Abstract
As the most common cause of progressive cognitive decline in humans, Alzheimer’s disease (AD) has been intensively studied, but the mechanisms underlying its profound synaptic dysfunction remain unclear. Here we confirm that exposing wild-type mice to an enriched environment (EE) facilitates signaling in the hippocampus that promotes long-term potentiation (LTP). Exposing the hippocampus of mice kept in standard housing to soluble Aβ oligomers impairs LTP, but EE can fully prevent this. Mechanistically, the key molecular features of the EE benefit are an upregulation of miRNA-132 and an inhibition of histone deacetylase (HDAC) signaling. Specifically, soluble Aβ oligomers decreased miR-132 expression and increased HDAC3 levels in cultured primary neurons. Further, we provide evidence that HDAC3 is a direct target of miR-132. Overexpressing miR-132 or injecting an HDAC3 inhibitor into mice in standard housing mimics the benefits of EE in enhancing hippocampal LTP and preventing hippocampal impairment by Aβ oligomers in vivo. We conclude that EE enhances hippocampal synaptic plasticity by upregulating miRNA-132 and reducing HDAC3 signaling in a way that counteracts the synaptotoxicity of human Aβ oligomers. Our findings provide a rationale for prolonged exposure to cognitive novelty and/or epigenetic modulation to lessen the progressive effects of Aβ accumulation during human brain aging.
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease and produces a syndrome of progressive cognitive and behavioral decline in older humans. Although three decades of intensive molecular research have yielded major insights into early pathogenic events, including the linked roles of amyloid β-protein (Aβ) and tau accumulation, a safe and effective disease-modifying therapeutic still lies ahead. Growing evidence from experimental and clinical studies suggests that an enriched environment (EE) may be an effective strategy to delay and ameliorate AD and AD-like deficits in rodent models (Prado Lima et al., 2018). The cognitive-enhancing and anti-neurodegenerative effects of EE are well-documented in rodent models of neurological diseases (e.g., Nithianantharajah and Hannan, 2006; Hannan, 2014; Griñan-Ferré,et al., 2016; Stuart et al., 2017). It is assumed that the benefits of EE are driven primarily through increased neuronal plasticity. However, the molecular mechanisms by which EE may prevent or delay AD-type pathogenic events remain unclear.
We previously demonstrated a beneficial effect of EE against oligomeric Aβ (oAβ), including soluble oligomers isolated from typical AD brains, in wild-type (WT) mice that have no genetic diathesis for Aβ deposition (Li et al., 2013). Our work showed that levels of the synaptic proteins PSD95 and CREB were significantly increased after EE exposure and decreased after human Aβ oligomer treatment. miRNAs are well-known to contribute to the regulation of protein synthesis by modulating post-transcriptional programs. It has been reported that certain miRNAs were regulated in opposite directions between EE and AD rodent models (Barak et al., 2013; Dehghani et al.,2018). For example, certain “memory” related miRNAs, such as miR-132, miR-124 and miR-34, can be altered in opposite ways during synaptic plasticity and in AD (Wei et al., 2017a; Hernandez-Rapp et al., 2017). Thus, analyzing miRNA alterations after EE exposure vs. Aβ oligomer treatment could provide new clues to the molecular mechanism of impaired memory.
Another pathway implicated in the regulation of gene expression is the epigenetic control mediated by histone modifications. For example, histone acetylation and deacetylation regulate gene transcription by altering chromatin structure and the resultant accessibility to transcription factors. Histone deacetylase (HDAC) inhibitors, which allow chromatin to relax and thus increase access of transcription factors to enhance gene expression, have been found to lessen memory deficits in AD transgenic mice (Fischer et al., 2007; Vadnal et al., 2012). Regulating H3/H4 acetylation of relevant gene promoters can enhance hippocampal long-term potentiation (LTP) (Fischer et al., 2007; Francis et al., 2009; Guan et al., 2009; Ricobaraza et al., 2012). However, most HDAC inhibitors that have been studied in AD mouse models are non-selective and may cause undesirable side effects. As most patients suffer the late-onset, largely “sporadic” (non-autosomal dominant) form of AD, the impact of HDAC inhibitors screened via transgenic AD mouse models may not be as relevant to sporadic AD. Further investigation of individual HDACs in AD pathogenesis, especially as regards ‘sporadic’ forms of AD, should provide critical insights toward developing more selective HDAC inhibitors. Here, using wild-type (wt) mice rather than transgenic mouse models and applying soluble Aβ oligomers, we find that EE and Aβ oligomers induce the opposite miRNA-132 and HDAC3 changes. Importantly, upregulating miR-132 or inhibiting HDAC3 mimics EE enhancement of hippocampal LTP and prevents LTP impairment induced by human Aβ oligomers.
