam of the skin of right inguinal area revealed skin with cutaneous adenocarcinoma, consistent with metastatic rectal adenocarcinoma, which consists of a single irregular piece of pale tan soft tissue measuring 0.8 × 0.6 × 0.3 cm. However, the rectal biopsies consisted of multiple tiny pieces of cream-tan soft irregular tissue measuring in aggregate 0.7 × 0.3 × 0.2 cm which revealed moderately differentiated rectal adenocarcinoma. Immunohistochemical stains were done as the assayed sample carried the mutation c.34G>T (p.G12C) in exon 2 of the K-RAS oncogene. No mutations were found in exons 2, 3, and 4 of the N-RAS oncogenes and in exons 11 and 15 of the BRAF gene.
Table 1.
Cases of rectal cancer with cutaneous metastasis (Dehal et al.).
| Author, year | Age, years | Sex | Histology | Stage | Primary cancer treatment | Interval,a months | Skin mets location | Skin mets morphology | Skin mets treatment | Survival (follow-up time in months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gray and Das, [7] 1989 | 79 | F | Adenocarcinoma | – | Radiation | 0b | Leg | Nodules | None | No (18) |
| Reed and Stoddard, [8] 1992 | 68 | F | Adenocarcinoma, poorly differentiated | – | LAR | 4 | Perineum | Nodules | APR | – |
| De Friend et al., [9] 1992 | 49 | F | Adenocarcinoma | III | LAR | 7 | Perineum | Nodules | WLE | – |
| Kauffman and Sina, [10] 1997 | 50 | M | Adenocarcinoma, signet ring | IV | LAR + ACR | 36 | Multiple | Plaques | None | No (3) |
| Adani et al., [11] 2001 | 70 | F | Adenocarcinoma | III | APR + AC | 36 | Leg | Nodules | CR | Yes (14) |
| Tsai et al., [12] 2002 | 47 | M | Adenocarcinoma, signet ring | III | APR + AC | 11 | Multiple | Nodules | C | No (4) |
| Melis et al., [13] 2002 | 41 | M | Adenocarcinoma | IV | NCR | 1 | Perineum | Plaques | None | – |
| Damin et al., [14] 2003 | 44 | M | Adenocarcinoma | II | LAR | 6 | Groin | Zosteriform | R | No (5) |
| Hayashi et al., [15] 2003 | 50 | M | Adenocarcinoma, mucinous | – | LAR | 4 | Perineum | Nodules | None | – |
| Sarid et al., [16] 2004 | 60 | F | Adenocarcinoma, mucinous | III | NR + LAR + ACR | 16 | Chest, abdomen | Ulcers | WLE | No (56) |
| Reuter et al., [17] 2006 | 69 | M | Adenocarcinoma | II | APR + ACR | 5 | Perineum | Plaques | None | No (6) |
| Tan et al., [18] 2006 | 70 | M | Adenocarcinoma, mucinous | IIIb | LAR + AC | 24 | Back | Nodules | WLE, C | – |
| Tan et al., [18] 2006 | 53 | F | Adenocarcinoma | IIIb | APR | 10 | Perineum | Nodules | WLE, CR | No (26) |
| Kilickap et al., [19] 2006 | 29 | M | Adenocarcinoma, signet ring | IIIa | LAR + APR + ACR | 14 | Chest wall, axilla | Nodules | WLE, C | Yes (4) |
| Gazoni et al., [20] 2008 | 55 | F | Adenocarcinoma, poorly differentiated | IV | Colostomy + CR | 0b | Perineum | – | CR | No (3) |
| Gazoni et al., [20] 2008 | 66 | M | Adenocarcinoma, poorly differentiated | IV | Colostomy + CR | 0b | Perineum | – | CR | No (4) |
| Gazoni et al., [20] 2008 | 68 | M | Adenocarcinoma, poorly differentiated | IV | Colostomy + CR | 0b | Thigh, axilla | – | CR | No (3) |
| Gazoni et al., [20] 2008 | 72 | M | Adenocarcinoma | IV | Colostomy + CR | 0b | Perineum | – | CR | No (5) |
| Gazoni et al., [20] 2008 | 65 | M | Adenocarcinoma | IV | Colostomy + CR | 0b | Perineum | – | CR | No (7) |
| Gazoni et al., [20] 2008 | 78 | M | Adenocarcinoma | IV | Stent + CR | 0b | Perineum | – | CR | No (1) |
| McWeeney et al., [21] 2009 | 72 | M | Adenocarcinoma | III | Ileostomy + NCR | 6 | Perineum | Nodules | WLE | – |
| Saladzinskas et al., [22] 2010 | 64 | M | Adenocarcinoma, mucinous | IIa | NR + LAR | 42 | Face | Ulcers | WLE | Yes (7) |
| Ismaili et al., [23] 2011 | 50 | F | Adenocarcinoma, signet ring | IV | None | 0b | Multiple | Zosteriform | None | No (1) |
