Abstract
Background
Biochemical, hematological and histological changes are major observable clinical and pathological factors associated with Diabetes mellitus. Derangement in the levels of these parameters increases the risk of the development of complications. In another hand, gastrointestinal intolerance due to the development of lactic acidosis on the gastrointestinal tract and the intestinal microbiome is the toxic side effect of various synthetic antidiabetic agents. The use of Kigelia africana fruit extract for the treatment of diabetes has been scientifically validated. This study therefore aimed at investigating changes in the biochemical, hematological and histological parameters as well as the determination of the functional groups present in the hexane fraction of the fruit.
Methods
The fruits were extracted with ethanol and partitioned with n-hexane to obtain the hexane fraction. Diabetic rats induced with streptozotocin (STZ) were divided into 5 groups of 5 animals each and treated with 100, 200 and 400 mg/kg body weight (BW) hexane fraction alongside reference standard; glibenclamide. Fasting blood glucose levels and their body weights were monitored weekly. Animals were sacrificed at the end of 28-day treatment. Blood, liver, and kidney were collected for biochemical, hematological and histopathological analyses. Fourier transform infrared resonance (FTIR) spectroscopic analysis was carried out on the hexane fraction for functional group determination.
Results
The hexane fraction of K. africana fruit extract decreased fasting blood glucose (FBG) levels significantly with ameliorative effects on the hematological parameters such as packed cell volume (PCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and red blood cells (RBC) etc. There were significant regenerative differences in the biochemical activities as well as the renal cortex and midzone sections of the rat's kidney and liver when compared with untreated diabetic rats. The presence of polyphenolic functional groups via FTIR analysis suggested high antioxidant activities of the fruit extract.
Conclusion
The use of Kigelia africana fruit extracts protects against biochemical, hematological and histological changes that are injurious to diabetic patients. Therefore, Kigelia africana fruit is a good hepatic- and nephroprotective agent and has a hemato-protective ability.
Keywords: Diabetes mellitus, Kigelia africana, Streptozotocin, FTIR, Hyperglycemia, Metformin, Chemistry, Food science, Agricultural science, Biological sciences, Health sciences
Diabetes mellitus; Kigelia africana; Streptozotocin; FTIR; Hyperglycemia; Metformin; Chemistry; Food Science; Agricultural Science; Biological Sciences; Health Sciences
1. Introduction
Diabetes mellitus (DM) is a metabolic disorder characterized by an excessive increase in blood glucose levels. In 2017, diabetes statistics has reached 451 million adults worldwide with a projected increase of up to 693 million by the year 2045 [1]. It is approximately estimated that more than 25 million people are affected by diabetes in Africa, with about 69.2% diabetic cases undiagnosed and the number is projected to be more than 40 million by 2045 [2, 3, 4]. Elevation of blood glucose levels without intervention results in macro- and microvascular complications and a major cause of end-stage renal disease worldwide leading to long term diabetic complications [5, 6]. The progression of diabetic complications is marked by renal structural abnormalities such as glomerular hypertrophy, mesangial matrix expansion, and thickening of a tubular glomerular basement with abnormal pathological values of albumin, creatinine in the plasma, and kidneys as well as that of the liver function tests [7, 8]. With different breakthroughs in the development of drugs used in the treatment of diabetes, limitations are, however, the toxic side effects with derangements in biochemical, hematological, and histological parameters associated with the progression [9, 10] together with the generation of free radicals implicated in the pathophysiology of the disease [11].
Moreover, the usage of the various synthetic chemical compounds, especially sulphonylureas and biguanides, over a while can induce severe side effects with hypoglycemia being the first line of attack on diabetic patients [12]. Furthermore, a review of the literature revealed changes in biochemical, hematological, and histopathological parameters of diabetic patients on oral antidiabetic agents. For example, a recently concluded study on oral metformin intake suggested a close monitoring of diabetic patients from the development of lactic acidosis. Metformin intolerance on the gastrointestinal tract and intestinal microbiome remains the toxic side effect [13, 14]. This information has created an appetite for the development of more effective drugs and this has led to the exponential increase in the application of herbal remedies to serve as a cheaper alternative therapy with high efficacy and reduced or complete absence of cytotoxic side effect based on the recommendation of World Health Organization on the evaluation of therapeutic plants because of their long traditional use with little side effects [15] for effective management of Diabetes mellitus.
Medicinal plants are used in several countries to manage different metabolic diseases and are thought to be cheaper than allopathic drugs. They are even available and affordable to many, especially in developing countries such as Nigeria [16]. There are over 400 local plants that have been reported to be able to treat Diabetes mellitus as reported by Ramachandran et al. [17]. One of such plants is Kigelia africana (Lam.) Benth. Kigelia africana (Family: Bignoniaceae) has a wide-spread distribution in the West, South and Central Africa. It is known as the sausage or cucumber tree [18] and is used traditionally in diabetes management. The uniqueness of the plant is in its huge and sausage-like fruits. It has an average weight of between 4 to 10 kg and hangs from a fibrous and long stalk [19]. The fruit wall is woody with distinctly marked lenticel at the surface and can be said to be an indehiscent fruit. It has grey-brown coloration with numerous seeds at maturation. The fruit is medicinal and employed in the treatment of several diseases and ailments such as tapeworm, ringworm, malaria, boils, eczema, psoriasis, malignant melanoma and solar keratosis [18]. High levels of antioxidant activities of the fruit extracts were reported in an earlier study with hexane fraction regarded as the most potent fraction [20]. In vivo studies on the antidiabetic effects of the fruit extracts have not been reported. Therefore, this study investigated changes in biochemical, hematological, and histopathological parameters of the hexane fraction of the fruit as well as the functional groups present, to ascertain the antidiabetic and ameliorative effects of Kigelia africana fruit in a 28-day study in STZ-induced diabetic rats.
2. Material and methods
2.1. Chemicals used
Streptozocin (STZ) was procured from MedChemExpress, New Jersey, USA and diagnostic kits for the biochemical assays were obtained from Randox Laboratory Limited, UK. All other analytically graded chemicals used were purchased from Sigma Aldrich, USA.
2.2. Fruit collection
The fruits of K. africana (Lam.) Benth. were collected from Ayegunle-Ekiti, Ekiti State, Nigeria on Longitude 70 50′ 42.0″N and Latitude 50 06′ 36.0″E. The fruit samples were identified at the Herbarium Laboratory, Obafemi Awolowo University with a voucher specimen (IFE-17801) and was deposited at the Department of Botany, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria.
2.3. Preparation and fractionation of the fruit extracts
The fruits (1 kg) were air-dried and extracted three times with 7.5 L of 70% v/v ethanol to obtain 111.11 g of the ethanolic crude extract (ECE) after concentrating at 40 °C with BUCHI Rotavapor R-210 rotary evaporator at the Department of Chemistry, University of South Africa, South Africa. Based on the earlier report of Fagbohun et al. [20], liquid-liquid partitioning was carried out using n-hexane from 100 g of ECE to give a yield of 60.40% after freeze-drying with Labconco free zone 1L Freeze dryer system at the Department of Biochemistry, University of South Africa, South Africa. The hexane fraction was stored at 4 °C for further analyses.
2.4. Animals
Male Wistar rats with weights between 120 - 130 g from Animal House, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, Nigeria were obtained. These experimental animals were kept under standard conditions (12 h dark/light cycle, 25 ± 2 °C) and satiated with standard pellet diet (Ladokun feeds, Ibadan, Oyo State, Nigeria) and water ad libitum. The experimental protocol was according to the guidelines of Laboratory Animal Care adopted from NIH Publication No. 85-23 principles (NIH Publication Revised, 1985) and ethical approval was obtained from the Institute of Public Health, Obafemi Awolowo University, Ile-Ife, Nigeria. The experimental rats were fasted without food after acclimatization but had access to water for 12 h. Acute toxicity tests were carried on male Wistar rats to determine the toxicity levels of Kigelia africana fruits.
2.5. Induction of diabetes
Fasting blood glucose (FBG) level was determined in the blood drawn from the retro-orbital plexus of the animals using a Fine-Test glucometer (Infopia Co., Ltd., Kyunggi, Korea) for the initial measurement of the rat's glucose levels. Thereafter, the rats were induced by a single intraperitoneal injection for three consecutive days with freshly prepared streptozotocin (STZ) dissolved in 0.01 M citrate buffer (pH 4.5, 60 mg/kg BW). The animals were immediately maintained on 5% glucose solution with the aid of feeding bottles to overcome the initial hypoglycemic phase normally associated with the induction of chemical diabetes and allowed to stabilize for two weeks [21]. Rat feeds were returned to the animals after the administration of streptozotocin. The determination of fasting blood glucose was repeated 48 h after streptozotocin administration and only animals with blood glucose levels higher than 200 mg/dL were assigned to various groups and used for the study.