Methods and materials
Animals
The Harvard Medical School Standard Committee on Animals approved all experiments involving mice used for electrophysiology and biochemical assays. All mice (male and female) contained a mixed background of C57Bl/6 and 129. Animals were housed in a temperature-controlled room on a 12-h light/12-h dark cycle and had ad libitum access to food and water.
Environmental enrichment
Mice at age 30 days were randomly divided into groups of 4-6 placed into either a standard housing (SH) or an enriched environment (EE) (Li et al., 2013). SH is a common housing cage (25x20x15 cm). EE cages are larger (60x38x20 cm) and contain 2 running wheels and multiple plastic toys/objects of varying shapes and colors. The toys were changed daily. The mice were housed either under SH or EE starting at 4 weeks for another 4 weeks.
Cellular Aβ preparations
Secreted human Aß peptides were collected and prepared from the conditioned media (CM) of a CHO cell line (7PA2) that stably expressed human APP751 containing the V717F AD mutation (Podlisny et al., 1995). Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum, 1% penicillin/streptomycin, 2 mM L-glutamine, and 200 mg/ml G418 for selection. Upon reaching ~95% confluence, the cells were washed and cultured overnight (~15 h) in serum-free medium. CM was collected, spun at 1500 × g to remove dead cells and debris, and stored at 4°C. The CM was concentrated 10-fold with a YM-3 Centricon filter (Walsh et al., 2005). Aliquots of concentrated 7PA2 CM were stored at −80°C. Secreted Aβ1-x, Aβx-42 or Aβx-42 ELISA assays utilized the Meso Scale Discovery (MSD) platform and reagents from Meso Scale (Rockville, MD), as we detailed (Li et al., 2018).
Preparation of Aβ oligomers
Aβ1-42 was purchased from rPeptide (Bogart, GA). Aβ oligomers were generated as described by our previous study (Tong et al., 2017). Briefly, 1 mg lyophilized Aβ1-42 was dissolved in 222 μL HFIP to 1 mM and placed on ice for 5–10 min. Then the solution was aliquoted, and HFIP was evaporated. The peptide was stored at −80 °C. Twenty-four hours before use, Aβ1-42 was dissolved in dimethylsulfoxide and sonicated for 2 min. Oligomeric Aβ peptide was obtained by diluting this stock solution to 100 μM in PBS and incubating at 4 °C for 24 h.
Hippocampal slice preparation
Mice (C57BL/6 x 129) in either SH or EE cages were euthanized with isoflurane at 8 wk or at 7 mos of age. Brains was quickly removed and submerged in ice-cold oxygenated sucrose-replaced artificial cerebrospinal fluid (ACSF) cutting solution (206 mM sucrose, 2 mM KCl, 1.25 mM NaH2PO4, 1 mM CaCl2, 5 mM MgCl2, 26 mM NaHCO3, 11 mM D-glucose, pH 7.4, 315 mOsm). Transverse slices (350 μm thickness) from the middle portion of each hippocampus were cut with a vibroslicer. After dissection, slices were incubated in ACSF that contained the following (in mM): 124 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 2.5 CaCl2, 26 NaHCO3, 10 D-glucose, pH 7.4, 310 mOsm, in which they were allowed to recover for at least 90 min before recording. A single slice was then transferred to the recording chamber and submerged beneath continuously perfusing ACSF that had been saturated with 95% O2 and 5% CO2. Slices were incubated in the recording chamber for 20 min before stimulation under room temperature (~26°C).
Electrophysiological recordings
We used standard procedures to record field excitatory postsynaptic potentials (fEPSP) in the CA1 region of the hippocampus. A bipolar stimulating electrode (FHC Inc., Bowdoin, ME) was placed in the Schaffer collaterals to deliver test and conditioning stimuli. A borosilicate glass recording electrode filled with ACSF was positioned in stratum radiatum of CA1, 200~300 μm from the stimulating electrode. fEPSP in the CA1 region was induced by test stimuli at 0.05 Hz with an intensity that elicited a fEPSP amplitude 40-50% of maximum. Test responses were recorded for 30-60 min prior to beginning the experiment to assure stability of the response. Once a stable test response was attained, experimental treatments were added to the 10 mL ACSF perfusate, and a baseline was recorded for an additional 30 min. To induce regular LTP, two consecutive trains (1 s) of stimuli at 100 Hz separated by 20 s were applied to the slices, a protocol (HFS) that induced LTP lasting approximately 1.5 hr in wild-type mice of this genetic background. A weak-HFS that one train of 100 Hz stimulation with an intensity of 35% of maximum was also applied to the brain slices. The field potentials were amplified 100x using an Axon Instruments 200B amplifier and digitized with Digidata 1322A. Data were sampled at 10 kHz and filtered at 2 kHz. Traces were obtained by pClamp 9.2 and analyzed using the Clampfit 9.2 program. LTP values reported throughout were measured at 60 min after the conditioning stimulus unless stated otherwise. Two-tailed Student’s t-test and one-way analysis of variance (ANOVA) were used to determine statistical significance.
