Abstract
Background:
Approximately 38% of patients with colorectal cancer will develop isolated liver metastases. Sidedness of colon tumor is identified in non-metastatic and unresected metastatic cancers as predictive of survival, yet its dedicated analysis in resected liver metastases is minimal. Our primary aim was to assess whether left-sided primary tumors improve prognosis in stage IV cancer patients undergoing curative-intent liver metastasectomy; it was hypothesized that it would.
Methods:
This is a retrospective, observational cohort study from 1996 to 2016 in a single tertiary-care facility. Survival from diagnosis was calculated via Kaplan-Meier method and compared between the right and left sides via log-rank analysis.
Results:
Median survival differs significantly between colorectal tumors of the right and left origins after hepatic metastasectomy in 612 patients. In patients with right-sided tumors, median survival from diagnosis was 4.5 years (IQR 4.1–5.3), and 6.3 years (IQR 5.6–6.9) in those with left tumors (HR 1.5, 95% CI 1.38–1.60, p < 0.001).
Conclusion:
As in studies on earlier-stage or unresected metastatic disease, tumor sidedness is an important prognostic factor in patient survival with liver metastasectomy. Clinical risk scores should include side of primary tumor. Further work is needed to determine the molecular basis for this difference.
Introduction
In patients who develop metastatic colorectal cancer, between 27% and 40% will involve the liver, and the liver is the only site of metastasis in up to 38% of patients.1–3 Survival can be favorable in patients amenable to resection.4–7 Despite strides in surgical therapy, however, recurrence occurs in up to 75% of patients.8–12 In fact, only 17–22% of patients with isolated liver metastases achieves a cure after liver resection, defined as ≥10 years’ survival.11–14 Current efforts to predict survival are still limited.3,11,12,15,16 Novel predictors of survival are therefore necessary to determine potential benefit from surgical resection of metastases.17–21 Primary tumor sidedness as a predictor of survival is currently an area of great interest. Data have emerged in the unresectable metastatic and resected primary settings, suggesting the left side is protective overall19,22–33; however, outcomes for patients with resectable metastatic disease specifically are limited to smaller retrospective or subset analyses and a single-institution study.2,27,29,34,35
Our primary aim in this study is to assess whether sidedness of the primary tumor has prognostic significance for stage IV CRC patients undergoing curative-intent liver metastasectomy. We hypothesized that, like prior studies, tumors originating from the left colon in our population would confer greater overall survival (OS) in resected hepatic metastases. Secondary questions included the effects of patient, surgery, and tumor characteristics on OS. We further expected that earlier stage at diagnosis, fewer established risk factors, younger age, lack of comorbidities, and no tobacco or ethanol use would correlate with improved survival. Finally, we hypothesized that there would be survival differences dependent on the year of the colon and liver resections, given the advancements in medical and surgical therapies over the past two decades.
Methods
A database of patients undergoing hepatectomy at Duke University Medical Center (DUMC) was collected both retrospectively and prospectively between 1996 and 2016. We identified 612 patients from this group who underwent liver metastasectomy for stage IV colorectal cancer. Chart review through the electronic medical record was undertaken to collect demographic and clinical information. The state-mandated institutional Tumor Registry was also queried for date of last contact. Approval from the DUMC Investigational Review Board was obtained prior to performing this analysis.
Patients who had incomplete debulking were excluded, as they did not undergo curative surgery. Ablative or liver-directed therapies alone were not considered metastasectomies and were also excluded. In patients who underwent staged hepatectomies, of which there were fewer than 5, or repeat hepatectomies, the first metastasectomy performed at DUMC was the surgery used in analysis.
Demographic data included gender, race, age at diagnosis, any current or former tobacco and ethanol use, and Charlson-age comorbidity index (CaCI) at the time of hepatectomy. Cancer characteristics included final tumor-node-metastasis and American Joint Committee on Cancer (AJCC) 7th edition staging36 determined within 6 months of diagnosis or onset of disease-specific symptoms (e.g., rectal bleeding), disease-free interval (from diagnosis of colon cancer to diagnosis of liver metastasis), Fong score,11,15 and neoadjuvant/adjuvant therapies for both initial colon surgery and hepatic metastasectomy. These therapies were constrained to the most-recent therapy tolerated for at least 4 weeks and received within 6 months perioperatively; the entire 4 weeks did not have to be within the 6-month time period. Surgical data comprised the interval between colon resection and liver resection, estimated blood loss (EBL) of procedure, and complications/death within 90 days. Right sidedness was defined as tumor location from cecum through transverse colon, excluding appendix. The side of the liver in hepatectomy was not examined in this study, so all mentions of side refer to the colon and the primary tumor. Sidedness in patients with multiple primary tumors was assigned to the tumor with the highest T stage, as it would be more likely to metastasize.37 Death from CRC was considered if patient had documentation of hospice care or notes indicating decline in status/progression of disease prior to date of death. Subjects were stratified by year of primary CRC resection and by year of metastasectomy into blocks of five years to account for effect of advances in treatment.