2.6. Experimental design
Thirty [30] male Wistar rats were assigned into 6 groups consisting of 5 animals each. Twenty-five [25] male Wistar rats in the experimental group were induced with Streptozotocin. Group A was non-diabetic rats which received 0.5 ml of citrate buffer. Group B was diabetic rats which received 0.5 ml of citrate buffer while groups C - E were diabetic rats which received 0.5 ml corresponding to 100, 200 and 400 mg/kg body weight (BW) of the hexane fraction of K. africana fruit. Group F served as the control group and constituted diabetic rats which received 0.5 ml corresponding to 10 mg/kg BW of glibenclamide (reference drug). All animals were given their respective intervention and treatment for 28 days. Fasting blood glucose (FBG) levels were determined on the 1, 7, 14, 21 and 28 days in fasting condition. Changes in the body weight of all animals were recorded and presented in a graph. The experimental animals were sacrificed at the end of the study. Liver, kidney and blood were removed and stored at -80 °C until use for biochemical, hematological and histopathological studies.
2.7. Evaluation of hematological parameters
Blood drawn through cardiac puncture was mixed by Blood Rolling Mixer (Microfield Instrument, England) and analyzed for various hematological parameters such as WBCs (x 103 μL−1), lymphocyte (%), monocyte (%), RBCs (x 106 μL−1), hemoglobin concentration (g/dL), mean corpuscular hemoglobin (pg), mean corpuscular volume (fL), mean corpuscular hemoglobin concentration (g/dL) and packed cell volume (%) using H18 Light Automated Hematological System Analyzer (SFRI Medical Diagnostics, France) at the Department of Hematology and Immunology, Obafemi Awolowo University, Nigeria.
2.8. Assessment of biochemical parameters
Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were assessed by the method of Reitman and Frankel [22] as modified by Tietz [23] with the absorbance read at 546nm. Lactate dehydrogenase (LDH) activity was measured by the method described by Ababio et al. [24] with the change in absorbance measured at 340nm due to the appearance of reduced nicotinamide adenine dinucleotide (NAD) which was proportional to LDH activity. Creatinine concentration was evaluated by Jaffe's method of alkaline picrate formation as described by Chawla [25] where creatinine reacts with picric acid in alkaline solution and the amount of complex formed which was proportional to creatinine concentration was read at 520nm while urea concentration was measured by the method of Weatherburn [26] as described and modified by Mbaka et al. [27] at 546nm. All analyses were carried out in the plasma, liver and/or kidney. For all the analyses, the excised organs were perfused with a chilled saline solution containing 0.9% sodium chloride (NaCl) at 40 °C, blotted dry, weighed and homogenized with corresponding buffers while the supernatant of 10% (w/v) homogenates of the liver and kidney prepared in 0.05 M of phosphate buffer pH 7.4 and centrifuged at 4000 rpm for 20 min, was used for measuring the activity of the corresponding enzymes.
2.9. Histopathological analyses
Portions of the tissues from the liver and kidney were used for histological studies. The tissues from the sacrificed animals were fixed in 10% neutral buffered formosaline solution, dehydrated in increasing concentrations of absolute ethanol and embedded into paraffin wax. A portion of 5 μm thickness tissues was sectioned using a Bright B5143 LEICA rotary microtome (Huntington, England). The sections were then floated in a water bath and dried at 40 °C before de-waxing in xylene and subsequently stained with hematoxylin and eosin (H&E) dye. Afterward, the sections were mounted in Distrene Plasticizer Xylene (DPX) with a coverslip and examined under LEICA DM 750 microscope interfaced with a LEICA ICC50W camera (Leica Microsystems, Germany) at the Department of Anatomy and Cell Biology, Obafemi Awolowo University, Obafemi Awolowo University, Ile-Ife, Nigeria.
2.10. FTIR spectroscopic analysis of functional groups
The hexane fraction was subjected to Fourier transform infrared resonance (FTIR) spectrometer of universal absorbance transmittance resonance (ATR) on a Perkin-Elmer Frontier FTIR spectrometer serial #100858 at Nanotechnology and Water Sustainability Research Unit of the College of Science Engineering and Technology, University of South Africa, South Africa, and data acquisition was carried out using spectrum software version 6.1. The measurements were performed between 25 and 27 °C and recorded from 4500 to 450 cm−1 with a spectral resolution of turnover of 4 cm−1. The frequencies of peaks were compared to the reference literature [28] to evaluate the functional groups present in the hexane fraction of Kigelia africana fruit extract.
2.11. Statistical analysis
The statistical program ‘XLSTAT’ and ‘GraphPad Prism’ were employed for the analyses. Results were expressed as Mean ± SEM for 5 animals per group and evaluated by one-way analysis of variance (ANOVA). ANOVA was followed by Tukey's as post hoc multiple comparison test with significance taken at p ≤ 0.05. Pattern recognition method; hierarchical cluster analysis (an unsupervised learning method) was employed for data collection as described by Patras et al. [29].
3. Results
3.1. Acute toxicity studies on Kigelia africana fruit extract
The acute toxicity study of Kigelia africana Fruit in Wistar rats was carried out according to the method of Lorke [30]. From the result shown in Table 1, there was no record of mortality within 24 h of treatment with the extract and the result of this study suggested that the LD50 is greater than 5000 mg/kg BW. Again, no death was recorded among all the dose groups throughout the two weeks experimental period. The LD50 result was in contrast to the report of Sharma and Marwa, (2013) who concluded that the fruit is toxic after administering the fruit to Wistar Rats with liver induced by CCl4. Furthermore, a dose-dependent weight loss occurred but the weight variations noticed among the extract-treated groups (Table 1) were not found to be significant (p > 0.05) when compared with the control group.
Table 1.
Acute toxicological effects of Kigelia africana fruit extracts in wistar rats.
| Experiment | Dose (mg/kg B.W.) | No of Death After 24 h | Weight gain (g) |
|---|---|---|---|
| Phase 1 | 10 | 0/3 | 150.33 ± 0.76∗ |
| 100 | 0/3 | 171.83 ± 1.08∗ | |
| 1000 | 0/3 | 173.50 ± 5.06∗ | |
| Control | 0 | 0/3 | 155.02 ± 3.19∗ |
| Phase 2 | 1600 | 0/1 | 165.33 ± 1.03 |
| 2900 | 0/1 | 170.48 ± 1.95 | |
| 5000 | 0/1 | 180.53 ± 0.33 |
Data are expressed as Mean ± SEM, (n = 3).
Weight values in phase 2 (where n < 3) were not compared due to absence of measure of variability.
Test significance was done in rows. Same superscripts indicate no significant difference (p < 0.05).
3.2. Effects of the hexane fraction (HF) of K. africana on body weight
The effect of the hexane fraction of K. africana fruit on the percent increase in body weight is shown in Table 2. An increase was mostly noticed in group D with a 42.28 ± 0.03% increase. A remarkable decrease in percent bodyweight was also seen in the diabetic group (group B) which was not significant when compared with the normal control group (group A).
Table 2.
Effect of hexane fraction of Kigelia africana fruit on weight of rats.
| Treatment | Body Weight (g) |
||
|---|---|---|---|
| Initial Body Weight (g) | Final Body Weight (g) | Percentage Increase in Body Weight | |
| Group A | 124.11 ± 1.28 | 164.79 ± 0.92 | 32.78 ± 0.36b |
| Group B | 125.47 ± 1.74 | 160.49 ± 1.06 | 27.91 ± 0.68a |
| Group C | 128.93 ± 0.77 | 167.21 ± 0.87 | 29.69 ± 0.10a |
| Group D | 123.24 ± 1.69 | 175.35 ± 1.72 | 42.28 ± 0.03d |
| Group E | 125.17 ± 1.66 | 164.53 ± 0.54 | 31.45 ± 1.12b |
| Group F | 127.61 ± 1.04 | 175.81 ± 0.83 | 37.75 ± 0.21c |
Values are presented as Mean ± SEM (n = 5). Means ± SEM with different superscript letters (a-d) within the column indicate a difference (p ≤ 0.05).
Keys: Group A = Normal Control Group with Citrate Buffer Only; Group B = Diabetic Group Only; Group C = Diabetic Group +100 mg/kg B. W. Hexane Fraction of Kigelia africana Fruit Extract; Group D = Diabetic Group +200 mg/kg B. W. Hexane Fraction of Kigelia africana Fruit Extract; Group E = Diabetic Group +400 mg/kg B. W. Hexane Fraction of Kigelia africana Fruit Extract; Group F = Diabetic Group +10 mg/kg B. W. Glibenclamide.
3.3. Effects of the HF on fasting blood glucose of STZ-Induced diabetic rats
After the STZ induction, there was a surge in the blood glucose level in the different groups with an average mean of 341.61 mg/dL. A significant reduction was seen in groups C, D, E, and F treated with the hexane fraction as well as glibenclamide when compared with the normal group (group A). After 28 days of treatment, the mean fasting blood glucose levels were 246.20 ± 12.88, 223.40 ± 51.02, 145.60 ± 8.52 and 110.60 ± 2.89 mg/dL respectively for groups C to F. As shown in Table 3, the reduction was in a dose-dependent manner with significant differences at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001 between groups B, C, D, and F. The pattern of alteration showed that Kigelia africana fruit can effectively reduce the level of sugar in vivo.
Table 3.
