Abstract
Skull-based neuroendocrine carcinomas are rare tumors with only a few case reports in literature. Here we present an unusual case of primary neuroendocrine carcinoma of the skull in 60-year-old male which was proven on surgical biopsy. The imaging features of this rare tumor along with differential diagnosis are discussed with brief review of the literature.
Keywords: Neuroendocrine, Skull base, Middle cranial fossa
Introduction
Carcinoids are biologically heterogeneous group of neuroendocrine tumors with a spectrum ranging from benign indolent to aggressive metastatic tumors. They belong to the category of amine precursor uptake and decarboxylase tumors, or apudomas. The most common sites for primary locations are the gastrointestinal and respiratory tracts; however, any organ can be involved [1]. Primary skull base neuroendocrine tumors are rarely mentioned in the literature. We report a rare case of a pathologically proven NET of skull base of middle cranial fossa. Extensive metastatic workup revealed no other primary lesion, indicating this to be the primary site of involvement.
Case report
A 60-year-old male presented with history of on and off episodes of right ear discharge since 6 years, for which the patient had received multiple courses of antibiotics. Presently the patient still had ear discharge with right-sided facial pain and weakness. On examination, there was evidence of right aural polyp with active serosanguinous discharge. Also he had decreased pinprick sensation on the right side of his face with weakness. For further assessment patient was referred for computed tomography (CT) and magnetic resonance imaging (MRI) of brain including the skull base.
MRI of the skull base demonstrated a large heterogeneously lobulated mass lesion centered at the lateral right middle cranial fossa involving the petrous apex. The lesion was seen along the course of right trigeminal nerve extending superiorly from the Meckel's cave and inferiorly along the mandibular division with resultant widening of foramen ovale assuming a dumbbell configuration [Fig. 1A-D]. Antero-superiorly the lesion was seen abutting the posterolateral aspect of right cavernous sinus and cavernous ICA. Inferiorly the lesion is seen to encase the petrous and adjoining cervical ICA with no luminal compromise [Fig. 3A-C]. Extension to infratemporal fossa was seen. Laterally the lesion is seen to extend to mastoid temporal bone and tympanic cavity. Labyrinthine, tympanic, and mastoid segments of facial nerve showed thickening with accentuated enhancement suggesting perineural spread. Discrete nodular enhancement was also seen within right IAC along VII-VIII nerve complex [Fig. 2A-D].
Fig. 1.
(A-D) Demonstrates T2 hyperintense and T1 isointense large heterogeneously lobulated mass lesion centered at the lateral right middle cranial fossa involving the petrous apex (thick arrow). The lesion was seen along the course of right trigeminal nerve extending superiorly from the Meckel's cave and inferiorly along the mandibular division with resultant widening of foramen ovale assuming a dumbbell configuration (notched arrow). Laterally the lesion was seen extending to mastoid temporal bone and tympanic cavity (thin arrow).
Fig. 3.
(A-C)MR angiography images showing encasement of right cervical and petrous portions of right ICA with medial displacement of cavernous portion.
Fig. 2.
(A-D) Post contrast images showing encasement of right cervical ICA with extension to infratemporal region (notched arrows). Heterogeneous enhancement of the petrous apex lesion was seen (thick arrows). Diffuse nodular thickening with accentuated enhancement of labyrinthine, tympanic, and mastoid segments facial nerve seen (thin arrows).
CT shows ill-defined permeative lytic destruction involving petrous, mastoid portions of right temporal bone, and greater wing of sphenoid with associated dense sclerosis. Complete ossicular erosion was seen with loss of pneumatization and sclerosis in rest of the mastoid air cells [Fig. 4A-D].
Fig. 4.
(A-D) Axial CT images showing permeative lytic destruction of petrous, mastoid portions of right temporal bone and greater wing of sphenoid with associated dense sclerosis (thick arrows). Complete ossicular erosion (thin arrows) was seen with loss of pneumatization and sclerosis in rest of the mastoid air cells (notched arrows).
In view of long history with repeated episodes of ear discharge and present MRI and CT findings, possibility of skull base tumor over osteomyelitis was considered. Further evaluation with whole body 18-fluoro-deoxy-glucose positron emission tomography (FDG PET) was performed. On PET scan, the lesion was non FDG avid with no other metabolically active lesion was identified elsewhere [Fig. 5A-B].
Fig. 5.