Survival from diagnosis by sidedness of primary tumor was plotted via Kaplan-Meier method using the survival package in R. Time from diagnosis was chosen to provide similar context to data published on survival in all patients with CRC. Univariate analysis of OS between the two sides was determined by log-rank analysis. Comparison of demographic differences between groups was made with Wilcoxon rank sum test for continuous and χ2 for categorical variables. For the secondary questions, Cox proportional hazards (CPH) model was used to determine effect of initial stage (I-IV), demographics, tumor characteristics, etc. on time-to-event between groups. This was first performed by backward selection of significant predictors, including side, then confirmed via forward selection. The predictors found to be stable between backward and forward selection were then tested via CPH for interaction with primary tumor side. Log-rank was then used to compare OS between sides by each significant predictor. All calculations were performed using R. A p-value <0.05 was considered significant. No adjustment for p-value was made in the secondary group analyses, as those data were exploratory.
Results
The majority of patients had a left-sided primary tumor (n = 386, 63%), and most subjects were male (n = 361, 59%). Most patients (n = 498, 81%) were white, and the racial break-down by side was equal (p = 0.290). The median age at diagnosis was younger for left-sided cancers (median 55 years, IQR 48–64 vs 62 years, IQR 54–69, p < 0.001). Other demographic and clinical information were clinically equivalent across groups, including tobacco and ethanol use, specific comorbidities, overall CaCI, Fong score, disease-free interval, and stage at diagnosis. Neoadjuvant chemotherapy prior to primary resection was higher in the left group (101, 26% vs 16, 7%, p < 0.001). Surgery data was clinically comparable between sides, including EBL, complications, multiple metastasectomies, synchronous liver and colon surgery, and hepatectomy prior to colectomy. The right-sided group had a slightly shorter, though non-significant, interval between colon and liver surgery at a median of 344 days (IQR 189–694, vs 409 days IQR 167–835, p = 0.164) (Table 1).
Table 1.
Patient demographics and clinical characteristics. Targeted chemotherapy refers to immunologic agents. Multiple metastasectomies indicates more than one procedure. Resection interval between primary and metastatic resections excludes synchronous or liver-first surgeries. Charlson Index excludes cancer measures. Disease-free interval excludes values ≤30 days as those timeframes are likely not truly disease free
| 612 | 226 | 37% | 386 | 63% | ||||
|---|---|---|---|---|---|---|---|---|
| (rectum | 165 | 27%) | ||||||
| Race | White | 498 | 81% | 179 | 79% | 319 | 83% | 0.290 |
| Black | 88 | 14% | 37 | 16% | 51 | 13% | ||
| American Indian | 9 | 1% | 4 | 2% | 5 | 1% | ||
| Asian | 4 | 1% | 3 | 1% | 1 | 0.3% | ||
| Unspecified | 13 | 2% | 3 | 1% | 10 | 3% | ||
| Median (IQR) | 58 | 49 – 66 | 62 | 54–69 | 55 | 48 – 64 | 1.98e-10 | |
| Tobacco use | current | 81 | 13% | 27 | 12% | 54 | 14% | 0.422 |
| previous | 228 | 37% | 87 | 39% | 141 | 36% | ||
| current | 213 | 35% | 78 | 35% | 135 | 35% | 0.327 | |
| previous | 66 | 11% | 20 | 9% | 46 | 12% | ||
