Key Points
Pathology findings of renal biopsy in LCCN predict renal outcomes independently of the hematologic response.
In patients with MM and biopsy-proven LCCN, quality of renal response correlates with overall survival.
Abstract
Light chain cast nephropathy (LCCN) in multiple myeloma often leads to severe and poorly reversible acute kidney injury. Severe renal impairment influences the allocation of chemotherapy and its tolerability; it also affects patient survival. Whether renal biopsy findings add to the clinical assessment in predicting renal and patient outcomes in LCCN is uncertain. We retrospectively reviewed clinical presentation, chemotherapy regimens, hematologic response, and renal and patient outcomes in 178 patients with biopsy-proven LCCN from 10 centers in Europe and North America. A detailed pathology review, including assessment of the extent of cast formation, was performed to study correlations with initial presentation and outcomes. Patients presented with a mean estimated glomerular filtration rate (eGFR) of 13 ± 11 mL/min/1.73 m2, and 82% had stage 3 acute kidney injury. The mean number of casts was 3.2/mm2 in the cortex. Tubulointerstitial lesions were frequent: acute tubular injury (94%), tubulitis (82%), tubular rupture (62%), giant cell reaction (60%), and cortical and medullary inflammation (95% and 75%, respectively). Medullary inflammation, giant cell reaction, and the extent of cast formation correlated with eGFR value at LCCN diagnosis. During a median follow-up of 22 months, mean eGFR increased to 43 ± 30 mL/min/1.73 m2. Age, β2-microglobulin, best hematologic response, number of cortical casts per square millimeter, and degree of interstitial fibrosis/tubular atrophy (IFTA) were independently associated with a higher eGFR during follow-up. This eGFR value correlated with overall survival, independently of the hematologic response. This study shows that extent of cast formation and IFTA in LCCN predicts the quality of renal response, which, in turn, is associated with overall survival.
Visual Abstract
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1917.
Disclosures
Associate Editor Hervé Avet-Loiseau served as a speaker or a member of a speakers bureau for Amgen Inc., Celgene Corporation/Bristol-Myers Squibb, Janssen Pharmaceuticals, Inc., Sanofi, and Takeda Pharmaceuticals North America, Inc. and received grants for clinical research from Amgen Inc., Celgene Corporation/Bristol-Myers Squibb, Sanofi, and Takeda Pharmaceuticals North America, Inc. CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC and the authors declare no relevant financial relationships.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe clinical and pathological features of light chain cast nephropathy (LCCN) in patients with multiple myeloma (MM), according to a retrospective review
Determine the predictive value for outcomes of clinical and pathological features of LCCN in patients with MM, according to a retrospective review
Identify clinical implications of the predictive value regarding outcomes of clinical and pathological features of LCCN in patients with MM, according to a retrospective review
Release date: May 21, 2020; Expiration date: May 21, 2021
Introduction
Acute kidney injury (AKI) is a common complication in symptomatic multiple myeloma (MM),1-4 and light chain cast nephropathy (LCCN) is the most frequent cause.5-7 LCCN is a myeloma-defining event8,9 that results from the precipitation of monoclonal free light chains (FLCs) with Tamm-Horsfall protein (THP) in distal tubules leading to tubular obstruction, interstitial inflammation, and, eventually, irreversible interstitial fibrosis and tubular atrophy (IFTA).10 Histologically, LCCN is characterized by intratubular proteinaceous casts by light microscopy, typically showing light chain (LC) restriction by immunofluorescence.
Controversy exists over the benefits of renal biopsy in patients with renal impairment and MM. The International Myeloma Working Group (IMWG) has suggested that this procedure is not necessary when the diagnosis of LCCN is likely (ie, in patients presenting with high serum FLC levels and predominant LC proteinuria). However, histologic confirmation may be helpful for patients with unrelated confounding conditions, such as diabetes, or when significant albuminuria exists.11 In addition, a study of MM with LCCN found a significant proportion of patients with coexisting nephropathies, including light chain deposition disease (LCDD) and light chain (AL) amyloidosis.6 The authors also found that the number of casts and the severity of tubular atrophy were associated with renal outcomes in the subgroup of hematologic responders.
The introduction of novel agents has increased survival in MM, even among patients with severe renal impairment.12-14 However, poor renal function may lower drug tolerance and limit treatment options, particularly high-dose melphalan followed by autologous stem cell transplantation, considered standard therapy in young patients.15,16 Renal response depends on the achievement of rapid and deep hematologic response17 and is associated with improved overall survival (OS), although not all series have confirmed this effect.3,4,18,19 Because very few studies provided renal pathology data, whether renal histology may predict renal outcome remains unknown. We conducted a multicenter retrospective cohort study of 178 patients with MM and biopsy-proven LCCN to assess reproducibility of histologic findings and evaluate their correlation with clinical presentation and renal outcomes.
Methods
Patient selection
Patients were selected from renal biopsy databases from 10 centers after approbation from local institutional review boards. The databases were queried for histologic diagnoses of LCCN between February 2000 and January 2018. Inclusion criteria were: (1) established diagnosis of MM according to the 2014 IMWG criteria9; (2) at least one cast with typical appearance according to light microscopy; (3) at least one cast staining for anti-κ or anti-λ by immunofluorescence or immunohistochemistry; and (4) sufficient follow-up to assess hematologic response. Research was conducted in accordance with the Declaration of Helsinki.
Clinical data set
Demographic characteristics, baseline clinical and biological data, antimyeloma therapy, use of plasma exchange or high cutoff hemodialysis, hematologic response (including FLC reduction after the first cycle17 and best FLC response), best estimated glomerular filtration rate (eGFR) value, and patient survival data were collected. The presence of precipitating factors for AKI, including infection, use of nephrotoxic drugs or contrast media, and hypercalcemia, was recorded.
Pathology
Standard processing of renal biopsy specimens for light microscopy and immunofluorescence was performed, including systematic staining with antibodies to κ and λ LCs. Glass slides were independently reviewed by 2 pathologists. Sixteen pathologists participated in the scoring. Scoring definitions, instructions, and scoring sheets were generated and distributed to collect a detailed pathology data set.
Pathology variables were defined as detailed in Table 1 and illustrated in Figure 1. Using all available slides, a semi-quantitative histologic assessment of glomerular and tubulointerstitial lesions was performed. On the most representative periodic acid–Schiff or trichrome-stained section, the extent of cast formation was assessed by calculating the mean and highest number of casts per square millimeter in the cortex and, separately, in the medulla. The mean number of casts per square millimeter was defined as the total number of casts divided by the estimated area of cortex or medulla. The highest number of casts was defined as the highest number of casts in one 20× field, adjusted for 1 mm2. The presence of other monoclonal immunoglobulin–related renal lesions was recorded.
Table 1.
