Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
Occurrence of chronic health conditions, not transplant receipt, is related to symptom prevalence in pediatric hematologic malignancy survivors.
Abstract
Patient-reported outcomes among survivors of pediatric hematopoietic stem cell transplant (HSCT) are understudied. We compared symptom prevalence, health-related quality of life (HRQOL), and risk factors in adult survivors of childhood hematologic malignancies treated with HSCT to those treated with conventional therapy and noncancer controls. Survivors of childhood hematologic malignancies (HSCT N = 112 [70% allogeneic, 30% autologous]; conventionally treated N = 1106) and noncancer controls (N = 242) from the St. Jude Lifetime Cohort Study completed surveys assessing 10 symptom domains and SF-36 HRQOL summary scores. Chronic health conditions (CHCs) were validated by clinical assessment. Multivariable logistic regression reveals that compared with noncancer controls, HSCT survivors endorsed a significantly higher symptom prevalence in sensation (OR = 4.7, 95% confidence interval [CI], 2.6-8.4), motor/movement (OR = 4.3, 95% CI, 1.6-11.0), pulmonary (OR = 4.6, 95% CI, 1.8-11.8), and memory domains (OR = 4.8, 95% CI, 2.5-9.2), and poorer physical HRQOL (OR = 6.9, 95% CI, 2.8-17.0). HSCT and conventionally treated survivors had a similar prevalence of all symptom domains and HRQOL (all P > .05); however, HSCT survivors had a significantly higher cumulative prevalence for specific symptoms: double vision (P = .04), very dry eyes (P < .0001), and trouble seeing when wearing glasses (P < .0001). Occurrence of organ-specific CHCs, instead of transplant receipt, was significantly associated with a higher prevalence of all symptom domains (all P < .05) in adult survivors of childhood cancer, except for pain and anxiety domains. This study found that patient-reported outcomes were equally impaired between HSCT and conventionally treated survivors, but poorer in both groups compared with noncancer controls. Poor patient-reported outcomes in all survivors of childhood hematologic malignancies correlated with the presence of CHCs, whether treated with conventional therapy or HSCT.
Visual Abstract
Introduction
Hematopoietic stem cell transplant (HSCT) is used for some children and adolescents with hematologic malignancies.1,2 Progress in transplant technology (eg, donor matching, alternative donor source, conditioning regimens), and supportive care have significantly improved the post-HSCT survival.3,4 However, survivors are at risk for developing chronic graft-versus-host disease (GVHD) and other chronic health conditions (CHCs) that may contribute to late morbidity.3,5-8 The number and severity of CHCs increase in the years following therapy completion, adversely affecting the quality and duration of their survival.7,9
Assessing patient-reported outcomes (eg, symptom prevalence and health-related quality of life [HRQOL]) provides unique health information perceived by cancer survivors that is complementary to traditional clinical end points (eg, disease stage, survival).10-12 Several studies have explored symptom phenotypes and HRQOL in survivors of adult-onset cancer,11 pediatric cancer,12-16 and adults treated with HSCT.10,17,18 We previously reported that ∼80% of adult survivors of childhood cancer experienced multiple symptoms decades after a diagnosis of pediatric cancers.12 We also observed that survivors, compared with individuals without cancer history, had a higher symptom burden that was associated with more CHCs and impaired HRQOL.11 Given the elevated risk of CHCs in survivors of pediatric HSCT,7,9 routine symptom assessment becomes clinically important as symptom phenotypes may indicate the new onset of adverse health problems.
Studies investigating symptom and HRQOL issues in pediatric HSCT survivors are limited by short follow-up duration (often 5-15 years),19-22 assessment of limited symptom domains (mostly pain or fatigue),23,24 or inclusion of small2,20,22,24 or heterogeneous23-25 samples. Although symptoms reflect the manifestation of CHCs, few studies have evaluated the association of CHCs and patient-reported outcomes in HSCT survivors. Therefore, conflicting findings have been reported when comparing symptom prevalence between survivors of pediatric hematologic malignancy treated with and without HSCT. Some studies noted a similar prevalence of pain,22,23 fatigue,21 and anxiety/depression,22,23 whereas others reported higher prevalence of pain in HSCT survivors compared with conventionally treated survivors.21,24
The first objective of this study was to compare symptom prevalence and HRQOL in long-term (≥20 years since diagnosis) survivors of childhood hematologic malignancies treated with HSCT to survivors treated with conventional therapies and noncancer controls, respectively. We hypothesized that HSCT survivors would in general have a higher symptom prevalence and poorer HRQOL compared with conventional therapy survivors and noncancer controls. The second objective was to identify risk factors for elevated symptom prevalence and poor HRQOL, with a focus on the influence of CHCs. We hypothesized that associations of higher symptom prevalence and poorer HRQOL related to HSCT experience (ie, receipt of HSCT vs conventional therapy) would be significantly related to the occurrence of CHCs, particularly for associations of symptom prevalence with CHCs from the same organ system. In contrast to previous studies that collected CHC data through self-reports,20,22,26,27 we conducted medical assessments to evaluate CHCs.28
Patients and methods
Study sample
This cross-sectional study used data collected from adult survivors of childhood cancer enrolled in the St. Jude Lifetime Cohort Study, a retrospective cohort study with prospective follow-ups established to investigate etiologies of late effects related to pediatric cancer therapies.28,29 The study sample consisted of cancer survivors who received conventional therapy for a hematologic cancer, a subgroup of whom also underwent HSCT. Survivors received comprehensive medical assessments per the Children’s Oncology Group Long-Term Follow-up Guidelines.30,31 Additionally, community controls having no history of cancer were included as a comparison group.
Data collection
Eligible survivors were: (1) ≥18 years of age at the time of participation; (2) treated for a hematologic malignancy, including acute myeloid leukemia, acute lymphoblastic leukemia, chronic myeloid leukemia, Hodgkin lymphoma, non-Hodgkin lymphoma, and myelodysplastic syndromes, at St. Jude Children’s Research Hospital; and (3) had survived ≥10 years after the completion of cancer therapies between January 1, 1982, and June 30, 2005. Eligible noncancer controls were: (1) ≥18 years of age at the time of participation; (2) non–first-degree relatives or friends of St. Jude patients, or any volunteer not associated with St. Jude; and (3) not treated for a childhood cancer.
Among 1965 potentially eligible participants, 1218 survivors (112 with HSCT [70% allogeneic, 30% autologous] and 1106 with conventional therapy only) and 242 noncancer controls who completed questionnaires and medical assessments at St. Jude Children’s Research Hospital were included (supplemental Figure 1, available on the Blood Web site). The study protocol was approved by St. Jude’s institutional review board, and all participants provided written informed consent for evaluations.
Measurement
Symptom assessments comprised 37 items recommended by the Children’s Oncology Group Long-Term Follow-up Guidelines31 that were used in our previous publication.12 Items assessed 10 domains: sensation (8 items), motor/movement (4 items), cardiac symptoms (3 items), pulmonary symptoms (2 items), pain (4 items), fatigue (2 items), nausea (1 item), memory (1 item), anxiety (6 items), and depression (6 items) (supplemental Table 1). The presence of any symptom item within a specific domain indicated presence of that symptom domain. The Medical Outcomes Study 36-Item Short-Form Health Survey was used to measure HRQOL. Physical and mental component summary scores (PCS and MCS) were calculated and normalized to a mean of 50 and a standard deviation (SD) of 10. A threshold ≤40 (1 SD below the norm) was used to indicate poor physical and mental HRQOL.