| Balta et al., [24] 2012 | 46 | M | Adenocarcinoma, mucinous | IIIb | Colostomy | 12 | Perineum | Ulcers | None | – |
| de Miguel Valencia et al., [25] 2013 | 55 | M | Adenocarcinoma, mucinous | IIIb | NCR + APR + AC | 18 | Multiple | Nodules | None | No (—) |
| Ozgen et al., [26] 2013 | 65 | M | Adenocarcinoma | IIa | NCR + LAR + ACR | 18 | Perineum | Nodules | CR | Yes (12) |
| Akpak et al., [27] 2013 | 47 | F | Adenocarcinoma | IV | APR | 36 | Perineum | Ulcers | WLE + CR | – |
| Dehal et al., [28] 2015 | 47 | M | Adenocarcinoma | IV | CR | 1 | Perineum | Nodules | R | Yes (12) |
– = data not reported; AC = adjuvant chemotherapy; ACR = adjuvant chemoradiation; APR = abdominoperineal resection; C = chemotherapy; CR = chemoradiation; F = female; LAR = low anterior resection; M = male; mets = metastasis; NCR = neoadjuvant chemoradiation; NR = neoadjuvant radiation; R = radiation; WLE = wide local excision.
Interval between cancer treatment/diagnosis and skin metastasis presentation.
In those patients, skin metastasis was the first sign of the underlying malignancy. Therefore, there was no interval between the primary cancer diagnosis and the onset of the skin metastasis.
Table 2.
Additional cases of rectal cancer with cutaneous metastasis (current study).
| Author, year | Age, years | Sex | Histology | Stage | Primary cancer treatment | Interval,a months | Skin mets location | Skin mets morphology | Skin mets treatment | Survival (follow-up time in months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Nasti G et al., [29] 2007 | 76 | F | Adenocarcinoma | IIIb | NCR | 0a | Parotid skin and Frontal face | Nodules | None | No (15) |
| Tranchart et al., [30] 2008 | 59 | F | Adenocarcinoma, well differentiated | IIa | LP + TME + ISR | 14a | Perianal | Nodules | WLE + C | No (16) |
| Tranchart et al., [30] 2008 | 70 | M | Adenocarcinoma, well differentiated | IIa | NCR + P + TME | 10a | Perianal | Nodules | WLE | Yes (22) |
| Goris et al., [31] 2011 | 79 | M | Adenocarcinoma | – | Resection (-) | 36a | Pubis, penis and scrotum | Nodule | None | No (6) |
| Balta et al., [32] 2013 | 84 | F | Adenocarcinoma | IV | NCR | – | Occipital | Nodule | NCR | Yes (-) |
| Miguel Valencia et al., [33] 2013 | 55 | M | Adenocarcinoma, well differentiated, mucinous | III | NCR + APR | – | Pectoral | Nodules | No | No (-) |
| Kitahara et al., [34] 2014 | 52 | M | Adenocarcinoma, moderately differentiated | IIc | CR + TPE | – | Perineum | Nodules | Extended TPE | No (36) |
| Kitahara et al., [34] 2014 | 38 | M | Adenocarcinoma, moderately differentiated | IV | CR + TPE | – | Perineum | Nodules | Extended TPE | Yes (60) |
| Kitahara et al., [34] 2014 | 50 | F | Adenocarcinoma, poorly differentiated | IIc | CR + APR | – | Perineum | Nodules | APR | Yes (24) |
| Yazilitas et al., [35] 2015 | 50 | F | Adenocarcinoma | – | NC + resection + ACR | 7a | Forehead | Nodule | None | -(24) |
| Liasis, L. et al., [36] 2016 | 61 | M | Adenocarcinoma, poorly differentiated | IIa | NCR + APR + ACR | 2a | Perineum | Ulcer | APR | Yes (60) |
| Wang et al., [37] 2017 | 76 | F | Adenocarcinoma, poorly differentiated | IIIc | LAR | 0b | Back, Gingiva | Nodules | None | No (3) |
| Hamid et al., [38] 2017 | 75 | F | Adenocarcinoma, well differentiated | – | NCR + P + TME | 14a | Perianal | Nodules | None | – |
| Yagnik et al., [3] 2018 | 38 | M | Adenocarcinoma | IV | DLC + C | 24a | Penis and pubic | Nodule and ulcer | None | No (2) |
| Current study 2019 | ||||||||||
| 45 | M | Adenocarcinoma, moderately differentiated | IV | DLC | 0b | Groin, Perineum | Ulcer | None | No (1) | |
