Abstract
Purpose
To report the first case of a patient with chronic thyroid eye disease (TED) treated with teprotumumab.
Observations
A 50-year-old female with a 3-year history of Graves’ disease presented with bilateral exophthalmos greatest on the left side. She was followed for 2 years with stable proptosis measurements (23mm OD, 28mm OS). Her clinical activity score (CAS) was 1 and there were no examination findings reflective of active inflammation. The patient underwent systemic treatment with teprotumumab and despite chronic TED and low CAS, she had notable improvement in proptosis (18mm OD, 22mm OS) and decrease in extraocular muscle volume as noted on orbital imaging.
Conclusion and importance
This case report suggests that teprotumumab may be used in patients with chronic TED and low CAS. Improvement in the proptosis and reduction in extraocular muscle volume suggest that teprotumumab may alter disease course even in patients with inactive or quiescent TED.
Keywords: Thyroid eye disease (TED), Proptosis, Clinical activity score (CAS), Teprotumumab, Inactive disease, Quiescent disease, Fibrotic disease
1. Introduction
Thyroid eye disease (TED) is an autoimmune condition with multiple ophthalmic manifestations and psychosocial consequences.1,2 TED has been described to follow Rundle's curve with an active inflammatory phase followed by disease quiescence.3 It is generally accepted that the active phase is when the course and outcome of TED can be altered by interventions. Previously reported treatment options include various courses of corticosteroid therapy, orbital radiotherapy, and more recently monoclonal antibodies such as rituximab.4,5 Teprotumumab, a monoclonal antibody directed against insulin-like growth factor I receptor (IGF-IR) was recently approved by the US Food and Drug Administration (FDA) for the medical management treatment of TED and in the phase 3 clinical trial, only patients with active TED and a clinical activity score (CAS) of 4 or greater were enrolled.6,7 Once TED has reached quiescence, surgical rehabilitation is considered if orbital manifestations of the disease persist.8 During the chronic or fibrotic stage of the disease, medical therapies are generally considered ineffective. In this case report, we describe our early experience with a patient with longstanding TED who opted for non-surgical treatment with teprotumumab.
2. Case report
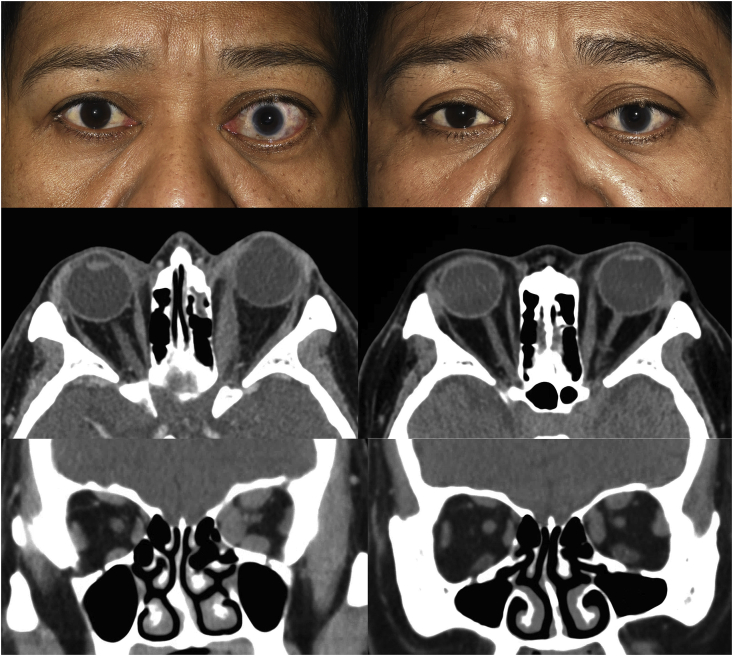
A 50-year-old female was referred for evaluation of asymmetric proptosis of the left eye with an associated retrobulbar ache. She had noted changes to her eyes of at least three years duration, and her symptoms had been stable for two years. Her medical history was significant for 3 years of medically controlled Graves’ disease on methimazole and a 40 pack-year history of cigarette use with a reduction to 2–4 cigarettes daily over the preceding year. Her ophthalmic history was significant for childhood trauma in the left eye with associated amblyopia. Visual acuity was 20/40 OD and 20/800 OS with the presence of a left relative afferent pupillary defect. Color testing was 8/8 in the right and 0/8 in the left eye. External examination showed asymmetric proptosis measuring 23mm OD and 28mm OS (Fig. 1, left column) but no lagophthalmos. Dilated fundus examination revealed left optic nerve pallor and peripapillary scarring. The were no inflammatory findings and the clinical activity score (CAS) was 1, attributed to her retrobulbar pain. Computed tomography of the orbit showed enlarged extraocular muscles with tendon sparing, greater on the left (Fig. 1, left column).