Medical assessment data were used to categorize 168 specific CHCs using a modified Common Terminology Criteria for Adverse Events (CTCAE) grading as asymptomatic/mild (grade 1), moderate (grade 2), severe/disabling (grade 3), or life-threatening (grade 4).28 CHCs were dichotomized as at least severe (grades 3-4) or not (no diagnosed condition or grades 1-2). This study focused on 7 CHC groups that were found to be more prevalent among survivors treated with HSCT compared with conventional therapy, including cardiovascular, endocrine, gastrointestinal, neurological, ocular, pulmonary, and reproductive disorders.7 A specific CHC group was considered as present if any condition under that group was present.
Important risk factors contributing to symptom presence and poor HRQOL were examined, including sociodemographic variables and cancer-/HSCT-related treatments. Sociodemographic variables were self-reported, including sex, race/ethnicity (white, non-Hispanic vs other), educational attainment (below college vs college or above), and marital status (married/living with a partner vs single/divorced/other status). Cancer therapy details were abstracted from medical records inclusive of years since cancer diagnosis, type of HSCT (autologous vs allogeneic), type and cumulative dose of chemotherapy and radiation therapy, and transplant-related variables (disease status at transplant and intensity of transplant experience). Disease status was classified as first complete remission, second or subsequent complete remission, and relapsed or progression. Intensity of transplant experience was classified as low (autologous), intermediate (allogeneic without the occurrence of chronic GVHD), and severe (allogeneic with the occurrence of chronic GVHD).17
Statistical analysis
Student t and χ2 tests were conducted to compare differences in symptom prevalence and poor HRQOL for HSCT vs conventionally treated survivors and for HSCT survivors vs noncancer controls. Cumulative prevalence of specific symptoms and HRQOL at the domain and item levels were estimated by referring the time since cancer diagnosis to the presentation of individual symptom items or domains, and poor HRQOL at the time of survey.11 Discrepancy in cumulative prevalence rates between HSCT and conventionally treated survivors were also compared. Multivariable logistic regression models were performed to estimate the odds of symptom prevalence and poor HRQOL for HSCT vs conventional therapy survivors, and for HSCT vs noncancer controls with an adjustment for the previously mentioned risk factors. In this modeling, cancer therapy was not included because we (1) aimed to identify risk factors of poor patient-reported outcomes using a parsimonious model and (2) hypothesized that the occurrence of CHCs has direct effects on patient-reported outcomes, whereas cancer therapy has indirect effects on patient-reported outcomes through the influence of CHCs. However, we also conducted additional analysis by adding therapy variables to the parsimonious model to evaluate robustness of the findings among 2 models. Additionally, a multivariable logistic regression was performed to calculate the odds of symptom prevalence and poor HRQOL for allogeneic vs autologous survivors. Multivariable logistic regression was also performed to test associations of the aforementioned HSCT-related variables with symptom prevalence and poor HRQOL among HSCT survivors only. All analyses were performed using SAS v9.4. Statistically significant differences were decided by P < .05 (2-sided).
Results
Table 1 summarizes the characteristics of study participants. The mean ages at assessment among survivors treated with HSCT, those treated with conventional therapy, and noncancer controls were 28.4, 29.2, and 35.1 years, respectively. The mean years since cancer diagnosis was 18.5 for survivors treated with HSCT and 19.9 for those treated with conventional therapy.
Table 1.
Characteristics of study participants
| Characteristics | HSCT survivors (N = 112) | Conventional therapy survivors (N = 1106) | Noncancer controls (N = 242) | HSCT survivors vs conventional therapy survivors | HSCT survivors vs noncancer controls |
|---|---|---|---|---|---|
| P | P | ||||
| Age (y) at survey, mean ± SD (range) | 28.4 ± 5.9 (18.8-43.2) | 29.2 ± 6.2 (18.3-47.6) | 35.1 ± 10.4 (18.1-70.0) | .132 | <.001 |
| Sex, n (%) | .741 | .555 | |||
| Female | 55 (49.1) | 525 (47.5) | 140 (52.6) | ||
| Male | 57 (50.9) | 581 (52.5) | 126 (47.4) | ||
| Race/ethnicity, n (%) | .026 | .03 | |||
| White, non-Hispanic | 83 (74.1) | 914 (82.6) | 203 (83.9) | ||
| Other | 29 (25.9) | 192 (17.4) | 39 (16.1) | ||
| Diagnosis, n (%) | <.001 | NA | |||
| Acute lymphoblastic leukemia | 23 (20.5) | 649 (58.7) | NA | ||
| Acute myeloid leukemia | 44 (39.3) | 42 (3.8) | NA | ||
| Lymphoma (Hodgkin, non-Hodgkin) | 18 (16.0) | 413 (37.3) | NA | ||
| Other | 27 (24.1) | 2 (0.2) | NA | ||
| Age (y) at diagnosis, mean ± SD (range) | 9.8 ± 5.3 (0.5-18.8) | 9.4 ± 5.5 (0.2-21.8) | NA | .425 | NA |
| Time (y) since diagnosis, mean ± SD (range) | 18.5 ± 4.2 (11.3-28.5) | 19.9 ± 5.1 (10.5-32.9) | NA | .002 | NA |
| Treatment era, n (%) | <.001 | NA | |||
| 1980-1989 | 24 (21.4) | 503 (45.5) | NA | ||
| 1990-1999 | 76 (67.9) | 502 (45.4) | NA | ||
| 2000 and after | 12 (10.7) | 101 (9.1) | NA | ||
| Education, n (%) | .039 | .559 | |||
| <College | 63 (56.3) | 730 (66.0) | 127 (52.9) | ||
| ≥College | 49 (43.8) | 376 (34.0) | 113 (47.1) | ||
| Annual household income, n (%) | .553 | .016 | |||
| <$20 000 | 62 (59.0) | 568 (54.1) | 93 (39.6) | ||
| $20 000-$39 999 | 22 (21.0) | 241 (23.0) | 46 (19.6) | ||
| $40 000-$59 999 | 9 (8.6) | 134 (12.8) | 43 (18.3) | ||
| >$60 000 | 12 (11.4) | 107 (10.2) | 53 (22.6) | ||
| Marital status, n (%) | .017 | <.001 | |||
| Single/divorced/other | 72 (64.3) | 580 (52.4) | 76 (31.4) | ||
| Married/living with partner | 40 (35.7) | 526 (47.6) | 166 (68.6) | ||
| Health insurance, n (%) | .713 | .036 | |||
| Insured | 84 (75.0) | 811 (73.5) | 204 (84.3) | ||
| Uninsured | 28 (25.0) | 294 (26.6) | 38 (15.7) | ||
| Independent living, n (%) | .009 | <.001 | |||
| Living independently | 63 (56.3) | 755 (68.3) | 205 (84.7) | ||
| Live dependently | 49 (43.8) | 349 (31.6) | 37 (15.3) | ||
| Radiation treatment, n (%) | |||||
| Total body irradiation | 82 (73.1) | 0 (0) | NA | <.001 | NA |
| Cranial/spinal | 9 (8.0) | 278 (25.1) | NA | <.001 | NA |
| Chest | 2 (1.8) | 17 (1.5) | NA | .840 | NA |
| Pelvic/abdominal | 4 (3.6) | 35 (3.2) | NA | .816 | NA |
| Chemotherapy, n (%) | |||||
| Alkylators | 111 (99.1) | 743 (67.2) | NA | <.001 | NA |
| Anthracyclines | 87 (77.7) | 941 (85.1) | NA | .04 | NA |
| Antimetabolites | 111 (99.1) | 955 (86.4) | NA | <.001 | NA |
| Dexamethasone | 35 (31.3) | 234 (21.2) | NA | .014 | NA |
| Epipodophyllotoxin | 699 (63.2) | 80 (71.4) | NA | .084 | NA |
| High-dose methotrexate | 37 (33.0) | 654 (59.1) | NA | <.001 | NA |
| Prednisone | 46 (41.1) | 939 (84.9) | NA | <.001 | NA |
| Vincristine | 45 (40.2) | 973 (88.0) | NA | <.001 | NA |
| Type of HSCT, n (%) | |||||
| Allogeneic | 79 (70.1) | NA | NA | NA | NA |
| Autologous | 33 (29.5) | NA | NA | NA | NA |
| Chronic GVHD among allogeneic HSCT survivors, n (%) | |||||
| Yes* | 25 (31.6) | NA | NA | NA | NA |
| No | 54 (68.4) | NA | NA | NA | NA |
| Intensity of transplant experience, n (%)† | |||||
| Low | 33 (29.5) | NA | NA | NA | NA |
| Intermediate | 52 (46.4) | NA | NA | NA | NA |
| Severe | 27 (24.1) | NA | NA | NA | NA |
| Relapse, n (%) | <.001 | ||||
| Yes | 24 (21.4) | 505 (45.7) | NA | NA | |
| No | 88 (78.6) | 601 (54.3) | NA | NA | |
| Second tumor, n (%) | .004 | ||||
| Yes | 28 (25.0) | 141 (12.8) | NA | NA | |
| No | 84 (75.0) | 965 (87.3) | NA | NA |
NA, nonapplicable.
