SUMMARY
Human and mouse thermal physiology differ due to dissimilar body sizes. Unexpectedly, in mice we found no ambient temperature zone where both metabolic rate and body temperature were constant. Body temperature began increasing once cold-induced thermogenesis was no longer required. This result reproduced in male, female, C57BL/6J, 129, chow-fed, diet-induced obese, and ob/ob mice as well as Trpv1−/−;Trpm8−/−;Trpa1−/− mice lacking thermal sensory channels. During the resting-light phase, the energy expenditure minimum spanned ~4°C of ambient temperature, whereas in the active-dark phase it approximated a point. We propose the concept of a thermoneutral point (TNP), a discrete ambient temperature below which energy expenditure increases and above which body temperature increases. Humans do not have a TNP. As studied, the mouse TNP is ~29°C in light phase and ~33°C in dark phase. These observations inform how thermoneutrality is defined and how mice are used to model human energy physiology and drug development.
In Brief
Škop et al. show that the mouse dark-phase thermoneutral zone is a thermoneutral point (TNP), defined as a discrete ambient temperature below which energy expenditure increases and above which body temperature increases. The mouse TNP changes diurnally by ~4°C. Thus, studying mice strictly “at thermoneutrality” is not feasible.
Graphical Abstract
INTRODUCTION
By 1790, Lavoisier had recognized that energy expenditure of mammals increased in the cold (Lusk, 1928, reprinted 1976), and by 1876, Voit showed that it increased in a hot environment (Rubner, 1902, translated 1982). The ambient temperatures where metabolic rate is at a minimum is the usual definition of the thermoneutral zone (TNZ). A TNZ was depicted at least as early as 1934 (Kleiber and Dougherty, 1934), and the concept was advanced by Scholander’s classic studies (Scholander et al., 1950) and provides the framework for understanding thermal physiology (Cannon and Nedergaard, 2011; Gordon, 2012; Kleiber, 1975; Mount, 1973).
The TNZ is critical for understanding the thermal biology differences between mice and humans, which arise due to the 3,000-fold difference in body weight. Mice at typical housing temperatures (20°C–22°C) live below thermoneutrality, and about half of their total energy expenditure (TEE) is devoted to maintaining core body temperature (Tb). In contrast, humans generally live in a thermoneutral micro-environment, and their Tb is supported by the “waste” heat byproduct of metabolic processes. Human thermal biology is more organized around heat dissipation, rather than generation or conservation (Ganeshan and Chawla, 2017; Gordon, 1993; Maloney et al., 2014; Reitman, 2018). One can remove this difference by studying mice at thermoneutrality (Feldmann et al., 2009; Fischer et al., 2018; Karp, 2012; Maloney et al., 2014; Overton, 2010; Reitman, 2018), although some have suggested using a cooler environment (Keijer et al., 2019; Speakman and Keijer, 2013). Thus, it is necessary to understand the characteristics of the mouse TNZ.
Energy expenditure has guided the definition of thermoneutrality, with the TNZ determined from the thermoregulatory response curve (of TEE versus ambient temperature [Ta]), which has three regions (e.g., Figure 4C; Kleiber, 1975). In the center is the TNZ, where metabolic rate is minimal and constant. When measured in the awake, resting, and postabsorptive state, this is the basal metabolic rate (BMR). Heat loss in the TNZ is controlled chiefly by regulating blood flow to superficial sites (Cannon and Nedergaard, 2011; Gordon, 2012; Kingma et al., 2012; Romanovsky, 2018; Romanovsky et al., 2002). Below the TNZ, as the environment becomes cooler, metabolic rate increases linearly, and this line is the energy required to maintain Tb, the “thermostatic heat requirement,” with the increment over the BMR being cold-induced thermogenesis. The intersection of the BMR and thermostatic heat requirement lines is the lower critical temperature (Tlc), demarcating the transition between energy-requiring and energy-neutral thermoregulatory mechanisms. At a Ta above the TNZ, energy expenditure increases due to energy-consuming cooling mechanisms, transitioning at the upper critical temperature (Tuc). This curve shape is widely applicable in thermal ecology and is valid for cities (Hill et al., 2013) as well as organisms, including mice and humans (Ganeshan and Chawla, 2017; Gordon, 2017; Lichtenbelt et al., 2014; Nedergaard and Cannon, 2014; Speakman, 2013).
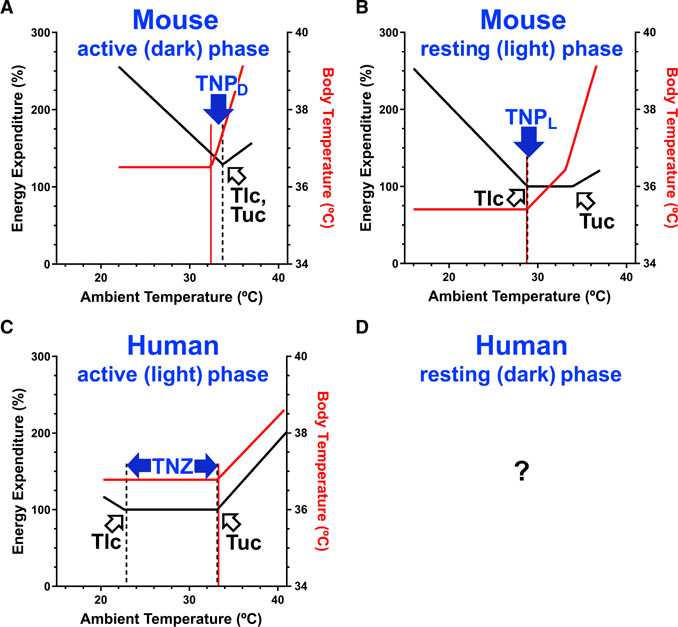
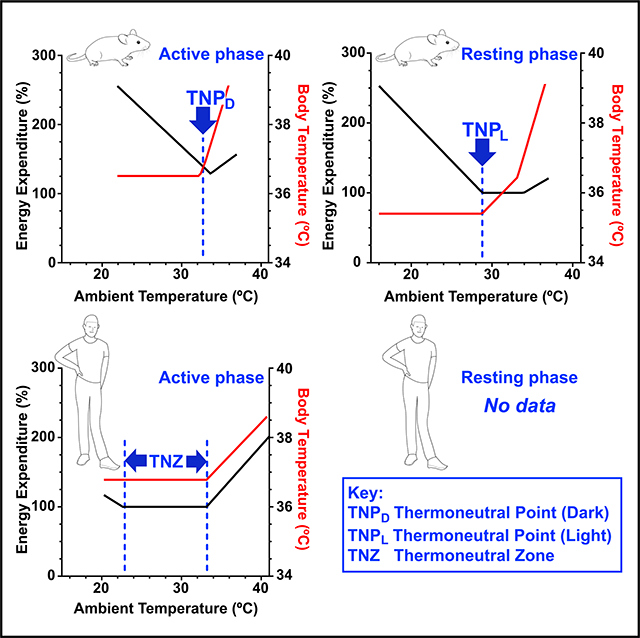
Figure 4. Thermoneutrality in Humans Compared to Mice.
(A and B) Mice have a thermoneutral point (TNP), a discrete ambient temperature below which energy expenditure increases and above which body temperature increases. The mouse TNP depends on the body temperature and, thus, the phase of the diurnal cycle; higher in the active-dark phase (TNPD) (A) and lower in the resting-light phase (TNPL) (B). Energy expenditure 100% is 0.23 kcal/h. Equation parameters are in Table S1. (C and D) In contrast, humans have a multi-degree thermoneutral zone (TNZ) in the active-light phase (C), determined by the Tlc (lower critical temperature; breakpoint in energy expenditure) and the Tuc (upper critical temperature; second breakpoint in energy expenditure). Humans do not have an active-phase TNP. We are not aware of comparable human studies in the resting phase (D). Body temperature data for lightly clothed humans are from McConnell and Yagloglou (1925). Energy expenditure data for lightly clothed humans are derived from Brychta et al. (2019) and McConnell and Yagloglou (1925); 100% is 72 kcal/h. Naked men have a 4°C warmer Tlc and a 44% steeper slope below the Tlc (Hill et al., 2013).