Fig. 1.
Left column: external preoperative and computed tomography of the orbit showing asymmetric left proptosis before treatment. Right column: external photograph and computed tomography of the orbit showing improvement in proptosis and reduction in extraocular muscle size after three infusions of teprotumumab.
The patient was followed for 2 years with stable measurements and continued to be bothered by left-sided eye pain and disfiguring proptosis affecting her daily activities of life. She was offered orbital decompression but was deemed to be a suboptimal surgical candidate by the anesthesia team because of poor adherence to methimazole and inadequately controlled hypertension. At this point, teprotumumab was recently approved by the FDA for thyroid eye disease and she was offered this treatment for her chronic TED with the understanding that the clinical trial only evaluated active disease with CAS 4 or greater patients.
An 8-cycle treatment of teprotumumab infusion at 3-week intervals was scheduled and after the second infusion, the patient noted marked improvement in retrobulbar pain, reduction of proptosis and eyelid retraction. Exophthalmometry after the second infusion measured 18mm OD and 23mm OS. After the third infusion and at the time of this report, exophthalmometry measured 17mm OD and 22mm OS and repeat orbital imaging demonstrated reduction in extraocular muscle size compared to pretreatment (Fig. 1, right column). The patient also noted reduction in periorbital soft-tissue swelling post infusion. With resolution of her orbital pain, the CAS was 0. The patient did report some fatigue after infusions which spontaneously resolved but no other infusion-related side effects.
3. Discussion
Thyroid eye disease (TED) is a challenging and complex autoimmune condition that may be both vision-threatening and disfiguring. The mainstay of treatment for TED has been medical management during the active phase with corticosteroids and recently with teprotumumab. Management of chronic TED, thought to represent end-stage, fibrotic disease, is typically surgical rehabilitation. In this study, we report the early results of treatment of a chronic TED patient with 2 years of stable eye disease and CAS of 1 who demonstrated marked improvement clinically and radiographically.
Post-teprotumumab imaging demonstrates the proptosis improvement resulted from reduction in the extraocular muscle size and possibly orbital fat volume. The patient also reported improvement of soft tissue swelling around the eyebrows which is consistent with the soft-tissue expansion seen with TED and suggests that the IGF-IR pathway may play a role in these periorbital changes.9,10 As this patient had stable disease and clinical measurements for 2 years, the rapid improvement in proptosis and periorbital soft tissue swelling is likely attributable to teprotumumab and not natural history of the disease. Additionally, the patient's tobacco use was stable over the last year and less likely to contribute to the improvement seen after teprotumumab. The quantifiable improvement in extraocular muscle size also suggests that teprotumumab may modify the disease course even in patients with chronic TED. Of note, our patient did not have any history of corticosteroid or radiotherapy treatment in the management of her TED.
The rapid proptosis improvement noted in our case was seen after the second infusion of teprotumumab and resembles the early response seen in the phase 3 clinical trial.6,7 The long term outcomes of the phase 3 patients are still pending and at the time of this report, our patient has received 3 out of 8 planned treatments. Further improvements in globe position and durability of treatment will be addressed in future studies and may have implications for medication dosing.
The role of teprotumumab in the management of compressive optic neuropathy (CON) compared to standard treatments with corticosteroids, radiotherapy and orbital decompression is unclear.11,12 Reduction of proptosis and extraocular muscle size was demonstrated in this case and the teprotumumab clinical trial but with the management of CON, timing is of the essence to prevent permanent vision loss. In our case, proptosis reduction was noted after the second dose which corresponds to 6 weeks after initiation of teprotumumab. The role of teprotumumab in the management with CON remains to be determined and should be the subject of a future randomized clinical trial.