| Median (IQR) | 2 | 1 – 3 | 2 | 1 −3 | 2 | 1 – 3 | 7.71e-5 | |
| Previous MI | 39 | 6% | 21 | 10% | 18 | 5% | 0.058 | |
| CHF | 5 | 1% | 2 | 1% | 3 | 1% | 1 | |
| Peripheral arterial disease | 17 | 3% | 7 | 3% | 10 | 3% | 0.910 | |
| Cerebrovascular disease | 18 | 3% | 11 | 5% | 7 | 2% | 0.056 | |
| Dementia | 1 | 0.2% | 1 | 0.5% | 0 | 0% | 0.786 | |
| Chronic pulmonary disease | 68 | 11% | 21 | 10% | 47 | 12% | 0.336 | |
| Connective tissue disease | 8 | 1% | 4 | 2% | 4 | 1% | 0.687 | |
| PUD | 20 | 3% | 10 | 5% | 10 | 3% | 0.319 | |
| Hemiplegia | 1 | 0.2% | 1 | 0.5% | 0 | 0% | 0.786 | |
| Moderate or severe renal disease | 9 | 1% | 5 | 2% | 4 | 1% | 0.413 | |
| DM requiring medication | 81 | 13% | 35 | 16% | 46 | 12% | 0.511 | |
| Liver disease | 10 | 2% | 5 | 2% | 5 | 1% | 0.594 | |
| Leukemia/lymphoma | 2 | 0.3% | 1 | 0.5% | 1 | 0.3% | 1 | |
| I | 17 | 3% | 7 | 3% | 10 | 3% | 0.446 | |
| II | 65 | 11% | 21 | 9% | 44 | 11% | ||
| III | 173 | 28% | 66 | 29% | 107 | 28% | ||
| IV | 333 | 54% | 127 | 56% | 206 | 53% | ||
| unknown | 24 | 4% | 5 | 2% | 19 | 5% | ||
| T4 | 79 | 13% | 29 | 13% | 50 | 13% | ||
| node positive | 471 | 77% | 158 | 70% | 313 | 81% | ||
| colectomy | 117 | 19% | 16 | 7% | 101 | 26% | 4.84e-8 | |
| metastasectomy | 349 | 57% | 124 | 55% | 225 | 58% | 0.684 | |
| colectomy | 475 | 78% | 183 | 81% | 292 | 76% | 0.038 | |
| metastasectomy | 357 | 58% | 115 | 51% | 242 | 63% | 0.002 | |
| colectomy | 28 | 5% | 10 | 4% | 18 | 5% | 1 | |
| metastasectomy | 170 | 28% | 69 | 31% | 101 | 26% | 0.285 | |
| colectomy | 150 | 25% | 64 | 28% | 86 | 22% | 0.114 | |
| metastasectomy | 159 | 26% | 55 | 24% | 104 | 27% | 0.494 | |
| colectomy | 169 | 28% | 72 | 32% | 97 | 25% | 0.089 | |
| metastasectomy | 256 | 42% | 97 | 43% | 159 | 41% | 0.739 | |
| 60 | 10% | 20 | 9% | 40 | 10% | 0.641 | ||
| Liver first | 13 | 2% | 4 | 2% | 9 | 2% | ||
| Resection interval (d) | Median (IQR) | 399 | 188–793 | 344 | 189–694 | 409 | 167–835 | 0.164 |
| DFI (>30d) | Median (IQR) | 496 | 267–879 | 481.5 | 170–821 | 502 | 293–882 | 0.704 |
| Fong Score | Median (IQR) | 2 | 2–3 | 2 | 2 – 3 | 2 | 2 – 3 | 0.0359 |
| Estimated blood loss (mL) | Median (IQR) | 300 | 200–550 | 350 | 200–550 | 300 | 200–550 | 0.582 |
| Multiple operations | 63 | 10% | 19 | 8% | 44 | 11% | 0.299 | |
| Complication | Any | 189 | 31% | 75 | 33% | 114 | 30% | 0.394 |
| Infection | 100 | 16% | 40 | 18% | 60 | 15% | 0.504 | |
| Pleural effusion | 51 | 8% | 22 | 10% | 29 | 7% | 0.419 | |
| Hemorrhage | 38 | 6% | 13 | 6% | 25 | 6% | 0.853 | |
| Bile leak | 30 | 5% | 8 | 4% | 22 | 6% | 0.317 | |
| Death | 22 | 4% | 11 | 5% | 11 | 3% | 0.285 | |
| Pneumonia | 13 | 2% | 6 | 3% | 7 | 2% | 0.685 | |
| Cardiac | 12 | 2% | 5 | 2% | 7 | 2% | 0.967 | |
| Liver failure | 11 | 2% | 4 | 2% | 7 | 2% | 1 | |
| Renal failure | 10 | 2% | 3 | 1.4% | 7 | 2% | 0.899 | |
| VTE | 9 | 1.5% | 4 | 1.8% | 5 | 1.3% | 0.902 | |
| GI bleed | 8 | 1.3% | 4 | 1.8% | 4 | 1.0% | 0.687 | |
| PVT | 2 | 0.3% | 1 | 0.5% | 1 | 0.3% | 1 | |
| Fistula | 1 | 0.2% | 0 | 0% | 1 | 0.3% | 1 |
IQR = interquartile range, MI = myocardial infarction, CHF = congestive heart failure, PAD = peripheral arterial disease, PUD = peptic ulcer disease, DM = diabetes mellitus, VTE = venous thromboembolism, GI = gastrointestinal, PVT = portal venous thrombus.