Real-time quantitative RT-PCR
Total RNA from primary cultures and mouse hippocampal slice was extracted with miRCURY RNA isolation kit (Exiqon), according to the manufacturer’s instructions. Reverse transcription and qPCR were performed as previous (Wei et al., 2017b). Briefly, miRCURY Universal cDNA Synthesis kit II (Exiqon) and PrimeScript RT Master Mix (Takara) were used to reverse transcribe miRNA and mRNA, respectively, and cDNAs were amplified with LNA primers for miRNA or DNA oligos for mRNA. Relative RNA levels were normalized to U6 snRNA and miR-103a for miRNAs, or to Actb for mRNAs. Primers for mRNAs:
| mRNA | Forward primer | Reverse primer |
|---|---|---|
| Actb | GGCACCACACCTTCTACAATG | GGTACGACCAGAGGCATACA |
| Hdac1 | ACACTAACGAGTACCTGGAGAAG | GTCAGAGGAGCAGATGGAGATG |
| Hdac2 | TGATGGAGATGTACCAGCCTAGC | TAGCCTCCTCCACCGAGCAT |
| Hdac3 | TCCAGCCAGTCATCAGCCAGGT | TCGATCACAGCCCAGGGAGTCA |
| Hdac8 | CAGCATCTGCGACTCCCTTGTGA | AGGCATCAGTGTGGAAGGTGGC |
Lentivirus production and stereotaxic brain injections
For lentivirus production, the miR-132-expressing PL13-pSyn-mmu-miR-132-IRES2-EGFP (El Fatimy et al., 2018), or control PL13-pSyn-IRES2-EGFP plasmid were co-transfected with packaging psPAX2 plasmids and VSV-G envelope-expressing plasmid (Addgene plasmids #12259 and #12260), and the viruses were then concentrated by additional ultracentrifugation at 25,000 rpm 4°C overnight. Lentivirus titers were determined by qPCR and functional titer was further determined by serial dilutions in the HEK293T cells, using GFP fluorescence. Positive cells were counted, and the titer was estimated using the following formula: titer (TU/mL) = number of transduced cells in day 1 x percentage of fluorescent positive cells x 1,000/volume of lentivirus used (mL). The lentivirus expressing miR-132 (LV-miR132) or empty vector (LV-control) were stereotactically injected at 6x103 TU to the CA1 region of the right hippocampus (Bregma coordinates: 2.5 mm posterior, 1.7 mm lateral, and 1.8 mm ventral) P–A, 0.5 mm; C–L, 1.7 mm; and D–V, 2.3 mm) of C57BL/6J mice. The animals were randomized to the LV-miR132and LV-control groups.
Validation of miR-132 targets by Luciferase Reporter Assay.
Full-length 3′ UTR sequence of mouse Hdac3 was cloned into psiCHECK2 plasmid (Promega, C8021), downstream of renilla luciferase, using XhoI and NotI. Binding site of miR-132-3p was predicted by RNAhybrid and starBase. Mutations in the miR-132 seed binding site (Fig. 5A) were introduced to these constructs using the QuikChange Multi Site-Directed Mutagenesis Kit (Agilent). Four hundred nanograms of the psiCHECK-based constructs were co-transfected with miRIDIAN miRNA mimics (at 25nM final concentration; Dharmacon), using Lipofectamine 2000 (Invitrogen), to the HEK293T cells grown in 96-well plates. Two days after transfections, luciferase luminescence was measured using the Dual-Glo Luciferase Assay System (Promega, E2920) and detected with Infinite F200 plate reader (TECAN). Renilla luminescence was normalized with that of firefly and the signals were presented as renilla/firefly relative luminescence.