Among 25 survivors having chronic GVHD, 23 with past and 2 with active chronic GVHD at the time of study.
Intensity of transplant experience: low (autologous), intermediate (allogeneic without the occurrence of chronic GVHD), and severe (allogeneic with the occurrence of chronic GVHD).
Table 1 also reports that a majority of HSCT survivors (67.9%) were treated between 1990 and 1999, and near equal numbers of conventional therapy survivors (45.5%, 45.4%) were between 1980 and 1989 and between 1990 and 1999. The conventional therapy group had more survivors treated for acute lymphoblastic leukemia (58.7%) and lymphoma (37.3%), whereas the HSCT group had more survivors treated for acute myeloid leukemia (39.3%). Among 79 allogeneic HSCT survivors, 23 had a history of past chronic GVHD and 2 had active chronic GVHD at the time of evaluation. More HSCT survivors received alkylators, antimetabolites, dexamethasone, and total body irradiation (all P < .05), whereas more conventionally treated survivors received anthracyclines, high-dose methotrexate, prednisone, vincristine, and cranial/spinal radiation (all P < .05). For HSCT survivors, more participants had a shorter elapsed time since cancer diagnosis and history of second cancer compared with nonparticipants. For conventionally treated survivors, more participants were female, and had shorter elapsed time since diagnosis, history of second cancer and relapse, and received treatment with cranial radiation, but fewer received chest radiation, anthracyclines, antimetabolites, dexamethasone, and high-dose methotrexate compared with nonparticipants (all P < .05; supplemental Table 2).
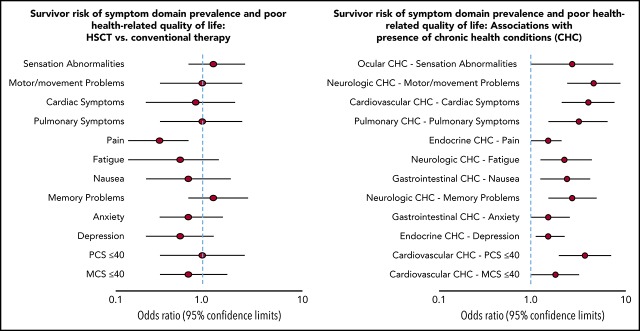
Table 2 shows that the most prevalent symptom domains in HSCT and conventional therapy survivors, respectively, were pain (64.3%, 74.1%), sensation abnormalities (39.3%, 33.0%), anxiety (29.5%, 34.9%), and memory problems (28.6%, 25.0%). Approximately 64% of HSCT and 62% of conventionally treated survivors experienced symptoms in multiple domains. However, the prevalence of all symptom domains and poor PCS/MCS between survivors of HSCT and conventional therapy were not significantly different (all P > .05). Compared with noncancer controls, HSCT survivors had an elevated risk for abnormalities related to sensation (odds ratio [OR]: 4.7; 95% confidence interval [CI], 2.6-8.4), motor/movement (OR: 4.3; 95% CI, 1.6-11.0), cardiac (OR: 2.5; 95% CI, 1.1-5.6) and pulmonary symptoms (OR: 4.6; 95% CI, 1.8-11.8), fatigue (OR: 3.1; 95% CI, 1.4-7.0), memory problems (OR: 4.8; 95% CI, 2.5-9.2), anxiety (OR: 2.1; 95% CI, 1.2-3.6), and poor PCS (OR: 6.9; 95% CI, 2.8-17.0). Among 112 HSCT survivors, significant associations of having past or active chronic GVHD with symptom prevalence and poor HRQOL were only found in the sensation domain (OR: 4.2; 95% CI, 1.5-12.1), especially salient by the indication of having the symptom of very dry eyes (OR: 4.9; 95% CI, 1.8-13.3).
Table 2.
Prevalence of symptom domains and poor HRQOL among overall HSCT survivors, conventional therapy survivors, and noncancer controls
| Symptoms and HRQOL | HSCT survivors | Conventional therapy survivors | Noncancer controls | HSCT survivors vs conventional therapy survivors | HSCT survivors vs noncancer controls |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | OR (95% CI)* | OR (95% CI)* | |
| Symptom prevalence | |||||
| Sensation abnormalities | 44 (39.3) | 365 (33.0) | 45 (18.6) | 1.5 (1.0-2.3) | 4.7 (2.6-8.4) |
| Motor/movement problems | 14 (12.5) | 149 (13.5) | 8 (3.3) | 1.0 (0.5-1.8) | 4.3 (1.6-11.0) |
| Cardiac symptoms | 15 (13.4) | 150 (13.6) | 16 (6.6) | 1.0 (0.6-1.8) | 2.5 (1.1-5.6) |
| Pulmonary symptoms | 15 (13.4) | 152 (13.7) | 9 (3.7) | 1.0 (0.6-1.8) | 4.6 (1.8-11.8) |
| Pain | 72 (64.3) | 819 (74.1) | 159 (65.7) | 0.7 (0.4-1.0) | 1.4 (0.8-2.3) |
| Fatigue | 15 (13.4) | 195 (17.6) | 18 (7.4) | 0.7 (0.4-1.4) | 3.1 (1.4-7.0) |
| Nausea | 15 (13.4) | 152 (13.7) | 27 (11.2) | 1.0 (0.6-1.8) | 1.3 (0.7-2.7) |
| Memory problems | 32 (28.6) | 276 (25.0) | 19 (7.9) | 1.2 (0.8-1.9) | 4.8 (2.5-9.2) |
| Anxiety | 33 (29.5) | 386 (34.9) | 44 (18.2) | 0.8 (0.5-1.3) | 2.1 (1.2-3.6) |
| Depression | 27 (24.1) | 341 (30.8) | 45 (18.6) | 0.8 (0.5-1.2) | 1.7 (0.9-2.9) |
| Multiple (≥2) symptoms | 65 (63.7) | 648 (61.9) | 88 (36.8) | 1.1 (0.7-1.7) | 3.0 (1.9-4.9) |
| Poor HRQOL | |||||
| PCS ≤40 | 19 (17.0) | 156 (14.1) | 13 (5.4) | 1.5 (0.9-2.5) | 6.9 (2.8-17.0) |
| MCS ≤40 | 20 (17.9) | 252 (22.8) | 35 (14.5) | 0.8 (0.5-1.3) | 1.5 (0.5-2.9) |
CI, confidence interval; MCS, mental component summary; OR, odds ratio; PCS, physical component summary.