There has been ample investigation of mouse thermal biology at or below the Tlc (Abreu-Vieira et al., 2015; Fischer et al., 2016a, 2016b; Garami et al., 2011; Golozoubova et al., 2004; Högberg et al., 2006; Mount, 1971; Pertwee and Tavendale, 1977; Selman et al., 2001; Speakman and Rossi, 1999). The studies at higher Ta are fewer and, although some include Tb data, it was not analyzed in detail (Gordon, 1985; Herrington, 1940; Klaus et al., 1998; Meyer et al., 2004; Oufara et al., 1987; Pennycuik, 1967). Here, we combine the measurement of metabolic rate and Tb over a range of Ta in various types of mice in light and dark phases. Our unexpected findings lead us to propose the concept of the thermoneutral point (TNP) and to discuss the definition of the TNZ for mice and in a more general manner.
RESULTS
Mouse Tb Begins to Increase at the Tlc
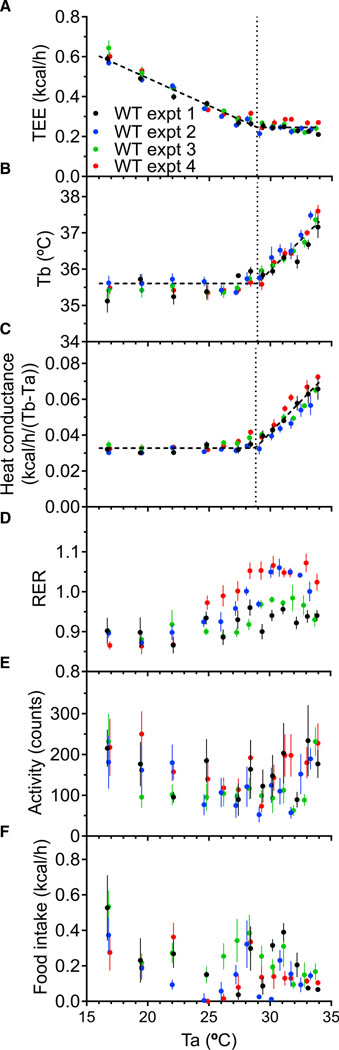
We measured the effect of Ta on TEE in the control cohorts from four independent experiments during the light phase (Figure 1). Concordant with prior observations, TEE decreased linearly with increasing Ta until a plateau was reached (Figure 1A). The Ta breakpoint (denoted the TlcEE [Tlc determined from TEE versus Ta analysis]), below which all heat preservation mechanisms are maximally recruited, was 28.90°C ± 0.15°C. No increase in TEE above this level was observed at warmer Ta, up to 34°C. The whole-body heat conductance (Mount, 1971) was constant at low Ta and then increased, with a Ta breakpoint, the Tlccond [Tlc determined from conductivity vs Ta analysis], of 28.78°C ± 0.08°C (Figure 1C). Thus, TlcEE and Tlccond agree remarkably well.
Figure 1. Effect of Ambient Temperature (Ta) on Thermal Physiology.
(A–F) Male C57BL/6J mice were studied in four independent experiments during light phase, with measurements of total energy expenditure (TEE) (A), body temperature (Tb) (B), heat conductance (C), respiratory exchange ratio (RER) (D), physical activity (E), and food intake (F). Lines and breakpoints (indicated by vertical lines) were calculated by mixed model regression analysis. For visual clarity, only Ta plateau mean ± SEM data points are depicted (each from 65 min, 5 sampling cycles, see also Figure S1).
See Table S1 for regression parameters and n.
The Tb response to a range of Ta produced a surprising result. As expected, at cooler Ta, the Tb was stable (35.60°C ± 0.07°C). However, at warmer Ta, the Tb increased, reaching 37.5°C at Ta = 33.7°C, with a slope of 0.337°C ± 0.029°C of Tb/°C of Ta (Figure 1B). Remarkably, the Ta breakpoint where the Tb started to increase (Tbinc) was at 28.92°C ± 0.11°C, coincident with the Tlc. No Ta range with both a minimum TEE and non-elevated Tb was detected.
We also measured the respiratory exchange ratio (RER), which was similar to the food quotient at cooler Ta and increased at warmer Ta (Figure 1D). Physical activity varied, sometimes greatly, both within and between experiments (Figure 1E). Because physical activity is a modest contributor to TEE (Abreu-Vieira et al., 2015; Moruppa, 1990; O’Neal et al., 2017; Virtue et al., 2012), we did not incorporate activity into these analyses. Food intake was higher in the cold and was variable (Figure 1F). We also did not incorporate the thermic effect of food into the analyses, due to its variability, small magnitude, and unknown time lag between food ingestion and metabolic rate increase in mice.
Thus, four independent cohorts demonstrate that Tb increases at the Tlc. There was no range of Ta over which both TEE and Tb were constant. Because TlcEE, Tlccond, and Tbinc all occur at the same Ta, we refer to this Ta as the TNP, which we define as the discrete Ta below which TEE increases and above which Tb increases.
Effect of Ta in Mice with Altered Thermal Physiology
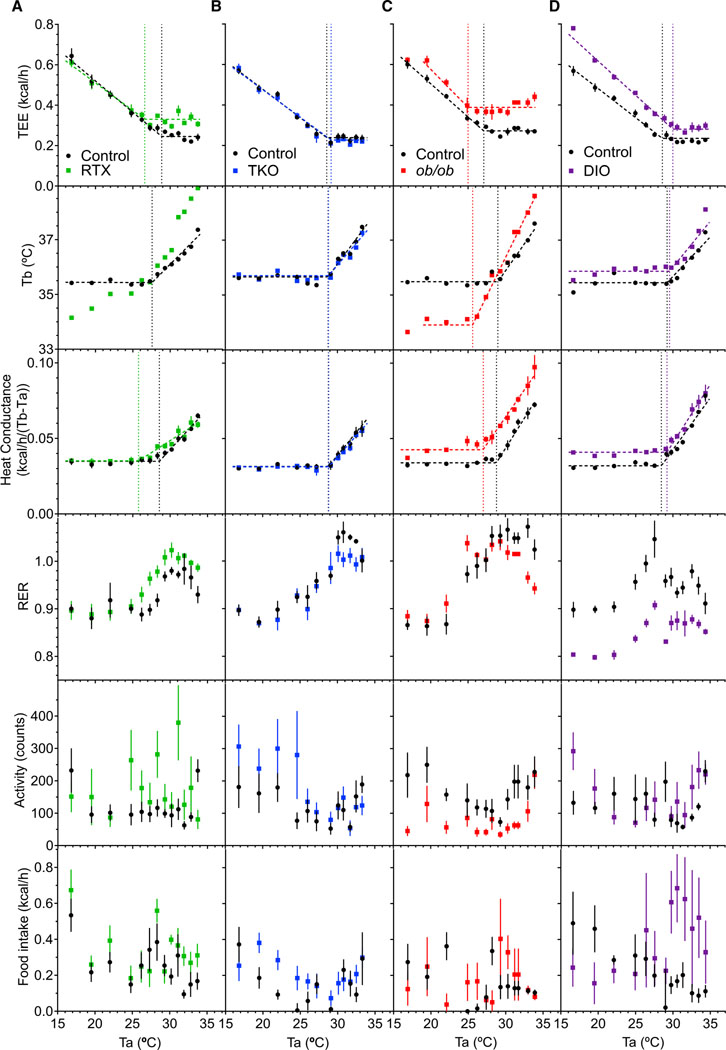
We extended the analysis to mouse models with altered thermal physiology, starting with resiniferatoxin (RTX)-treated mice, which have disrupted thermal sensing due to neonatal neuronal ablation (Cavanaugh et al., 2011; Sándor et al., 2009). At 22°C, the baseline Tb of RTX-treated mice was similar to that of controls, with no difference in light-phase Tb, dark-phase Tb, or diurnal rhythm (Figure S2). However, the RTX-treated mice showed significantly greater variation in Tb, with a 3.58°C ± 0.06°C span between the 5th and 95th percentiles, compared to a 2.86°C ± 0.11°C Tb span in controls (p = 0.0001).When exposed to different Ta, the RTX-treated mice were somewhat poikilothermic: at cooler Ta they were cooler than controls and at higher Ta they were warmer (Figure 2A). The TlcEE and Tlccond (26.59°C ± 0.48°C and 25.77°C ± 0.48°C, respectively) were 2°C–3°C cooler than controls. The plateau metabolic rate of the RTX-treated mice was elevated, possibly due to both increased heat loss and the increased Tb. Because there was no Ta range over which the Tb of RTX-treated mice was constant, it is not possible to calculate a Tbinc.