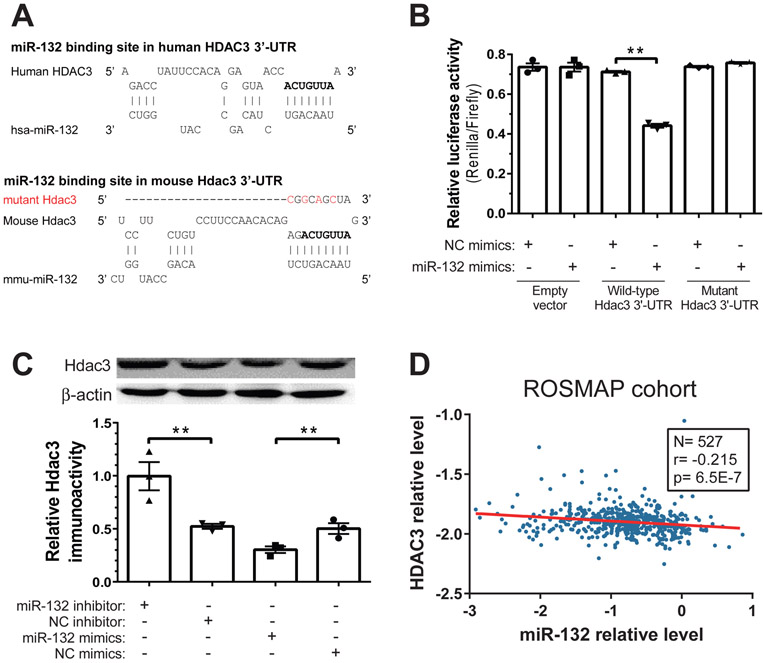
Figure 5. HDAC3 is a direct target of miR-132-3p.
(A) The predicted binding site of miR-132-3p in the 3’-UTR of HDAC3 mRNA in human and mouse, which is also observed by CLIP-Seq datasets. The conserved seed binding site is shown in bold. As a negative control, this seed binding region was disrupted by four mutations (labeled in red) in the mutant vector. (B) The effects of miR-132-3p on HDAC3 3’-UTR were examined by co-transfection of the dual-luciferase reporter system with either miR-132-3p mimics or negative control (NC) mimics. Overexpression of miR-132-3p specifically downregulated the Renilla reporter for HDAC3 3’-UTR but not for the mutant version. N=3. (C) The effect of miR-132-3p on HDAC3 protein level in neurons. Primary mouse cortical neurons were transfected with miR-132-3p inhibitor, NC inhibitor, mimics or NC mimics, and Aβ1-42 (1 μM) was added 24 hr later. Total protein was extracted after another 24 hr, and the HDAC3 protein was quantified by Western blot, with β-actin as the reference protein. N=3. (D) HDAC3 mRNA relative levels are negatively correlated with miR-132 relative levels in postmortem brain tissues from the ROSMAP cohort (N=527). miR-132 microarray data were normalized to miR-99a, and HDAC3 RNAseq data were normalized to GAPDH following log-transformation. Spearman correlation, p<0.001. All data are means ± S.E.M; *, p<0.05; **, p<0.01
Western blotting assay
Primary mouse neurons, cultured as previous (Wei et al., 2017b), were transfected with miRIDIAN miRNA mimics (25nM; Dharmacon) or LNA inhibitors (50nM; Exiqon), using Lipofectamine 2000. Total protein was isolated 24 hr later using RIPA buffer. Protein concentrations were determined using the bicinchoninic acid (BCA) assay. SDS-PAGE and immunoblot were performed as previous (Zhang et al., 2017). Briefly, equal amounts of proteins were resolved via 12% SDS-PAGE and transferred to 0.45 um PVDF membranes. After blocking with 5% BSA, membranes were incubated with 1:1000 diluted primary antibodies (Hdac3 (Santa Cruz Biotechnology, #sc-376957); β-actin (Beyotime Biotechnology, #AA128)), followed by HRP-conjugated secondary antibodies. The blots were developed by enhanced chemiluminescence (Beyotime) and quantified by ImageJ.
Fluorescence in situ hybridization of miR-132
Fluorescence in situ hybridization kits and probes were obtained from FOCOFISH (Guangzhou, China). The sequence of the miR-132 probe was: 5’DIG-CGACCATGGCTGTAGACTGTTA -3’DIG. Briefly, the neurons were incubated with 4% paraformaldehyde for 15 min at 37°C. Then neurons were incubated with 200 μl of hybridization buffer inside a water bath (55°C) for 2 hours, then probes were diluted 50 times, denatured at 85°C for 3 min, balanced at 37°C for 2 min, and added to neurons. Hybridization was carried out in a pre-warmed humidified box kept in a 42°C incubator for 15-18 hours. Finally, DAPI was used to stain the cell nuclei, and the sections were examined using a laser scanning confocal microscope (Zeiss, Germany).