This report suffers from shortfalls seen in other single case studies and raises several questions. Can results from a single report be extrapolated to other chronic TED cases? What is the durability of treatment? Additionally, what is teprotumumab's role to treat disease recurrence? What are the characteristics of patients who are non-responders to teprotumumab? How does orbital decompression play a role in the management of TED? How does teprotumumab compare to corticosteroids or other immunomodulators? Lastly, what changes occur at the ultrastructural level of the orbital tissues to account for improvement in the fibrotic phase of the disease. Expanded use and future studies will address these important considerations.
4. Conclusions
Teprotumumab may be a viable treatment modality for the management of TED patients with low CAS and chronic disease. Proptosis reduction and improvement in extraocular muscle size suggests that teprotumumab may alter disease course even in patients with quiescent TED.
Patient consent
Consent to publish this case report has been obtained from the patient(s) in writing.
Funding
This work was supported by grants from Kathleen and Steve Flynn and the Bell Charitable Foundation and an unrestricted departmental grant from Research to Prevent Blindness (RPB). The sponsor or funding organization had no role in the design or conduct of this research.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
BSK and DOK are consultants for Horizon Therapeutics.
DJO has no financial disclosures.
Acknowledgements
None.
References
- 1.Bartley G.B., Fatourechi V., Kadrmas E.F. Clinical features of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121(3):284–290. doi: 10.1016/s0002-9394(14)70276-4. [DOI] [PubMed] [Google Scholar]
- 2.Farid M., Roch-Levecq A.C., Levi L., Brody B.L., Granet D.B., Kikkawa D.O. Psychological disturbance in graves ophthalmopathy. Arch Ophthalmol. 2005;123(4):491–496. doi: 10.1001/archopht.123.4.491. [DOI] [PubMed] [Google Scholar]
- 3.Bartley G.B. Rundle and his curve. Arch Ophthalmol. 2011;129(3):356–358. doi: 10.1001/archophthalmol.2011.29. [DOI] [PubMed] [Google Scholar]
- 4.Strianese D. Efficacy and safety of immunosuppressive agents for thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34(4S Suppl 1):S56–S59. doi: 10.1097/IOP.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 5.Bartalena L., Krassas G.E., Wiersinga W. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves' orbitopathy. J Clin Endocrinol Metab. 2012;97(12):4454–4463. doi: 10.1210/jc.2012-2389. Graves’ orbitopathy. J Clin Endocrinol Metab 2012;97:4454-63. [DOI] [PubMed] [Google Scholar]
- 6.Smith T.J., Kahaly G.J., Ezra D.G. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748–1761. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas R.S., Kahaly G.J., Patel A. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341–352. doi: 10.1056/NEJMoa1910434. [DOI] [PubMed] [Google Scholar]
- 8.Ediriwickrema Lilangi S., Korn Bobby S., Kikkawa Don O. Orbital decompression for thyroid-related orbitopathy during the quiescent phase. Ophthalmic Plast Reconstr Surg. 2018;34(4S):S90–S97. doi: 10.1097/IOP.0000000000001119. July/August. [DOI] [PubMed] [Google Scholar]
- 9.Papageorgiou K.I., Hwang C.J., Chang S.H. Thyroid-associated periorbitopathy: eyebrow fat and soft tissue expansion in patients with thyroid-associated orbitopathy. Arch Ophthalmol. 2012;130(3):319–328. doi: 10.1001/archopthalmol.2011.1271. [DOI] [PubMed] [Google Scholar]
- 10.Mimura M., Yang P.T., Ko A.C., Korn B.S., Kikkawa D.O. Analysis of periorbital soft tissue in thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2020;36(1):30–33. doi: 10.1097/IOP.0000000000001450. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey K.J., Kazim M. Radiotherapy for active thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34(4S Suppl 1):S98–S104. doi: 10.1097/IOP.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 12.Kikkawa D.O., Pornpanich K., Cruz R.C., Jr., Levi L., Granet D.B. Graded orbital decompression based on severity of proptosis. Ophthalmology. 2002;109(7):1219–1224. doi: 10.1016/s0161-6420(02)01068-0. [DOI] [PubMed] [Google Scholar]