Figure 2. Effect of Ambient Temperature in Mice with Altered Thermal Physiology.
(A–D) Thermal biology of resiniferatoxin-treated (RTX) (A), Trpv1−/−;Trpm8−/−;Trpa1−/− (TKO) (B), ob/ob (C), and diet-induced obese (DIO) (D) mice.
See Figure 1 legend for details and Table S1 for regression parameters and n.
We next studied Trpv1−/−;Trpm8−/−;Trpa1−/− (TKO) mice with germline ablation of three temperature-sensing channels. In TKO mice, the light-phase Tb, dark-phase Tb, Tb diurnal rhythm, Tb span, and body weight were not significantly different from controls (Figure S2; Table S1). The TKO mice also did not differ from controls in the Ta dependence of their Tb, TEE, or conductance (Figure 2B). Thus, deletion of thermal sensory channels did not detectably alter the TNP or other measured thermal physiology parameters, in marked contrast to RTX-treated mice.
Thermal Physiology in Leptin-Deficient and Obese Mice
Leptin-deficient (ob/ob) mice do not sense their energy stores and, thus, behave as if they are starving: increasing food intake, reducing activity, lowering Tb, and becoming obese (Fischer et al., 2016b; Högberg et al., 2006; Trayhurn and James, 1978; Figure 2C; Table S1). At 22°C, the Tb of ob/ob mice was reduced compared to controls (by 0.76°C ± 0.32°C in light and 0.87°C ± 0.25°C in dark phase) with an intact diurnal rhythm (Figure S2). Tb was approximately constant over Ta of 19°C–25°C but declined more at 16°C. The TlcEE (25.00°C ± 0.34°C) and Tbinc (25.61°C ± 0.19°C) were similarly reduced, by ~2°C–3°C. Thus, ob/ob mice coordinately orchestrate their thermal physiology to regulate Tb, aiming for a lower target Tb (“set point”). Similar to the controls, ob/ob mice have a TNP.
Diet-induced obese (DIO) mice had higher Tb and lower Tb span than the controls (Figure S2); we have not observed a higher Tb and lower Tb span in other DIO cohorts (Abreu-Vieira et al., 2015). The RER of DIO mice was lower, reflecting the lower food quotient (Figure 2D). TEE of DIO mice was higher at all Ta. The TlcEE (29.99°C ± 0.20°C), Tlccond (29.21°C ± 0.12°C), and Tbinc (29.55°C ± 0.13°C) occurred at similar Tas, which are slightly higher than the chow-fed controls. Thus, DIO mice also do not have a Ta range over which TEE and Tb are constant.
These data suggest that despite differences in values of thermal biology parameters, the thermal behavior of TKO, ob/ob, and DIO mice obey the same basic principles governing thermal biology. None of the mice had a range of Ta over which both TEE and Tb were constant.
Thermal Biology at Higher Tas
We next investigated if TEE increases at higher Ta (>34°C). Indeed, TEE increased, beginning at Ta = 33.91°C ± 0.29°C (TEE_R [breakpoint of the TEE versus Ta graph, where TEE starts to rise with Ta]; Figure S3A). Tb did not increase linearly, so data were fitted using two Ta breakpoints. One breakpoint was 28.76°C ± 0.13°C (Tbinc). The second was at 33.15°C ± 0.08°C (Tb_R), above which the Tb increased steeply, 0.780°C ± 0.035°C Tb/°C Ta (Figure S3B). The TEE and steep Tb increase were accompanied by increased physical activity (Figure S3E), which may partially explain the TEE increase. The high physical activity, low food intake (Figure S3F), and sharp Tb increases indicate that above Ta ~33°C–34°C, Tb regulatory mechanisms are overwhelmed and the mice are under a qualitatively different and severe heat stress.
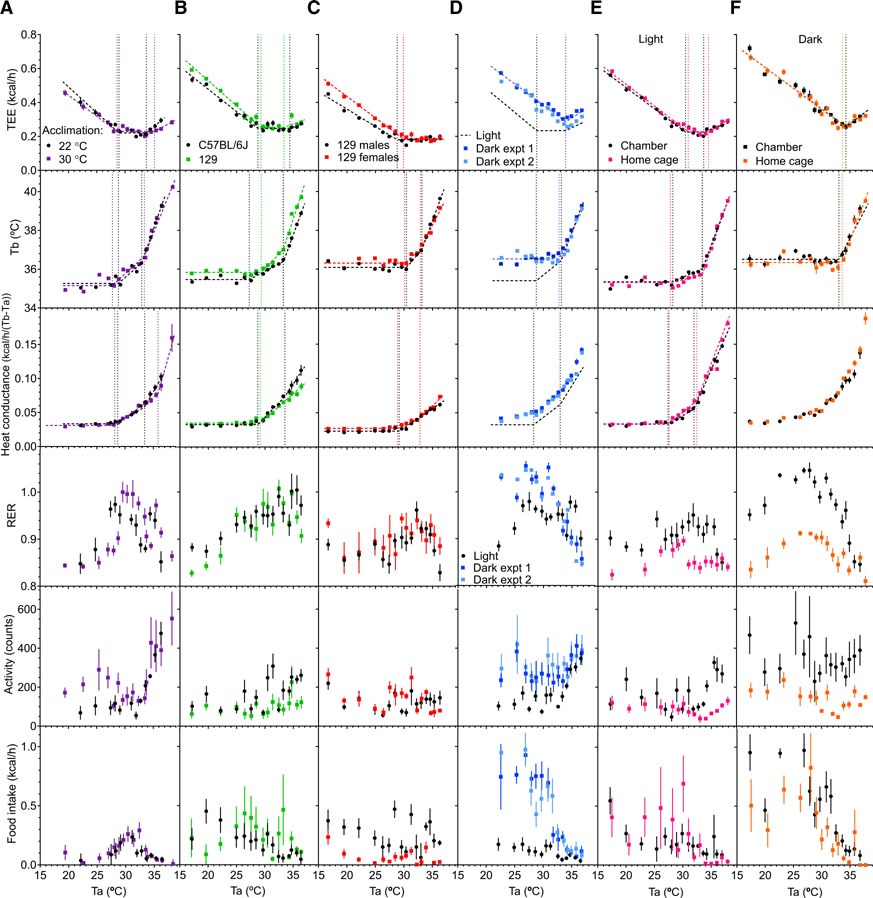
Mice were housed at 30°C for 10 days for warm acclimation. After acclimation, light-phase Tb at 30°C was 36.08°C ± 0.14°C (versus 35.49°C ± 0.08°C in unacclimated mice at 22°C, p = 0.0099) and dark-phase Tb was 36.80°C ± 0.11°C (versus 36.49°C ± 0.07°C in unacclimated mice at 22°C, p = 0.052). Although TEE_R increased slightly, there was no clear effect of thermal acclimation on TlcEE, Tlccond, or Tbinc (Figure 3A; Table S1). There was also no major effect on thermal parameters of genotype or sex (Figures 3B and 3C; Table S1).
Figure 3. Thermal Biology at Higher Ambient Temperatures.
(A) Effect of warm acclimation (30°C for 10 days) versus controls kept at 22°C.
(B) Effect of C57BL/6J versus 129 genotype.
(C) Effect of sex in 129 mice; male versus female.
(D) Light phase (from Figure S3) compared to dark phase.
(E) Light phase comparison of chamber and home cage.
(F) Dark phase comparison of chamber and home cage. Mice were studied during light phase (except D and F). See Figure 1 legend for details and Table S1 for regression parameters and n.
Different Thermal Biology during the Dark Phase
We next investigated thermal biology during the dark (active) phase. As expected, the mice were more active, ate more, and had a ~1°C higher Tb (Figures 3D and S2). In addition, the TEE versus Ta graph was strikingly different from the light phase, with the minimum TEE being restricted to a very narrow Ta range, approximating a point. This dark-phase TlcEE (33.94°C ± 0.14°C) occurred coincident with the higher light-phase breakpoint (TEE_R). The Tb versus Ta graph also showed a single breakpoint (32.77°C ± 0.13°C), coincident with the higher light-phase breakpoint (Tb_R), with the slope above this point (0.62°C ± 0.03°C Tb/°C Ta) similar to the slope above the light phase Tb_R. The Tb breakpoint may be at a slightly lower Ta than the TEE breakpoint. We designate the single dark-phase breakpoint as the TNPD. The conductance versus Ta plot was curvilinear, without clear linear portions. These data demonstrate fundamental differences between light and dark phase thermal biology. In dark phase, the minimum TEE zone, the TNZ, approximates a point (the TNPD), above which both TEE and Tb increase.