– = data not reported; AC = adjuvant chemotherapy; ACR = adjuvant chemoradiation; APR = abdominoperineal resection; C = chemotherapy; CR = chemoradiation; F = female; LAR = low anterior resection; M = male; mets = metastasis; NCR = neoadjuvant chemoradiation; NR = neoadjuvant radiation; R = radiation; WLE = wide local excision, DLC = diversion loop colostomy, TPE = total pelvic exenteration, LP = laparoscopic proctectomy, TME = total mesorectal excision, ISR = intersphincteric resection, P = proctectomy, O = oopherctomy.
cDescribed in this article.
Interval between cancer treatment/diagnosis and skin metastasis presentation.
In those patients, skin metastasis was the first sign of the underlying malignancy. Therefore, there was no interval between the primary cancer diagnosis and the onset of the skin metastasis.
Advanced-stage disease was found in most of the patients (stage III in 13 patients and stage IV in 15 patients). Surgical intervention, either low anterior resection, diversion, or abdominoperineal resection, was performed, with or without neoadjuvant or adjuvant chemoradiation. 25 patients had recurrent skin metastasis, recurring in less than 2 years in average. In some cases, skin metastasis was the first sign of the underlying malignancy. Skin metastases appeared in several sites, the most common of which was the perineum. Most patients received treatment for skin metastasis in different modalities and vital status was reported for 33 patients. Among these, 23 died.
Only 32 patients reported for follow-up. In average, the period of time between the diagnosis of skin metastasis and death was about 14.8 months (median 7, range 1–60). Multiple cases of isolated cutaneous rectal metastases with no evidence of visceral disease were reported. Also, in a limited number of patients, the cutaneous lesion could occur before the metastasis of any other organ.
Up to date, there is no optimal or standardized strategy with limitation in the management of malignant cutaneous metastasis, but some chemotherapy regimens (including cisplatin, oxaliplatin, irinotecan, capecitabine, and 5-FU) showed good results in control symptoms and increasing survival rate where, for example, combinations of infusional 5FU/LV with irinotecan (FOLFIRI) or FOLFOX helped extend the survival to more than 20 months if compared to surgical intervention [4,28,37].
Also, regimens were reported by Tournigand et al. which prolonged the median survival times, as follows: FOLFIRI followed by FOLFOX to 21.5 months and FOLFOX followed by FOLFIRI to 20.6 months [3,43]. However, multiple cutaneous metastases or unresectable lesions patients can consider systemic chemotherapy. Very limited margins or wide local excision and reconstruction can be considered for isolated skin lesions as recommended in many studies [37,44,45].
4. Conclusion
Patient and relatives education, thorough clinical examination during follow-up, and a high index of suspicion are highly recommended with patients who have colorectal cancer or at high risk to develop it. Additional research looking for proper management of such lesions is needed.
Declaration of Competing Interest
All authors have nothing to disclose.
Sources of funding
All authors listed below have no source of funding to disclose.
Ethical approval
There is no ethical approval was obtained as it’s a case report but a written consent was taken from the family as the patient passed away.
Consent
A written consent was taken from the family as the patient passed away.
Author contribution
Riyadh Hakami: study concept or design.
Turki Alshammari: study concept or design.
Mohammed alali: data collection, data analysis, interpretation, writing the paper.
Sulaiman AlShammari: data collection, writing the paper.
Zyad Alyahya: data collection.
Mohammed Ayesh: data interpretation (radiology part).
Khaled AlSaad: data analysis interpretation (pathology part).
Alaa Abduljabbar: study concept, design, writing the paper.
Research studies
Our paper is a case report, no registration was done for it.