Results
Inhibiting HDAC activity mimics the EE enhancement of hippocampal LTP
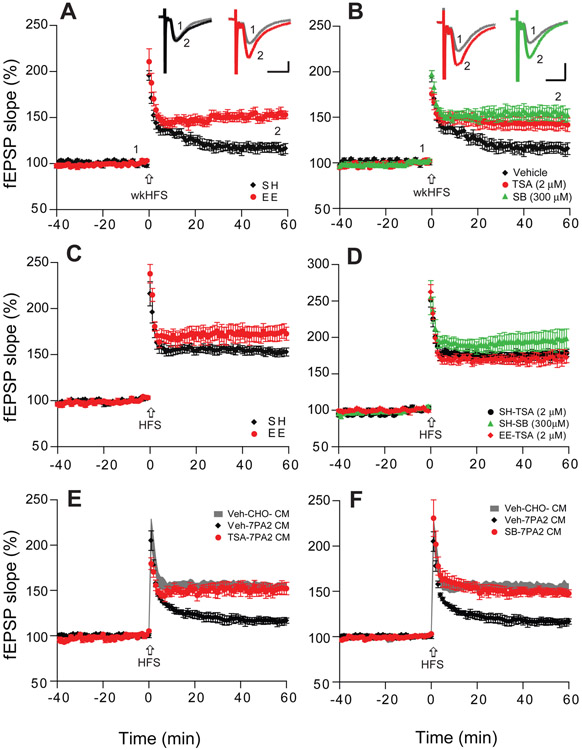
Rodents exposed to EE show improved learning and memory function, and this may alleviate signs of certain brain diseases (e.g., Alzheimer’s or Huntington’s disease) and posttraumatic stress disorder (Nithianantharajah and Hannan 2006; Fischer .2016; de la Tremblaye et al.,2018). HDAC inhibitors have been reported to result in comparable neuro-restorative effects as EE training in some rodent models of neurodegenerative diseases (Fischer and Peduzzi, 2007; Fischer et al., 2007; Govindarajan et al., 2011 ). We previously found that wt mice exposed to EE had significantly augmented hippocampal LTP (Li et al., 2013). To further assess EE-enhanced LTP, we used a sub-threshold LTP induction protocol: a weak high-frequency stimulation (wkHFS) failed to induce LTP in slices of wt mice housed conventionally [i.e., in standard housing (SH)] but produced robust LTP in slices of EE mice (117 ± 5%, n=8 slices/N=6 mice, vs 152 ± 5%, n=9 slices/N=6 mice, p<0.001) (Fig. 1A). To ascertain whether HDAC inhibitors could reproduce this EE effect, we first tested a pan-HDAC inhibitor, Trichostatin-A (TSA, 2 μM) (Levenson et al., 2004) by a 40-min perfusion onto hippocampal slices of wt mice kept in SH. The wkHFS protocol induced a significant LTP (143 ± 7%, n=8 slices/N=4 mice, vs. 113 ± 12% for vehicle alone, n=8 slices/N=4 mice, p<0.001) (Fig. 1B). Another HDAC inhibitor, sodium butyrate (SB, 300 μM) (Levenson et al., 2004), also produced a significant LTP in slices of SH mice (154 ± 7%, n=7 slices/N=4 mice, Fig. 1B). These results further confirmed previous reports that pan-HADC inhibitors enhanced hippocampal LTP (Levenson et al.,2004; Vecsey et al., 2007; Guan et al., 2009; Gräff et al., 2014). To confirm that HDAC inhibitors enhance hippocampal LTP like EE does (Li et al., 2013), we next recorded the regular-strength, HFS-induced LTP in SH vs. EE mice and observed that EE significantly enhanced this hippocampal LTP (SH: 154 ± 4%, n=9 slices/N=5 mice; vs EE: 176 ± 4%, n=9 slices/N=6 mice; p<0.001) (Fig. 1C). In line with our hypothesis, TSA and SB each significantly increased HFS-induced LTP magnitude in SH mice (TSA: 176 ± 4%, n=8 slices/N=4 mice, Fig. 1D-black; SB:196 ± 13%, n=9 slices/N=4 mice, Fig. 1D-green). Importantly, TSA had no further effect on slices of EE mice (171 ± 8%, n=10 slices/N=6 mice) (Fig. 1D-red), suggesting that the LTP enhancement induced in EE-mediated signaling pathway and HDAC inhibition effect may share similar mechanisms. Perfusing SH slices with soluble human Aβ oligomers (~1-2 nM) from the conditioned media (CM) of 7PA2 cells [CHO cells stably expressing the hAPP-V717F AD mutant (Podlisny et al., 1995)] fully inhibited HFS-induced LTP, as expected (Li et al., 2011, western blotting of 7PA2 CM were shown on Supplementary Fig. S1), but SH slices co-treated with TSA still allowed a significant and normal LTP (veh: 116 ± 4%, n=8 slices/N=6 mice, vs. TSA: 151 ± 7%, n=8 slices/N=6 mice, p<0.001) (Fig. 1E). The same benefit was seen with SB-treated SH slices (149 ± 4%, n=8 slices/N=6 mice) (Fig. 1F). These results are in line with previous observations that acute application of HDAC inhibitors can enhance hippocampal LTP in brain slices (Levenson et al., 2004; Vecsey et al., 2007; Guan et al., 2009; Hanson et al., 2013) and prevent hippocampal LTP impairment induced by soluble Aβ oligomers, similar to what EE exposure does (Li et al., 2013).