Age/sex-adjusted ORs.
Table 3 shows that the risks of having cardiovascular, gastrointestinal, ocular, pulmonary, and reproductive CHCs among survivors treated with HSCT were significantly higher (all P < .05) than conventionally treated survivors and noncancer controls. Approximately, 47% of HSCT and 22% of conventional therapy survivors had multiple CHCs. Table 4 shows that the difference in prevalence of symptom domains and poor HRQOL between HSCT and conventionally treated survivors was not statistically significant (all P > .05) based on multivariable models. Longer time since cancer diagnosis was associated with a higher prevalence of some symptom domains (eg, sensation abnormalities, memory problems) and poor PCS for HSCT survivors vs conventionally treated survivors; however, the differences were not statistically significant (all P > .05) (supplemental Figure 2). In contrast, longer time since cancer diagnosis was associated with significantly higher prevalence of specific symptom items for survivors of HSCT vs conventional therapy, including double vision (OR: 2.8; P = .04), very dry eyes (OR: 3.4; P < .0001), and trouble seeing when wearing glasses (OR: 2.9; P < .0001) (supplemental Figure 3). Lower educational attainment and single/divorced marital status were significantly associated with elevated risks for motor/movement problems, pulmonary symptoms, memory problems, depression, and poor MCS (all P < .05).
Table 3.
Prevalence of chronic health conditions among overall HSCT survivors, conventional therapy survivors, and noncancer controls
| Chronic health conditions (CTCAE grades ≥3) | HSCT survivors | Conventional therapy survivors | Noncancer controls | HSCT survivors vs conventional therapy survivors | HSCT survivors vs noncancer controls |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | OR (95% CI)* | OR (95% CI)* | |
| Cardiovascular | 17 (15.2) | 89 (8.1) | 12 (5.0) | 2.3 (1.3-4.1) | 7.4 (2.8-19.3) |
| Endocrine | 29 (25.9) | 422 (38.2) | 91 (37.6) | 0.6 (0.4-0.9) | 0.8 (0.5-1.3) |
| Gastrointestinal | 20 (17.9) | 117 (10.6) | 23 (9.5) | 2.1 (1.2-3.6) | 3.6 (1.7-7.7) |
| Neurology | 9 (8.1) | 83 (7.5) | 15 (6.2) | 1.1 (0.6-2.3) | 1.6 (0.6-4.1) |
| Ocular | 22 (19.6) | 12 (1.1) | 2 (0.8) | 23.2 (11.0-48.7) | 47.2 (9.6-231.7) |
| Pulmonary | 17 (15.2) | 60 (5.4) | 17 (7.0) | 3.5 (1.9-6.3) | 2.8 (1.3-6.1) |
| Reproductive | 60 (53.6) | 203 (18.4) | 18 (7.4) | 5.6 (3.7-8.4) | 37.1 (16.0-86.0) |
| Multiple (≥2) conditions | 53 (47.3) | 241 (21.8) | 38 (15.7) | 3.2 (2.2-4.8) | 4.8 (2.9-8.0) |
Age/sex-adjusted ORs.
Table 4.
Multivariable logistic regression for risks of symptom domain prevalence and poor HRQOL between overall HSCT and conventional therapy survivors by accounting for chronic health conditions
| Risk factors | Sensationabnormalities | Motor/movementproblems | Cardiacsymptoms | Pulmonarysymptoms | Pain | Fatigue |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Treatment group | ||||||
| Overall HSCT vs conventional therapy survivors* | 1.2 (0.8-2.0) | 1.0 (0.5-1.9) | 0.9 (0.4-1.7) | 1.0 (0.5-1.9) | 0.5 (0.3-0.8) | 0.7 (0.3-1.3) |
| Age at survey | 1.1 (1.0-1.1) | 1.1 (1.0-1.1) | 1.1 (1.0-1.1) | 1.1 (1.0-1.1) | 1.0 (1.0- 1.1) | 1.1 (1.0-1.1) |
| Sex | ||||||
| Female vs male* | 1.3 (1.1-1.7) | 1.5 (1.0-2.1) | 2.4 (1.7-3.5) | 1.5 (1.0-2.0) | 1.7 (1.3-2.3) | 1.9 (1.4-2.5) |
| Race/ethnicity | ||||||
| White, non-Hispanic vs other* | 1.4 (1.0-2.0) | 1.0 (0.6-1.5) | 0.9 (0.6-1.4) | 0.8 (0.5-1.2) | 1.3 (0.9-1.8) | 1.1 (0.7-1.7) |
| Education | ||||||
| <College vs ≥college* | 1.3 (1.0-1.8) | 1.7 (1.2-2.6) | 1.5 (1.1-2.3) | 1.6 (1.1-2.3) | 1.8 (1.3-2.4) | 2.3 (1.6-3.3) |
| Marital status | ||||||
| Single/divorced/other vs married/living with partner* | 1.0 (0.8-1.4) | 1.6 (1.1-2.3) | 1.0 (0.7-1.4) | 1.7 (1.2-2.4) | 0.8 (0.6-1.1) | 0.9 (0.7-1.3) |
| Cardiovascular† | 1.3 (0.8-1.9) | 1.2 (0.7-2.1) | 3.0 (1.8-4.9) | 1.2 (0.7-2.1) | 1.5 (0.9-2.7) | 1.2 (0.7-2.0) |
| Endocrine† | 1.4 (1.1-1.8) | 1.9 (1.4-2.7) | 1.0 (0.7-1.4) | 1.2 (0.9-1.8) | 1.4 (1.0-1.8) | 1.5 (1.1-2.1) |