Pathology definitions for scoring
| LCCN: Histological diagnosis of LCCN was defined as follows: (1) at least one cast with typical appearance of LC cast by light microscopy; and (2) at least one cast staining for anti-κ or anti-λ conjugate by immunofluorescence or immunohistochemistry |
| LC cast: Tubular cast that meets ≥2 of these 3 criteria: (1) periodic acid–Schiff negative and/or polychromatophilic on trichrome and/or eosinophilic on hematoxylin-eosin and/or Congo red positive; (2) fractured and/or sharp edged and/or brittle and/or crystalline appearance; or (3) cellular and/or giant cell reaction around the cast |
| Glomerular definitions* |
| THP in the Bowman’s space: Identification of THP in the Bowman’s space |
| Tubulointerstitial definitions* |
| Tubular atrophy and interstitial fibrosis: Thickened tubular basement membranes, flattened epithelial cells, expanded interstitium with fibrosis in the cortex. <10% (0), 10%-24% (1), 25%-50% (2), >50% (3) |
| Interstitial inflammation: Increased interstitial leukocytes in the cortex. None (0), focal <50% of the cortex (1), diffuse ≥50% (2) assessed for the cortex, and separately for the medulla |
| Acute tubular injury: Cortical tubular epithelial flattening and loss of brush border, or necrosis of tubular epithelial cells, denudation of the tubular basement membranes |
| Interstitial edema: Expansion of the interstitium by edema |
| Tubulitis: Infiltrating mononuclear cells in the tubular epithelium of nonatrophic and atrophic tubule |
| THP extravasation: Identification of THP extravasation into the interstitium |
| Giant cell reaction around casts: Identification of at least one multinucleated histiocyte surrounding an LC cast |
| Tubular rupture: Interruption in the tubular basement membrane secondary to dilatation, to the presence of an LC cast or inflammation |
| Interstitial giant cell reaction: Identification of a least one multinucleated histiocyte in the interstitium |
| Extent of cast formation definitions assessed for the cortex, and separately for the medulla† |
| Estimated area (square millimeter): Total number of 20× fields of cortex/medulla multiplied by the area of one 20× field in square millimeter |
| Mean number of casts per square millimeter: Total number of casts in the cortex/medulla divided by the estimated area of cortex/medulla |
| Highest number of casts per square millimeter: Highest number of LC casts in one 20× field divided by the area of one 20× field in square millimeter |
| Vascular definitions* |
| Arteriosclerosis: Thickening of the intima with fibrosis or duplication of the elastic lamina. Absent (0), vascular narrowing of <25% of luminal area (1), 25%-50% of luminal area (2), >50% of luminal area (3) |
| Arteriolar hyalinosis: Accumulation of hyaline material in the arteriolar wall. Absent (0), mild (1), moderate (2), severe (3) |
Evaluation based on all available slides.
Evaluation based on the most representative periodic acid–Schiff or trichrome-stained slide.
Figure 1.
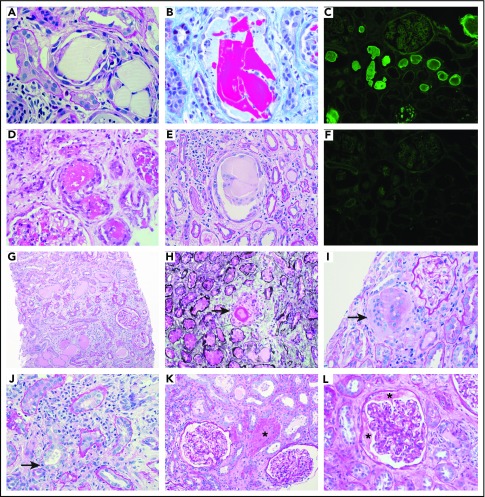
Pathology illustrations. Intraluminal LC casts with the typical appearance. By light microscopy: (A) sharp-edged periodic acid–Schiff negative casts surrounded by a cellular reaction (magnification ×600), (B) fractured cast on trichrome stain (magnification ×600), (D) eosinophilic granular casts on hematoxylin and eosin stain (magnification ×400), and (E) multinucleated giant cell reaction around a fractured cast (magnification ×400). By immunofluorescence: (C) positive staining with anti-κ (magnification ×100) and (F) negative staining with anti-λ (magnification ×100). Images of pathology features that were recorded: (G) extensive interstitial fibrosis and tubular atrophy with scarce mononuclear interstitial inflammation (periodic acid–Schiff, magnification ×100), (H) tubular rupture with the interruption of the tubular basement membrane (arrow) (Jones methenamine silver, magnification ×400), (I) interstitial giant cells (arrow) (periodic acid–Schiff, magnification ×400), (J) interstitial inflammation with tubulitis (arrow) (periodic acid–Schiff, magnification ×600), (K) THP extravasation into the interstitium (asterisk) (periodic acid–Schiff, magnification ×200), and (L) THP protein in the Bowman’s space (asterisks) (periodic acid–Schiff, magnification ×400).
For variables with a divergent categorical score, discrepancies were solved by discussion to generate a consensus score. For the evaluation of continuous variables, differences between the counts >25% were reviewed simultaneously. Otherwise, the average of the 2 reviewers was used.
Definitions
Hematologic response was defined according to the IMWG criteria20,21: stringent complete response (CR), CR, very good partial response (VGPR), partial response, stable disease, and progressive disease. Because not all patients underwent a bone marrow biopsy, we merged stringent CR, CR, and near CR, defined as negative serum and urine immunofixation, without bone marrow biopsy examination. IMWG hematologic response was assessed retrospectively in patients who presented before 2006. When FLCs were not available, hematologic response was based on serum and urine immunofixation and protein electrophoresis.
eGFR was calculated by using the Modification of Diet in Renal Disease four-variable formula. AKI stage was determined according to the Acute Kidney Injury Network (AKIN) criteria, using the preexisting serum creatinine value,22 and, if not available, using the best serum creatinine value during follow-up if the corresponding eGFR was >60 mL/min/1.73 m2, as a reflection of preexisting creatinine.
Renal outcomes included best eGFR value during follow-up, in which individuals who remained dialysis dependent had their best eGFR set at 10 mL/min/1.73 m2. We also recorded those who remained dialysis dependent. Renal responses were also determined according to the IMWG criteria,11 as follows: CR (best eGFR ≥60 mL/min/1.73 m2 when baseline eGFR <50 mL/min/1.73 m2), partial response (best eGFR 30-59 mL/min/1.73 m2 best when baseline eGFR <15 mL/min/1.73 m2), and minor response (best eGFR 15-29 mL/min/1.73 m2 or 30-59 mL/min/1.73 m2 in those with baseline eGFR <15 or 15-29 mL/min/1.73 m2, respectively).11
For outcome analyses, only individuals who received at least 1 chemotherapy cycle and completed at least 2 months of follow-up were considered.
Statistical analyses
Normally distributed variables are expressed as mean ± standard deviation and compared by using the Student t test, one-way analysis of variance, or Pearson correlation. Nonparametric variables are expressed by using median with interquartile range or percentages and compared by using the Mann-Whitney, Kruskal-Wallis, or Spearman test. Proportions are compared by using the Pearson χ2 test. Analyses were performed by using SPSS software version 11 (IBM SPSS Statistics, IBM Corporation, Armonk, NY) and R software (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria).
Agreement assessment was performed between local and central review (V.R.). We used Gwet’s agreement coefficient (GAC) for dichotomous variables and the intraclass correlation coefficient for ordinal or continuous variables.23 The GAC has been validated in simulation approaches and is more robust compared with the classic κ score for dichotomous outcomes.24 By convention, a GAC or intraclass correlation coefficient value <0.40 indicates poor interrater reliability; 0.40 to 0.59 is moderate, and ≥0.60 is good.25,26
Correlations between pathology variables were conducted by using the phi, Cramer’s V, or Spearman test, as appropriate. Given the number of possible comparisons between pathology variables, the Holm-Bonferroni method was used to minimize the risk of type I error.27,28
To test the associations between clinicopathologic variables and renal outcomes, we categorized continuous variables into groups, defined according to clinically relevant cutoffs or by tertiles rounded to the simplest value. Variables associated with best eGFR by univariate analyses were tested for independence by using a stepwise linear regression. Variables associated with dialysis dependency were tested by using multivariate Cox proportional hazards regression, and variables associated with renal response according to IMWG were tested by using a logistic regression grouping complete, partial, and minor responses together. Hazard ratios and odds ratios (ORs) are reported with 95% confidence intervals (CIS).
OS according to renal and hematologic responses was compared by using Kaplan-Meier curves. Renal responses were categorized in groups of <15, 15 to 29, 30 to 44, 45 to 59, and ≥60 mL/min/1.73 m2.11 A time-dependent Cox proportional hazards analysis was used to investigate if the best eGFR was associated with OS independently from hematologic response in which survival before hematologic response was allocated to the “no remission” group. We were not able to define a time-dependent expression of the best eGFR.