Effect of hexane fraction of Kigelia africana fruit on fasting blood glucose levels.
| Treatment | Fasting Blood Glucose (mg/dL) |
||
|---|---|---|---|
| Initial FBS (mg/dL) | Final FBS (mg/dL) | Percentage Output (%) | |
| Group A | 77.80 ± 3.47 | 95.20 ± 1.25 | 22.37 ± 2.22↑ |
| Group B | 262.40 ± 3.03 | 480.40 ± 4.82α, β, ɣ | 83.08 ± 1.79↑ |
| Group C | 341.61 ± 13.77 | 246.20 ± 12.88 α, β, ɣ | 27.92 ± 0.89↓ |
| Group D | 460.20 ± 31.92 | 223.40 ± 51.02 α, β | 51.46 ± 0.90↓ |
| Group E | 339.00 ± 12.05 | 145.60 ± 8.52 | 57.05 ± 2.47↓ |
| Group F | 401.80 ± 20.05 | 110.6 ± 2.89 α | 72.47 ± 1.89↓ |
Values are presented as Mean ± SEM (n = 5). Means ± SEM with different superscript letters (α, β, ɣ) within the column indicate significant difference at p ≤ 0.05, p ≤ 0.01 and p ≤ 0.001 respectively and (↑↓) within the column indicate a percentage increase and decrease.
Keys: Group A = Normal Control Group with Citrate Buffer Only; Group B = Diabetic Group Only; Group C = Diabetic Group +100 mg/kg B. W. Hexane Fraction of Kigelia africana Fruit Extract; Group D = Diabetic Group +200 mg/kg B. W. Hexane Fraction of Kigelia africana Fruit Extract; Group E = Diabetic Group +400 mg/kg B. W. Hexane Fraction of Kigelia africana Fruit Extract; Group F = Diabetic Group +10 mg/kg B. W. Glibenclamide.
3.4. Effects of the HF on hematological parameters
The hematological profile as shown in Table 4 and Figure 1 employed hierarchical cluster analysis (HCA) to generate the similarity clusters. A significant difference at p < 0.05 was found in the levels of WBC, MCHC, and PCV when the normal control group (group A) animals were compared with all other groups (groups B – F). Seven clusters were generated from HCA analysis and this revealed the similarities between red blood cell and hemoglobin, lymphocyte with monocyte and white blood cell, MCV, and MCH while MCHC was predicted by white blood cells and red blood cells parameters.
Table 4.
Effects of the hexane fraction of Kigelia africana fruit extract on hematological parameters of STZ-Induced diabetic rats.
| Groups | WBC (x 103) μL−1 | LYM (%) | MON (%) | RBC (x 106) μL−1 | HGB (g/dL) | MCV (fL) | MCH (pg) | MCHC (g/dL) | PCV (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 5.33 ± 0.7 | 35.63 ± 0.43 | 6.97 ± 0.39 | 5.03 ± 0.79 | 15.17 ± 1.68 | 59.13 ± 0.72 | 21.07 ± 0.14 | 34.03 ± 0.53 | 44.58 ± 1.48 | ||
| B | 14.10 ± 2.6∗ | 60.00 ± 0.13∗ | 9.37 ± 0.49∗ | 7.63 ± 0.67∗ | 16.2 ± 1.69 | 60.00 ± 0.88 | 21.07 ± 0.41 | 135.2 ± 81.75∗ | 11.99 ± 0.12∗ | ||
| C | 10.80 ± 0.57∗ | 64.2 ± 1.63∗ | 8.4 ± 0.76 | 6.92 ± 2.55 | 14.27 ± 0.74 | 59.17 ± 0.59 | 20.53 ± 0.30 | 34.8 ± 0.52 | 41.00 ± 1.03 | ||
| D | 13.70 ± 2.12∗ | 68.43 ± 2.35∗ | 8.8 ± 0.26 | 7.44 ± 4.64 | 16.13 ± 1.21 | 59.07 ± 0.59 | 21.6 ± 0.42 | 36.63 ± 1.05 | 44.03 ± 1.78 | ||
| E | 10.63 ± 2.29∗ | 62.07 ± 2.09∗ | 9.03 ± 0.78 | 8.24 ± 0.06∗ | 17.37 ± 0.14 | 57.03 ± 0.41 | 21.03 ± 0.03 | 36.97 ± 0.24 | 47.36 ± 2.02 | ||
| F | 8.43 ± 1.27∗ | 24.79 ± 19.72 | 7.97 ± 0.15 | 7.84 ± 0.45 | 16.67 ± 1.16 | 60.23 ± 0.17 | 21.17 ± 0.31 | 35.2 ± 0.43 | 46.98 ± 2.02 | ||
| Limits | 2.5–10.5 | 20–40 | 1–15 | 3.5–5.5 | 11.0–16.0 | 80–99 | 26–32 | 32–36 | 40–52 | ||
Data are expressed as Mean ± SEM; ∗Significant difference at p < 0.05; WBC = White Blood Cells Count; RBC = Red Blood Cells Count; LYM = Lymphocyte Count; MON = Monocyte Count; HGB = Hemoglobin Count; MCV = Mean Corpuscular Volume; MCH = Mean Corpuscular Hemoglobin; MCHC = Mean Corpuscular Hemoglobin Concentration; PCV = Packed Cell Volume.
Figure 1.
Hierarchical cluster analysis of haematological parameters.
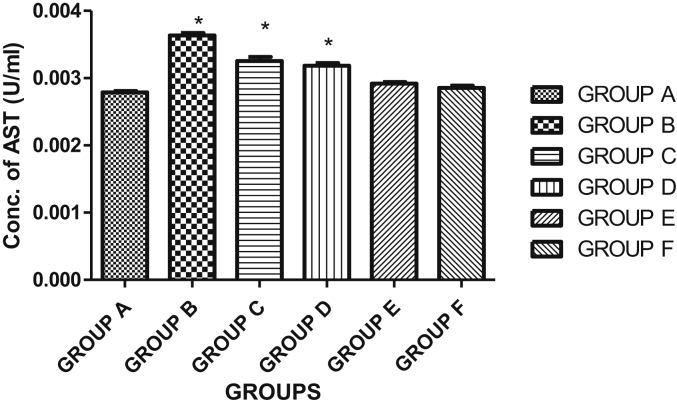
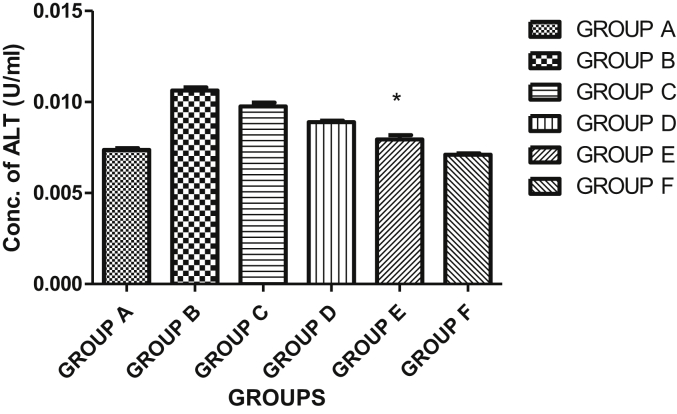
3.5. Effects of HF of Kigelia africana fruit extract on aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in plasma
The result showed a significant difference (p < 0.05) in the plasma AST when the hexane fraction of Kigelia africana fruit extract was compared in groups A-D. Generally, there was a significant decrease in the aspartate aminotransferase (AST) activity in the plasma of the control group when compared with group D (diabetic group +200 mg/kg BW hexane fraction of Kigelia africana fruit extract, group E (diabetic group +400 mg/kg BW hexane fraction of Kigelia africana fruit extract) and group F (diabetic group + glibenclamide) animals. The result showed a decrease in AST activity when compared to the normal control group. Group C showed an increase in AST when compared with the normal control group as shown in Figure 2. As shown in Figure 3, the activity of alanine aminotransferase (ALT) activity revealed a significant difference at p < 0.05 in the plasma of group B (diabetic group only), group C (diabetic group +100 mg/kg BW hexane fraction of Kigelia africana fruit extract) and group D (diabetic group +200 mg/kg BW hexane fraction of Kigelia africana fruit extract) when compared with the normal control group (group A). However, there was no statistically significant difference at p < 0.05 for group E and F when compared with the normal control group (group A).
Figure 2.
Effect of the hexane fraction of Kigelia africana fruit extract on the aspartate aminotransferase (AST) activity in the plasma. Keys: Group A = normal control group with distilled water only; group B = diabetic group only; group C = diabetic group +100 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group d = diabetic group +200 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group E = diabetic group +400 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group F = diabetic group +10 mg/kg B. W. Glibenclamide.
Figure 3.
Effect of the hexane fraction of Kigelia africana fruit extract on the alanine aminotransferase (ALT) in the plasma. Keys: Group A = normal control group with distilled water only; group B = diabetic group only; group C = diabetic group +100 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group d = diabetic group +200 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group E = diabetic group +400 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group F = diabetic group +10 mg/kg B. W. Glibenclamide.