Synchronous presentation increased over time and was the most common presentation in the last block (13 of 16 stage IV in colon resection block, p < 0.001; 22 of 35in liver resection block, p = 0.0399). The most common origin of right tumors was cecal (n = 85, 38%), and the origin of most left tumors was rectosigmoid (n = 339, 88%) (Fig. 1).
Figure 1.

Origin of Primary Tumor. The majority of right tumors are found in cecum and the majority of left in sigmoid or rectum. Color on proportion bar corresponds with location on colon diagram. Numbers indicate absolute values of tumors in that location. Volume of color on diagram does not correlate with number. Unspecified right or left tumors included in proportion bar represented in background of colon schematic
There was a significant difference in survival between the right- and left-sided primary tumor, favoring the left side (HR = 1.5 ± 0.11, p < 0.001) (Fig. 2). This difference persisted even if the rectum was excluded from the left group (HR = 1.6 ± 0.13, p < 0.001), as there was no difference in survival between the rectum and rest of the left colon (p = 0.292). Median survival was accordingly 4.5 years (IQR 4.1–5.3) in the right-sided group and 6.3 years (IQR 5.6–6.9) in the left, for an overall median survival of 5.6 years (IQR 5.2–6.2). Approximately 30% of all patients survived at least 10 years from diagnosis: ~35% in the left-sided group and ~25% in the right-sided group (Fig. 3a). From date of metastasectomy, this difference also holds (HR 1.45 ± 0.11, p < 0.001) (Fig. 3b).
Figure 2.
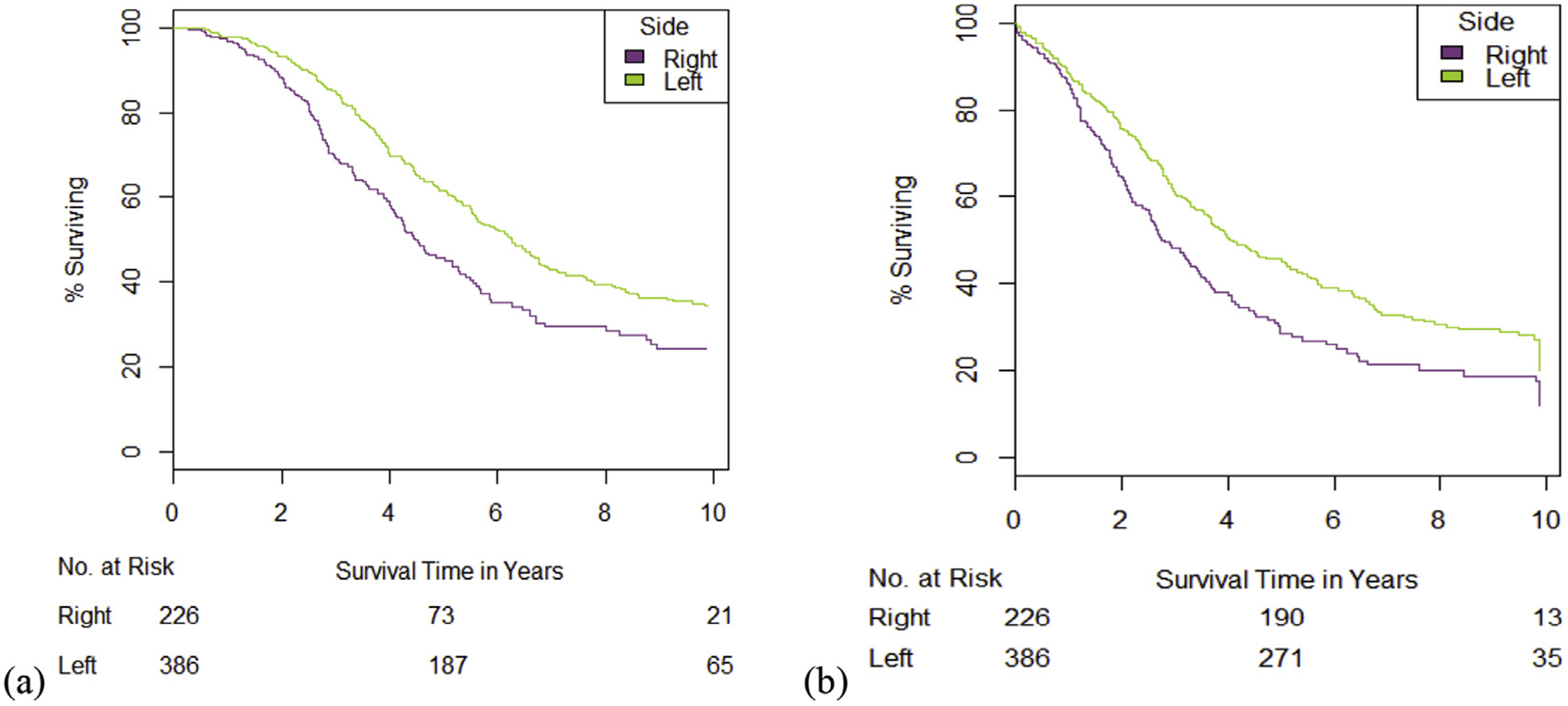
Survival by Side of Primary Tumor. There is a significant difference between sides, whether measuring survival from diagnosis of primary tumor (a) or from resection of liver metastases (b). Patients were censored by date of last follow-up. Rectal tumors are included with left-sided tumors
Figure 3.