Results
Baseline clinical characteristics
Demographic and myeloma characteristics, renal presentation, and AKI risk factors at the time of LCCN diagnosis in the 178 patients are shown in Table 2. Mean age was 66 ± 11 years, and median time between MM diagnosis and LCCN diagnosis was 2 (0-25) days. Ninety-two percent of biopsy samples were obtained before first-line therapy and 8% at myeloma relapse. The median serum level of involved FLC was 5010 (2780-10 504) mg/L, with median bone marrow plasma cell infiltration of 50% (27%-75%) and a β2-microglobulin level of 13 (7-22) mg/L. Baseline mean eGFR was 13 ± 11 mL/min/1.73 m2. Eighty-two percent of patients had AKIN stage 3, and 83 subjects (47%) required hemodialysis at presentation. Median level of proteinuria was 2.3 (1.2-4.4) g/d, and median albuminuria was 9% (5%-16%). Five patients (2.8%) experienced renal biopsy complications, including retroperitoneal hematomas (n = 4) and arteriovenous fistula (n = 1).
Table 2.
Clinical characteristics at time of diagnosis of LCCN
| Characteristic | Patients (n = 178) |
|---|---|
| Demographic characteristics | |
| Age, y | 66 ± 11 |
| Ethnicity, % white, AA, Asian, Middle Eastern, other | 90, 6, 1, 1, 2 |
| Female sex, % | 45 |
| Medical history | |
| Hypertension, % | 40 |
| Congestive heart failure, % | 2.3 |
| Vascular disease, % | 16 |
| Diabetes, % | 14 |
| Myeloma characteristics | |
| Prior chemotherapy for myeloma, % | 7.3 |
| Type of myeloma, % | |
| IgG | 26 |
| IgA | 21 |
| IgD | 3 |
| IgM | 2 |
| LC only | 48 |
| Size of monoclonal spike, g/L | 12 (3-32) |
| FLC level, mg/L | 5010 (2780-10 504) |
| κ/λ, % | 47/53 |
| Bone marrow plasma cells % | 50 (27-75) |
| Hemoglobin, g/L | 92 ± 17 |
| LDH, U/L | 270 ± 145 |
| β2-microglobulin, mg/L | 13 (7-22) |
| Presence of lytic bone lesions, % | 63 |
| Renal characteristics | |
| eGFR before LCCN, mL/min/1.73 m2 | 76 ± 27 |
| eGFR, mL/min/1.73m2 | 13 ± 11 |
| AKIN stage (0, 1, 2, 3) | 1, 5, 12, 82 |
| Dialysis dependence, % | 47 |
| Albumin, g/L | 34 ± 6 |
| Albuminuria, % | 9 (5-16) |
| Proteinuria, g/d | 2.2 (1.2-4.4) |
| Additional risk factors for AKI | |
| Infection at diagnosis of LCCN, % | 24 |
| Nephrotoxic drugs, % | 27 |
| Contrast media, % | 5.1 |
| Hypercalcemia, % | 24 |
| Any additional risk factor for AKI, % | 61 |
Missing data were as follows: size of monoclonal spike, 15%; FLC, 5%; % bone marrow plasma cells, 18%; lactic acid dehydrogenase (LDH), 21%; β2-microglobulin, 33%; lytic lesions, 11%; eGFR before LCCN, 29%.
AA, African American; Ig, immunoglobulin.
Pathology findings
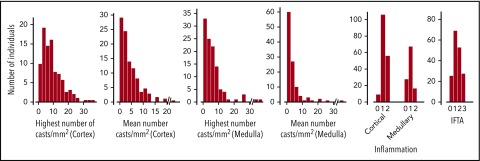
Biopsy findings are detailed in Figure 2. The median number of glomeruli was 11 (8-20). Median proportion of global and segmental glomerulosclerosis was 9% and 14%, respectively. THP was found in the Bowman space in 6.2% of cases. Cortical and medullary inflammation, usually mild, was found in 95% and 75% of cases. Acute tubular injury, tubulitis, giant cell reaction around casts, and tubular rupture were observed in 94%, 82%, 60%, and 62% of cases. Forty-six percent and 52% of patients had moderate to severe IFTA and arteriosclerosis. The interstitium showed giant cells or THP extravasation in 19% and 32% of cases.
Figure 2.
Pathology findings. There was no cortex in 3 biopsy specimens and no medulla in 67 (38%), where no highest or mean number of casts could be determined.
The mean and highest numbers of casts per square millimeter were 3.2 (1.4-6.4) and 7.8 (3.9-12.6) in the cortex, and 2.2 (0.8-4.5) and 4.1 (2.0-7.8) in the medulla. Only 7 patients had <5 cortical or medullary casts within a total surface area of 6.1 (4.8-10.2) mm2. Eleven patients (6.2%) had associated LCDD, with nodular glomerulosclerosis and/or thickening of tubular basement membranes and linear LC staining along basement membranes according to immunofluorescence. Four patients (2.2%) had concomitant AL amyloidosis, with Congo red–positive deposits showing typical ultrastructural fibrillary organization. Focal interstitial infiltration by neoplastic plasma cells was found in 3 patients. Other clinically relevant findings were cryoglobulinemic glomerulonephritis (n = 1), fibrillary glomerulonephritis (n = 1), and crystalline LC proximal tubulopathy (n = 2).
Intercorrelations between pathology findings are detailed in supplemental Table 1 (available on the Blood Web site). The highest and mean number of casts per square millimeter were strongly intercorrelated in the cortex (r = 0.90) and medulla (r = 0.92).
The GAC and intraclass correlation coefficient scores between pathology variables showed good reproducibility (scores >0.6), except for arteriosclerosis (0.55), giant cell reaction around casts (0.59), tubular rupture (0.47), and interstitial edema (0.34) (Table 3).
Table 3.
Pathology agreement between reviewers
| Pathology findings | ICC | GAC |
|---|---|---|
| Ratio of globally sclerosed glomeruli | 0.84 | |
| Ratio of glomeruli with segmental sclerosis | 0.42 | |
| Interstitial fibrosis and tubular atrophy, 0-1-2-3 | 0.61 | |
| Cortical interstitial inflammation, 0-1-2 | 0.60 | |
| Medullary interstitial inflammation, 0-1-2 | 0.73 | |
| THP in Bowman’s space, yes/no | 0.92 | |
| Acute tubular injury, yes/no | 0.72 | |
| Interstitial edema, yes/no | 0.34 | |
| Tubulitis, yes/no | 0.61 | |
| Giant cell reaction around casts, yes/no | 0.59 | |
| Tubular rupture, yes/no | 0.47 | |
| THP extravasation, yes/no | 0.60 | |
| Interstitial giant cell reaction, yes/no | 0.80 | |
| Highest number of cortical cast/mm2 | 0.73 | |
| Mean no. of cortical cast/mm2 | 0.75 | |
| Highest no. of medullary cast/mm2 | 0.90 | |
| Mean no. of medullary cast/mm2 | 0.92 | |
| Arteriolosclerosis, 0-1-2-3 | 0.66 | |
| Arteriosclerosis, 0-1-2-3 | 0.55 |
A GAC score of 0.59 corresponds to an absolute agreement of 79% between the 2 pathologists.
ICC, intraclass correlation coefficient.
Correlations between baseline eGFR and clinicopathologic findings
Higher age, β2-microglobulin, involved FLC level, lactic acid dehydrogenase, and bone marrow plasma cell infiltration were associated with lower eGFR at diagnosis; higher proteinuria was associated with slightly higher eGFR (Table 4). Medullary inflammation, giant cell reaction around casts, and higher number of casts in the cortex or medulla correlated with lower baseline GFR. Similar associations were found by using the AKIN stage (3 vs other) or hemodialysis at presentation as outcomes (data not shown).
Table 4.