3.6. Effects of HF of Kigelia africana fruit extract on aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities in the liver
The result showed a statistical difference (p < 0.05) in the liver AST of group D when compared with group A which is the normal control group (Figure 4). Generally, there was no significant decrease in the aspartate aminotransferase (AST) activity in the liver of groups B, C, E and F of the experimental animals when compared to the normal control group. The same trend was recorded in the liver ALT activities of normal control and the Kigelia africana fruit extract treated rats (Figure 5).
Figure 4.
Effect of the hexane fraction of Kigelia africana fruit extract on the aspartate aminotransferase (AST) activity in the liver. Keys: Group A = normal control group with distilled water only; group B = diabetic group only; group C = diabetic group +100 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group d = diabetic group +200 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group E = diabetic group +400 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group F = diabetic group +10 mg/kg B. W. Glibenclamide.
Figure 5.
Effect of the hexane fraction of Kigelia africana fruit extract on the alanine aminotransferase (ALT) activity in the liver. Keys: Group A = normal control group with distilled water only; group B = diabetic group only; group C = diabetic group +100 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group d = diabetic group +200 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group E = diabetic group +400 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group F = diabetic group +10 mg/kg B. W. Glibenclamide.
3.7. Effects on lactate dehydrogenase activity in the plasma
Lactate dehydrogenase concentration in the plasma in Figure 6 revealed significant difference at p < 0.05 when group A with compared to groups B and C. However, the result showed that the highest lactate dehydrogenase activity was seen in group B (diabetic group only).
Figure 6.
Effect of the hexane fraction of Kigelia africana fruit extract on LDH activity in the plasma. Keys: Group A = normal control group with distilled water only; group B = diabetic group only; group C = diabetic group +100 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group d = diabetic group +200 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group E = diabetic group +400 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group F = diabetic group +10 mg/kg B. W. Glibenclamide.
3.8. Effects on creatinine concentration in the plasma and kidney
Creatinine concentration in the plasma as represented in Figure 7 showed a significant difference when the normal control group was compared with groups B, C, D and E at p < 0.05. However, Group B (diabetic group only) has the highest concentration of creatinine when compared with the rest of the groups. The same trend was repeated in the liver as shown in Figure 8. Group B likewise had the highest creatinine concentration when compared with the rest of the group.
Figure 7.
Effect of the hexane fraction of Kigelia africana fruit extract on creatinine concentration in the plasma. Keys: Group A = normal control group with distilled water only; group B = diabetic group only; group C = diabetic group +100 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group d = diabetic group +200 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group E = diabetic group +400 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group F = diabetic group +10 mg/kg B. W. Glibenclamide.
Figure 8.
Effect of the hexane fraction of Kigelia africana fruit extract on creatinine concentration in the kidney. Keys: Group A = normal control group with distilled water only; group B = diabetic group only; group C = diabetic group +100 mg/kg B. W. Hexane fraction of Kigelia africana fruit Extract; Group d = diabetic group +200 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group E = diabetic group +400 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group F = diabetic group +10 mg/kg B. W. Glibenclamide.
3.9. Effects on urea concentration in the plasma and kidney
Urea level in the plasma as shown in Figure 9 was significantly different when the normal control group was compared with groups B, C, D and E at p < 0.05. Group B (diabetic group only) has the highest concentration of urea when compared with the rest of the groups. The same trend was repeated in the kidney as shown in Figure 10 while group B was also observed to show the highest concentration of urea when compared with the rest of the group.
Figure 9.
Effect of the hexane fraction of Kigelia africana fruit extract on urea concentration in the plasma. Keys: Group A = normal control group with distilled water only; group B = diabetic group only; group C = diabetic group +100 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group d = diabetic group +200 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group E = diabetic group +400 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group F = diabetic group +10 mg/kg B. W. Glibenclamide.
Figure 10.
Effect of the hexane fraction of Kigelia africana fruit extract on urea concentration in the kidney. Keys: Group A = normal control group with distilled water only; group B = diabetic group only; group C = diabetic group +100 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group d = diabetic group +200 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group E = diabetic group +400 mg/kg B. W. Hexane fraction of Kigelia africana fruit extract; group F = diabetic group +10 mg/kg B. W. Glibenclamide.
3.10. Morphological and histopathological findings
3.10.1. Effects of Kigelia africana hexane fraction on general liver histomorphology of streptozotocin-induced diabetic rats
Liver sections of normal control group (group A) as presented in Figure 11 revealed normal hepatic features identified histologically as the cord-like arrangement of hepatocytes with round euchromatic nuclei having prominent nucleoli. Hepatic microcapillary networks (the sinusoids) were radially apparent. Endothelial cells of the sinusoids were well localized in sinusoidal spaces of Disse in this group. The hepatocellular arrangement was severely disrupted in diabetic rats with features like cord-like hepatocytes derangement, very few radially apparent fenestrated sinusoids, cytoplasmic degeneration evident by intracytoplasmic vacuolations, lymphocytic infiltration and vesicles around the nucleus of hepatocytes, and nuclear changes such as karyopyknosis and karyorrhexis. However, groups D, E, F showed some normal features as presented by the normal control rats with other mild degenerative changes such as pyknosis.
Figure 11.
Representative light micrographs of midzone of sections of Rats Liver tissues subjected to H&E staining. Observe: A – features typical of intact liver cells such as intact cord of hepatocyte (H), round euchromatic nuclei with prominent nucleoli (white circles), radially apparent sinusoids (S) and sinusoidal endothelial cells nucleus within the perisinusoidal spaces of Disse (SEC). B, C – deranged cellular architecture evident by disrupted cord-like hepatocytes arrangement, very few radially apparent sinusoids (S); cytoplasmic degeneration evident by intracytoplasmic vacuolations (blue circles), lymphocytic infilteration (brown circle) and vesicles around the nucleus of hepatocytes and nuclear changes such as karyopyknosis (black circle), karyorrhexis (yellow circle). D, E, F – show cord-like hepatocytes arrangement (H), radially apparent sinusoids (S), some normal nucleus of hepatocytes (white circles) and some degenerative changes such as karopyknosis (black circle). Karyorrhexis was not observed. Scale bar - 50μm.
3.10.2. Effects of Kigelia africana hexane fraction on general renal cortex histomorphology of streptozotocin-induced diabetic rats
The cortex of the normal control rat kidney, indicated in Figure 12, showed an intact corpuscular framework with parietal epithelial cells of simple squamous epithelium evident by a flat nucleus surrounding a fluid-filled urinary space within which the glomerulus was suspended. The nucleus of the glomerular cells (capillary endothelial, podocytes and mesangial cells) distinguishable by their location was compactly arranged. Cells lying directly adjacent to the Bowman's space, recognized as podocytes with epithelial appearance appeared normal. The endothelial cells as seen lying close to capillary lumen also appeared intact. Capillary luminal and convoluted tubules arrangement and staining appear normal in this group evident by more intensely eosinophilic proximal tubules cells and more open and clear distal tubules lumen. However, the glomerular tuft of diabetic rats (group B) became atrophied with a consequent reduction in cell types of the renal corpuscle (podocytes, mesangial cells, Bowman's capsule cells, capillary endothelial cells) and widened urinary space. The capillary lumen of these atrophied glomeruli were ill-defined and tubular simple cuboidal epithelial cells appeared degenerated in this group. Rats treated with low-dose extract had focal degeneration of glomerular tuft and occluded urinary space which was a result of the proliferation of the cell types of the renal corpuscle. Occlusion of microcapillary networks and focal degeneration of renal corpuscle were also observed in groups treated with high dose extract and negative control rats (group F) evident by atrophied glomeruli in these groups. The severity of degenerative change was more in group E.
Figure 12.
Representative light micrographs showing renal cortex of Wistar rats. Observe: A - two intact renal corpuscles each of which consists of an outer covering of simple squamous epithelium (BC) surrounding a fluid-filled space (US) within which is suspended a glomerular tuft (G) and well-defined capillary lumen (CL). B – two atrophied glomerular tuft (G), widened urinary space (US), ill-defined capillary Lumina, reduction in cell types of the renal corpuscle (podocytes, mesangial cells, Bowman's capsule cells, capillary endothelial cells) tubular epithelial degeneration (asterisks). C – two of four renal corpuscles show minimal glomerular tuft atrophy (G) and well-defined capillary lumen (CL), widened urinary spaces (US). The other two appeared normal. D – two normal renal corpuscles and tubules. E – two of four renal corpuscles had atrophic changes of glomerular tuft (G), one showed complete sclerotic corpuscles (GS) with little or no urinary space and occluded capillary lumen (CL). Others showed partial degeneration. F – three renal corpuscles with minimally widened urinary spaces in two (US – upper L-R), and one intact corpuscle (LR). Scale bar - 50μm.