Guarantor
Riyadh Hakami: Drriyadhhakami@gmail.com.
Turki Alshammari: turki.md84@gmail.com.
Alaa Abduljabbar: aabduljabbar@kfshrc.edu.sa.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgement
None exists.
Contributor Information
Riyadh Hakami, Email: Drriyadhhakami@gmail.com.
Mohammed N. Alali, Email: drmo7ammed2@gmail.com.
Turki Alshammari, Email: turki.md84@gmail.com.
Sulaiman AlShammari, Email: dr.sulimaan@gmail.com.
Zyad Alyahya, Email: zyadalyahya@gmail.com.
Mohammed Ayesh, Email: msashorafa@gmail.com.
Khaled AlSaad, Email: kalsaad@kfshrc.edu.sa.
Alaa Abduljabbar, Email: aabduljabbar@kfshrc.edu.sa.
References
- 1.Ferlay J., Soerjomataram I., Ervik M. International Agency for Research on Cancer; Lyon, France: 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. [Google Scholar]
- 2.Arnold M., Sierra M.S., Laversanne M. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Kemal Y., Odabaşı E.A., Kemal Ö., Bakırtaş M. Cutaneous metastasis of colon adenocarcinoma. Turk. J. Surg. 2018;34(3):237–239. doi: 10.5152/turkjsurg.2017.3298. Published 2018 Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yagnik V.D. Penile and multiple cutaneous metastases over the pubic region from a rectal adenocarcinoma: an uncommon case. Tzu Chi Med. J. 2018;30(1):44–46. doi: 10.4103/tcmj.tcmj_73_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madabhavi Irappa. Multiple subcutaneous nodules leading to diagnosis of Colon Cancer. Middle East J. Digest. Dis. (MEJDD), [S.l.] 2018;10(3):188–191. doi: 10.15171/mejdd.2018.109. ISSN 2008-5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nambiar Sudheer, Karippot Asha. Multiple cutaneous metastases as initial presentation in advanced colon cancer. Case Rep. Gastrointest. Med. 2018;2018 doi: 10.1155/2018/8032905. Article ID 8032905, 3 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray M., Das S. An unusual presentation of colorectal carcinoma. Br. J. Clin. Pract. 1989;43(9):344–345. [PubMed] [Google Scholar]
- 8.Reed M.W., Stoddard C.J. Cutaneous perianal recurrence of cancer after anterior resection using the EEA stapling device. Ann. R. Coll. Surg. Engl. 1992;74(4):301–302. [PMC free article] [PubMed] [Google Scholar]
- 9.De Friend D.J., Kramer E., Prescott R., Corson J., Gallagher P. Cutaneous perianal recurrence of cancer after anterior resection using the EEA stapling device. Ann. R. Coll. Surg. Engl. 1992;74(2):142–143. [PMC free article] [PubMed] [Google Scholar]
- 10.Kauffman C.L., Sina B. Metastatic inflammatory carcinoma of the rectum: tumor spread by three routes. Am. J. Dermatopathol. 1997;19(5):528–532. doi: 10.1097/00000372-199710000-00107. [DOI] [PubMed] [Google Scholar]
- 11.Adani G.L., Marcello D., Anania G. Subcutaneous right leg metastasis from rectal adenocarcinoma without visceral involvement. Chir. Ital. 2001;53(3):405–407. [PubMed] [Google Scholar]
- 12.Tsai H.L., Huang Y.S., Hsieh J.S., Huang T.J., Tsai K.B. Signet-ring cell carcinoma of the rectum with diffuse and multiple skin metastases—a case report. Kaohsiung J. Med. Sci. 2002;18(7):359–362. [PubMed] [Google Scholar]
- 13.Melis M., Scintu F., Marongiu L., Mascia R., Frau G., Casula G. Inflammatory cutaneous metastasis from rectal adenocarcinoma: report of a case. Dis. Colon Rectum. 2002;45(4):562–563. doi: 10.1007/s10350-004-6239-4. [DOI] [PubMed] [Google Scholar]
- 14.Damin D.C., Lazzaron A.R., Tarta C., Cartel A., Rosito M.A. Massive zosteriform cutaneous metastasis from rectal carcinoma. Tech. Coloproctol. 2003;7(2):105–107. doi: 10.1007/s10151-003-0019-3. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi H., Shimizu T., Shimizu H. Scrotal metastases originating from colorectal carcinoma. Clin. Exp. Dermatol. 2003;28(2):226–227. doi: 10.1046/j.1365-2230.2003.01232_4.x. [DOI] [PubMed] [Google Scholar]