Clinicopathological correlations with eGFR at LCCN diagnosis
| Variable | Baseline eGFR (mL/min/1.73 m2) | P |
|---|---|---|
| Age, y | ||
| ≤60 | 16 ± 15 | .04 (≤60 compared with >60) |
| 60-70 | 12 ± 9 | |
| >70 | 11 ± 9 | |
| LCCN at myeloma relapse | ||
| Absent | 13 ± 12 | .73 |
| Present | 15 ± 9 | |
| Proteinuria, g/d | ||
| ≤2.0 | 12 ± 9 | .03 (>4 g/d compared with ≤4 g/d) |
| 2.1-4.0 | 12 ± 9 | |
| >4.0 | 17 ± 16 | |
| Free light chains, mg/L | ||
| ≤3500 | 15 ± 11 | .04 (trend test) |
| 3501-7000 | 14 ± 14 | |
| >7000 | 11 ± 10 | |
| Lactic acid dehydrogenase, U/L | ||
| ≤200 | 16 ± 15 | .03 (trend test) |
| 201-400 | 12 ± 9 | |
| >400 | 11 ± 9 | |
| β2-microglobulin, mg/L | ||
| ≤10.0 | 22 ± 17 | <.001 (trend test) |
| 10.1-20.0 | 12 ± 8 | |
| >20.0 | 7 ± 6 | |
| Bone marrow plasma cells, % | ||
| ≤33% | 16 ± 11 | .05 |
| 34-66 | 13 ± 14 | |
| >66% | 11 ± 8 | |
| Medulla interstitial inflammation, 0 vs 1-2+ | ||
| 0 | 20 ± 20 | .04 |
| 1 to ≥2 | 12 ± 9 | |
| Giant cell reaction around casts, yes/no | ||
| Absent | 16 ± 15 | .003 |
| Present | 11 ± 7 | |
| Cortex: highest number of casts/mm2 | ||
| ≤5 | 18 ± 17 | <.001 (trend test) |
| 5-10 | 11 ± 7 | |
| >10 | 12 ± 9 | |
| Cortex: mean number of casts/mm2 | ||
| ≤2 | 18 ± 16 | <.001 (trend test) |
| 2-5 | 12 ± 8 | |
| >5 | 11 ± 8 | |
| Medulla: highest number of casts/mm2 | ||
| ≤3 | 20 ± 19 | <.001 (trend test) |
| 3-7 | 12 ± 10 | |
| >7 | 10 ± 9 | |
| Medulla: mean number of casts/mm2 | ||
| ≤1.5 | 19 ± 18 | <.001 (trend test) |
| 1.5-3.0 | 13 ± 11 | |
| >3.0 | 11 ± 9 |
Treatments and outcomes
Five patients did not complete 1 cycle of treatment, and 19 died within 2 months of diagnosis. In the 154 patients available for outcome analysis, median follow-up duration was 22 (11-44) months. Most patients received bortezomib or immunomodulatory drug–based regimens at first (82%) or at second (100%) line (Table 5). FLC removal (plasma exchange or high-cutoff dialysis) and high-dose melphalan followed by autologous stem cell transplantation were performed in 36% and 39% of cases, respectively.
Table 5.
Treatment, hematology, and nephrology outcomes in treated individuals (n = 154)
| Duration of follow-up, month | 22 (11-44) |
| First-line chemotherapy, n (%) | |
| Bortezomib-based therapy | 93 (60) |
| IMiD-based therapy* | 15 (10) |
| Combination bortezomib and IMiDs | 19 (12) |
| Alkylating-based (without bortezomib or IMiDs) | 9 (5.8) |
| Vincristine-doxorubicin-dexamethasone | 13 (8.4) |
| Dexamethasone only | 5 (3.2) |
| Plasma exchange or high-cutoff hemodialysis | 36 |
| Second-line chemotherapy, n (%) | |
| None | 79 (51) |
| Proteasome inhibitors | 26 (17) |
| IMiD-based therapy | 34 (22) |
| Combination proteasome inhibitors and IMiDs | 15 (9.7) |
| Autologous stem cell transplantation | 39 |
| Hematologic response, % | |
| Near CR or better | 38 |
| Very good partial response | 31 |
| Partial response | 22 |
| Stable disease or worse | 9 |
| Light chain response | |
| 60% reduction in FLCs after the first cycle, % | 73 |
| FLC ≤ 500 mg/L, % | 82 |
| Best serum FLC level, mg/L | 38 (13-319) |
| Best FLC reduction, % | 99 (93-99) |
| Renal response | |
| Best eGFR, mL/min/1.73m2 | 43 ± 30 |
| Dialysis dependency, % | 26% |
| IMWG renal response criteria, % | |
| Complete, partial, minor, none | 28, 21, 21, 30 |
Cohort of subjects with at least 2 months’ follow-up and 1 complete chemotherapy cycle were included. Missing data were as follows: best hematologic remission status (3%), best serum FLC level (13%), best FLC reduction (15%), >60% reduction in serum FLC level after 1 initial cycle of chemotherapy (18%), and AKIN stage improvement (16%).
IMiD, immunomodulatory drug.
Includes thalidomide, lenalidomide, and pomalidomide.
Hematologic response VGPR or better occurred in 69% of patients after a median of 5 (3-8) months (near CR occurred in 38% of cases and VGPR in 31%) (Table 5). Seventy-three percent had a ≥60% reduction in involved FLC level after 1 cycle. Median best FLC reduction was 99% (93%-99%), with a median FLC level of 38 (13-319) mg/L.
The best eGFR value was 43 ± 30 mL/min/1.73 m2. It occurred at 5 (0-9) months, when 138 of 154 patients were still being followed up. Among 83 patients who required dialysis at presentation, 64 were included in the follow-up analyses after excluding early deaths and untreated patients. Seven additional individuals required dialysis during follow-up. Of these 71 patients, 56% (40 of 71) remained dialysis dependent. Overall, 26% (40 of 154) of the population remained dialysis dependent at last follow-up.
The overall survival was 37 (9-69) months, and there were 103 deaths that occurred within a median of 13 (3-34) months after LCCN diagnosis. Of the remaining 75 patients, 17 were lost to follow-up (9.6%), and 58 patients were still being followed up at the time of data collection.