3.11. Fourier Transform Infrared Spectroscopy of HF of Kigelia africana fruit
The infrared spectroscopic study of the representative spectra in the mid-infrared region (4000-400 cm−1) for the hexane fraction of Kigelia africana fruit as shown in Figure 13 and Table 5 had characteristic peaks seen in the spectra with specific functional groups. The peaks were as follows: 1062 cm−1, 1744 cm−1, 1749 cm−1, 2906 cm−1 and 2933 cm−1. The 3700-3584 cm−1 and 3550-3200 cm−1 peaks were specific to the alcohol, O–H stretching groups (medium and strong). The peaks having the values of 2260-2222 cm−1 corresponded to the nitrile (CΞN stretching, weak) and specific for the nitriles. The 3000-2800 cm−1 peaks corresponded to N–H stretching and specific for the amine salts while the 1086 cm−1 peak corresponded to the C–H groups and specific for the aromatic nucleus. The 1086 cm−1 peak matched up to the ketone group. The 862-864 cm−1 peaks corresponded to the R-NH2 and specific for primary amine while the 761 cm−1 peak corresponded to the inner deformation δ C–H. The wavenumbers for p-coumaric acid at 669, 1124, 1171, 1508 and 1638 cm−1 were also noticed.
Figure 13.
FTIR spectra of hexane fraction of Kigelia africana fruit.
Table 5.
General band assignments of the fourier transform infrared spectroscopy (FTIR) spectra of the hexane fraction of Kigelia africana fruit extract.
| Characteristic Absorption (cm−1) | %T | Tentative Assignments |
|---|---|---|
| 3700–3584 | 99.27 | Alcohol, O–H Stretching, Medium-Sharp, Free |
| 3550–3200 | 92.98 | Alcohol, O–H Stretching, Strong, Broad, intermolecular bonded |
| 3300–2500 | 82.92 | Carboxylic acid, O–H Stretching, usually centred on 3000 cm−1 |
| 3000–2800 | 90.55 | Amine Salt, N–H Stretching, Strong-Broad |
| 2260–2222 | 98.29 | Nitrile, CΞN stretching, Weak |
| 2160–2120 | 98.06 | Azide, N=N=N stretching, Strong |
| 2065–2083 | 98.1 | N=N anti symmetric stretch or C–N double bond or triple bond |
| 1815–1785 | 97.97 | C=O Stretching, S, |
| 1750–1735 | 87.1 | Esters, C=O Stretching, Strong, 6-membered lactone |
| 1658–1648 | 88.85 | Alkane, C=C stretching, Medium, vinylidene |
| 1653–1624 | 88.79 | C=O stretch in enol form |
| 1639 | 88.07 | N–H in primary amide or C=O β-Ketone of C=O stretch |
| 1629 | 88 | N–H in primary amide |
| 1617 | 89.06 | Vinyl carbon |
| 1610 | 88.56 | Vinyl carbon |
| 1550–1500 | 94.41 | N–O stretching |
| 1508–1534 | 93.07 | Benzene ring in aromatic compounds |
| 1406–1404 | 82.02 | C–N in primary amide |
| 1420–1330 | 81.96 | Alcohol, O–H Bending, Medium |
| 1310–1250 | 81.26 | Aromatic esters, C–O Esters |
| 1342–1266 | 75.6 | Aromatic amine, C–N Stretching |
| 1250–1282 | 75.66 | Ar-O in alky and aryl ethers |
| 669,1124,1171, 1508, 1638 | 95 | p-Coumaric acid |
| 1172 | 74.82 | C–OH in alcohols of C–O stretch |
| 1099–1109 | 65.41 | C–O–H in secondary and tertiary alcohols |
| 1015–1022 | 51.74 | CH–O–H in cyclic alcohols |
| 1044 | 82.4 | Ketone group |
| 1086 | 95 | Aromatic nucleus, C–H relation |
| 905–936 | 74.22 | Carbon ring in cyclic compounds |
| 862–864 | 74.74 | R-NH2 – in primary amine |
| 761 | 67.76 | CH2 out of plane |
| 669 | 57.38 | C–OH in alcohols of C–O–H bending |
| 607 | 50.39 | N–C=O in amides |
FTIR = Fourier Transform Infrared Spectroscopy; %T = %Transmittance.
4. Discussion
The reason for the high prevalence of morbidity and mortality seen in diabetic patients is the complications associated with the disease evident by the derangements in the biochemical, histopathological and hematological parameters as diabetes progresses [31]. As much as the search for the cure is met with brick walls, the management of blood glucose levels is the strategy targeted by different mechanistic studies regarding diabetes. The application of tradomedical plants is proving to be the solution to the researches of Scientists with their use dated back to the last century. Previous studies carried out on the phytochemical composition of Kigelia africana fruit [20] reported the presence of terpenoids, tannins, glycosides, and polyphenols. The presence of these phytochemicals, as well as alkaloids in the hexane fraction, was the highlight of the study and suggestive of its use in in vivo studies. The bioactive compounds present in this plant as previously reported and as depicted in the FTIR study may be responsible for the hypoglycemic effect observed in this study. The infrared spectroscopic spectra highlighted valence vibration of C=O, through strips between 1815-1785 cm−1, 1750-1735 cm−1 and 1653-1624 cm−1 which are specific for flavonoids while 3700-3584 cm−1, 3550-3200 cm−1 and 3300-2500 cm−1 peaks are specific for the polyphenol class. Previous studies have established the antioxidant and anti-inflammatory activity of p-coumaric acid which was found in abundance in the hexane fraction of the fruit in this study [32, 33]. The FTIR spectral analysis thereby confirmed the presence of phenolic compounds in the hexane fraction of Kigelia africana fruit investigated and was in agreement with the report of Chen et al. [34], Sinelli et al. [35] and Kannan et al. [36] in their studies on the use of FTIR spectroscopy in confirmatory analysis towards the determination of polyphenols in plants.
Of interest is the significant reduction in PCV of group B (diabetic group only). This could be due to a reduced red blood cell count (anemia) in which there might be a decreased level of hemoglobin concentration or hypochromia. As seen in the hierarchical cluster analysis of the hematological parameters of this study, there is a direct relationship between red blood cells and hemoglobin whereby an increased red blood cell count is proportional to an increase in hemoglobin concentration and vice versa. Persistent hyperglycemia progresses the pathology of cardiac damage through changes in hematological parameters if left untreated [37]. The present study assessed the effects of the hexane fraction of Kigelia africana fruit on the hematological parameters always altered in diabetic conditions. In agreement with the research conducted by Ludidi et al. [38], no significant differences were observed between MCV and MCH of untreated diabetic rats. With the results of previous literature survey showing an increase in mean arterial blood pressure in STZ-induced diabetic rats followed by a further increase in rupturing rate of erythrocytes, our study concluded that the antidiabetic effect of Kigelia africana fruit may have buffered malondialdehyde (MDA) concentration thereafter attenuating lipid peroxidation which were especially seen in the hexane fraction of some traditional plants in previous studies [20] due to abundance of lipids in RBC membrane thereby maintaining the fluidity in facilitating deformability without rupturing [39]. Consequently, diabetic patients with complications are characterized with a decrease in hemoglobin concentration, MCH, and MCHC paramount in erythrocyte function and these changes can cause rapid initiation of apoptosis in damaged erythrocytes, decreasing the oxygen-carrying capacity due to increase in hemolysis rate and thus decreasing their lifespan; a condition resulting in reduced PCV which was noticed in group B (diabetic group) of this study. However, the treated groups were seen to have increased levels of hemoglobin concentration, mean corpuscular hemoglobin concentration (MCHC) and PCV (packed cell volume). The reason could be the antidiabetic effect of the fruit. Furthermore, the increase in the levels of WBC and lymphocyte count of this study could be as a result of the damaging effects of STZ.
Schrier et al. [40] proposed that plasma creatinine is a better indicator when compared to urea in the first phase of the toxicity of the kidney. Sodipo et al. [41] reported higher creatinine levels in renal dysfunction and muscle injury. Animals that were treated with the extract of the K. africana fruit had significantly lowered creatinine in the kidney and plasma corresponding to the concentration of urea. In a previous toxicity assessment, the extract administration resulted in a significant decrease in urea and creatinine levels thereby suggesting that the fruit has a nephroprotective potential. The STZ renal damage inducement is a familiar and well-replicated experiment model for the study of the effects of potential nephroprotective drugs and agents. The animals in the group administered with the hexane fraction of K. africana fruit extracts showed significant improvement in the plasma creatinine and urea when compared with the normal control groups. These improvements are a likely indication or signs that the fraction was possibly reversing damages to kidney cells resulting from STZ.
Cells release lactate dehydrogenase (LDH) into the systemic circulation after tissue damage or erythrocyte hemolysis. Accordingly, measurement of activities of marker enzymes (notably LDH, AST and ALT activities) in the plasma, liver and/or kidney is a reliable diagnostic parameter for ascertaining inherent-, biological- and xenobiotic-induced systemic toxicity [42, 43, 44]. Elevated plasma LDH activity is a reliable clinical indicator of organ injuries, especially those adversely affected by DM pathology and chemical toxicity, namely, the heart, liver, and muscle [45, 46, 47]. The relatively raised level of LDH activity was an obvious indication of systemic toxicity occasioned by tissue necrosis as exemplified in STZ-induced DM rats (group B). In clinical diagnosis, LDH activity is relatively low in plasma in the absence of cellular injuries, whereas the raised level of plasma LDH activity is a reflection of tissue infarction and/or necrosis [45, 46, 47] and parallels the number of necrotic cells [48]. Previous reports have shown that tissue necrosis, as a result of alloxan/streptozotocin intoxication in concert with the pathophysiology of DM, contributed to organ injuries and were a reflection of raised levels of plasma LDH activity in DM rats [48, 49]. All forms of liver disease lead to an increase in membrane-bound aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) in blood and liver [50]. This is due to the release of cell membrane fragments into the circulation. K. africana fruits suppressed the GGT activities in the plasma of all treated animals. Pancreas specific diseases such as diabetes, pancreatic cancer, and pancreatitis can be life-threatening [51]. STZ, a diabetogenic drug in animals is unique in its special affinity for the islet cells of the pancreas and has been reported to produce irreversible damage to pancreatic β-cells [52].