Generality of the Observations
One limitation of our data is that by testing many Tas, an individual Ta is represented by few data points. To address this, all the data were used in each regression analysis. To probe the effect of a longer time (24 h) at each Ta, we re-analyzed previous data (Abreu-Vieira et al., 2015) by using segmented regression and obtained similar results as with more rapid Ta transitions (Table S1). We also tested more typical vivarium conditions with a second indirect calorimetry system by using standard “home cages” with bedding. The same mice were studied in both the original CLAMS and the home cage systems under both light-phase and dark-phase conditions (Figures 3E and 3F). The data and parameters calculated from the two systems agreed remarkably well. Thus, the observations do not depend on the specific calorimetry system or caging conditions, supporting the robustness and generality of the observations.
DISCUSSION
We measured TEE at various Tas with continuous Tb monitoring in mice and found no range of Tas over which both TEE and Tb were constant (Figure 4). Mouse thermal physiology was also different between the dark and light periods. During the dark phase, a single transition (TNPD) occurred in both the Tb and TEE, at a Ta of ~33°C. Further investigation is needed to determine if the Tb increase started at the TEE minimum or just below it. At Tas below the TNPD, Tb was constant and TEE increased, whereas above the TNPD, both Tb and TEE increased.
In contrast, during the light phase there were two breakpoints. Above ~29°C (the TNPL [light phase TNP]), Tb increased gently (~0.3°C Tb/°C Ta) and TEE was constant. Above ~33°C (the TNPD), the TEE increased and the Tb increased more rapidly, with similar slopes in the light and dark phases (~0.7°C Tb/°C Ta). This indicates that between TNPL and TNPD, the Tb target (or “set”) point is regulated upward from the lower light-resting phase Tb to the warmer dark-active phase Tb. The modestly increasing Tb illustrates a mixed approach in thermal physiology, dissipating some and storing some of the excess heat, reducing the demand for heat loss. Once Tb reaches the TNPD, heat loss mechanisms are insufficient and overwhelmed.
The utility of the TNP concept is its explicit incorporation of Tb, which is more variable in mammals with small body sizes. Prior data in gerbils (Pan et al., 2014), hamsters (Zhao et al., 2014), and mice (Fischer et al., 2016b; Meyer et al., 2004) showing an increase in Tb at a lower Ta than the increase in metabolic rate are consistent with our observations. Very small mammals have a minimal thermal shell, so vasoconstriction and vasodilation have a relatively modest effect and occur over a narrow Ta span. Although we (Reitman, 2018) and others (Ganeshan and Chawla, 2017; Gordon, 2017; Lichtenbelt et al., 2014; Nedergaard and Cannon, 2014; Speakman, 2013) have depicted mice having a broad TNZ, the dark-phase TNZ being a point is a consequence of the small body size.
Relationship between the TNP and TNZ
The TNZ is formally defined as “the Ta range in which temperature regulation is achieved only by control of sensible heat loss, i.e., without regulatory changes in metabolic heat production or evaporative heat loss” (Bligh and Johnson, 1973; IUPS, 2001) and does not include Tb. We define TNP as a discrete Ta below which TEE increases and above which Tb increases, explicitly incorporating Tb.
Because the TNZ and TNP are defined differently, there is not a constant relationship between them. In the mouse dark phase, the TNZ is a point and the same as the TNPD. In contrast, in the mouse light phase (assuming Tb is regulated by non-evaporative heat loss, which we did not measure), TEE is constant over ~4°C, defining a TNZ. Because Tb is gradually increasing in this TNZ, the lower bound of the TNZ (the Tlc) is coincident with the TNPL.
No Evidence for a TNP in Humans
Not all homeotherm organisms will have a TNP. For example, humans have an active phase TNZ with a Tlc of 21°C –23°C (lightly clothed; Brychta et al., 2019) to 26°C–27°C (naked; Hill et al., 2013; reviewed in Brychta and Chen, 2017; Figure 4C). Fewer studies have measured both TEE and Tb in a range of warm conditions (Bradbury et al., 1967; Hardy and Du Bois, 1940; Hardy and Stolwijk, 1966; Houghton et al., 1929; McConnell and Yagloglou, 1925; Rubner, 1902, translated 1982). From data in McConnell and Yagloglou, (1925), we estimate an “effective temperature” Tuc of ~33°C for lightly clothed men (the effective temperature is determined from air velocity and wet and dry bulb temperatures and is equal or below the dry bulb temperature), which is also where Tb started to increase (Figure 4C). Thus, the human active-light phase TNZ (lightly clothed) is ~21°C–23°C to ~33°C and there is no TNP.
The human inactive-dark phase has a lower Tb (Refinetti, 2010) and TEE, so compared to the active-light phase, the Tlc and Tuc will be similar or slightly lower, the TNZ similar or slightly broader, and, again, no TNP is expected. However, we are not aware of human studies of both Tb and TEE during a range of nighttime Ta.
TNZ Definition
A mouse housed in the light phase at 33°C is physiologically different from one housed at 29°C; yet, both could be at thermoneutrality under the current TNZ definition. Should the TNZ definition be revised? One could rephrase the TNZ definition as “the Ta range between the Tlc and Tuc,” focusing on the Tlc and Tuc individually. The current Tlc definition, the Ta below which energy-expending processes are required to maintain Tb, or “the Ta below which the rate of metabolic heat production of a resting thermoregulating tachymetabolic animal must be increased by shivering and/or nonshivering thermogenesis in order to maintain thermal balance,” (IUPS, 2001) needs no reconsideration.
The Tuc is commonly defined as the Ta above which metabolic rate starts to increase (Ganeshan and Chawla, 2017; Gordon, 2017; Lichtenbelt et al., 2014; Nedergaard and Cannon, 2014; Romanovsky, 2018; Speakman, 2013) or “the Ta above which the rate of evaporative heat loss of a resting thermoregulating animal must be increased … in order to maintain thermal balance” (IUPS, 2001). Evaporative heat dissipation, typically by respiratory or cutaneous water loss, is the main cooling mechanism at warm Ta and the only one when Ta is higher than Tb (although mice can groom saliva onto skin, this indicates severe heat stress and mice do not routinely use evaporative heat loss) (Adolph, 1947; Hainsworth, 1967; Perissin et al., 2000; Roberts et al., 1974; Szymusiak and Satinoff, 1981). However, increases in evaporative heat loss, metabolic rate, and Tb may each start at different Tas (Baldo et al., 2015; Cooper et al., 2018; McKechnie et al., 2017; O’Connor et al., 2017; Talbot et al., 2017). Humans increase evaporative loss at a lower Ta than the rise in metabolic rate and Tb (Gagge et al., 1967; Hardy and Du Bois, 1940).
The above Tuc definitions do not consider Tb, which is an important factor in small species. We suggest a consideration of Tb in the Tuc definition, for example, making the Tuc the highest Ta above which any of the following occurs: (1) resting metabolic rate increases, (2) rate of evaporative heat loss increases, or (3) Tb increases. Use of the Tb increase as a Tuc definition has been opposed to avoid confusion with the upper temperature survival limit (IUPS, 2001). However, in species or situations using little evaporative heat loss, the Tb increase seems like a reasonable option for the Tuc.
Our results underscore that Tb must be measured to fully understand energy homeostasis, particularly in small organisms.
Using Mice to Model Human Physiology
Understanding mouse thermal physiology improves the use of mice to model human physiology. Mice are typically housed below, whereas humans effectively live at, thermoneutrality. One option is to nullify cold-induced thermogenesis by studying mice at thermoneutrality, often using 30°C (Feldmann et al., 2009; Fischer et al., 2018; Karp, 2012; Maloney et al., 2014; Overton, 2010; Reitman, 2018). Another suggestion is that because human TEE is typically about 1.7 × BMR, one should choose a Ta (25°C–27°C) to study mice where their TEE is also 1.7 × BMR (Keijer et al., 2019; Speakman and Keijer, 2013). However, based on how mouse BMR is measured, others suggest that mice at 30°C are already at 1.8 × BMR (Fischer et al., 2018, 2019).