Factors associated with renal outcomes
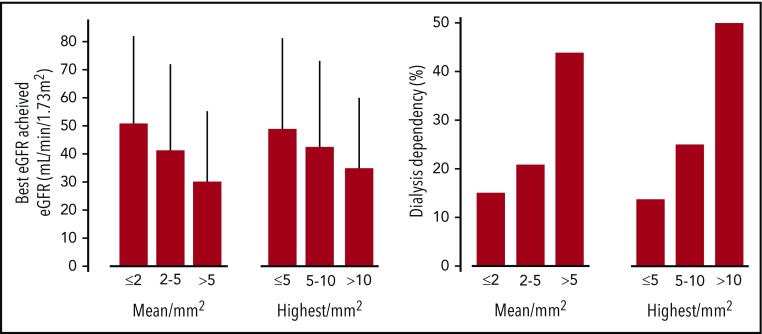
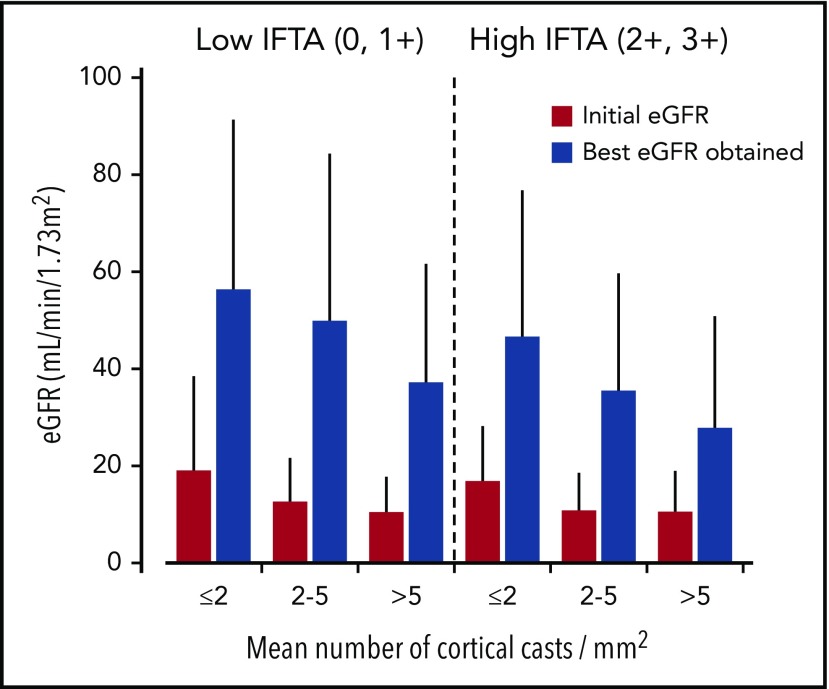
Older age, lower initial eGFR, LCCN diagnosed at myeloma relapse, higher baseline FLC level, and β2-microglobulin levels were strongly associated with a lower eGFR during follow-up (Table 6). Achievement of hematologic response VGPR or better, ≥60% reduction of the involved FLCs after 1 cycle, and lower involved FLC level during follow-up correlated with a higher follow-up eGFR value. We found no correlation between the presence of risk factors for AKI at presentation and the best eGFR. Giant cell reaction around casts, greater IFTA, and associated AL amyloidosis or LCDD were associated with lower follow-up eGFR. THP extravasation correlated with higher follow-up eGFR (57 ± 35 vs 36 ± 26 mL/min/1.73 m2; P < .001), although these patients had higher baseline eGFR (16 ± 14 vs 12 ± 10 mL/min/1.73 m2; P = .07) and less frequent moderate to severe IFTA (33% vs 55% in patients without THP extravasation; P = .01). eGFR and dialysis outcomes according to different methods of assessment of the extent of cast formation are shown in Figure 3. eGFR increased to 32 ± 24 mL/min/1.73 m2 in patients with mean number of casts >5/mm2 (44% dialysis dependent) vs 52 ± 33 mL/min/1.73 m2 (16% dialysis-dependent) in those with ≤2 casts/mm2. We found similar results when considering only patients who achieved ≥60% reduction in FLCs after 1 cycle of chemotherapy (n = 103).
Table 6.
Univariate and multivariate correlations with the best eGFR obtained during follow-up
| Variable | Univariate (mL/min/1.73 m2) | P (trend test) | Multivariate standardized β | P |
|---|---|---|---|---|
| Age, y | ≤60: 56 ± 37 | <.001 | −0.274 | .002 |
| 61-70: 38 ± 29 | ||||
| >70: 38 ± 23 | ||||
| LCCN at myeloma relapse | No: 44 ± 31 | .002 | — | |
| Yes: 23 ± 15 | ||||
| Presenting eGFR, mL/min/1.73 m2 | ≥30: 65 ± 36 | <.001 | — | |
| 15-29: 60 ± 30 | ||||
| <15: 36 ± 26 | ||||
| Baseline serum FLC level, mg/L | ≤3500: 48 ± 39 | .03 | — | |
| 3501-7000: 41 ± 34 | ||||
| >7000: 35 ± 28 | ||||
| β2-microglobulin, mg/L | <10: 57 ± 36 | <.001 | −0.365 | <.001 |
| 10-20: 50 ± 25 | ||||
| >20: 25 ± 21 | ||||
| 60% reduction in serum FLC level after the first cycle | Present: 50 ± 29 | .001 | * | |
| Absent: 31 ± 31 | ||||
| Best hematologic response | VGPR or better: 49 ± 30 | <.001 | 0.21 (VGPR or better vs PR or worse) | .01 |
| PR: 38 ± 30 | ||||
| Stable disease or worse: 22 ± 20 | ||||
| Best serum FLC level, mg/L | ≤25: 58 ± 31 | <.001 | * | |
| 26-500: 42 ± 30 | ||||
| >500: 25 ± 19 | ||||
| Interstitial fibrosis or tubular atrophy | 0 to ≥1: 48 ± 32 | .01 | −0.20 | .02 |
| 2 to ≥3: 36 ± 26 | ||||
| THP extravasation | Present: 57 ± 35 | <.001 | — | |
| Absent: 36 ± 26 | ||||
| Giant cell reaction around casts | Present: 38 ± 28 | .02 | — | |
| Absent:50 ± 33 | ||||
| Cortex: mean no. of casts/mm2 | ≤2: 52 ± 33 | <.001 | −0.23 | .008 |
| 2-5: 42 ± 30 | ||||
| >5: 32 ± 24 | ||||
| Medulla: mean no. of casts/mm2 | ≤1.5: 56 ± 33 | <.001 | † | |
| 1.5-3.0: 41 ± 27 | ||||
| >3.0: 33 ± 26 | ||||
| Glomerular amyloidosis or LCDD | Present: 21 ± 16 | <.001 | — | |
| Absent: 44 ± 31 |
Patients with a minimum 2 months’ follow-up and one cycle of treatment were included (n = 154). We included all the variables associated by univariate analysis in the multivariate model except for “60% reduction in serum FLC level after the first cycle” and “best FLC” because they are closely interrelated by definition to “best hematologic response” (*). We also excluded the “mean number of medullary casts” since the medulla was absent in 40% (†).
NR, no response; PR, partial response.
Figure 3.
Renal outcomes according to mean and highest number of casts in the cortex.
Although LCCN occurring at myeloma relapse was not associated with lower baseline eGFR, it was strongly associated with a lower best eGFR (23 ± 15 vs 44 ± 31 mL/min/1.73 m2; P = .002) and lower hematologic response rate (30% with VGPR or better compared with 72%; P = .006). Patients who presented with LCCN at myeloma relapse had a greater proportion of giant cell reaction around casts (40% vs 17%; P = .03), although they had similar amounts of IFTA and casts.
Variables selected according to univariate analysis were compared in a multivariate model by using a stepwise method (Table 6). We only considered the best hematologic response, since a 60% reduction in FLCs after the first cycle and the best FLC level were closely interrelated. Age, β2-microglobulin level, best hematologic response, mean number of cortical casts per square millimeter, and degree of IFTA were independently associated with best eGFR during follow-up. Findings were identical when considering the highest instead of the mean number of casts per square millimeter. The interrelations between number of casts and IFTA with baseline and best follow-up eGFR are illustrated in Figure 4.
Figure 4.
Interrelation between independent pathology findings with the initial and best follow-up eGFR.
Factors associated with dialysis dependency were a lower initial eGFR, presence of any risk factor of AKI, higher β2-microglobulin, the absence of a hematologic response VGPR or better, greater number of casts per square millimeter, and presence of concomitant amyloidosis or LCDD. A multivariate Cox regression using these variables identified β2-microglobin (hazard ratio, 1.03 per mg/L increase; 95% CI, 1.01-1.04; P < .001), mean number of cortical casts per square millimeter (hazard ratio, 2.21 per increase in tertile; 95% CI, 1.26-3.90; P = .006), and failure of achieving hematologic response VGPR or better (hazard ratio, 4.38; 95% CI, 1.81-10.57; P = .001) as independently associated with dialysis dependency.
When considering the IMWG renal response criteria, merging complete, partial, and minor responses, lower β2-microglobulin level, absence of any risk factor of AKI, achievement of hematologic response VGPR or better, lower number of casts per square millimeter, lower severity of arteriolosclerosis, and absence of amyloidosis or LCDD were associated with a higher probability of renal response by univariate analysis. In the multivariate model, β2-microglobulin (OR, 0.96; 95% CI, 0.93-0.98; P = .003), mean number of cortical casts per square millimeter (OR, 0.40 per increase in tertile; 95% CI, 0.19-0.87; P = .02), and achieving VGPR or better (OR, 8.40; 95% CI, 2.41-29.29; P = .001) were independently associated with renal response.