- 16.Sarid D., Wigler N., Gutkin Z., Merimsky O., Leider-Trejo L., Ron I.G. Cutaneous and subcutaneous metastases of rectal cancer. Int. J. Clin. Oncol. 2004;9(3):202–205. doi: 10.1007/s10147-004-0389-1. [DOI] [PubMed] [Google Scholar]
- 17.Reuter J., Bruckner-Tuderman L., Braun-Falco M. Epidermotropic scrotal metastasis of colorectal cancer. Int. J. Colorectal Dis. 2007;22(9):1133–1134. doi: 10.1007/s00384-006-0140-7. [DOI] [PubMed] [Google Scholar]
- 18.Tan K.Y., Ho K.S., Lai J.H. Cutaneous and subcutaneous metastases of adenocarcinoma of the colon and rectum. Ann. Acad. Med. Singapore. 2006;35(8):585–587. [PubMed] [Google Scholar]
- 19.Kilickap S., Aksoy S., Dinçer M., Saglam E.A., Yalçin S. Cutaneous metastases of signet cell carcinoma of the rectum without accompanying visceral involvement. South. Med. J. 2006;99(10):1137–1139. doi: 10.1097/01.smj.0000221633.71021.ac. [DOI] [PubMed] [Google Scholar]
- 20.Gazoni L.M., Hedrick T.L., Smith P.W. Cutaneous metastases in patients with rectal cancer: a report of six cases. Am. Surg. 2008;74(2):138–140. [PubMed] [Google Scholar]
- 21.McWeeney D.M., Martin S.T., Ryan R.S., Tobbia I.N., Donnellan P.P., Barry K.M. Scrotal metastases from colorectal carcinoma: a case report. Cases J. 2009;2(1):111. doi: 10.1186/1757-1626-2-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saladzinskas Z., Tamelis A., Paskauskas S., Pranys D., Pavalkis D. Facial skin metastasis of colorectal cancer: a case report. Cases J. 2010;3:28. doi: 10.1186/1757-1626-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismaili Z., Dekhay S., Moussaoui A., Jahid A. Primary gastric, duodenal, and rectal signet ring cell carcinoma revealed by cutaneous metastasis. Endoscopy. 2011;43(Suppl UCTN):E209–210. doi: 10.1055/s-0030-1256399. [DOI] [PubMed] [Google Scholar]
- 24.Balta I., Vahaboglu G., Karabulut A.A. Cutaneous metastases of rectal mucinous adenocarcinoma mimicking granuloma inguinale. Intern. Med. 2012;51(17):2479–2481. doi: 10.2169/internalmedicine.51.7802. [DOI] [PubMed] [Google Scholar]
- 25.de Miguel Valencia M.J., Fraile González M., Yagüe Hernando A. [Cutaneous metastases of rectal cancer] An Sist Sanit Navar. 2013;36(3):557–561. doi: 10.4321/s1137-66272013000300021. [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- 26.Ozgen A., Karakaya E., Bozdoğan N. Scrotal skin metastasis from rectum adenocarcinoma. Rare Tumors. 2013;5(4):e60. doi: 10.4081/rt.2013.e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akpak Y.K., Dandin Ö., Gün I., Atay V., Haholu A. A rare case of vulvar skin metastasis of rectal cancer after surgery. Int. J. Dermatol. 2014;53(6):e337–8. doi: 10.1111/ijd.12230. [DOI] [PubMed] [Google Scholar]
- 28.Dehal A., Patel S., Kim S., Shapera E., Hussain F. Cutaneous metastasis of rectal cancer: a case report and literature review. Perm. J. 2016;20(1):74–78. doi: 10.7812/TPP/15-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasti G., Facchini G., Caraglia M., Franco R., Mura A., Staiano M., Budillon A., Iaffaioli R.V., Ottaiano A. Concomitant occurrence of facial cutaneous and parotid gland metastases from rectal cancer after preoperative chemoradiotherapy. Onkologie. 2007;30:324–326. doi: 10.1159/000102538. [DOI] [PubMed] [Google Scholar]
- 30.Tranchart H., Benoist S., Penna C. Dis. Colon Rectum. 2008;51:1850. doi: 10.1007/s10350-008-9338-9. [DOI] [PubMed] [Google Scholar]