How do our results inform studying mice at thermoneutrality? By the classic energy expenditure TNZ definition, in the light phase, the TNPL to TNPD range is the TNZ. However, to avoid a Tb increase, mice in the light phase should be studied at the TNPL. In the dark phase, the TNZ is the TNPD, so mice should be studied at the TNPD. Thus, to minimize thermal physiology perturbations, mice would be housed at their TNP on a diurnal cycle (~29°C in light phase and ~33°C in dark phase, a four-degree change every 12 hours).
This analysis suggests that studying mice strictly at thermoneutrality is nearly impossible. Achieving sufficiently rapid and strict Ta control is difficult. The recommended mouse facility Ta is 20°C–26°C (National Research Council, 2011) and vivariums typically do not control Ta more tightly than ±1°C. Additionally, choosing the target Ta requires knowing the TNPs, which depend on experimental conditions: mouse Tlc (a mouse TNP surrogate) are reported to range from 24°C to 32°C (Speakman and Keijer, 2013). Variables that may affect mouse energy homeostasis and the TNP include genetics (e.g., ob/ob); relative humidity and air circulation (Gagge and Gonzalez, 1996); bedding (Gordon, 1993); group versus single housing (Gordon, 2017; Mount and Willmott, 1967); body weight (Speakman and Keijer, 2013); sex, and acclimation, noise, and stress in the vivarium.
We hypothesize that a Ta for chronically housing mice to better model human thermal biology would be just below the TNPL, while strenuously avoiding exceeding it. Under our conditions, a Ta of ~28°C–29°C might be a reasonable choice for wild-type mice. This allows the mouse to self-regulate energy expenditure and Tb, while minimizing cold-induced thermogenesis and allowing for the real-world practicalities of environmental thermal control. A fail safe would include monitoring Tb to ensure that it is not increasing.
A Ta of 30°C has been commonly used as a thermoneutral Ta. Experiments at this Ta increase the adiposity of mice, such as that induced by a high-fat diet (Feldmann et al., 2009) or with ablated UCP1 (Feldmann et al., 2009) or type 2 deiodinase (Castillo et al., 2011). Conversely, treatment with dinitrophenol (Goldgof et al., 2014) or a β3-adrenergic agonist (Xiao et al., 2015) reduced body weight at 30°C but not at 22°C. Other physiology that is different at 30°C includes worse vascular inflammation and atherosclerosis (Giles et al., 2016; Tian et al., 2016) and reduced tumor growth and improved immune response to tumors (Hylander et al., 2019; Kokolus et al., 2013). When using a Ta of 30°C, the physiologic effects could be due to reduced brown adipose tissue (BAT) activity, energy expenditure, food intake, or sympathetic tone; increased Tb; and/or other mechanisms. For example, an elevated Tb stimulates the immune system, and this could augment an inflammatory state, affecting response to infection (Evans et al., 2015). A warm environment prevents heat loss, precluding hypothermic states (Ganeshan et al., 2019).
In contrast, a high Ta (30°C) can be useful for studying acute physiology, such as the effect of a drug to increase energy expenditure. Mice have robust thermogenic mechanisms that are attenuated by housing at or above the Tlc. Thus, acutely moving mice from the customary 20°C–22°C to at or above the TNPL sensitizes the detection of a drug effect, preventing confounding by a compensatory reduction in cold-induced thermogenesis. A light-phase Ta of 30°C seems reasonable for this purpose.
In summary, we emphasize the concept of a TNP, below which TEE increases and above which Tb increases. In the mouse, the dark-phase and light-phase TNPs differ by ~4°C, raising questions about how to study the mouse at thermoneutrality. This knowledge informs the use of mice to model human energy physiology and drug development.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Marc Reitman (marc. reitman@nih.gov).
Materials Availability
This study did not generate new unique reagents, mouse lines, or other material.
Data Code Availability
The data and SAS code generated during this study are available for download at Open Science Framework: https://osf.io/r5nfs/.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Mice at 3–10 months of age were singly housed at 21–22°C with a 12:12-h dark:light cycle (lights on at 0600) in a clean, conventional facility with Teklad paper bedding (7099-TEK-fresh, Envigo, Indianapolis, IN) with water and chow (NIH-07 Envigo Inc, Madison, WI; 3.1 metabolizable kcal/g, food quotient 0.909) provided ad libitum. Experiments were approved by the NIDDK Institutional Animal Care and Use Committee (protocol K016-DEOB-17). Control (WT) mice were male C57BL/6J (#000664; Jackson Laboratories, Bar Harbor, ME). Leptin-deficient (#000632; Jackson Laboratories) ob/ob male and wild-type 129 (#002448) male and female mice were obtained from Jackson Laboratories (Bar Harbor, ME). Diet induced obese (DIO) C57BL/6J (#000664; Jackson Laboratories, Bar Harbor, ME) male mice were prepared by high fat diet (60 cal% from fat, D12492, Research Diets, New Brunswick, NJ; 5.24 metabolizable kcal/g, food quotient 0.793) feeding for 4 months starting at 8 weeks of age. Mice number, age, and body weight for each experiment are in Table S1.
Treatment with resiniferatoxin (RTX; 20 mM in ethanol, diluted with saline; 50 μl of 20, 40, then 80 μM s.c. on 3 consecutive days, starting at 3–7 days of age), a potent TRPV1 agonist, was used to ablate neonatal TRPV1-positive neurons in C57BL/6J male mice. Successful ablation was confirmed by the lack of wiping movements after administration of 20 μl of 0.1% capsaicin into the eye and by the lack of licking hind paws in a hot plate test (Cavanaugh et al., 2011; Sándor et al., 2009).
Trpv1−/−;Trpm8−/−;Trpa1−/− triple knockout (TKO) male mice were provided by Dr. Alexander Chesler, NCCIH, bred from the single gene knockout mice (Bautista et al., 2006, 2007; Caterina et al., 2000). TRPV1, TRPM8, and TRPA1, contribute to sensing hot, cool, and possibly cold, respectively. Mice with single deletions exhibit loss of Tb response to cognate ligands (Tan and Knight, 2018). However, their reported baseline Tb phenotype is subtle: increased Tb span in Trpv1−/− (Szelényi et al., 2004), slightly reduced Tb at cool Ta in Trpm8−/− (Reimúndez et al., 2018), and none described for Trpa1−/− mice (Zygmunt and Hogestatt, 2014).
C57BL/6J male mice were warm-acclimated (30°C for 10 days) versus controls kept at 22°C. While a longer acclimation time was not tested, human adaptation to heat stress is near complete by one week (Périard et al., 2015), one week produces major changes in mouse BAT (Clayton and McCurdy, 2018), and 6 months at 30°C versus 21°C versus 4°C revealed no differences in a Scholander analysis (Fischer et al., 2016a). Thus, the 10-day adaptation to 30°C probably produces maximal or near-maximal acclimation.
METHOD DETAILS
Indirect calorimetry systems
TEE, respiratory exchange ratio (RER), food intake (floor feeder), and physical activity (infrared beam break as total activity, 0.5 inch spacing) were measured with an indirect calorimetry system (CLAMS using Oxymax software v5.52, Columbus Instruments, Columbus, OH). Tb was measured simultaneously by telemetry using G2 E-Mitter transponders implanted intraperitoneally (Starr Life Sciences, Oakmont, PA). Whole body heat conductance was calculated as TEE/(Tb-Ta) (Mount, 1971). Mice were housed individually with ad libitum access to food and water in chambers without bedding or nesting material (2.5 L volume, flow rate 0.5 L/min, sampling flow 0.4 L/min, settle time 55 s, measure time 5 s, each chamber sampled every 13 min, giving 5 sampling cycles per 65 min interval). The food intake and physical activity were measured per 13 minute interval. All 12 calorimetry chambers were housed in a single temperature-controlled environmental chamber. Ta was continuously monitored (U12–012 data logger, Onset, Bourne, MA) in an empty calorimetry chamber.
A second, ‘home cage’, indirect calorimetry system was used when noted (CLAMS-HC using Oxymax v5.52, Columbus Instruments). In this system mice were housed individually with ad libitum access to food (hanging feeder) and water in Tecniplast 1284 cages with ~95 g of wood chip bedding (7090 Teklad sani-chips, Envigo, Indianapolis, IN) with measured physical activity (infrared beam break as total activity, 1 inch spacing) and continual monitoring of Ta in each cage. Calorimetry parameters are: 7.75 L volume, 0.9 L/min flow rate, 0.6 L/min sampling flow, 15 s settle time, 5 s measure time, with each chamber sampled every 260 s. Thus, the food intake and physical activity were measured per 260 s interval, giving 14 sampling cycles per 61 min interval (Figures 3E and 3F). The activity and food intake measurements are not directly comparable between the chamber and home cage indirect calorimetry systems.