In this study, STZ-induced diabetic rats had an altered islet histomorphology, reduced islands of β-cell populations and necrotic changes, predominantly, karyopyknosis. This aligns with several other reports where insulin deficiency was reported to occur due to β-cells destruction by STZ. Portha et al. [53] reported that STZ diabetes is caused by the specific necrosis of the pancreatic β-cells. A similar report where intracellular entry of STZ leads to hypertrophy of β-cells Golgi apparatus within 1 h, and cell pyknosis follows shortly all of which was in consonance with this study [54]. STZ inflicts its hyperglycemic action by methylation of DNA and also acts as a donor of nitric oxide [54]. Nitric oxide has been reported to be an influencer of human body physiology and pathophysiology, including cytotoxic and cytoprotective effects [51, 55]. The effects generated depend on the cell type and concentration [54]. Pancreatic β-cells exposed to inflammatory cytokines or hyperglycemia produce high concentrations of nitric oxide. β-cells have been reported to be particularly sensitive to damage by nitric oxide and free radicals because of their low levels of free radical scavenging enzymes thus promoting cell death [56]. Several studies have highlighted the importance of interactions among the different islet cells in hormone production [57, 58]. Glucagon was reported to enhance insulin secretion [59, 60]. The extensive damage to the periphery cells in this study could also point to the STZ effect on peripherally-placed cells thus preventing communication among islet cells that enhance the maintenance in the physiological balance of insulin production.
The hexane fraction of K. africana fruit extracts at the low and middle doses administered showed significant ameliorative activities by restoring islet circular to oval appearance, a cord-like arrangement of islet cells with close apposition to capillaries, increase in the number of cells and nuclei at the islet core recognized as β-cells. These effects could be as a result of the antioxidant potentials of the hexane fraction. Olubunmi et al. [61] reported that the hexane fraction of Kigelia africana fruit showed an increase in antioxidant activity in a dose-dependent manner. Antioxidants are known to reduce the oxidative stress of the physiological system caused by an uncontrolled generation of reactive oxygen species in the cells. It can also be inferred that the hexane fraction of Kigelia africana possesses an antihyperglycemic effect. One major mechanism of the four reported ways by which plants exert antihyperglycemic activities is the regeneration of β-cells [62]. However, the highest dose of Kigelia africana extract caused an atrophic change in the endocrine pancreas. This could also be a pointer to the toxic effect of the extract at this dose.
Zonal evaluation of hepatic changes in diabetic rats revealed the predominant mid-zonal hepatocellular injury in Wistar rats of group B followed by C, D, then E and F. Morphological injuries observed across the experimental groups include an altered pattern of cord-like arrangement of hepatocytes, altered radial arrangement of sinusoids, intracytoplasmic vacuolations and karyo-necrotic changes such as karyopyknosis and karyorrhexis. Maintenance of blood glucose levels within a normal range requires several cell types, including a highly significant role of hepatocytes and the parenchymal liver cells [63]. They play a very significant role under hormonal condition, acting as both glucose reservoir and manufacturer [63]. Hepatocytes respond as demanded to either feeding or fasting conditions by storing or producing glucose. In the fasting state, the effects of glucagon avoid hypoglycemia which it achieves by stimulating gluconeogenesis and glycogenolysis and initiating hepatic glucose release. After a meal, insulin prevents hyperglycemia by actively suppressing hepatic gluconeogenesis and glycogenolysis and facilitating hepatic glycogen synthesis [63]. Hepatocyte death automatically impairs this function. Considering the foregoing, pancreatic β-cell death correlates with impaired liver function and vice versa. Kierszenbaum et al. [64] considered a cell to be dead when it's no longer performing the required function thus validating the reason for hepatocellular death observed in this study. This result agrees with an earlier report by Rodríguez et al. [65]. They also reported cell death and inflammation in the liver of STZ-induced diabetic rats. Hexane fraction of Kigelia africana mitigated these effects with the best response observed at the middle dose given. This could be as a result of the antidiabetic potentials of this fraction at this concentration.
In addition, this study showed that STZ-induced diabetes in rats was associated with specific renal alterations such as a reduction in cell types of the renal corpuscle (podocytes, mesangial cells, Bowman's capsule cells, capillary endothelial cells), atrophied glomerular tuft, widened urinary space, ill-defined capillary lumens, and tubular epithelial degeneration. Cells are the basic structural and functional units of all multicellular organisms with specific functions associated with specific structural components and domains within the cell [66]. Under diabetic conditions, all cell types of the renal corpuscles, tubules, and interstitium including capillary endothelial cells, tubulointerstitial cells, tubular epithelial cells, podocytes, and mesangial cells can be affected [67]. Moreover, any injury and dysfunction affecting a cell type extends to all renal cell types and affects renal function [67]. Podocytes are specialized visceral epithelial cells with an important role in the glomerular filtration barrier [66]. Loss of podocytes has been associated with diabetic nephropathy [68]. Nakamura et al. [69] reported that hyperglycemia results in a break off of podocytes, a visceral epithelial cell from the glomerular basement membrane with possible detection in the urine of diabetic patients and it worsens as the disease progresses from normoalbuminuria to microalbuminuria and finally to macroalbuminuria. This could be brought about by reactive oxygen species [70]. It was previously reported that hyperglycemia induces generation of reactive oxygen species (free radicals) through the nicotinamide adenine dinucleotide phosphate (NADP) oxidase and free radical production initiates apoptotic death of podocytes. The reversed effect exhibited by the extract could be a result of its free radical scavenging potentials thereby augmenting the endogenous antioxidant.
Another significant morphological derangement observed in experimental groups B and E in this study is the occlusion of the glomerular capillary lumen. Previously, Abrass [71] reported that one major characteristic of diabetic nephropathy which is an abnormal condition seen in patients with Diabetes mellitus is the expansion of the mesangial matrix that ultimately occludes glomerular capillaries. This is similar to the observation of this study. The properties of the glomerular filter are dependent on three structures which are the endothelial cell lining of the glomerular capillaries, the glomerular basement membrane with connective tissue makeup and the visceral epithelial cells of the Bowman's capsule [72]. Mesengium, occupied by extracellular matrix secreting intraglomerular mesangial cells is an intercapillary meshwork of contractile, smooth muscle-like cells embedded in the amorphous extracellular matrix [73]. It provides central support for the glomerular capillaries [71]. Expansion of the mesangium, not accompanied by proportional enlargement of the capillary bed, results in compression and loss of filtration surface, with a decline in the single-nephron glomerular filtration rate [74].
A major biochemical event that happens in long-standing untreated hyperglycemia is the glycosylation of structural proteins resulting in an alteration of perivascular mesangial cells and this may contribute to albuminuria of diabetes [76, 77]. In other words, expansion of mesangium occurs as a result of the accumulation of proteins normally present in the mesangial matrix, and new interstitial collagens and ECM proteins which later manifest as the occlusion of adjacent capillary loops [77, 78]. This explains the reason for capillary occlusion observed in this study. Results from this study established that the hexane fraction mitigates this action which it does probably by augmenting endogenous antioxidants thus preventing oxidative damage with the more potent activity exhibited at the middle dose. Flavonoids, a phenolic compound with high antioxidant activities have been reported to be venoactive with the potentials to restore the delineable nature of the capillaries [79].
5. Conclusion
The current study has shed more light on some beneficial properties of K. africana fruit administration which will serve as a step further in authenticating the consumption. The presence of polyphenolic functional groups earmarked the antioxidant properties and the results presented appear to show improvement of the antioxidant status on antidiabetic properties. This stemmed from the regeneration seen in the histological features of treated diabetic rats. The administration of the fruit could be used for the treatment of diseases associated with persistent hyperglycemia. Further studies regarding gene expression will expand indigenous knowledge in a wide spectrum range for easy access to the management of Diabetes mellitus. Kigelia africana fruit has protective potentials on the kidney and the liver as well as in the blood.
Declarations
Author contribution statement
O.F. Fagbohun: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
P. Awoniran: Performed the experiments; Analyzed and interpreted the data.
O.O. Babalola and F.K. Agboola: Contributed reagents, materials, analysis tools or data.
T.T.A.M. Msagati: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
There is no additional information for this paper.
Acknowledgements
The authors wish to appreciate the support from Professor Ayobami Salami; the Vice-Chancellor of First Technical University, Ibadan, Oyo State, Nigeria whose interest in research made this work a reality. We also wish to thank the staff of Nanotechnology and Water Sustainability Research Unit, College of Science Engineering and Technology, University of South Africa (UNISA), Florida Park, Johannesburg, South Africa.