Figure 1. HDAC inhibitors enhance hippocampal LTP and occlude the LTP effect of EE.
(A) A weak high frequency stimulation (wkHFS, arrow) that usually fails to induce a significant hippocampal LTP in CA1 of hippocampal slices of mice kept in standard housing (SH, black, n=8 slices from 6 mice, i.e. n=8/6) can induce a persistent LTP in slices of mice housed in an enriched environment (EE, red, n=9/6). (B) The non-selective HDAC inhibitors, Trichostatin-A (TSA, 2 μM, red, n=8/4) and sodium butyrate (SB, 300 μM, green, n=7/4) enable the weak HFS to induce a significant LTP in slices of SH mice. (C) LTP recorded in CA1 after regular HFS is significantly enhanced in the slices of EE (n=9/6) vs. SH mice (n=9/5). (D) Treating slices with TSA (black, n=8/4) or SB (green, n=9/4) each mimics the EE effect of enhancing LTP in SH mice, and TSA occludes any further enhancement of LTP in EE mice (red). (E) TSA treatment of slices (red, n=8/6) prevents Aβ oligomer-rich 7PA2 CM from impairing LTP. (F) SB treatment of slices likewise prevents 7PA2 CM oligomers from impairing LTP (n=8/6). Inset traces are typical field excitatory postsynaptic potentials (fEPSPs) recorded before (gray) and after (black, red or green) HFS for each condition. Horizontal calibration bars: 10 ms; vertical bars: 0.5 mV.
miRNA-132/212 are upregulated in hippocampus of mice kept in EE
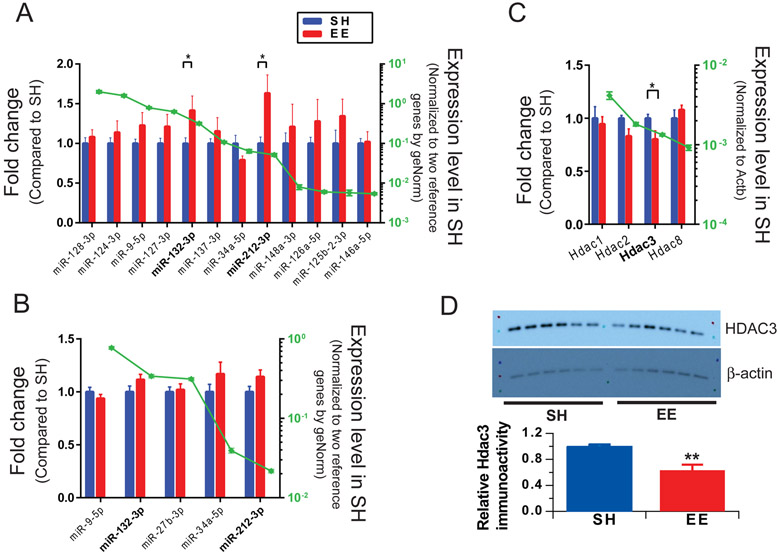
Certain miRNAs are changed in mice housed in EE and may be involved in regulating synaptic activity (e.g., Benito et al., 2018; Wei et al.,2017a). To test whether our EE paradigm also changed certain neuronal miRNAs, we quantified 12 miRNAs that were repeatedly implicated in synaptic function and/or memory (Barak et al., 2013; Garza-Manero et al., 2014; Li et al., 2014; Saab et al., 2014; Sim et al., 2014) and consistently detected in mouse brain slices. An initial comparison of the brains of 12 EE and 12 SH mice demonstrated that the expression of miRNA-132-3p and miRNA-212-3p were significantly increased, and all other miRNAs tested (34-5p, 124-3p, 125b-2-3p, 126a-5p, 127-3p, 128, 137-3p, 146a-5p, 148a-3p and 9-5p) were unchanged after 4 weeks of EE exposure (Fig. 2A). Several other relevant miRNAs were examined, but their detection levels are not higher than qPCR background signal. To further substantiate that the miR-132/212 play a role in our EE paradigm, we trained another 3 groups of mice (6 mice for EE and 6 mice for SH in each group); all demonstrated that the miR-132/212 were upregulated in EE vs. SH mice (Supplementary Fig. S2).