Mice were acclimated to the chambers or cages for 3 days at 22°C (30°C acclimated mice were acclimated to chambers at 30°C) before each study.
Ambient temperature changes setup
Previously, we studied mice for 24 h at each Ta (Abreu-Vieira et al., 2015). We compared that procedure (using Ta of 16, 22, 26, and 32°C) to a protocol where Ta was changed multiple times/day, similar to that used by Fischer et al. (2016a). In the current protocol, Ta was changed periodically during the light phase, using the data from the final 65 minutes (5 data points) of a given Ta, where it is plateauing. The TEE, Tb, and heat conductance determined from the two protocols agreed well (Figure S1A). The physical activity, food intake, and RER varied more and agreed less well between the methods. We concluded that the shorter protocol is suitable for further studies.
We evolved protocols with: i) measurements between 0900 and 1600, during light phase (1900 to 0400, during dark phase), to avoid altered physiology associated with phase change, ii) using multiple consecutive days in the chambers, with the overnight Ta = 25°C (30°C in case of 30°C acclimated mice), and iii) using a 2 h interval for Ta changes of 1°C, with longer interval times for larger Ta changes. Protocols for Ta changes setups are sumarized in Table S2 and an example of temperature changes measured in cage is in Figure S1B.
For visual clarity the points in Figures 1, 2, 3, and S3 represents mean Ta and mean value of parameter from last 65 minutes before Ta change, whereas the regression analyses used data from the full intervals (all points from gray shaded areas in Table S2; excluding only data where Ta changed steeply, > 2°C/h).
Body temperature telemetry
G2 E-Mitter transponders (Starr Life Sciences, Oakmont, PA) were implanted intraperitoneally under isoflurane anesthesia (5% induction, 1.2% maintenance; Baxter Healthcare Corporation, Deerfield, IL) with Prevail (flunixin meglumine) analgesia (2.2 mg/kg sc at operation and daily for two days). Mice were studied at least one week after surgery. Tb was continuously measured by ER4000 energizer/receivers and 1 min means collected with VitalView software (Starr Life Sciences, Oakmont, PA), or by indirect calorimetry systems (Columbus Instruments, Columbus, OH)
QUANTIFICATION AND STATISTICAL ANALYSIS
The thermal biology parameters were evaluated by segmented linear regression with Ta as the independent variable and individual mice as random effect. The dependent variable is either TEE, Tb, or heat conductance. In experiments with Ta < 34°C, data were fit to two-segment models with four parameters: slope of line 1, breakpoint Ta, value for dependent variable at breakpoint, and slope of line 2. For TEE versus Ta, the slope of line 2 was fixed to zero and the breakpoint is where the TEE stops decreasing and plateaus (the TlcEE). For heat conductance versus Ta, the slope of line 1 was fixed to zero and the breakpoint is where the heat conductance begins to increase (the Tlccond). For Tb versus Ta, the slope of line 1 was fixed to zero and the breakpoint is where the Tb begins to increase (the Tbinc).
In light-phase experiments including higher Ta (> 34°C), the data were fitted to three-segment models, with six parameters: slope of line 1, breakpoint 1 Ta, value for dependent variable at breakpoint 1, slope of line 2, breakpoint 2 Ta, and slope of line 3. For TEE versus Ta, the slope of line 2 was fixed to zero, breakpoint 1 is where the TEE stops decreasing and plateaus (TlcEE), and breakpoint 2 is where the TEE starts increasing (the TEE_R). For Tb versus Ta, the slope of line 1 was fixed to zero, breakpoint 1 is where the Tb begins to increase (Tbinc), and breakpoint 2 is where the rate of Tb rise increases (the Tb_R).
For dark-phase experiments including Ta > 34°C, the TEE data were fit to two-segment models with no slope restrictions. Only one TEE breakpoint (the TEE_R) was used because adding a second breakpoint did not improve the fit. For Tb versus Ta, the slope of line 1 was fixed to zero and the breakpoint is where Tb begins to increase (the Tb_R). The conductance parameters were not evaluated by segmented regression because the conductance versus Ta plot was curvilinear, without distinct linear portions.
The defended Tb was calculated as the intercept of TEE versus Ta in the first segment, where Ta < TlcEE. Statistical analyses were conducted using PROC NLMIXED (SAS version 9.4; SAS Institute, Cary, NC, USA). Data are presented as mean ± SE. Statistical significance was declared at p < 0.05. See Supplementary Materials for further details.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Resiniferatoxin | Tocris, Bristol, UK | Cat# 1137 |
| Capsaicin | Sigma, St. Louis, MO, USA | Cat# 360376 |
| Isoflurane (Forane) | Baxter Healthcare Corporation, Deerfiled, IL, USA | Cat# 10019-360-40 |
| Prevail (flunixin meglumine) | VetOne, Boise, ID, USA | Cat# 502018 |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | Jackson Laboratories, Bar Harbor, ME, USA | Cat# 000664 |
| ob/ob mice | Jackson Laboratories, Bar Harbor, ME, USA | Cat# 000632 |
| 129 mice | Jackson Laboratories, Bar Harbor, ME, USA | Cat# 002448 |
| Trpv1−/−;Trpm8−/−;Trpa1−/− triple knockout mice (TKO) | Dr. Alexander Chesler, NCCIH | (Bautista et al., 2006, 2007; Caterina et al., 2000) |
| Software and Algorithms | ||
| SAS v 9.4 | SAS Institute, Cary, NC, USA | N/A |
| Oxymax software v 5.52 | Columbus Instruments, Columbus, OH, USA | N/A |
| VitalView v 5.0 | Starr Life Sciences, Oakmont, PA, USA | N/A |
| Graph pad v 8.1.0 | GraphPad Software, San Diego, CA, USA | N/A |
| Other | ||
| CLAMS | Columbus Instruments, Columbus, OH, USA | Sn#110117 |
| CLAMS-HC | Columbus Instruments, Columbus, OH | Sn# 190192 |
| HOBO Temperature data logger | Onset Computer Corporation, Bourne, MA, USA | Cat# U12-012 |
| Mice cages | Tecniplast USA, West Chester, PA | Cat# 1284 |
| Body temperature telemetry system - Implants | Starr Life Sciences, Oakmont, PA, USA | Cat# G2 E-Mitter |
| Body temperature telemetry system - Energizer/receivers | Starr Life Sciences, Oakmont, PA, USA | Cat# ER4000 |
| Chow diet | Envigo Inc, Madison, WI, USA | Cat# NIH-07 |
| High fat diet | Research Diets, New Brunswick, NJ, USA | Cat# D12492 |
| Paper bedding | Envigo, Indianapolis, IN, USA | Cat# 7099-TEK-fresh |
| Sani-Chips bedding | Envigo, Indianapolis, IN, USA | Cat# 7090 Teklad sani-chips |
Highlights.
We develop the thermoneutral point (TNP) concept to describe mouse thermoregulation
Energy expenditure increases below and body temperature increases above the TNP
The mouse TNP is 29°C in light phase and 33°C in dark phase, a diurnal change of 4°C
Studying mice strictly “at thermoneutrality” is not feasible
ACKNOWLEDGMENTS
We thank Dr. Alexander Chesler for the TKO mice and advice; Alice Franks, Yuning Huang, and Jun Feranil for experimental contributions; and Drs. Gustavo Abreu-Vieira, Kong Chen, Aaron Cypess, Ramó n Piñol, Sushil Rane, and Lee Weinstein for helpful comments. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (ZIA DK075062, ZIA DK075063, ZIA DK075064, and ZIA DK070002).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.03.065.