References
- 1.WHO Mortality Database (Online Database) World Health Organization; Geneva: 2017. http://apps.who.int/healthinfo/statistics/mortality/causeofdeath_query/ [Google Scholar]
- 2.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Unwin N., Gan D., Whiting D. The IDF Diabetes Atlas: providing evidence, raising awareness and promoting action. Diabetes Res. Clin. Pract. 2010;87:2–3. doi: 10.1016/j.diabres.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Herman W.H., Ye W., Griffin S.J., Simmons R.K., Davies M.J., Khunti K. Early detection and treatment of type 2 diabetes reduce cardiovascular morbidity and mortality: a simulation of the results of the AngloDanish-Dutch study of intensive treatment in people with screen-detected diabetes in primary care (ADDITION-Europe) Diabetes Care. 2015;38(8):1449–1455. doi: 10.2337/dc14-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loghmani E.S. Nutrition therapy for overweight children and adolescents with Type 2 Diabetes. Curr. Diabetes Rep. 2005;5(5):385–390. doi: 10.1007/s11892-005-0098-9. [DOI] [PubMed] [Google Scholar]
- 6.Kishore L., Singh R. Ameliorative effect of Cephalandra indica homeopathic preparation in STZ induced diabetic nephropathy rats. J. Ayurveda Integr. Med. 2018 doi: 10.1016/j.jaim.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vleming L.J., Baelde J.J., Westendorp R.G., Daha M.R., van Es L.A., Bruijn J.A. The glomerular deposition of PAS positive material correlates with renal function in human kidney diseases. Clin. Nephrol. 1997 Mar;47(3):158–167. PMID: 9105762. [PubMed] [Google Scholar]
- 8.Alsaad K.O., Herzenberg A.M. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J. Clin. Pathol. 2007;60(1):18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers A.C., D’Alessio D. Endocrine pancreas and pharmacotherapy of diabetes mellitus and hypoglycaemia. In: Brunton L.B., Bruce C., Knollman B., editors. Goodman and Gilman’s the Pharmacological Basis of Therapeutics 12thedition. Mc Graw Hill; New York: 2011. pp. 1237–1274. [Google Scholar]
- 10.Oyedemi S.O., Adewusi E.A., Aiyegoro O.A., Akinpeanolu D.A. Antidiabetic and hematological effect of aqueous extract of stem bark of Afzelia africana (Smith) on streptozocin-induced diabetic wistar rats. Asian Pac. J. Trop. Biomed. 2011;1:353–358. doi: 10.1016/S2221-1691(11)60079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinmoladun A.C., Farombi E.O., Omoniyi O. 2014. Antidiabetic Botanicals and Their Potential Benefits in the Management of Diabetes Mellitus. [DOI] [Google Scholar]
- 12.Kuroe M., Ohori S., Takatsuki S., Miyashita T. Nest-site selection by the harvest mouseMicromys minutus in seasonally changing environments. Acta Theriol. 2007;52(4):355–360. [Google Scholar]
- 13.Wadhar K.A., Magdum C.S., Patil S.S., Naikwade N.S. Antidiabetic potential and Indian medicinal plants. J. Herb. Med. Toxicol. 2008;2:45–50. [Google Scholar]
- 14.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher E.B., Thorpe C.T., McEvoy DeVellis B., DeVellis R.F. Healthy coping, negative emotions, and diabetes management. Diabetes Educat. 2007;33(6):1080–1103. doi: 10.1177/0145721707309808. [DOI] [PubMed] [Google Scholar]
- 16.Okine A., Hanada M., Aibibula Y., Okamoto M. Ensiling of potato pulp with or without bacterial inoculants and its effect on fermentation quality, nutrient composition and nutritive value. Anim. Feed Sci. Technol. 2005;121:329–343. [Google Scholar]
- 17.Ramachandran V., Mandal D., Payyavala U., Sagai P.D., Muthureddy N.S., Shanish A. Hypoglycemic activity of Asparagus racemosus on streptozotocin-induced diabetic in rats. Adv. Appl. Sci. Res. 2011;2(3):179–185. [Google Scholar]
- 18.Atawodi S.E., Olowoniyi O.D. Pharmacological and therapeutic activities of Kigelia africana (lam.) Benth. Ann. Res. Rev. Biol. 2015;5(1):1–17. [Google Scholar]
- 19.Azu O.O., Duru F.I.O., Osinubi A.A., Oremosu A.A., Noronha C.C., Elesha S.O., Okanlawon A.O. Histomorphometric effects of Kigelia africana (Bignoniaceae) fruit extract on the testis following short-term treatment with cisplatin in male Sprague–Dawley rats. Middle East Fertil. Soc. J. 2010;15(3):200–208. [Google Scholar]
- 20.Fagbohun O.F., Babalola O.O., Agboola F.K., Joseph J.S., Malindisa S., Msagati T.A. Evaluation of phytochemicals, antioxidants, trace Elements in Kigelia africana fruit Extracts and chemical profiling analysis using UHPLC-qTOF-MS2 spectrometry. Biol. Trace Elem. Res. 2019 doi: 10.1007/s12011-019-01869-2. [DOI] [PubMed] [Google Scholar]
- 21.Sikarwar M.S., Patil M.B. Antidiabetic activity of Crateva nurvala stem bark extracts in alloxan-induced diabetic rats. J. Pharm. Bioall Sci. 2010;2:18–21. doi: 10.4103/0975-7406.62700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 23.Tietz N.W. third ed. W.B. Saunders, Co.; Philadelphia: 1995. Clinical Guide to Laboratory Tests (ELISA) pp. 22–23. [Google Scholar]
- 24.Ababio G.K., Adu-Bonsaffoh K., Narh G., Morvey D., Botchway F. Effects of lactate dehydrogenase (LDH) in preeclampsia. Clin. Med. Biochem. 2017;3:129. [Google Scholar]
- 25.Chawla R. Serum total protein and albumin-globulin ratio. In: Chawla R., editor. Methods and Interpretations. Jaypee Brothers Medical Publishers; New Delhi, India: 1999. pp. 106–118. [Google Scholar]
- 26.Weatherburn M.W. Urease-berthelot colorimetric method for in vitro determination of urea. Anal. Chem. 1967;39:971–974. [Google Scholar]
- 27.Mbaka G.O., Ogbonnia S.O., Oyeniran K.J., Awopetu P.I. Effect of Raphia hookeri seed extract on blood glucose, glycosylated haemoglobin and lipid profile of alloxan induced diabetic rats. J. Adv. Med. Med. Res. 2010;21:621–635. [Google Scholar]
- 28.Stuart B. Wiley Online Library; 2005. Infrared Spectroscopy. [Google Scholar]
- 29.Patras A., Brunton N.P., O’Donnell C., Tiwari B.K. Effect of thermal processing on anthrocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010;21:3–11. [Google Scholar]
- 30.Lorke D. A new approach to practical acute toxicity testing. Acute Toxic. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 31.Rashid U., Khan M.R., Sajid M. Antioxidant, anti-inflammatory and hypoglycemic effects of Fagonia olivieri DC on STZ-nicotinamide induced diabetic rats - in vivo and in vitro study. J. Ethnopharmacol. 2019;242:112038. doi: 10.1016/j.jep.2019.112038. [DOI] [PubMed] [Google Scholar]
- 32.Rice-Evans C., Miller N., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2(4):152–159. [Google Scholar]
- 33.Svobodová A., Psotová J., Walterová D. Natural Phenolics in the prevention of UV-induced skin damage: a review. Biomed. Pap. 2003;147:137–145. [PubMed] [Google Scholar]
- 34.Chen H., Karne R.J., Hall G., Campia U., Panza J.A., Cannon R.O. High dose oral vitamin C partially replenishes vitamin C levels in patients with type 2 diabetes and low vitamin C levels but does not improve endothelial dysfunction or insulin resistance. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H137–H145. doi: 10.1152/ajpheart.00768.2005. [DOI] [PubMed] [Google Scholar]
- 35.Sinelli N., Casiraghi E., Barzaghi S., Brambilla A., Giovanelli G. Near infrared (NIR) spectroscopy as a tool for monitoring blueberry osmo–air dehydration process. Food Res. Int. 2011;44(5):1427–1433. [Google Scholar]
- 36.Kannan A., Senthil K. A study on drug utilization of oral hypoglycemic agents in type-2 diabetic patients. Asian J. Pharmaceut. Clin. Res. 2011;4(4):1–10. [Google Scholar]
- 37.Thomas M.C., MacIsaac R.J., Tsalamandris C., Power D., Jerums G. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care. 2003;26(4):1164–1169. doi: 10.2337/diacare.26.4.1164. [DOI] [PubMed] [Google Scholar]
- 38.Ludidi A., Baloyi M.C., Khathi A., Sibiya N.H., Ngubane P.S. The effects of Momordica balsamina methanolic extract on haematological function in streptozotocin-induced diabetic rats: effects on selected markers. Biomed. Pharmacother. 2019;116:108925. doi: 10.1016/j.biopha.2019.108925. [DOI] [PubMed] [Google Scholar]