Figure 2. Environmental enrichment increases expression of specific miRNAs and decreases the levels of HDAC3 mRNA in the hippocampus of wild-type mice.
(A) miRNA expression levels of mice after 4 weeks EE exposure relative to littermates in SH. N=12 mice per condition. (B) Hippocampal miRNAs were quantified after just 2-week EE exposure. N=8 mice per condition. (C) Hippocampal HDAC mRNAs were quantified after 4-week EE exposure vs. SH mice. Approximately estimated abundance for each RNA was shown in green dot scaled on the right y-axis. (D) Representative Western blots showing HDAC3 in hippocampal tissue from SH and EE mice. β-actin is a loading control. Bars: mean levels (N = 6) of HDAC3 normalized to the values in SH mice. Error bars, s.e.m. Statistical analysis by one-way ANOVA test: * p<0.05, ** p < 0.01.
To test whether the miRNA expression changes we observed in EE correlated specifically with enhanced hippocampal LTP, we exposed mice to EE for just 2 weeks and then recorded LTP and quantified miRNAs levels. This brief EE exposure did not enhance hippocampal LTP significantly (Supplementary Fig. S3), nor did it alter miRNAs (Fig. 2B). This result suggests that EE enhancement of LTP may require upregulation of miR-132/212. Although these two miRNAs share a common seed sequence and thus overlapping downstream functions, miR-132 has approximately five- to ten-fold higher abundance than miR-212 (green dots in Fig. 2A-B) and thus is focused upon in the following experiments.
Because we found above that HDAC inhibition can mimic the effects of EE, we asked whether the hippocampal levels of certain HDACs were also changed. We focus on class I HDACs (that include HDAC1, HDAC2, HDAC3 and HDAC8), because they are targeted by TSA and SB. Two critical HDACs, HDAC1 (the first identified mammalian HDAC protein) and HDAC3 (the only class I HDAC located in both the nucleus and the cytoplasm (Yang et al., 2002)) were analyzed. We harvested hippocampi from mice exposed to EE for 4 weeks vs. mice of the same age and gender kept in SH and quantified the levels of certain HDAC mRNAs. Interestingly, Hdac3 was significantly decreased while Hdac1, Hdac2 and Hdac8 mRNA levels remained unchanged (Fig. 2C). To further check the protein level of HDAC3 in the hippocampus of EE and SH mice, we performed quantitative western blotting, the HDAC3 expression of EE mice was found to significantly decreasing in compare to that of SH mice (Fig. 2D). These results demonstrated that the HDAC3 mRNA and protein levels decreased in EE mice.