Other Predictors of Survival. While neoadjuvant chemotherapy within 6 months of metastasectomy clearly affects survival (a), there do not appear to be differences between adjuvant and no adjuvant therapy within 6 months of surgery (b). The large number of patients with unknown-therapy status in the cohort does have a decreased survival compared to those who are known. (c) Any complication persistently decreased survival over the studied timeframes
Of the variables listed in Table 1, the predictors for survival in the overall cohort selected by both backward and forward selection were year of primary tumor resection, surgery interval, at least one complication, and neo- or adjuvant therapy around metastasectomy. Although not greatly different, the predictors varied somewhat from the overall cohort for each side. Both were affected by year of primary tumor resection, complication, and neoadjuvant before metastasectomy; and CaCI, surgery interval, complication, and adjuvant therapy after metastasectomy affected the right alone. The hazard ratios for survival were still similar to overall survival between sides when controlling for year of primary resection (e.g., between 2010 and 2014 HR = 1.94 ± 0.20, p < 0.001), complication (HR = 1.26 ± 0.18, p = 0.182), or neoadjuvant therapy (HR = 1.57 ± 0.15, p = 0.001). In the overall cohort, patients who underwent neoadjuvant therapy prior to metastasectomy overwhelmingly were diagnosed with stage IV cancer (236 of 331, 72% vs 108 of 253, 43% stages I-III), which is reflected in the worse overall median survival (4.8 years, 95% CI 4.4–5.3, vs 6.6 years, 95% CI 5.9–8.0, p < 0.001 by log-rank analysis) (Fig. 4a). Patients undergoing adjuvant therapy after primary resection also had metastases at diagnosis most frequently (213 of 349, 61%). Patients who had unknown chemotherapy status (e.g., lost to follow-up or not recorded) had worse median survival (4.2 years, 95% CI 3.4–5.6, vs 6.3 years, 95% CI 5.3–7.8, p < 0.001) (Fig. 4b). Presence of one or more of the complications listed in Table 1 was associated with decreased median survival (4.6 years, 95% CI 3.9–5.4 vs 6.2 years, 95% CI 5.6–6.8, p < 0.001), a difference persisting many years after hepatectomy (Fig. 3c). The relationship between sidedness of primary tumor and survival continued to hold after adjusting for these five covariates (p > 0.1 for all five). Survival between sides was still significantly different when controlling for neoadjuvant therapy (p = 0.006), as well as in the groups with no complication (p < 0.001; Fig. 3c), surgical intervals of <1 year (p < 0.001) or 1–2 years (p = 0.010), no primary resection (p = 0.002), and primary resection between 2010 and 2014 (p < 0.001).
Figure 4.

Interactions of Sidedness and Confounding Variables. When controlling for complication (a), there was still a significant difference in survival between side of primary tumor. Disease-free intervals (simplified into blocks of less than one year and one year or more) (b) and Fong scores (here represented as less than 3 and 3 or more) (c) likewise do not affect differences in survival between side of primary tumor
Disease-free interval was not a significant predictor by backward or forward selection, and it did not obviate survival differences between sides; survival in left-sided tumors was significantly greater whether disease-free interval was less than one year or not (HR 1.54 + 0.15, p < 0.001 vs 1.46 + 0.17, p = 0.16, Fig. 4b). Likewise, survival differences were still better in left-sided group when controlling for Fong scores less than three or three and greater (HR 1.36 ± 0.15, p = 0.029 vs 1.78 ± 0.18, p < 0.001, Fig. 4c).