REFERENCES
- Abreu-Vieira G, Xiao C, Gavrilova O, and Reitman ML (2015). Integration of body temperature into the analysis of energy expenditure in the mouse. Mol. Metab. 4, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolph EF (1947). Tolerance to heat and dehydration in several species of mammals. Am. J. Physiol. 151, 564–575. [DOI] [PubMed] [Google Scholar]
- Baldo MB, Antenucci CD, and Luna F (2015). Effect of ambient temperature on evaporative water loss in the subterranean rodent Ctenomys talarum. J. Therm. Biol. 53, 113–118. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, and Julius D (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, and Julius D (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208. [DOI] [PubMed] [Google Scholar]
- Bligh J, and Johnson KG (1973). Glossary of terms for thermal physiology. J. Appl. Physiol. 35, 941–961. [DOI] [PubMed] [Google Scholar]
- Bradbury PA, Fox RH, Goldsmith R, Hampton IF, and Muir AL (1967). Resting metabolism in man at elevated body temperatures. J. Physiol. 189, 61P–62P. [PubMed] [Google Scholar]
- Brychta RJ, and Chen KY (2017). Cold-induced thermogenesis in humans. Eur. J. Clin. Nutr. 71, 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brychta RJ, Huang S, Wang J, Leitner BP, Hattenbach JD, Bell SL, Fletcher LA, Perron Wood R, Idelson CR, Duckworth CJ, et al. (2019). Quantification of the Capacity for Cold-Induced Thermogenesis in Young Men With and Without Obesity. J. Clin. Endocrinol. Metab. 104, 4865–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J (2011). Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 214, 242–253. [DOI] [PubMed] [Google Scholar]
- Castillo M, Hall JA, Correa-Medina M, Ueta C, Kang HW, Cohen DE, and Bianco AC (2011). Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes 60, 1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, and Julius D (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Brá z JM, Shah NM, Julius D, and Basbaum AI (2011). Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J. Neurosci. 31, 10119–10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton ZS, and McCurdy CE (2018). Short-term thermoneutral housing alters glucose metabolism and markers of adipose tissue browning in response to a high-fat diet in lean mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 315, R627–R637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CE, Withers PC, Munns SL, Geiser F, and Buttemer WA (2018). Geographical variation in the standard physiology of brushtail possums (Trichosurus): implications for conservation translocations. Conserv. Physiol 6, coy042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SS, Repasky EA, and Fisher DT (2015). Fever and the thermal regulation of immunity: the immune system feels the heat. Nat. Rev. Immunol. 15, 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, and Nedergaard J (2009). UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9, 203–209. [DOI] [PubMed] [Google Scholar]
- Fischer AW, Csikasz RI, von Essen G, Cannon B, and Nedergaard J (2016a). No insulating effect of obesity. Am. J. Physiol. Endocrinol. Metab. 311, E202–E213. [DOI] [PubMed] [Google Scholar]
- Fischer AW, Hoefig CS, Abreu-Vieira G, de Jong JMA, Petrovic N, Mittag J, Cannon B, and Nedergaard J (2016b). Leptin Raises Defended Body Temperature without Activating Thermogenesis. Cell Rep. 14, 1621–1631. [DOI] [PubMed] [Google Scholar]
- Fischer AW, Cannon B, and Nedergaard J (2018). Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol. Metab. 7, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AW, Cannon B, and Nedergaard J (2019). The answer to the question “What is the best housing temperature to translate mouse experiments to humans?” is: thermoneutrality. Mol. Metab. 26, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagge AP, and Gonzalez RR (1996). Mechanisms of heat exchange: biophysics and physiology In Handbook of Physiology, Fregly MJ and Blatteis CM, eds. (Environmental Physiology; ), pp. 45–84. [Google Scholar]
- Gagge AP, Stolwijk JA, and Hardy JD (1967). Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ. Res. 1, 1–20. [DOI] [PubMed] [Google Scholar]
- Ganeshan K, and Chawla A (2017). Warming the mouse to model human diseases. Nat. Rev. Endocrinol. 13, 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan K, Nikkanen J, Man K, Leong YA, Sogawa Y, Maschek JA, Van Ry T, Chagwedera DN, Cox JE, and Chawla A (2019). Energetic Trade-Offs and Hypometabolic States Promote Disease Tolerance. Cell 177, 399–413.e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garami A, Pakai E, Oliveira DL, Steiner AA, Wanner SP, Almeida MC, Lesnikov VA, Gavva NR, and Romanovsky AA (2011). Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J. Neurosci. 31, 1721–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles DA, Ramkhelawon B, Donelan EM, Stankiewicz TE, Hutchison SB, Mukherjee R, Cappelletti M, Karns R, Karp CL, Moore KJ, and Divanovic S (2016). Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wild type C57BL/6 mice. Mol. Metab. 5, 1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldgof M, Xiao C, Chanturiya T, Jou W, Gavrilova O, and Reitman ML (2014). The chemical uncoupler 2,4-dinitrophenol (DNP) protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. J. Biol. Chem. 289, 19341–19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golozoubova V, Gullberg H, Matthias A, Cannon B, Vennström B, and Nedergaard J (2004). Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol. Endocrinol. 18, 384–401. [DOI] [PubMed] [Google Scholar]
- Gordon CJ (1985). Relationship between autonomic and behavioral thermoregulation in the mouse. Physiol. Behav. 34, 687–690. [DOI] [PubMed] [Google Scholar]
- Gordon CJ (1993). Temperature regulation in laboratory rodents (Cambridge University Press; ). [Google Scholar]
- Gordon CJ (2012). Thermal physiology of laboratory mice: Defining thermoneutrality. J. Therm. Biol. 37, 654–685. [Google Scholar]
- Gordon CJ (2017). The mouse thermoregulatory system: Its impact on translating biomedical data to humans. Physiol. Behav. 179, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth FR (1967). Saliva spreading, activity, and body temperature regulation in the rat. Am. J. Physiol. 212, 1288–1292. [DOI] [PubMed] [Google Scholar]
- Hardy JD, and Du Bois EF (1940). Differences between men and women in their response to heat and cold. Proc. Natl. Acad. Sci. USA 26, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JD, and Stolwijk JA (1966). Partitional calorimetric studies of man during exposures to thermal transients. J. Appl. Physiol. 21, 1799–1806. [DOI] [PubMed] [Google Scholar]
- Herrington LP (1940). The heat regulation of small laboratory animals at various environmental temperatures. Am. J. Physiol. 129, 123–139. [Google Scholar]
- Hill RW, Muhich TE, and Humphries MM (2013). City-scale expansion of human thermoregulatory costs. PLoS One 8, e76238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högberg H, Engblom L, Ekdahl A, Lidell V, Walum E, and Alberts P (2006). Temperature dependence of O2 consumption; opposite effects of leptin and etomoxir on respiratory quotient in mice. Obesity (Silver Spring) 14, 673–682. [DOI] [PubMed] [Google Scholar]
- Houghton FC, Teague WW, Miller WE, and Yant WP (1929). Thermal exchange between the human body and its atmospheric environment. Am. J. Physiol. 88, 386–406. [Google Scholar]
- Hylander BL, Gordon CJ, and Repasky EA (2019). Manipulation of Ambient Housing Temperature To Study the Impact of Chronic Stress on Immunity and Cancer in Mice. J. Immunol. 202, 631–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUPS (2001). Glossary of terms for thermal physiology. Jpn. J. Physiol. 51, 245–280. [Google Scholar]
- Karp CL (2012). Unstressing intemperate models: how cold stress undermines mouse modeling. J. Exp. Med. 209, 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijer J, Li M, and Speakman JR (2019). What is the best housing temperature to translate mouse experiments to humans? Mol. Metab. 25, 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma B, Frijns A, and van Marken Lichtenbelt W (2012). The thermoneutral zone: implications for metabolic studies. Front. Biosci. (Elite Ed.) 4, 1975–1985. [DOI] [PubMed] [Google Scholar]
- Klaus S, Münzberg H, Trüloff C, and Heldmaier G (1998). Physiology of transgenic mice with brown fat ablation: obesity is due to lowered body temperature. Am. J. Physiol. 274, R287–R293. [DOI] [PubMed] [Google Scholar]
- Kleiber M (1975). The Fire of Life (Robert E Krieger Publishing Company; ). [Google Scholar]