- 39.Kalaycı D., Arpacı A.H., Çomu F.M., Güneş I., Beşkardeş E., Kurtipek Ö., Arslan M., Dikmen B. Investigation of the effects of sevoflurane and Desflurane on erythrocytedeformability in transient hyperglycemia. Gazi Med. J. 2017;29:2147. [Google Scholar]
- 40.Schrier R.W., Wang W., Poole B., Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J. Clin. Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sodipo O.A., Abdulrahman F.I., Sandabe U.K. Biochemical kidney function with aqueous fruit extract of Solanum macrocarpum (Linn) in albino rats chronically administered triton-X to induce hyperlipidemia. Afr. J. Med. Med. Sci. 2012;3:93–98. [Google Scholar]
- 42.Pérez-Trueba G., Ramos-Guanche C., Martínez-Sánchez B., Márquez-Hernández I., Giuliani A., Martínez-Sánchez G. Protective effect of gossypitrin on carbon tetrachloride-induced in vivo hepatotoxicity. Redox Rep. 2003;8(4):215–221. doi: 10.1179/135100003225002718. [DOI] [PubMed] [Google Scholar]
- 43.Petlevski R., Hadzija M., Bajalo J.L., Juretic D. Effects of acarbose on alanine aminotransferase and aspartate aminotransferase activities in the liver of control and diabetic CBA mice. Acta Pharmacol. 2006;56(1):87–93. [PubMed] [Google Scholar]
- 44.Pitocco D., Tesauro M., Alessandro R., Ghirlanda G., Cardillo C. Oxidative stress in diabetes: implications for vascular and other complications. Int. J. Mol. Sci. 2013;4(11):21525–21550. doi: 10.3390/ijms141121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Claudino M., Ceolin D.S., Alberti S., Cestari T.M., Spadella C.T., Rubira-Bullen I.R.F. Alloxan-induced diabetes triggers the development of periodontal disease in rats. PLoS One. 2007;2(12):e1320. doi: 10.1371/journal.pone.0001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das M., Barua N. Pharmacological activities of Solanum melongena linn. (Brinjal plant) Int. J. Green Pharmacol. 2013;7(4):274–277. [Google Scholar]
- 47.Chikezie P.C., Ojiako A.O., Ogbuji C.A. Oxidative stress in Diabetes mellitus. Int. J. Biol. Chem. 2015;9:92–109. [Google Scholar]
- 48.Sanlioglu A.D., Altunbas H.A., Balci M.K., Griffih T.S., Sanlioglu S. Clinical utility of insulin and insulin analogs. Islets. 2013;5(2):67–78. doi: 10.4161/isl.24590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elsawy M., Emara E. The impact of ghrelin on oxidative stress and inflammatory markers on the liver of diabetic rats. Tanta Med. J. 2016;44(4):163–169. [Google Scholar]
- 50.Dhanya K.G., Thangavel M. Levels of lactase dehydrogenase, gamma-glutamyl transferase, glycogen, bilrubin and evaluation of the hepatoprotective activity of ethanol extract of Mimosa pudica. Int. J. Biotechnol. Biochem. 2017;13(4):351–359. [Google Scholar]
- 51.Martins I., Galluzzi L., Kroemer G. Hormesis, cell death and aging. Aging. 2011;3:821–828. doi: 10.18632/aging.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daniel L., Gustafson R.L. Page, in withrow and MacEwen's small animal clinical oncology (5th edition) Cancer Chemother. 2013;2:18–26. [Google Scholar]
- 53.Portha B., Levacher C., Picon L., Rosselin G. Diabetogenic effect of streptozotocin in the rat during the perinatal period. Diabetes. 1974;23(11):889–895. doi: 10.2337/diab.23.11.889. [DOI] [PubMed] [Google Scholar]
- 54.Francisco J.B., Carmen S.A., Gladys M., Cahuana R.T., Bernat S., Juan R.T. Regulation of pancreatic β-cell survival by nitric oxide. Islets. 2012;4(2):108–118. doi: 10.4161/isl.19822. [DOI] [PubMed] [Google Scholar]
- 55.Thomas D.D., Ridnour L.A., Isenberg J.S., Flores-Santana W., Switzer C.H., Donzelli S. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic. Biol. Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spinas G.A. The dual role of nitric oxide in islet ß-cells. News Physiol. Sci. 1999;14:49–54. doi: 10.1152/physiologyonline.1999.14.2.49. [DOI] [PubMed] [Google Scholar]
- 57.Halban P.A., Powers S.L., George K.L., Bonner-Weir S. Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregates with three-dimensional architecture typical of native islets. Diabetes. 1987;36(7):783–790. doi: 10.2337/diab.36.7.783. [DOI] [PubMed] [Google Scholar]
- 58.Bosco D., Orci L., Meda P. Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp. Cell Res. 1989;184:72–80. doi: 10.1016/0014-4827(89)90365-0. [DOI] [PubMed] [Google Scholar]
- 59.Wojtusciszyn M., Armanet P., Morel T., Berney B., Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008;51(10):1843–1852. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]
- 60.Jain R., Lammert E. Cell-cell interactions in the endocrine pancreas. Diabetes Obes. Metabol. 2009;11(4):159–167. doi: 10.1111/j.1463-1326.2009.01102.x. [DOI] [PubMed] [Google Scholar]
- 61.Olubunmi A., Stephen O.A., Essiet A., Charles B.A., Gabriel A.O. Chemical composition and anti-oxidant potentials of Kigelia pinnata root oil and extracts. Excli J. 2011;10:264–273. [PMC free article] [PubMed] [Google Scholar]
- 62.Jelodar G.A., Maleki M., Motadayen M.H., Sirus S. Effect of fenugreek, onion and garlic on blood glucose and histopathology of pancreas of alloxan-induced diabetic rats. Indian J. Med. Sci. 2005;59(2):64–69. [PubMed] [Google Scholar]
- 63.Klover P.J., Mooney R.A. Hepatocytes: critical for glucose homeostasis. Int. J. Biochem. Cell Biol. 2004;36:753–758. doi: 10.1016/j.biocel.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Kierszenbaum A. Elsevier Saunders; Philadelphia: 2012. Histology and Cell Biology - an Introduction to Pathology; pp. 23–35. [Google Scholar]
- 65.Rodríguez A., Chiti T., Rey A., Durán J. Forest die-off reduces soil C and N content and increases C stability in a Mediterranean woodland. Geoderma. 2019;359:113990. [Google Scholar]
- 66.Ross M.H., Pawlina W. sixth ed. Lippincott Williams and Wilkins; 2011. Histology: A Text and Atlas, with Correlated Cell and Molecular Biology; pp. 35–46. [Google Scholar]
- 67.Maezawa Y., Takemoto M., Yokote K. Cell biology of diabetic nephropathy: roles of endothelial cells, tubulointerstitial cells and podocytes. J. Diab. Invest. 2015;6:3–15. doi: 10.1111/jdi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalluri R. Proteinuria with and without renal glomerular podocyte effacement. J. Am. Soc. Nephrol. 2006;17:2383–2389. doi: 10.1681/ASN.2006060628. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura T., Ushiyama C., Suzuki S. Urinary excretion of podocytes in patients with Diabetic Nephropathy. Nephrol. Dial. Transplant. 2000;15:1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 70.Susztak K., Raff A.C., Schiffer M., Bottinger E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 71.Abrass C.K. Diabetic Nephropathy-Mechanisms of mesangial matrix expansion. West. J. Med. 2005;162:318–321. [PMC free article] [PubMed] [Google Scholar]
- 72.Lote C. Principles of Renal Physiology. Springer; Dordrecht: 2000. Glomerular filtration; pp. 34–46. [Google Scholar]
- 73.Menè P., Simonson M.S., Dunn M.J. Physiology of the mesangial cell. Physiol. Rev. 1989;69:1347–1423. doi: 10.1152/physrev.1989.69.4.1347. [DOI] [PubMed] [Google Scholar]
- 74.Zheng S., Noonan W.T., Metreveli N.S. Development of late-stage diabetic nephropathy in OVE26 diabetic mice. Diabetes. 2004;53:3248–3257. doi: 10.2337/diabetes.53.12.3248. [DOI] [PubMed] [Google Scholar]
- 76.Loeffler I., Hopfer U., Koczan D., Wolf G. Type VIII collagen modulates TGF-beta1-induced proliferation of mesangial cells. J. Am. Soc. Nephrol. 2011;22:649–663. doi: 10.1681/ASN.2010010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abrass C.K., Peterson C.V., Raugi G.J. Phenotypic expression of collagen types in mesangial matrix of diabetic and nondiabetic rats. Diabetes. 1988;37:1695–1702. doi: 10.2337/diab.37.12.1695. [DOI] [PubMed] [Google Scholar]
- 78.Steffes M.W., Osterby R., Chavers B., Mauer S.M. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1989;38:1077–1081. doi: 10.2337/diab.38.9.1077. [DOI] [PubMed] [Google Scholar]
- 79.Jargin S.V. Ethnopharmacology of Kigelia pinnata. J. Int. Ethnopharmacol. 2018;7(1):97–100. [Google Scholar]