Discussion
To date, this is the largest cohort of CRC hepatic metastasectomies examining the side of primary tumor as a prognostic factor. A left-sided primary CRC is associated with a survival advantage in patients undergoing curative hepatic metastasectomy in this group. This outcome supports data from other, smaller studies and sub-analyses of larger studies.2,27,29,34 The stages of cancer evaluated in other studies are predominantly lower stages. Most studies also examined survival over a range of stages, which likely overwhelms outcomes in the subset we examined for this project. Those studies that did examine stage IV cancer alone showed an overall survival benefit in all left-sided cancers in those who underwent metastasectomy.29,35 Those studies are, however, smaller than this one. Though the primary outcome of survival in this study was based on time from primary tumor diagnosis, the difference in survival was maintained when measuring from metastasectomy and when controlling for disease-free interval. Another study examining resected liver metastases specifically did not find a survival difference between sides but was also based on a smaller cohort.38 The survival advantages between the two sides have been posited to stem from different embryologic origin of the native bowel39 or from biomarker differences in the tumor types.20,35,38,40–43
Underlying disease biology is also a likely reason for difference in survival in surgical interval, as shorter intervals reflect quickly progressing tumors, though this was not a significant predictor in the left-sided group. Comparisons of survival between sided groups by predictor was, however, limited by the decrease in sample size. Surgical complications did affect both groups and may also have microbiologic implications that worsen tumor progression, such as inflammation and tumor seeding. The rate of patients presenting with synchronous liver metastases do not differ between sides, so these effects of worse initial disease did not affect the primary outcome.
Based on previous literature, we expected more of the other confounders to have been associated with overall survival for the cohort, but the large number of studied variables may have overpowered small individual effects. Although several of the predictors were significantly associated with survival in single-model analysis, we restricted the interaction models to those predictors stable between backward and forward selection group models to minimize the weaker confounders. While none of the secondary analyses, owing to their exploratory nature, had adjustments in p-value threshold to reduce Type I error, most p-values obtained were below the 0.05 threshold by two orders of magnitude. These analyses will provide the basis for development of future risk model. The current risk model examined in this study, the Fong score, did not diminish the difference in survival by sidedness of the primary tumor, indicating that sidedness should be a part in future risk model development.
This study was limited by a few considerations: a significant proportion of patients who undergo hepatic metastasectomy are referred to DUMC after their primary resection, or they are treated with adjuvant chemoradiotherapy at local facilities. Censorship therefore takes place much earlier than if they were followed at this institution, diminishing the power of this study. This missing data resulted in unclear effects of adjuvant chemotherapy on OS, but possible meaningful effects of coordination of care. Disease-specific survival was also therefore difficult to ascertain in many patients, and this aspect of survival was excluded from analysis.
Though multiple studies have demonstrated the effects of primary tumor site on recurrence and survival, consensus has yet to be reached on its importance. The majority of the literature showing survival difference, including this study, indicates left-sided cancers to have greater OS. This finding becomes more important with the recent rise in right-sided cancers, and the need for determining the molecular basis for their difference continues.44 Regardless of the cause, a significant proportion of patients will have liver metastases and must decide on a course of medical and/or surgical therapy. Patients with risk factors for early recurrence and short survival might benefit more from continued systemic therapy over surgical resection. The results of this study support making sidedness a relevant component of this preoperative discussion and decision making.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
This paper was presented at the Americas Hepatico-Pancreatico-Biliary Association Annual Meeting (Miami Beach, FL) on March 9, 2018 under the same title.
Conflicts of interest
None declared.