- Kleiber M, and Dougherty JE (1934). The influence of environmental temperature on the utilization of food energy in baby chicks. J. Gen. Physiol. 17, 701–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, and Repasky EA (2013). Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc. Natl. Acad. Sci. USA 110, 20176–20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenbelt Wv., Kingma B, van der Lans A, and Schellen L (2014). Cold exposure—an approach to increasing energy expenditure in humans. Trends Endocrinol. Metab. 25, 165–167. [DOI] [PubMed] [Google Scholar]
- Lusk G (1928). The Elements of the Science of Nutrition (Johnson Reprint Corporation; ). [Google Scholar]
- Maloney SK, Fuller A, Mitchell D, Gordon C, and Overton JM (2014). Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 29, 413–420. [DOI] [PubMed] [Google Scholar]
- McConnell WJ, and Yagloglou CP (1925). Basal metabolism as affected by atmospheric condiitons. Arch. Intern. Med. 36, 382–396. [Google Scholar]
- McKechnie AE, Gerson AR, McWhorter TJ, Smith EK, Talbot WA, and Wolf BO (2017). Avian thermoregulation in the heat: evaporative cooling in five Australian passerines reveals within-order biogeographic variation in heat tolerance. J. Exp. Biol. 220, 2436–2444. [DOI] [PubMed] [Google Scholar]
- Meyer CW, Klingenspor M, Rozman J, and Heldmaier G (2004). Gene or size: metabolic rate and body temperature in obese growth hormone-deficient dwarf mice. Obes. Res. 12, 1509–1518. [DOI] [PubMed] [Google Scholar]
- Moruppa SM (1990). Energy expenditure and locomotor activity in mice selected for food intake adjusted for body weight. Theor. Appl. Genet. 79, 131–136. [DOI] [PubMed] [Google Scholar]
- Mount LE (1971). Metabolic rate and thermal insulation in albino and hairless mice. J. Physiol. 217, 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount LE (1973). The concept of thermal neutrality In Heat Loss from Animals and Man: Assessment and Control, Monteith JL and Mount LE, eds. (Butterworths; ), pp. 425–439. [Google Scholar]
- Mount LE, and Willmott JV (1967). The relation between spontaneous activity, metabolic rate and the 24 hour cycle in mice at different environmental temperatures. J. Physiol. 190, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011). Guide for the Care and Use of Laboratory Animals (The National Academies Press; ). [PubMed] [Google Scholar]
- Nedergaard J, and Cannon B (2014). The browning of white adipose tissue: some burning issues. Cell Metab. 20, 396–407. [DOI] [PubMed] [Google Scholar]
- O’Connor RS, Wolf BO, Brigham RM, and McKechnie AE (2017). Avian thermoregulation in the heat: efficient evaporative cooling in two southern African nightjars. J. Comp. Physiol. B 187, 477–491. [DOI] [PubMed] [Google Scholar]
- O’Neal TJ, Friend DM, Guo J, Hall KD, and Kravitz AV (2017). Increases in Physical Activity Result in Diminishing Increments in Daily Energy Expenditure in Mice. Curr. Biol. 27, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oufara S, Barre H, Rouanet JL, and Chatonnet J (1987). Adaptation to extreme ambient temperatures in cold-acclimated gerbils and mice. Am. J. Physiol. 253, R39–R45. [DOI] [PubMed] [Google Scholar]
- Overton JM (2010). Phenotyping small animals as models for the human metabolic syndrome: thermoneutrality matters. Int. J. Obes. 34, S53–S58. [DOI] [PubMed] [Google Scholar]
- Pan Q, Li M, Shi YL, Liu H, Speakman JR, and Wang DH (2014). Lipidomics reveals mitochondrial membrane remodeling associated with acute thermoregulation in a rodent with a wide thermoneutral zone. Lipids 49, 715–730. [DOI] [PubMed] [Google Scholar]
- Pennycuik PR (1967). A comparison of the effects of a variety of factors on the metabolic rate of the mouse. Aust. J. Exp. Biol. Med. Sci. 45, 331–346. [DOI] [PubMed] [Google Scholar]
- Peériard JD, Racinais S, and Sawka MN (2015). Adaptations and mechanisms of human heat acclimation: Applications for competitive athletes and sports. Scand. J. Med. Sci. Sports 25, 20–38. [DOI] [PubMed] [Google Scholar]
- Perissin L, Facchin P, and Porro CA (2000). Diurnal variations in tonic pain reactions in mice. Life Sci. 67, 1477–1488. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, and Tavendale R (1977). Effects of delta9-tetrahydrocannabinol on the rates of oxygen consumption of mice. Br. J. Pharmacol. 60, 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinetti R (2010). The circadian rhythm of body temperature. Front. Biosci. 15, 564–594. [DOI] [PubMed] [Google Scholar]
- Reimúndez A, Fernández-Peña C, García G, Fernández R, Ordás P, Gallego R, Pardo-Vazquez JL, Arce V, Viana F, and Señarís R (2018). Deletion of the Cold Thermoreceptor TRPM8 Increases Heat Loss and Food Intake Leading to Reduced Body Temperature and Obesity in Mice. J. Neurosci. 38, 3643–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman ML (2018). Of mice and men—environmental temperature, body temperature, and treatment of obesity. FEBS Lett. 592, 2098–2107. [DOI] [PubMed] [Google Scholar]
- Roberts WW, Mooney RD, and Martin JR (1974). Thermoregulatory behaviors of laboratory rodents. J. Comp. Physiol. Psychol. 86, 693–699. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA (2018). The thermoregulation system and how it works. Handb. Clin. Neurol. 156, 3–43. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Ivanov AI, and Shimansky YP (2002). Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J. Appl. Physiol. 92, 2667–2679. [DOI] [PubMed] [Google Scholar]
- Rubner M (1902). The Laws of Energy Consumption in Nutrition. (Academic Press; ). [Google Scholar]
- Sándor K, Helyes Z, Elekes K, and Szolcsányi J (2009). Involvement of capsaicin-sensitive afferents and the Transient Receptor Potential Vanilloid 1 Receptor in xylene-induced nocifensive behaviour and inflammation in the mouse. Neurosci. Lett. 451, 204–207. [DOI] [PubMed] [Google Scholar]
- Scholander PF, Hock R, Walters V, Johnson F, and Irving L (1950). Heat regulation in some arctic and tropical mammals and birds. Biol. Bull. 99, 237–258. [DOI] [PubMed] [Google Scholar]
- Selman C, Lumsden S, Bünger L, Hill WG, and Speakman JR (2001). Resting metabolic rate and morphology in mice (Mus musculus) selected for high and low food intake. J. Exp. Biol. 204, 777–784. [DOI] [PubMed] [Google Scholar]
- Speakman JR (2013). Measuring energy metabolism in the mouse - theoretical, practical, and analytical considerations. Front. Physiol. 4, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, and Keijer J (2013). Not so nuanced: Reply to the comments of Gaskill and Garner on “Not so hot: Optimal housing temperatures for mice to mimic the environment of humans.”. Mol. Metab. 3, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, and Rossi FP (1999). No Support for Socio-Physiological Suppression Effect on Metbolism of Paired White Mice (Mus sp.). Funct. Ecol. 13, 373–382. [Google Scholar]
- Szelényi Z, Hummel Z, Szolcsányi J, and Davis JB (2004). Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur. J. Neurosci. 19, 1421–1424. [DOI] [PubMed] [Google Scholar]
- Szymusiak R, and Satinoff E (1981). Maximal REM sleep time defines a narrower thermoneutral zone than does minimal metabolic rate. Physiol. Behav. 26, 687–690. [DOI] [PubMed] [Google Scholar]
- Talbot WA, McWhorter TJ, Gerson AR, McKechnie AE, and Wolf BO (2017). Avian thermoregulation in the heat: evaporative cooling capacity of arid-zone Caprimulgiformes from two continents. J. Exp. Biol. 220, 3488–3498. [DOI] [PubMed] [Google Scholar]
- Tan CL, and Knight ZA (2018). Regulation of Body Temperature by the Nervous System. Neuron 98, 31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, Tangirala RK, Tontonoz P, and Chawla A (2016). Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell Metab. 23, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, and James WP (1978). Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflugers Arch. 373, 189–193. [DOI] [PubMed] [Google Scholar]
- Virtue S, Even P, and Vidal-Puig A (2012). Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell Metab. 16, 665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Goldgof M, Gavrilova O, and Reitman ML (2015). Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22°C. Obesity (Silver Spring) 23, 1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZJ, Chi QS, Liu QS, Zheng WH, Liu JS, and Wang DH (2014). The shift of thermoneutral zone in striped hamster acclimated to different temperatures. PLoS One 9, e84396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, and Hogestatt ED (2014). TRPA1. In Mammalian Transient Receptor Potential (TRP) Cation Channels, Nilius B and Flockerzi V, eds. (Springer; ), pp. 583–630. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.