| Gastrointestinal† | 1.3 (0.9-1.9) | 1.4 (0.9-2.3) | 1.2 (0.7-2.0) | 1.5 (0.9-2.4) | 1.3 (0.8-2.1) | 1.2 (0.7-1.9) |
| Neurology† | 1.9 (1.2-3.1) | 3.3 (2.0-5.5) | 1.4 (0.8-2.5) | 1.9 (1.1-3.2) | 1.3 (0.8-2.4) | 1.9 (1.2-3.2) |
| Ocular† | 2.2 (1.0-4.8) | 2.7 (1.1-6.9) | 1.0 (0.3-2.9) | 0.5 (0.1-1.7) | 2.3 (0.9-6.0) | 2.3 (0.9-5.6) |
| Pulmonary† | 1.0 (0.6-1.7) | 0.9 (0.4-1.7) | 3.0 (1.7-5.3) | 2.5 (1.4-4.3) | 1.8 (0.9-3.6) | 1.0 (0.5-1.8) |
| Reproductive† | 1.3 (1.0-1.8) | 0.8 (0.5-1.2) | 0.9 (0.6-1.4) | 1.0 (0.6-1.5) | 1.4 (1.0-2.0) | 1.4 (1.0-2.1) |
| Risk factors | Nausea | Memory problems | Anxiety | Depression | PCS (score ≤40) | MCS (score ≤40) |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Treatment group | ||||||
| Overall HSCT vs conventional therapy survivors* | 0.8 (0.4-1.6) | 1.2 (0.8-2.1) | 0.8 (0.5-1.4) | 0.7 (0.4-1.2) | 1.0 (0.5-2.0) | 0.8 (0.5-1.5) |
| Age at survey | 1.0 (1.0-1.0) | 1.0 (0.9-1.0) | 1.0 (1.0-1.1) | 1.1 (1.0-1.1) | 1.1 (1.1-1.1) | 1.1 (1.0-1.1) |
| Sex | ||||||
| Female vs male* | 1.9 (1.4-2.7) | 1.5 (1.2-2.0) | 1.3 (1.1-1.7) | 1.3 (1.0-1.6) | 1.5 (1.0-2.1) | 1.5 (1.1-2.0) |
| Race/ethnicity | ||||||
| White, non-Hispanic vs other* | 1.1 (0.7-1.7) | 1.8 (1.2-2.6) | 1.1 (0.8-1.6) | 0.9 (0.7-1.3) | 1.0 (0.6-1.5) | 1.1 (0.7-1.5) |
| Education | ||||||
| <College vs ≥college* | 1.4 (1.0-2.0) | 2.1 (1.5-2.8) | 1.3 (1.0-1.7) | 1.5 (1.1-2.0) | 2.6 (1.7-4.0) | 2.1 (1.5-3.0) |
| Marital status | ||||||
| Single/divorced/other vs married/living with partner* | 0.9 (0.6-1.3) | 1.5 (1.1-2.0) | 1.3 (1.0-1.7) | 2.0 (1.5-2.6) | 1.4 (0.9-2.0) | 1.6 (1.2-2.2) |
| Cardiovascular† | 1.2 (0.7-2.0) | 0.9 (0.6-1.5) | 1.1 (0.7-1.7) | 1.6 (1.0-2.4) | 2.8 (1.7-4.6) | 1.6 (1.0-2.5) |
| Endocrine† | 1.6 (1.1-2.3) | 1.2 (0.9-1.6) | 1.1 (0.9-1.4) | 1.4 (1.1-1.9) | 1.3 (0.9-1.9) | 1.4 (1.0-1.8) |
| Gastrointestinal† | 2.0 (1.2-3.1) | 1.1 (0.7-1.7) | 1.4 (1.0-2.1) | 1.3 (0.9-1.9) | 1.3 (0.8-2.1) | 0.9 (0.6-1.4) |
| Neurology† | 1.2 (0.6-2.2) | 2.2 (1.4-3.5) | 1.0 (0.6-1.6) | 1.3 (0.8-2.1) | 2.6 (1.6-4.4) | 1.6 (1.0-2.6) |
| Ocular† | 1.6 (0.6-4.2) | 0.7 (0.3-1.7) | 0.7 (0.3-1.6) | 0.6 (0.2-1.5) | 2.1 (0.8-5.4) | 0.9 (0.3-2.2) |
| Pulmonary† | 1.5 (0.8-2.7) | 2.0 (1.2-3.3) | 1.0 (0.6-1.7) | 1.1 (0.6-1.9) | 2.7 (1.6-4.8) | 1.5 (0.9-2.6) |
| Reproductive† | 1.5 (1.0-2.2) | 1.1 (0.8-1.6) | 1.1 (0.8-1.5) | 1.2 (0.9-1.7) | 1.3 (0.9-1.9) | 0.9 (0.6-1.3) |
Reference group.
CTCAE grades 3-4 vs none or 1-2.
The prevalence of abnormalities in symptom domains and HRQOL were significantly associated with the occurrence of specific CHCs (Table 4). Cardiovascular CHCs were associated with increased risks for cardiac symptoms (OR: 3.0; 95% CI, 1.8-4.9) and poor PCS (OR: 2.8; 95% CI, 1.7-4.6). Pulmonary CHCs were associated with a higher risk for pulmonary symptoms (OR: 2.5; 95% CI, 1.4-4.3), cardiac symptoms (OR: 3.0; 95% CI, 1.7-5.3), and poor PCS (OR: 2.7; 95% CI, 1.6-4.8). Neurological CHCs were associated with a higher risk for motor/movement problems (OR: 3.3; 95% CI, 2.0-5.5), memory problems (OR: 2.2; 95% CI, 1.4-3.5), and poor PCS (OR: 2.6; 95% CI, 1.6-4.4). After adding specific cancer therapy (chemotherapy agents, total body irradiation, and other radiation exposures) as covariates to the parsimonious models focusing on HSCT status (ie, receipt of HSCT vs conventional therapy) and CHCs, the occurrence of organ-specific CHCs was still significantly associated with poor patient-reported outcomes with similar magnitudes (all P < .05), whereas HSCT status and cancer therapy were not (all P > .05; supplemental Table 3).
Table 5 shows that HSCT recipients, compared with noncancer controls, were at elevated risks for abnormalities related to sensation (OR: 3.2; 95% CI, 1.5-6.8), motor/movement (OR: 4.3; 95% CI, 1.2-15.4), memory (OR: 3.7; 95% CI, 1.5-8.9) symptoms, and poor PCS (OR: 5.4; 95% CI, 1.6-18.6). Cardiovascular CHCs were associated with increased risks for cardiac symptoms (OR: 4.9; 95% CI, 1.6-15.1) and fatigue (OR: 3.4; 95% CI, 1.2-9.4). Pulmonary CHCs were associated with a higher risk for pulmonary (OR: 5.3; 95% CI, 1.8-15.6) and cardiac symptoms (OR: 3.6; 95% CI, 1.3-9.6).
Table 5.