(A-B) Whole body PET-CT images showing the lesion to be non FDG avid (compared to background brain parenchyma) with no other metabolically active lesion was identified elsewhere.
Biopsy samples were obtained from right external and middle ear via endoscopic method. Further second sample from middle cranial fossa lesion was obtained via frontotemporal extradural approach.
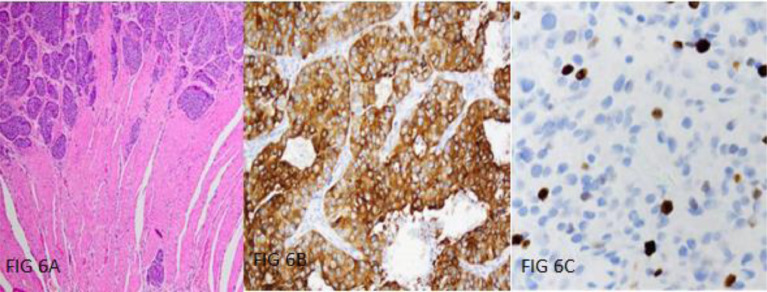
Histopathological analysis revealed fibrocollagenous tissue infiltrated by a malignant tumor composed of round to polygonal cells arranged in sheets and lobular pattern. The tumor cells had moderate to ample eosinophilic cytoplasm, round nuclei, and stippled chromatin. Calcification was seen with absence of necrosis in both specimens. Mitotic count was around 6 of 10 hpf in middle cranial fossa and <1/10 hpf in middle ear specimen. Further immunohistochemical analysis with neuroendocrine markers stained positive for chromogranin, synaptophysin and CK. Ki proliferating index was about 10%-12% in middle cranial fossa lesion and 2% in right middle ear lesion. Based on the above findings, the lesion was diagnosed as neuroendocrine carcinoma, well differentiated type (Grade I) [Fig. 6A-C].
Fig. 6.
(A-C)Histopathological analysis showing fibrocollagenous tissue infiltrated by a malignant tumor composed of round to polygonal cells arranged in sheets and lobular pattern. The tumor cells had moderate to ample eosinophilic cytoplasm, round nuclei, and stippled chromatin (A). Immunohistochemical analysis with neuroendocrine markers stained positive for chromogranin (B) with Ki proliferating index of about 2%-10%.
Discussion
Neuroendocrine tumors (NETs) originate from amine precursor uptaking and decarboxylation cells, which are called diffuse neuroendocrine system [2]. The incidence of intracranial neuroendocrine tumor in brain and middle ear is 0.8% and 2% respectively [3]. Neuroendocrine cells are naturally involved in the coordination of neurotransmitter-initiated synthesis and release of biologically active substances.
NETs can be categorized clinically as functional or nonfunctional depending on if increased hormone production is associated. Additionally, they can be categorized by anatomical location, and by their grade. Ki-67 is a function of the degree of proliferation because it is expressed in the actively dividing cells [4].
Primary skull base NETs are rare entity. Li et al described 2 cases of primary intracranial tumors centered in sellar/suprasellar and anterior cranial fossa respectively. They concluded that the mechanism, diagnosis, and treatment of NET are still challenging. Surgical resection followed by radiotherapy had demonstrated an effective treatment, and chemotherapy still needs researches to demonstrate its therapeutic efficiency [5].
Deshaies and Huang reported Central nervous system (CNS) carcinoid mimicking a meningioma. They concluded that intracranial carcinoid should be included in the differential diagnosis of dural-based, extra-axial brain lesions [6].
Westerveld et al described a case of an atypical carcinoid tumor of the sphenoid associated with multiple endocrine neoplasia type 1 and with bone metastases [7]. They also found that these tumors are more prone to local recurrence. They can erode through the walls of the sinus and are difficult to differentiate from other tumors, such as squamous cell carcinoma, neuroblastoma, pituitary adenoma, paraganglioma, lymphoma, and malignant melanoma [8]
Optimal work up of NETs requires use of a combination of conventional and somatostatin-based imaging techniques [9]. Cross-sectional and functional imaging play an important role in diagnosis, lesion characterization, and staging of skull base tumors. On imaging, there are no specific features of neuroendocrine carcinomas. Bone erosion on CT scans, hypointensity on T1WI, hyperintensity on T2WI, and homogeneous enhancement are general characteristics. Imaging findings similar to more common pathologies like glomus tumor, meningioma, schwannoma, and metastasis [10]. All these form part of differentials to be considered while evaluating the lesion based on its location in skull base.