References
- 1.Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder M et al. (1986) Haematogenous metastastic patterns in colonic carcinoma : an analysis of 1541 necropsies. J Pathol 150:195–203. [DOI] [PubMed] [Google Scholar]
- 2.Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D et al. (2015) Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 121:830–835. [DOI] [PubMed] [Google Scholar]
- 3.Brandi G, De Lorenzo S, Nannini M, Curti S, Ottone M, Dall’Olio FG et al. (2016) Adjuvant chemotherapy for resected colorectal cancer metastases: literature review and meta-analysis. World J Gastroenterol 22: 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neeff H, Hörth W, Makowiec F, Fischer E, Imdahl A, Hopt UT et al. (2009) Outcome after resection of hepatic and pulmonary metastases of colorectal cancer. J Gastrointest Surg 13:1813–1820. [DOI] [PubMed] [Google Scholar]
- 5.Mirnezami R, Moran BJ, Harvey K, Cecil T, Chandrakumaran K, Carr N et al. (2014) Cytoreductive surgery and intraperitoneal chemotherapy for colorectal peritoneal metastases. World J Gastroenterol 20: 14018–14032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silberhumer GR, Paty PB, Temple LK, Araujo RLC, Denton B, Gonen M et al. (2015) Simultaneous resection for rectal cancer with synchronous liver metastasis is a safe procedure. Am J Surg 209:935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silberhumer GR, Paty PB, Denton B, Guillem J, Gonen M, Araujo RLC et al. (2016) Long-term oncologic outcomes for simultaneous resection of synchronous metastatic liver and primary colorectal cancer Surgery (United States), 10–12. Elsevier; Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bramhall S, Gur U, Coldham C, Gunson B, Mayer A, McMaster P et al. (2003) Liver resection for colorectal metastases. Ann R Coll Surg Engl 85:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbas S, Lam V, Hollands M. (2011) Ten-year survival after liver resection for colorectal metastases: systematic review and meta-analysis [Internet] ISRN Oncol 2011:763211–763245. Available from: http://www.hindawi.com/isrn/oncology/2011/763245/%5Cnpapers3://publication/doi/10.5402/2011/763245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park M-S, Yi N-J, Son S-Y, You T, Suh S-W, Choi YR et al. (2014) Histopathologic factors affecting tumor recurrence after hepatic resection in colorectal liver metastases. Ann Surg Treat Res 87:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230: 309–318. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordlinger B, Guiguet M, Boudjema K, Bachellier P, Jaeck D. (1996) Surgical resection of colorectal carcinoma metastases to the liver. Cancer 77:1254–1262. [PubMed] [Google Scholar]
- 13.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M et al. (2007) Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 25:4575–4580. [DOI] [PubMed] [Google Scholar]
- 14.Zarour LR, Anand S, Billingsley KG, Bisson WH, Cercek A, Clarke MF et al. (2017) Colorectal cancer liver metastasis: evolving paradigms and future directions [Internet]. Elsevier Inc Cell Mol Gastroenterol Hepatol 3: 163–173. Available from: http://linkinghub.elsevier.com/retrieve/pii/S2352345X17300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreckenbach T, Malkomes P, Bechstein WO, Woeste G, Schnitzbauer AA, Ulrich F. (2015) The clinical relevance of the Fong and the Nordlinger scores in the era of effective neoadjuvant chemotherapy for colorectal liver metastasis [Internet]. Springer Japan Surg Today 45:1527–1534. Available from: 10.1007/s00595-014-1108-9. [DOI] [PubMed] [Google Scholar]
- 16.Ayez N, Lalmahomed ZS, van der Pool AEM, Vergouwe Y, van Montfort K, de Jonge J et al. (2011) Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy? [Internet] Ann Surg Oncol 18:2757–2763. Available from: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=21638093&retmode=ref&cmd=prlinks%5Cnpapers3://publication/doi/10.1245/s10434-011-1819-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jess P, Hansen IO, Gamborg M, Jess T. (2013) A nationwide Danish cohort study challenging the categorisation into right-sided and left-sided colon cancer [Internet] BMJ Open 3 e002608-. Available from: http://bmjopen.bmj.com/content/3/5/e002608.full?rss=1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bufill J (1990) Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 113:779–789. [DOI] [PubMed] [Google Scholar]
- 19.Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y. (2016) The worse prognosis of right-sided compared with left-sided colon cancers: a systematic review and meta-analysis. J Gastrointest Surg 20: 648–655. [DOI] [PubMed] [Google Scholar]
- 20.Tamas K, Walenkamp AME, de Vries EGE, van Vugt MATM, Beets-Tan RG, van Etten B et al. (2015) Rectal and colon cancer: not just a different anatomic site [Internet]. Elsevier Ltd Cancer Treat Rev 41: 671–679. Available from: 10.1016/j.ctrv.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. (2015) Is right-sided colon cancer different to left-sided colorectal cancer? - a systematic review [Internet]. Elsevier Ltd Eur J Surg Oncol 41:300–308. Available from: 10.1016/j.ejso.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Karim S, Brennan K, Nanji S, Berry SR, Booth CM. (2017) Association between prognosis and tumor laterality in early-stage colon cancer [Internet] JAMA Oncol, 1–7. Available from: http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2017.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warschkow R, Sulz MC, Marti L, Tarantino I, Schmied BM, Cerny T et al. (2016) Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer [Internet]. BMC Canc 16:554 Available from: http://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brungs D, Aghmesheh M, de Souza P, Ng W, Chua W, Carolan M et al. (2017) Sidedness is prognostic in locoregional colon cancer: an analysis of 9509 Australian patients [Internet] BMC Cancer 17:251 Available from: http://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P et al. (2017) Prognostic survival associated with left-sided vs right-sided colon cancer [Internet] JAMA Oncol 3:211 Available from: http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2016.4227. [DOI] [PubMed] [Google Scholar]