Multivariable logistic regression for risks of symptom domain prevalence and poor HRQOL between overall HSCT survivors and noncancer controls by accounting for chronic health conditions
| Risk factors | Sensationabnormalities | Motor/movementproblems | Cardiacsymptoms | Pulmonarysymptoms | Pain | Fatigue |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Treatment group | ||||||
| Overall HSCT survivors vs noncancer controls* | 3.2 (1.5-6.8) | 4.3 (1.2,15.4) | 2.0 (0.7-5.7) | 2.3 (0.6-8.0) | 0.7 (0.3-1.4) | 1.5 (0.5-4.7) |
| Age at survey | 1.1 (1.0-1.1) | 1.0 (1.0-1.1) | 1.0 (1.0-1.1) | 1.0 (1.0-1.1) | 1.1 (1.0-1.1) | 1.1 (1.0-1.1) |
| Sex | ||||||
| Female vs male* | 1.2 (0.7-2.1) | 0.6 (0.2-1.5) | 1.0 (0.4-2.2) | 0.6 (0.2-1.6) | 0.9 (0.6-1.5) | 1.5 (0.7-3.3) |
| Race/ethnicity | ||||||
| White, non-Hispanic vs other* | 1.2 (0.6-2.4) | 0.7 (0.2-2.3) | 0.8 (0.3-2.2) | 0.6 (0.2-1.5) | 1.5 (0.8-2.8) | 0.7 (0.3-1.8) |
| Education | ||||||
| <College vs ≥college* | 1.1 (0.6-1.9) | 2.2 (0.8-6.4) | 0.7 (0.3-1.7) | 1.0 (0.4-2.5) | 1.0 (0.6-1.6) | 1.4 (0.6-3.1) |
| Marital status | ||||||
| Single/divorced/other vs married/living with partner* | 1.3 (0.7-2.4) | 2.8 (0.9-8.7) | 2.3 (0.9-5.8) | 3.0 (1.0-8.7) | 0.8 (0.5-1.5) | 1.5 (0.6-3.6) |
| Cardiovascular† | 1.5 (0.6-3.6) | 1.8 (0.5-7.1) | 4.9 (1.6,15.1) | 0.9 (0.2-3.6) | 2.4 (0.7-8.0) | 3.4 (1.2-9.4) |
| Endocrine† | 1.5 (0.8-2.7) | 2.7 (1.0-7.3) | 0.5 (0.2-1.4) | 1.5 (0.6-4.1) | 1.2 (0.7-2.1) | 0.8 (0.3-2.0) |
| Gastrointestinal† | 2.0 (0.9-4.2) | 0.8 (0.2-3.1) | 3.4 (1.3-9.0) | 2.2 (0.7-6.5) | 0.9 (0.4-2.0) | 1.8 (0.7-4.9) |
| Neurology† | 1.6 (0.6-4.3) | NA | 0.3 (0.0-2.9) | 0.4 (0.1-3.7) | 3.9 (1.1-14.4) | 0.7 (0.2-3.5) |
| Ocular† | 2.2 (0.8-6.2) | 1.9 (0.5-8.0) | 1.2 (0.3-4.8) | 1.0 (0.2-4.5) | 4.5 (1.1-17.7) | 1.1 (0.3-4.5) |
| Pulmonary† | 1.4 (0.6-3.3) | 0.4 (0.1-2.3) | 3.6 (1.3-9.6) | 5.3 (1.8,15.6) | 1.5 (0.6-3.7) | 2.1 (0.8-5.9) |
| Reproductive† | 1.1 (0.6-2.3) | 0.8 (0.2-2.5) | 0.3 (0.1-1.0) | 1.7 (0.6-5.1) | 2.5 (1.1-5.6) | 1.5 (0.6-4.0) |
| Risk factors | Nausea | Memory problems | Anxiety | Depression | PCS (score ≤40) | MCS (score ≤40) |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Treatment group | ||||||
| Overall HSCT survivors vs noncancer controls* | 1.0 (0.4-2.6) | 3.7 (1.5-8.9) | 1.9 (0.9-3.9) | 1.5 (0.7-3.3) | 5.4 (1.6-18.6) | 1.1 (0.5-2.6) |
| Age at survey | 1.0 (0.9-1.0) | 1.0 (1.0-1.1) | 1.0 (1.0-1.0) | 1.0 (1.0-1.1) | 1.1 (1.0-1.2) | 1.0 (1.0-1.1) |
| Sex | ||||||
| Female vs male* | 1.6 (0.8-3.3) | 0.9 (0.4-1.7) | 1.3 (0.8-2.3) | 0.9 (0.5-1.6) | 1.3 (0.5-3.1) | 1.4 (0.8-2.7) |
| Race/ethnicity | ||||||
| White, non-Hispanic vs other* | 1.0 (0.4-2.4) | 0.9 (0.4-2.2) | 0.9 (0.5-1.7) | 0.7 (0.4-1.4) | 2.5 (0.6-9.6) | 1.5 (0.6-3.4) |
| Education | ||||||
| <College vs ≥college* | 1.4 (0.7-3.0) | 2.5 (1.1-5.3) | 1.5 (0.9-2.7) | 1.5 (0.8-2.7) | 2.2 (0.9-5.5) | 1.8 (1.0-3.5) |
| Marital status | ||||||
| Single/divorced/other vs married/living with partner* | 1.2 (0.5-2.5) | 3.7 (1.6-8.4) | 1.2 (0.6-2.3) | 2.2 (1.1-4.2) | 2.5 (0.9-6.5) | 1.6 (0.8-3.2) |
| Cardiovascular† | 1.2 (0.4-3.9) | 1.0 (0.3-3.0) | 0.8 (0.3-2.1) | 0.8 (0.3-2.2) | 2.8 (0.9-8.1) | 0.9 (0.3-3.0) |
| Endocrine† | 1.2 (0.6-2.4) | 0.6 (0.2-1.3) | 1.1 (0.6-1.9) | 2.2 (1.2-4.0) | 3.2 (1.3-8.0) | 1.4 (0.7-2.7) |
| Gastrointestinal† | 3.2 (1.4-7.5) | 3.5 (1.5-8.3) | 2.8 (1.3-5.8) | 1.2 (0.5-2.8) | 2.5 (0.9-6.5) | 1.4 (0.6-3.4) |
| Neurology† | 0.6 (0.1-2.8) | 2.4 (0.8-7.6) | 1.4 (0.5-3.8) | 1.0 (0.3-2.8) | 3.3 (1.0-11.2) | 1.6 (0.6-4.5) |
| Ocular† | 1.0 (0.2-3.9) | 0.5 (0.2-1.8) | 0.7 (0.2-2.0) | 0.5 (0.1-1.8) | 1.6 (0.4-6.1) | 0.4 (0.1-1.9) |
| Pulmonary† | 1.1 (0.4-3.3) | 1.9 (0.7-4.8) | 2.0 (0.9-4.5) | 2.3 (1.0-5.5) | 1.3 (0.4-4.1) | 1.8 (0.7-4.7) |
| Reproductive† | 1.2 (0.5-3.2) | 1.5 (0.6-3.5) | 1.1 (0.5-2.2) | 1.2 (0.6-2.7) | 0.9 (0.3-2.3) | 1.8 (0.8-4.2) |
Reference group.
CTCAE grades 3-4 vs none or 1-2.
Supplemental Tables 4 and 5 show that the type of HSCT received (allogeneic vs autologous) and most of the transplant-specific factors were not significantly associated with symptoms prevalence or poor HRQOL (all P > .05). Relapse/progression of hematologic malignancy was the only transplant factor significantly associated with a higher risk of more motor/movement problems (OR: 17.5; 95% CI, 2.2-138.2).
Discussion
In this large clinically assessed pediatric cancer survivor cohort, pain, sensation abnormalities, anxiety, and memory problems were the most prevalent symptom domains (>25%) endorsed by survivors treated with HSCT. However, the prevalence of impaired symptom domains and poor HRQOL in survivors treated with HSCT was similar to those treated with conventional therapy, but significantly higher than noncancer controls. The occurrence of organ-specific CHCs, rather than HSCT or transplant-related variables, were significant predictors of organ-related symptoms and poor HRQOL (eg, cardiovascular CHCs for cardiac symptoms; neurological CHCs for motor/movement symptoms). Additionally, socioeconomic vulnerability (eg, lower educational attainment, single/divorced marital status) explained variation in patient-reported outcomes.
As anticipated, HSCT survivors exhibited an excess prevalence of symptoms and impairment in HRQOL compared with noncancer controls, but surprisingly there was no difference in these outcomes between HSCT and conventionally treated survivors. This finding could be explained by several factors. First, the lack of difference is likely related to the relatively low prevalence of chronic GVHD experience (N = 25; 22.3%) in our HSCT group, which reflects a conservative approach in donor selection during the early years of our transplant program. Additionally, chronic GVHD in 23 HSCT survivors had been resolved at the time of assessment. A previous study found that although active chronic GVHD was significantly associated with severe adverse events in adult survivors of childhood cancer, health status in survivors with resolved chronic GVHD was equivalent to those who had never been diagnosed with GVHD.8 Second, patients surviving life-threatening conditions may adapt to disadvantaged circumstances, a phenomenon known as response shift.32,33 Evidence suggests that childhood HSCT survivors might change their conceptualizations and thresholds for the presence or severity of patient-reported outcomes over time.34,35 Third, HSCT recipients have lower survival rates36 or may have been too ill to enroll in this study because of transplant-related complications or higher prevalence of severe/disabling health status as compared with conventionally treated survivors.7,8,37 Therefore, the true prevalence of patient-reported outcome impairment in HSCT survivors might be underestimated.