- 31.Gbenou Goris, Christian Maximilien, Wahidy Tawfik, Llinares Karine, Cracco Dominique, Perrot Alain, Riquet Dominique. Atypical phimosis secondary to a preputial metastasis from rectal carcinoma. Case Rep. Oncol. 2011;4:542–546. doi: 10.1159/000334747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balta A.Z., Sücüllü İ., Özdemir Y., Dandin Ö. A rare clinical manifestation of rectal adenocarcinoma and synchronous scalp metastasis: a case report. Ulusal Cerrahi Dergisi. 2013;29(4):197–199. doi: 10.5152/UCD.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Miguel Valencia M.J., Fraile González M., Yagüe Hernando A., Oteiza Martínez F., Ciga Lozano M.A., Armendáriz Rubio P., de Miguel Velasco M., Ortiz Hurtado H. Cutaneous metastases of rectal cancer. Anales del Sistema Sanitario de Navarra. 2013;36(3):557–561. doi: 10.4321/S1137-66272013000300021. [DOI] [PubMed] [Google Scholar]
- 34.Kitahara T., Uemura M., Haraguchi N., Nishimura J., Shingai T., Hata T., Takemasa I., Mizushima T., Doki Y., Mori M. Successful treatment of rectal cancer with perineal invasion: three case reports. Mol. Clin. Oncol. 2014;2(4):497–500. doi: 10.3892/mco.2014.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yazilitas Dogan. Rectal cancer with metastasis to the face. Int. J. Hematol. Oncol. 2015;25:145–147. doi: 10.4999/uhod.15903. [DOI] [Google Scholar]
- 36.Liasis L., Papaconstantinou H.T. Colorectal cancer implant in an external hemorrhoidal skin tag. Proceedings (Bayl. Univ. Med. Cent.) 2016;29(2):194–195. doi: 10.1080/08998280.2016.11929414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D.Y., Ye F., Lin J.J., Xu X. Cutaneous metastasis: a rare phenomenon of colorectal cancer. Ann. Surg. Treat. Res. 2017;93(5):277–280. doi: 10.4174/astr.2017.93.5.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamid M., Majbar A.M., Hrora A., Ahallat M. Perineal skin recurrence on the site of Lone Star Retractor: case report. Surg. Case Rep. 2017;3(1):130. doi: 10.1186/s40792-017-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The National Saudi Cancer Registry (SCR) 2017. Saudi Health Council. [Google Scholar]
- 40.Krathen R.A., Orengo I.F., Rosen T. Cutaneous metastasis: a meta-analysis of data. South. Med. J. 2003;96:164–167. doi: 10.1097/01.SMJ.0000053676.73249.E5. [DOI] [PubMed] [Google Scholar]
- 41.Reingold I.M. Cutaneous metastases from internal carcinoma. Cancer. 1966;19:162–168. doi: 10.1002/1097-0142(196602)19:2<162::aid-cncr2820190204>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 42.Lookingbill D.P., Spangler N., Sexton F.M. Skin involvement as the presenting sign of internal carcinoma. A retrospective study of 7316 cancer patients. J. Am. Acad. Dermatol. 1990;22:19–26. doi: 10.1016/0190-9622(90)70002-y. [DOI] [PubMed] [Google Scholar]
- 43.Tournigand C., André T., Achille E., Lledo G., Flesh M., Mery-Mignard D. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J. Clin. Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 44.Wong C.Y., Helm M.A., Kalb R.E., Helm T.N., Zeitouni N.C. The presentation, pathology, and current management strategies of cutaneous metastasis. N. Am. J. Med. Sci. 2013;5:499–504. doi: 10.4103/1947-2714.118918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nesseris I., Tsamakis C., Gregoriou S., Ditsos I., Christofidou E., Rigopoulos D. Cutaneous metastasis of colon adenocarcinoma: case report and review of the literature. An. Bras. Dermatol. 2013;88(6 Suppl 1):56–58. doi: 10.1590/abd1806-4841.20132441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agha R.A., Borrelli M.R., Farwana R., Koshy K., Fowler A., Orgill D.P., For the SCARE Group The SCARE 2018 statement: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018;60:132–136. doi: 10.1016/j.ijsu.2018.10.028. [DOI] [PubMed] [Google Scholar]