Impact of first-line treatments on renal outcomes
In the follow-up cohort, we compared the respective effect of first-line chemotherapy with bortezomib, immunomodulatory drugs, and alkylating or vincristine-based regimens. Although the initial eGFR was similar (±1 mL/min/1.73 m2), the bortezomib group achieved a significantly higher best eGFR value (46 ± 31 mL/min/1.73 m2) compared with patients who received immunomodulatory drugs (33 ± 25 mL/min/1.73 m2) or alkylating/vincristine-based regimens (33 ± 30 mL/min/1.73 m2) (P = .02), with a lower rate of dialysis dependency (20% vs 40% and 46%, respectively; P = .004). Rates of a hematologic response VGPR or better were significantly higher in the bortezomib group (78% vs 60% and 29%; P < .001).
Among patients requiring hemodialysis at presentation, the best eGFR achieved was higher in those who received extracorporeal removal of FLCs, compared with those who did not (33 ± 27 vs 21 ± 20 mL/min/1.73 m2; P = .046). Moreover, they exhibited a trend for lower dialysis dependency (45% vs 60%) and higher hematologic response VGPR or better (77% vs 61%) (P = .25 and 0.16, respectively).
Finally, autologous stem cell transplantation was strongly associated with the best eGFR, as expected, because a preserved eGFR is a prerequisite for such therapy (data not shown).
Factors associated with the number of casts and IFTA
Given that 2 independent pathology findings were associated with the best eGFR during follow-up, we sought to determine which baseline clinical factors could foretell the number of casts and degree of IFTA.
The number of casts correlated with the degree of bone marrow infiltration, FLC level, and proteinuria. Patients with ≤2, 2 to 5, and >5 mean cortical casts per square millimeter had baseline FLC levels of 3910 (1605-7970), 4920 (2875-8495), and 6460 (4120-15650) mg/dL, respectively, indicating substantial overlap. No significant association was found between the degree of chronic tubulointerstitial changes and eGFR before the diagnosis of LCCN, baseline eGFR, age, comorbidities, previous chemotherapy for MM, or time interval between the diagnoses of MM and LCCN.
Associations between renal, hematologic response, and OS
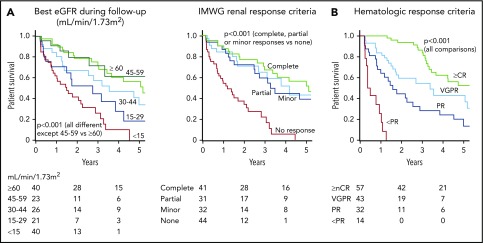
OS was strongly associated with the best eGFR value with a hazard ratio of death of 1.6 (1.3-1.9) per every 15 mL/min/1.73 m2 drop in eGFR below 45 mL/min/1.73 m2 (P < .001) (Figure 5A). When using the IMWG criteria, complete, partial, and minor renal responses were associated with a greater OS compared with no renal response (P < .001). However, there was no statistically significant difference in OS between types of renal response. Finally, the quality of hematologic response correlated with OS (P < .001) (Figure 5B).
Figure 5.
OS according to the renal and hematologic responses. (A) Renal response, (B) hematologic response. nCR, near CR; PR, partial response.
A multivariate Cox regression analysis using a time-dependent expression of hematologic response found the best eGFR associated with an independent hazard ratio of death of 1.4 (1.1-1.8) per every 15 mL/min/1.73 m2 drop in eGFR below 45 mL/min/1.73 m2 (P = .003). By contrast, baseline eGFR was not associated with increased OS, with a hazard ratio of death of 0.98 (95% CI, 0.96-1.01; P = .15) for every 1 mL/min/1.73 m2 increase in initial eGFR. Achieving VGPR or better was associated with a 0.5 (0.3-0.9) time-dependent hazard of death (P = .01).
Discussion
In this large multicenter retrospective study, renal pathology features in patients with LCCN correlated with both initial and follow-up renal function. The extent of cast formation and the degree of IFTA were significantly associated with renal recovery in multivariate analysis, independently to baseline clinical features and to hematologic response. We also found that achieving a renal response was associated with higher OS independently of the hematologic response. Importantly, initial clinical assessment was poorly representative of pathology, underscoring the value of performing a renal biopsy when managing patients with MM and severe AKI. Although these results are currently insufficient to recommend treatment adaptation based on renal pathology findings, future interventional studies dedicated to renal outcomes in LCCN should consider stratification according to the amount of casts and degree of IFTA.
In the previous series that focused on determinants of renal recovery in LCCN, 2 main factors were identified: the severity of renal impairment and the rapid achievement of a deep and sustained hematologic response.4,6,29-31 Even though deep FLC response is a required condition for renal recovery, it is not sufficient, and identifying reliable predictors of renal prognosis would help in the prognostication and management of LCCN. The present analysis confirms previously established factors associated with renal response: age <65 years, baseline eGFR, involved FLCs, and β2-microglobulin level were significantly associated with renal outcome. Hematologic response, either defined by the achievement of VGPR or better, or by ≥60% reduction of FLC level after 1 cycle of treatment, or by the lowest FLC level obtained, correlated with better renal outcome. Sixty-nine percent of patients experienced VGPR or better, a high response rate probably due to the large use of novel agents as first-line therapy.32 Supporting the importance of a rapid FLC reduction,17,33,34 we recorded a higher best eGFR in patients requiring hemodialysis at presentation when treated with extracorporeal removal of FLCs; these patients also showed a tendency toward better hematologic response. In our study, the maximal renal response expressed as the best eGFR and the best hematologic response occurred simultaneously at 5 months, reinforcing the value of the best eGFR as a reliable parameter of renal response.
To date, little information exists on the prognostic value of renal histologic features in LCCN. We performed an extensive morphologic assessment and applied a robust methodology with all renal biopsy samples reviewed independently by 2 pathologists, with good inter-reader reproducibility. We found that the extent of cast formation and, to a lesser extent, IFTA were associated with renal response independently of baseline clinical characteristics and depth of hematologic response. In pioneering studies,35,36 only extensive interstitial inflammation and fibrosis correlated with poor renal recovery. In a study by Johnson et al,37 the degree of cast formation was the strongest predictive factor of irreversible renal failure. However, these series were limited by their small size, the inclusion of diagnoses other than LCCN, and the absence of assessment of FLC response. In a cohort of 70 patients, Ecotière et al6 found that only in patients who achieved a hematologic response were the number of casts and the extent of tubular atrophy inversely proportional to the probability of renal response. Interstitial fibrosis was not significantly associated with renal outcome, possibly due to insufficient sample size.
IFTA reflect the chronic damage and are crucial determinants of renal outcome in most renal diseases.38-40 In the transition of AKI to CKD, recent observations showed that proximal tubule reabsorption of monoclonal FLCs promotes a pro-inflammatory/fibrotic state that develops early on with the kidney injury.41 THP released in interstitium has been shown to trigger inflammation by activating dendritic cells through Toll-like receptor 4 signaling42 and by promoting secretion of interleukin-1β by the NRLP3 inflammasome.43 We found that THP extravasation was associated with a higher follow-up eGFR, although it also correlated with higher initial eGFR and milder IFTA. These data suggest that uromodulin accumulation in the interstitium may reflect an early consequence of tubule obstruction by casts.