- 26.Meguid R a, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. (2011) Is there a difference in survival between right-versus left-sided colon cancers? Ann Surg 15:2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrag D, Weng S, Brooks G, Meyerhardt JA, Venook AP. (2016) The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol 34(Suppl. 3503). [Google Scholar]
- 28.Hussain M, Waqas O, Hassan U, Loya A, Akhtar N, Mushtaq S et al. (2016) Right-sided and left-sided colon cancers are two distinct disease entities: an analysis of 200 cases in Pakistan. Asian Pac J Cancer Prev APJCP 17:2545–2548. [PubMed] [Google Scholar]
- 29.Sasaki K, Andreatos N, Margonis GA, He J, Weiss M, Johnston F et al. (2016 Dec 1) The prognostic implications of primary colorectal tumor location on recurrence and overall survival in patients undergoing resection for colorectal liver metastasis [Internet] J Surg Oncol 114, 803–9. Available from: http://doi.wiley.com/10.1002/jso.24425. [DOI] [PubMed] [Google Scholar]
- 30.Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D et al. (2014) Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 121:830–835. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto Y, Hayashi N, Sakamoto Y, Ohuchi M, Tokunagam R, Kurashige J et al. (2015) Predictors of long-term survival in patients with stage IV colorectal cancer with multi-organ metastases: a single-center retrospective analysis Int J Clin Oncol 20:1140–1146. Springer; Japan. [DOI] [PubMed] [Google Scholar]
- 32.Ciombor KK, Goldberg RM. (2016) Primary tumor sidedness as prognostic and predictive biomarker in metastatic colorectal cancer [Internet] JAMA Oncol 3:1–2. Available from: http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2016.3777. [DOI] [PubMed] [Google Scholar]
- 33.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. (2010) Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 53:57–64. [DOI] [PubMed] [Google Scholar]
- 34.Venook AP, Niedzwiecki D, Innocenti F, Fruth B, Greene C, O’Neil BH et al. (2016) Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. p. suppl; abstr 3504. [Google Scholar]
- 35.Kamran SC, Clark JW, Zheng H, Borger DR, Blaszkowsky LS, Allen JN et al. (2018) Primary tumor sidedness is an independent prognostic marker for survival in metastatic colorectal cancer: results from a large retrospective cohort with mutational analysis. Cancer Med, 1–9 (February). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AJCC. Colon and rectum cancer staging (7th ed. (2009). [Google Scholar]
- 37.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier A-M. (2006) Epidemiology and management of liver metastases from colorectal cancer [Internet] Ann Surg 244:254–259. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1602156&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marques MC, Ribeiro HSC, Wílson L, Costa J, Jesus VHF de, Macedo MP de et al. (2018) Is primary sidedness a prognostic factor in patients with resected colon cancer liver metastases (CLM)? J Surg Oncol 117:858–863. [DOI] [PubMed] [Google Scholar]
- 39.Shen H (2015) Different treatment strategies and molecular features between right-sided and left-sided colon cancers [Internet] World J Gastroenterol 21:6470 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4458758&tool=pmcentrez&rendertype=abstract%5Cnhttp://www.wjgnet.com/1007-9327/full/v21/i21/6470.htm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muzny DM, Bainbridge MN, Chang K, Dinh HH, Drummond JA, Fowler G et al. (2012) Comprehensive molecular characterization of human colon and rectal cancer [Internet] Nature 487, 330–7. Available from: http://www.nature.com/doifinder/10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamauchi M,Morikawa T,Kuchiba A, Imamura Y, Qian ZR, Nishihara R et al. (2012) Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum [Internet] Gut 61:847–854. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3345105&tool=pmcentrez&rendertype=abstract%5Cnhttp://gut.bmj.com/lookup/doi/10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kataoka K, Kanazawa A, Nakajima A, Yamaguchi A, Arimoto A. (2015) Prognostic value of biomarkers in metastatic colorectal cancer patients [Internet]. Elsevier Inc J Surg Res 194:343–350. Available from: 10.1016/j.jss.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki K, Margonis GA, Wilson A, Kim Y, Buettner S, Andreatos N et al. (2016) Prognostic implication of KRAS status after hepatectomy for colorectal liver metastases varies according to primary colorectal tumor location [Internet] Ann Surg Oncol, 3736–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27352204. [DOI] [PubMed] [Google Scholar]
- 44.Siegel RL, Miller KD, Jemal A. (2016) Cancer statistics. CA Cancer J Clin 66:7–30. [DOI] [PubMed] [Google Scholar]