We found that having a history of past or active chronic GVHD was significantly associated with higher prevalence of sensation symptom domain (especially the symptom of very dry eyes), and receiving HSCT was significantly associated with higher cumulative prevalence of ocular symptoms (double vision, very dry eyes, and trouble seeing when wearing glasses), which were part of sensation domain. The underlying clinical causes are likely multifactorial. Ocular manifestations appear in 60% to 90% of patients with GVHD.38 Additionally, specific HSCT complications, including keratoconjunctivitis sicca, pseudomembranous conjunctivitis, corneal ulceration, and microvascular retinopathy, may contribute to the development of ocular symptoms.38
Virtually all adult survivors of childhood cancers develop at least 1 CHC, and the risk increases with the duration of time since therapy completion.27,39 In comparison with conventionally treated survivors, those treated with HSCT experience elevated risks for infections,9 dyslipidemia, lung disease, cataracts, osteonecrosis, and secondary malignancies.7 By accounting for the influence of CHCs, we found that prevalence of symptom domains was highly associated with CHCs from the same organ system (eg, cardiac), and the magnitude for associations of both symptom prevalence and poor HRQOL with HSCT status (eg, HSCT vs conventional therapy or noncancer) decreased. Specifically, excessive prevalence of pulmonary symptoms, anxiety, and fatigue was explained by the occurrence of specific CHCs rather than transplant per se, which suggests that the development of various CHCs following transplant underlies the pathway of poor patient-reported outcomes. Because symptom presence is a manifestation of CHCs, focusing on symptoms as early indicators of adverse health conditions is clinically relevant. Interestingly, undergoing a HSCT, rather than the occurrence of CHCs, was significantly associated with the prevalence of neuropsychological/cognitive symptoms (ie, sensation abnormalities, motor/movement problems, and memory problems). This finding indicates the failure to appreciate the importance of integrating patient-reported outcomes into evaluating morbidities during survivorship care.28
Although childhood cancer survivors develop multiple symptoms,12 the mechanisms behind cooccurring multiple symptoms in childhood cancer survivors remain understudied. Underlying biophysiological mechanisms (eg, endothelial dysfunction or systemic inflammation related to treatment exposures40-42) may contribute to the concurrent symptoms. Experience with chronic GVHD, a systemic inflammatory response by donors’ immune systems, resulting in multisystem organ damage in the host, may also lead to cooccurring multiple symptoms in survivors treated with HSCT and further affect HRQOL.10,21,43,44 Pain syndromes in cancer survivors related to the exposure to neurotoxic chemotherapeutic agents or ionizing radiation have been reported several years after completion of therapy.45 Both chronic pain and chronic GVHD have been associated with depressive symptoms in adult cancer survivors treated with HSCT.10,46 Although we identified disadvantaged sociodemographic status (eg, less than college education) as risk factors of symptom prevalence and poor HRQOL, other psychosocial factors not available in this study (eg, coping behaviors, cognitive appraisal, family support) may have influenced the development of psychological symptoms in cancer survivors.18,47
The goal of cancer survivorship care is not merely to identify and manage medical complications, but also to improve daily functional status and HRQOL. The findings of high symptom prevalence in several domains and poor HRQOL for adult survivors of childhood hematologic malignancies highlight the usefulness of implementing comprehensive symptom screening regularly to identify potential adverse health events and facilitate timely referral for early interventions, especially for HSCT survivors who have a substantial burden of CHCs. Strategies to improve HSCT survivorship care by developing a standardized symptom screening tool, using relevant symptom domains for evaluation, and determining a meaningful screening period are warranted.
Several limitations should be noted. First, our sample was recruited from adult survivors of childhood hematologic malignancies who were treated at a single institution. Our results may not be generalizable to other survivor populations. Second, we included a relatively small number of survivors treated with HSCT in the analyses. Although our study population represents 1 of the largest groups of long-term HSCT survivors with systematically clinically assessed outcomes, the overall size of the study sample does limit statistical power. Future clinical investigation of HSCT survivors will be important to validate and expand upon our findings. Third, the inclusion of survivors with heterogeneous cancer diagnoses may confound the comparison of patient-reported outcomes in HSCT and conventional therapy survivors. However, the adjustment of comprehensive treatment variables in our analyses addresses this concern. Fourth, because of the nature of cross-sectional study design, the causal relationship between symptom prevalence and CHC occurrence is unknown. Future longitudinal research is warranted to answer this question. Last, our symptom measures merely capture the attribute of symptom presence rather than frequency or severity. Future studies are needed to replicate our design by using comprehensive tools (eg, the Patient-Reported Outcomes version of the CTCAE48 with additional HSCT-specific items) to improve the accuracy and clinical relevance for symptom assessment.
In conclusion, poor patient-reported outcomes including symptom prevalence and poor HRQOL are concerning issues in survivors of childhood hematologic malignancies, whether treated with conventional therapy or HSCT. Symptom prevalence and poor HRQOL were more closely related to the occurrence of CHCs than the mode of therapy, at least among the heterogeneous group of survivors featured in this report. Routine screening of symptom phenotypes may help identify adverse health events in adult survivors of childhood hematologic malignancies, especially for survivors treated with HSCT who have a substantial burden of CHCs.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health, National Cancer Institute (U01CA195547, R01CA238368, and P30CA021765-33). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.-J.Y. and I.-C.H. undertook concept and design; L.L.R. and M.M.H. provided study materials; M.J.E., N.B., K.K.N., K.R.K., L.L.R., M.M.H., and I.-C.H. collected and assembled data; H.M.E., N.S.B., S.H., M.J.E., N.B., D.K.S., L.L.R., M.M.H., and I.-C.H. undertook data analysis and clinical interpretation; H.-J.Y. and I.-C.H. wrote the manuscript; and all authors edited the article and provided final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: I-Chan Huang, Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, MS-735, 262 Danny Thomas Pl, Memphis, TN 38105; e-mail: i-chan.huang@stjude.org.