We assessed the extent of cast formation by estimating the highest and the mean number of casts per square millimeter, separately in the cortex and the medulla. The number of casts seemed to reflect the disease burden, as patients with numerous casts exhibited higher bone marrow infiltration and higher FLC levels at presentation. However, the extent of cast formation could not be predicted by baseline level of the involved FLCs. Indeed, casts not only reflect the amount of monoclonal LC that reach the distal tubule lumen but also the affinity of LC for THP, which is determined by the structure of the LC hypervariable domain (complementarity-determining region 3) that binds to a specific 9 amino-acid region of THP.44 In the current study, both the mean and highest number of cortical casts per square millimeter were independently associated with renal outcomes. Therefore, in routine clinical practice, we propose to report only the highest number of casts per square millimeter in the cortex, using simple cutoffs of <5, 5 to 10, and >10 casts/mm2.
Renal recovery has been associated with improved OS in patients with LCCN.3,4,18,45,46 However, recent studies have questioned the validity of this relation in the era of novel antimyeloma agents.31,47,48 These studies were smaller, the definition of renal outcomes varied, initial eGFRs were higher, and the proportion with LCCN was unknown. de Vries et al31 studied 131 patients newly diagnosed with MM and renal impairment and found that renal recovery was not associated with a better OS, even in patients with severe renal impairment. However, only 52 patients had an eGFR <30 mL/min/1.73 m2, which contrasts with the initial eGFR of 13 mL/min/1.73 m2 in our study. The MM-013 trial also reported similar OS and progression-free survival in patients with and without renal recovery, although nonrenal responders showed a trend for shorter OS. In this study, patients had relapsed/refractory MM, most had chronic renal impairment, and only 48 patients had an initial eGFR <30 mL/min/1.73m2.47
Our data support the predictive value of renal improvement for OS, independently of the hematologic response, although we were not able to ascertain the rapidity of renal response and hence could not use a time-dependent expression of final eGFR. However, because improvement in renal function usually occurs within the first 6 months, a lead-time bias is unlikely.29,35,49 Our multivariate model indicates that the best eGFR during follow-up is more relevant than the presenting one. Figure 5A shows that achievement of renal response according to IMWG criteria was associated with higher OS. However, differences between complete, partial, and minor responses were not clear, as opposed to a strong dose-dependent effect of the best eGFR obtained. Because pathologic findings independently correlate with renal outcome, this scenario underscores the clinical value of renal biopsy as a predictive tool for renal recovery and OS.
Renal biopsy is also useful for the diagnosis of coexisting monoclonal immunoglobulin–related renal diseases, present in 12% of LCCN cases in our series, including AL amyloidosis and LCDD in 2.2% and 6.2% of patients, respectively. The association of LCCN with other monoclonal immunoglobulin–related renal diseases was observed in 16% of cases reported by Ecotière et al6 and 14% of those reported by Nasr et al.5 These patients require careful clinical assessment for extrarenal involvement that requires specific management. Furthermore, the addition of different renal lesions seems to influence renal prognosis in patients with MM. Zand et al50 reported that patients with pure LCDD have better renal prognosis than those with both LCDD and LCCN. In our study, patients with LCCN and coexisting amyloidosis or LCDD achieved lower eGFR during follow-up, compared with those with pure LCCN.
The methodology used in this study deserves further comments. We considered all individuals at onset, but for follow-up assessment, we excluded those in whom no improvement in renal function could be expected (ie, who died prematurely, or did not receive a minimum of 1 cycle of chemotherapy and 2 months of follow-up). To eliminate the effect of ongoing FLC injury on top of the initial biopsy findings, we also considered only those who achieved a ≥60% drop in FLC levels after 1 cycle17 and found the same findings according to univariate analysis. Inevitably, selecting these patients resulted in increased final best eGFR. This approach will have to be taken into account when comparing data from other cohorts.
Certain limitations must be addressed. In this retrospective study, antimyeloma regimens varied over time. It is possible that future treatments will lead to a greater renal recovery and modify the independent predictors identified here. The choice of the main predictive outcome, best eGFR, can occur at various times, and results may be different when considering eGFR at 1, 3, or 6 months. Nevertheless, this outcome proved superior to renal response according to the IMWG criteria, and other outcomes such as dialysis dependency identified similar predictors. Also, the decision to perform a renal biopsy in patients with AKI may have influenced the results of this study. Because it is not routinely performed in most centers, this overselection may have excluded individuals with poor prognosis or less rapid or severe AKI.
In conclusion, this study shows the importance of renal biopsy findings in predicting the renal response in LCCN. As in previous studies,33,51,52 our findings also confirm the safety of the renal biopsy in patients with MM, similar to that of other populations.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank their colleagues from the participating centers who provided insight and expertise that greatly assisted the research: CHU Poitiers (Poitiers, France), Mayo Clinic (Rochester, MN), University of Alabama at Birmingham (Birmingham, AL), Hôpital Necker-Enfants Malades (Paris, France), Hospital Fernando Fonseca (Lisbon, Portugal), University of Alberta (Edmonton, AB, Canada), Brigham and Women’s Hospital (Boston, MA), St. Giovanni Bosco Hospital (Turin, Italy), Azienda Ospedaliera G. Brotzu (Cagliari, Italy) and Hôpital Maisonneuve-Rosemont (Montréal, QC, Canada).
P.W.S. is supported by a Merit Award (2 I01 CX001326) from the US Department of Veterans Affairs Clinical Sciences R&D (CSRD) Service and a National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases George M. O'Brien Kidney and Urological Research Centers Program grant (5 P30 DK079337).
Footnotes
Requests for original data should be sent to the corresponding author (Virginie Royal; e-mail: virginie.royal@umontreal.ca).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.R., S.H.N., F.B., N.L., and S.T. designed the study; all authors participated in the acquisition of data; S.T. and V.R. analyzed the data, and created the figures and tables; V.R., S.T., F.B., and N.L. drafted the paper; and all authors revised and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Virginie Royal, Division of Pathology, Hôpital Maisonneuve-Rosemont, 5415 Boulevard de l’Assomption, Montréal, QC, Canada, H1T2M4; e-mail: virginie.royal@umontreal.ca.