As most carcinoid tumors express type 2 somatostatin receptors, scintigraphy is widely used as the primary imaging method for diagnosis, staging, and monitoring of carcinoid tumors [11]. FDG PET is used to detect malignancy for a variety of tumor types. Unfortunately, majority of NETs tend to be relatively metabolically inactive and fail to take up the tracer well [12]. However, high-grade NETs are more likely to demonstrate avid uptake of 18FDG, giving these scans utility in identifying tumors likely to display more aggressive behavior [13].
Finally the diagnosis of NET depends on the pathological characteristics and immunohistochemistry. Multidisciplinary approach is required for accurate diagnosis, lesion characterization, localization, staging, and monitoring treatment response.
Conclusion
Primary skull base NETs are rare. Hence it is essential to do a full body imaging using a combination of anatomic and functional imaging to rule out any additional lesions. Skull base metastases and other tumors should be part of the differential diagnosis when assessing patients with NET. Treatment should be conducted in a multidisciplinary manner with multiple modalities if required, including surgery, radiotherapy, and chemotherapy.
Declaration of Competing Interest
None declared under financial, general, and institutional competing interests.
References
- 1.Baxi A.J., Chintapalli K., Katkar A., Restrepo C.S., Betancourt S.L., Sunnapwar L. Multimodality imaging findings in carcinoid tumors: a head-to-toe spectrum. RadioGraphics. 2017;37:516–536. doi: 10.1148/rg.2017160113. [DOI] [PubMed] [Google Scholar]
- 2.Jing S., Xu Z., Wang Z. Pathological category and diagnosis of neuroendocrine tumor in nonneuroendocrine system. J Clin Exp Pathol. 2009;25:548–550. [Google Scholar]
- 3.Silveira F., Basile M.L., Kuga F.S., Próspero J.D., Paes R.A.P., Bernardi F.D.C. Neuroendocrine tumors: an epidemiological study of 250 cases at a tertiary hospital. Rev Assoc Med Bras. 2017;63(10):856–861. doi: 10.1590/1806-9282.63.10.856. 857. [DOI] [PubMed] [Google Scholar]
- 4.Klimstra D.S., Modlin I.R., Coppola D., Lloyd R.V., Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 5.Liu H., Wang H., Qi X., Yu C. Primary intracranial neuroendocrine tumor:two case reports. W J Surg Oncol. 2016;14:138. doi: 10.1186/s12957-016-0887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshaies E.M., Adamo M.A., Qian J., DiRisio D.A. A carcinoid tumor mimicking an isolated intracranial meningioma. J Neurosurg. 2004;101:858–860. doi: 10.3171/jns.2004.101.5.0858. [DOI] [PubMed] [Google Scholar]
- 7.Westerveld G.J., van Diest P.J., van Nieuwkerk E.B. Neuroendocrine carcinoma of the sphenoid sinus: a case report. Rhinology. 2001;39(1):52–54. [PubMed] [Google Scholar]
- 8.Hong S.L., Kim S.D., Roh H.J., Cho K.S. The sphenoid sinus: an unusual presentation of a typical carcinoid tumor. J Craniofac Surg. 2014;25(5):e483–e485. doi: 10.1097/SCS.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell, Howe Imaging in neuroendocrine tumors: an update for the clinician. Int J Endocr Oncol. 2015;2(2):159–168. doi: 10.2217/ije.14.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohannad I., Mohammad Y., Bohnen N., Eisbruch A., Parmar H. Primary carcinoid tumor of the skull base: case report and review of the literature. J Neuroimaging. 2010;20:390–392. doi: 10.1111/j.1552-6569.2008.00317. [DOI] [PubMed] [Google Scholar]
- 11.Reubi J.C. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80:51–56. doi: 10.1159/000080742. [DOI] [PubMed] [Google Scholar]
- 12.Sundin A., Eriksson B., Bergström M., Långström B., Öberg K., Örlefors H. PET in the diagnosis of neuroendocrine tumors. Ann. NY Acad. Sci. 2004;1014(1):246–257. doi: 10.1196/annals.1294.027. PubMed: 15153441. [DOI] [PubMed] [Google Scholar]
- 13.Pasquali C., Rubello D., Sperti C., Gasparoni P., Liessi G., Chierichetti F. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J. Surg. 1998;22:588–592. doi: 10.1007/s002689900439. PubMed: 9597933. [DOI] [PubMed] [Google Scholar]