REFERENCES
- 1.Flower A, Cairo MS. The evolution of allogeneic stem cell transplant for children and adolescents with acute myeloid leukemia. Clin Adv Hematol Oncol. 2017;15(1):52-62. [PubMed] [Google Scholar]
- 2.Sundberg KK, Wettergren L, Frisk P, Arvidson J. Self-reported quality of life in long-term survivors of childhood lymphoblastic malignancy treated with hematopoietic stem cell transplantation versus conventional therapy. Pediatr Blood Cancer. 2013;60(8):1382-1387. [DOI] [PubMed] [Google Scholar]
- 3.Faraci M, Békássy AN, De Fazio V, Tichelli A, Dini G; EBMT Paediatric and Late Effects Working Parties . Non-endocrine late complications in children after allogeneic haematopoietic SCT. Bone Marrow Transplant. 2008;41(suppl 2):S49-S57. [DOI] [PubMed] [Google Scholar]
- 4.Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013;50(3):185-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsons SK, Phipps S, Sung L, Baker KS, Pulsipher MA, Ness KK. NCI, NHLBI/PBMTC First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: health-related quality of life, functional, and neurocognitive outcomes. Biol Blood Marrow Transplant. 2012;18(2):162-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung W, Ahn H, Rose SR, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore). 2007;86(4):215-224. [DOI] [PubMed] [Google Scholar]
- 7.Eissa HM, Lu L, Baassiri M, et al. Chronic disease burden and frailty in survivors of childhood HSCT: a report from the St. Jude Lifetime Cohort Study. Blood Adv. 2017;1(24):2243-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood. 2011;118(5):1413-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow EJ, Cushing-Haugen KL, Cheng GS, et al. Morbidity and mortality differences between hematopoietic cell transplantation survivors and other cancer survivors. J Clin Oncol. 2017;35(3):306-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bevans M, El-Jawahri A, Tierney DK, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: the Patient-Centered Outcomes Working Group Report. Biol Blood Marrow Transplant. 2017;23(4):538-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang IC, Hudson MM, Robison LL, Krull KR. Differential impact of symptom prevalence and chronic conditions on quality of life in cancer survivors and non-cancer individuals: a population study. Cancer Epidemiol Biomarkers Prev. 2017;26(7):1124-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang IC, Brinkman TM, Kenzik K, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort study. J Clin Oncol. 2013;31(33):4242-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkman TM, Zhu L, Zeltzer LK, et al. Longitudinal patterns of psychological distress in adult survivors of childhood cancer. Br J Cancer. 2013;109(5):1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirchhoff AC, Krull KR, Ness KK, et al. Physical, mental, and neurocognitive status and employment outcomes in the childhood cancer survivor study cohort. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1838-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2396-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prasad PK, Hardy KK, Zhang N, et al. Psychosocial and neurocognitive outcomes in adult survivors of adolescent and early young adult cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2015;33(23):2545-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wingard JR, Huang IC, Sobocinski KA, et al. Factors associated with self-reported physical and mental health after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16(12):1682-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenzik K, Huang IC, Rizzo JD, Shenkman E, Wingard J. Relationships among symptoms, psychosocial factors, and health-related quality of life in hematopoietic stem cell transplant survivors. Support Care Cancer. 2015;23(3):797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel G, Bordigoni P, Simeoni MC, et al. Health status and quality of life in long-term survivors of childhood leukaemia: the impact of haematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40(9):897-904. [DOI] [PubMed] [Google Scholar]
- 20.Clarke SA, Skinner R, Guest J, et al. Clinical outcomes and health-related quality of life (HRQOL) following haemopoietic stem cell transplantation (HSCT) for paediatric leukaemia. Child Care Health Dev. 2011;37(4):571-580. [DOI] [PubMed] [Google Scholar]
- 21.Berbis J, Michel G, Chastagner P, et al. A French cohort of childhood leukemia survivors: impact of hematopoietic stem cell transplantation on health status and quality of life. Biol Blood Marrow Transplant. 2013;19(7):1065-1072. [DOI] [PubMed] [Google Scholar]
- 22.Schultz KA, Chen L, Chen Z, et al. Health conditions and quality of life in survivors of childhood acute myeloid leukemia comparing post remission chemotherapy to BMT: a report from the children’s oncology group. Pediatr Blood Cancer. 2014;61(4):729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanders JE, Hoffmeister PA, Storer BE, Appelbaum FR, Storb RF, Syrjala KL. The quality of life of adult survivors of childhood hematopoietic cell transplant. Bone Marrow Transplant. 2010;45(4):746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Löf CM, Winiarski J, Giesecke A, Ljungman P, Forinder U. Health-related quality of life in adult survivors after paediatric allo-SCT. Bone Marrow Transplant. 2009;43(6):461-468. [DOI] [PubMed] [Google Scholar]
- 25.Loiselle KA, Rausch JR, Bidwell S, Drake S, Davies SM, Pai AL. Predictors of health-related quality of life over time among pediatric hematopoietic stem cell transplant recipients. Pediatr Blood Cancer. 2016;63(10):1834-1839. [DOI] [PubMed] [Google Scholar]
- 26.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116(17):3129-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oeffinger KC, Mertens AC, Sklar CA, et al. ; Childhood Cancer Survivor Study . Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572-1582. [DOI] [PubMed] [Google Scholar]
- 28.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017;26(5):666-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landier W, Wallace WH, Hudson MM. Long-term follow-up of pediatric cancer survivors: education, surveillance, and screening. Pediatr Blood Cancer. 2006;46(2):149-158. [DOI] [PubMed] [Google Scholar]
- 31.Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, version 5.0. http://www.survivorshipguidelines.org/. Accessed October 2018.
- 32.Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. 1999;48(11):1507-1515. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz CE, Sprangers MA. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med. 1999;48(11):1531-1548. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz CE, Feinberg RG, Jilinskaia E, Applegate JC. An evaluation of a psychosocial intervention for survivors of childhood cancer: paradoxical effects of response shift over time. Psychooncology. 1999;8(4):344-354. [DOI] [PubMed] [Google Scholar]
- 35.Brinksma A, Tissing WJ, Sulkers E, Kamps WA, Roodbol PF, Sanderman R. Exploring the response shift phenomenon in childhood patients with cancer and its effect on health-related quality of life. Oncol Nurs Forum. 2014;41(1):48-56. [DOI] [PubMed] [Google Scholar]
- 36.Holmqvist AS, Chen Y, Wu J, et al. Assessment of late mortality risk after allogeneic blood or marrow transplantation performed in childhood. JAMA Oncol. 2018;4(12):e182453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson NG, Regillo C. Ocular manifestations of graft versus host disease. Curr Opin Ophthalmol. 2004;15(6):503-507. [DOI] [PubMed] [Google Scholar]
- 39.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynch Kelly D, Dickinson K, Hsiao CP, et al. Biological basis for the clustering of symptoms. Semin Oncol Nurs. 2016;32(4):351-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cleeland CS, Bennett GJ, Dantzer R, et al. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? A cytokine-immunologic model of cancer symptoms. Cancer. 2003;97(11):2919-2925. [DOI] [PubMed] [Google Scholar]
- 42.Lee BN, Dantzer R, Langley KE, et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11(5):279-292. [DOI] [PubMed] [Google Scholar]
- 43.Forinder U, Löf C, Winiarski J. Quality of life following allogeneic stem cell transplantation, comparing parents’ and children’s perspective. Pediatr Transplant. 2006;10(4):491-496. [DOI] [PubMed] [Google Scholar]
- 44.Reinfjell T, Tremolada M, Zeltzer LK. A review of demographic, medical, and treatment variables associated with health-related quality of life (HRQOL) in survivors of hematopoietic stem cell (HSCT) and bone marrow transplantation (BMT) during childhood. Front Psychol. 2017;8:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown MR, Ramirez JD, Farquhar-Smith P. Pain in cancer survivors. Br J Pain. 2014;8(4):139-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jim HS, Sutton SK, Jacobsen PB, Martin PJ, Flowers ME, Lee SJ. Risk factors for depression and fatigue among survivors of hematopoietic cell transplantation. Cancer. 2016;122(8):1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beeken RJ, Eiser C, Dalley C. Health-related quality of life in haematopoietic stem cell transplant survivors: a qualitative study on the role of psychosocial variables and response shifts. Qual Life Res. 2011;20(2):153-160. [DOI] [PubMed] [Google Scholar]
- 48.Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.