REFERENCES
- 1.Kyle RA. Multiple myeloma: review of 869 cases. Mayo Clin Proc. 1975;50(1):29-40. [PubMed] [Google Scholar]
- 2.Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J; The Nordic Myeloma Study Group . Renal function in newly diagnosed multiple myeloma—a demographic study of 1353 patients. Eur J Haematol. 1994;53(4):207-212. [DOI] [PubMed] [Google Scholar]
- 3.Bladé J, Fernández-Llama P, Bosch F, et al. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med. 1998;158(17):1889-1893. [DOI] [PubMed] [Google Scholar]
- 4.Knudsen LM, Hjorth M, Hippe E; Nordic Myeloma Study Group . Renal failure in multiple myeloma: reversibility and impact on the prognosis. Eur J Haematol. 2000;65(3):175-181. [DOI] [PubMed] [Google Scholar]
- 5.Nasr SH, Valeri AM, Sethi S, et al. Clinicopathologic correlations in multiple myeloma: a case series of 190 patients with kidney biopsies. Am J Kidney Dis. 2012;59(6):786-794. [DOI] [PubMed] [Google Scholar]
- 6.Ecotière L, Thierry A, Debiais-Delpech C, et al. Prognostic value of kidney biopsy in myeloma cast nephropathy: a retrospective study of 70 patients. Nephrol Dial Transplant. 2016;31(5):850. [DOI] [PubMed] [Google Scholar]
- 7.Montseny JJ, Kleinknecht D, Meyrier A, et al. Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol Dial Transplant. 1998;13(6):1438-1445. [DOI] [PubMed] [Google Scholar]
- 8.2014 International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma [in Japanese]. Nihon Rinsho. 2016;74(suppl 5):264-268. [PubMed] [Google Scholar]
- 9.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548. [DOI] [PubMed] [Google Scholar]
- 10.Hutchison CA, Batuman V, Behrens J, et al. ; International Kidney and Monoclonal Gammopathy Research Group . The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2011;8(1):43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, Sonneveld P, Leung N, et al. International Myeloma Working Group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol. 2016;34(13):1544-1557. [DOI] [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Delimpasi S, Katodritou E, et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol. 2014;25(1):195-200. [DOI] [PubMed] [Google Scholar]
- 13.Uttervall K, Duru AD, Lund J, et al. The use of novel drugs can effectively improve response, delay relapse and enhance overall survival in multiple myeloma patients with renal impairment. PLoS One. 2014;9(7):e101819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheid C, Sonneveld P, Schmidt-Wolf IG, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99(1):148-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antlanger M, Dust T, Reiter T, et al. Impact of renal impairment on outcomes after autologous stem cell transplantation in multiple myeloma: a multi-center, retrospective cohort study. BMC Cancer. 2018;18(1):1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Bernard R, Chodirker L, Masih-Khan E, et al. Efficacy, toxicity and mortality of autologous SCT in multiple myeloma patients with dialysis-dependent renal failure. Bone Marrow Transplant. 2015;50(1):95-99. [DOI] [PubMed] [Google Scholar]
- 17.Hutchison CA, Cockwell P, Stringer S, et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol. 2011;22(6):1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kastritis E, Anagnostopoulos A, Roussou M, et al. Reversibility of renal failure in newly diagnosed multiple myeloma patients treated with high dose dexamethasone-containing regimens and the impact of novel agents. Haematologica. 2007;92(4):546-549. [DOI] [PubMed] [Google Scholar]
- 19.Gonsalves WI, Leung N, Rajkumar SV, et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2015;5(3):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma [published correction appears in J Clin Oncol. 2005;23(25):6281]. J Clin Oncol. 2005;23(15):3412-3420. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346. [DOI] [PubMed] [Google Scholar]
- 22.Forni LG, Darmon M, Ostermann M, et al. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43(6):855-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zee J, Hodgin JB, Mariani LH, et al. Reproducibility and feasibility of strategies for morphologic assessment of renal biopsies using the Nephrotic Syndrome Study Network Digital Pathology Scoring System. Arch Pathol Lab Med. 2018;142(5):613-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol. 2008;61(Pt 1):29-48. [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. [PubMed] [Google Scholar]
- 26.Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. [DOI] [PubMed] [Google Scholar]
- 27.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Quan H, Ng J, Stepanavage ME. Some statistical methods for multiple endpoints in clinical trials. Control Clin Trials. 1997;18(3):204-221. [DOI] [PubMed] [Google Scholar]
- 29.Dimopoulos MA, Roussou M, Gkotzamanidou M, et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia. 2013;27(2):423-429. [DOI] [PubMed] [Google Scholar]
- 30.Dimopoulos MA, Richardson PG, Schlag R, et al. VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27(36):6086-6093. [DOI] [PubMed] [Google Scholar]
- 31.de Vries JC, Oortgiesen B, Hemmelder MH, et al. Restoration of renal function in patients with newly diagnosed multiple myeloma is not associated with improved survival: a population-based study. Leuk Lymphoma. 2017;58(9):1-9. [DOI] [PubMed] [Google Scholar]
- 32.Tandon N, Sidana S, Rajkumar SV, et al. Outcomes with early response to first-line treatment in patients with newly diagnosed multiple myeloma. Blood Adv. 2019;3(5):744-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bridoux F, Carron PL, Pegourie B, et al. ; MYRE Study Group . Effect of high-cutoff hemodialysis vs conventional hemodialysis on hemodialysis independence among patients with myeloma cast nephropathy: a randomized clinical trial. JAMA. 2017;318(21):2099-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung N, Gertz MA, Zeldenrust SR, et al. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int. 2008;73(11):1282-1288. [DOI] [PubMed] [Google Scholar]
- 35.Rota S, Mougenot B, Baudouin B, et al. Multiple myeloma and severe renal failure: a clinicopathologic study of outcome and prognosis in 34 patients. Medicine (Baltimore). 1987;66(2):126-137. [DOI] [PubMed] [Google Scholar]
- 36.Pasquali S, Zucchelli P, Casanova S, et al. ; Renal Immunopathology Group . Renal histological lesions and clinical syndromes in multiple myeloma. Clin Nephrol. 1987;27(5):222-228. [PubMed] [Google Scholar]
- 37.Johnson WJ, Kyle RA, Pineda AA, O’Brien PC, Holley KE. Treatment of renal failure associated with multiple myeloma. Plasmapheresis, hemodialysis, and chemotherapy. Arch Intern Med. 1990;150(4):863-869. [PubMed] [Google Scholar]
- 38.Sethi S, D’Agati VD, Nast CC, et al. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int. 2017;91(4):787-789. [DOI] [PubMed] [Google Scholar]
- 39.Yadav P, Hutchison CA, Basnayake K, et al. Patients with multiple myeloma have excellent long-term outcomes after recovery from dialysis-dependent acute kidney injury. Eur J Haematol. 2016;96(6):610-617. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava A, Palsson R, Kaze AD, et al. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol. 2018;29(8):2213-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ying WZ, Li X, Rangarajan S, Feng W, Curtis LM, Sanders PW. Immunoglobulin light chains generate proinflammatory and profibrotic kidney injury. J Clin Invest. 2019;129(7):2792-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Säemann MD, Weichhart T, Zeyda M, et al. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4-dependent mechanism. J Clin Invest. 2005;115(2):468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darisipudi MN, Thomasova D, Mulay SR, et al. Uromodulin triggers IL-1β-dependent innate immunity via the NLRP3 inflammasome. J Am Soc Nephrol. 2012;23(11):1783-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ying WZ, Allen CE, Curtis LM, Aaron KJ, Sanders PW. Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. J Clin Invest. 2012;122(5):1777-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morabito F, Gentile M, Mazzone C, et al. Safety and efficacy of bortezomib-melphalan-prednisone-thalidomide followed by bortezomib-thalidomide maintenance (VMPT-VT) versus bortezomib-melphalan-prednisone (VMP) in untreated multiple myeloma patients with renal impairment. Blood. 2011;118(22):5759-5766. [DOI] [PubMed] [Google Scholar]
- 46.Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Outcomes of newly diagnosed myeloma patients requiring dialysis: renal recovery, importance of rapid response and survival benefit. Blood Cancer J. 2017;7(6):e571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimopoulos M, Weisel K, van de Donk NWCJ, et al. Pomalidomide plus low-dose dexamethasone in patients with relapsed/refractory multiple myeloma and renal impairment: results from a phase II trial. J Clin Oncol. 2018;36(20):2035-2043. [DOI] [PubMed] [Google Scholar]
- 48.Dimopoulos M, Siegel D, White DJ, et al. Carfilzomib vs bortezomib in patients with multiple myeloma and renal failure: a subgroup analysis of ENDEAVOR. Blood. 2019;133(2):147-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finkel KW, Cohen EP, Shirali A, Abudayyeh A; American Society of Nephrology Onco-Nephrology Forum . Paraprotein-related kidney disease: evaluation and treatment of myeloma cast nephropathy. Clin J Am Soc Nephrol. 2016;11(12):2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zand L, Nasr SH, Gertz MA, et al. Clinical and prognostic differences among patients with light chain deposition disease, myeloma cast nephropathy and both. Leuk Lymphoma. 2015;56(12):3357-3364. [DOI] [PubMed] [Google Scholar]
- 51.Fish R, Pinney J, Jain P, et al. The incidence of major hemorrhagic complications after renal biopsies in patients with monoclonal gammopathies. Clin J Am Soc Nephrol. 2010;5(11):1977-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soares SM, Fervenza FC, Lager DJ, Gertz MA, Cosio FG, Leung N. Bleeding complications after transcutaneous kidney biopsy in patients with systemic amyloidosis: single-center experience in 101 patients. Am J Kidney Dis. 2008;52(6):1079-1083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.