Overexpression of miR-132 prevents impairment of hippocampal LTP by soluble Aβ oligomers
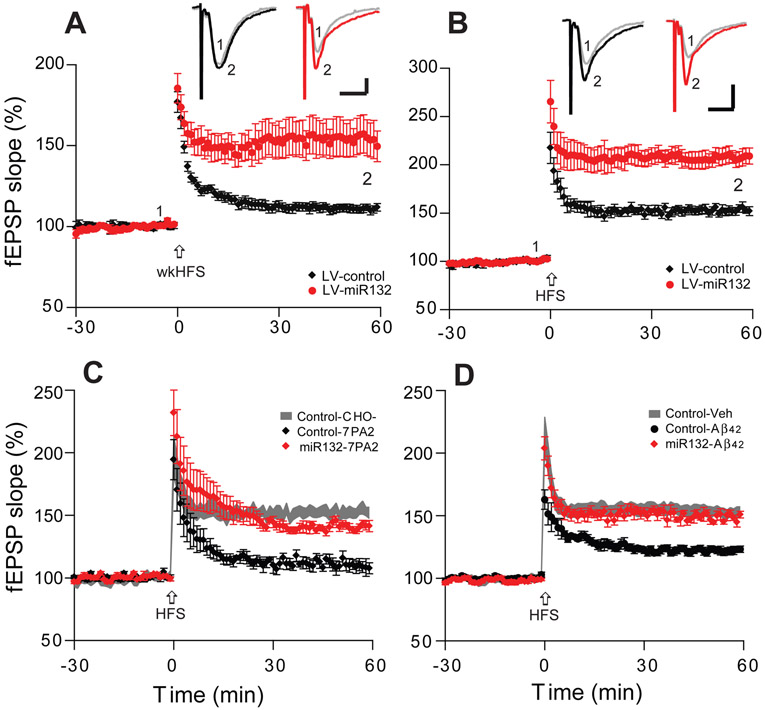
As our EE paradigm upregulated miR-132 expression, we asked whether overexpressing this microRNA could mimic the effects of EE. We produced a lentivirus expressing the mature miR-132 under the synapsin promoter (LV-miR132) and a control virus lacking the miR-132 gene. The optimized and titrated LV-miR132 or LV-control constructs were microinjected into the hippocampal CA1 areas of 6 months old wt mice via stereotactic neurosurgery (El Fatimy et al., 2018). Four weeks later, the mice were sacrificed, and the brains were sectioned for LTP recordings. Consistently with our previous results in EE-trained mice (Fig. 1A and El Fatimy et al., 2018), the miR-132 overexpressing SH mice (LV-miR-132) allowed a weak-HFS to induce a significant LTP (154 ± 11%, n=10/6; vs. LV-control 111 ± 3%, n=8/4, p<0.001) (Fig. 3A), while the basal neurotransmission and presynaptic function remain unaltered as shown by the input–output relationship between stimulation intensities and fEPSP slope in field recordings and paired-pulse facilitation, respectively (Supplementary Fig. S4) Moreover, the LV-miR-132 SH mice had markedly enhanced LTP magnitude under the full-strength HFS protocol (211 ± 9%, n=9/7; vs. LV-control 153 ± 4%, n=8/8, p<0.001) (Fig. 3B).
Figure 3. Hippocampal miR-132 overexpression significantly enhances LTP in SH mice and prevents its impairment by soluble Aβ oligomers.
(A, B) Hippocampal LTP induced by weak HFS (A) or regular HFS (B) in the CA1 region of hippocampal slices from LV-miR132 injected SH mice (red circles, n=7 slices/from 5 mice) vs. LV-control injected SH mice (black diamonds, n=7/4). (C) miR132 overexpression (red diamonds, n=7/4) prevents the ability of soluble Aβ oligomers (7PA2 CM) to impair HFS-induced LTP, whereas the LV-control vector does not (black diamonds, n=6/4). (D) The same as in (C) but using pure, aggregated synthetic Aβ1-42 (LV-control: black circles, n=7/4; LV-mi132: red diamonds, n=7/5). Inset traces are typical fEPSPs recorded before (gray) and after (black, red) HFS for each condition. Horizontal calibration bars: 10 ms; vertical bars: 0.5 mV.
To learn whether upregulating miR-132 ameliorated the adverse effects of Aβ oligomers on synaptic plasticity as shown for EE, we assessed the acute effects of soluble Aβ oligomers from several sources on hippocampal LTP. Soluble human Aβ oligomers in 7PA2 CM (vs. control CHO- CM) were applied to brain slices from the LV-miR-132 expressing vs. LV-control SH mice. Similar to our mice housed in EE, slices from miR-132 overexpressing brains were protected from oAβ-mediated LTP impairment (LV-miR-132: 141 ± 3%, n=9/6, vs. LV-control: 110 ± 5%, n=8/5, p<0.001) (Fig. 3C). To confirm this finding, we also tested aggregated synthetic Aβ1-42 (200 nM). This pure Aβ1-42 significantly impaired LTP in LV-control expressing SH mice (122 ± 3%, n=8), while the LTP inhibition was almost fully prevented in the miR-132 overexpressing SH mice (LV-miR132: 149 ± 3%, n=9/6) (p<0.001) (Fig. 3D). Our results thus far suggest that overexpressing miR-132 for 4 weeks early in life ameliorates the inhibition of hippocampal LTP by soluble Aβ oligomers, allowing a normal LTP. The beneficial effects of miR-132 overexpression were associated with improvement of synaptic proteins expression as the levels of PSD95 and CREB significantly increased (Supplementary Fig. S5).
Soluble Aβ oligomers increase HDAC3 levels and reduce miR-132 expression in cultured neurons
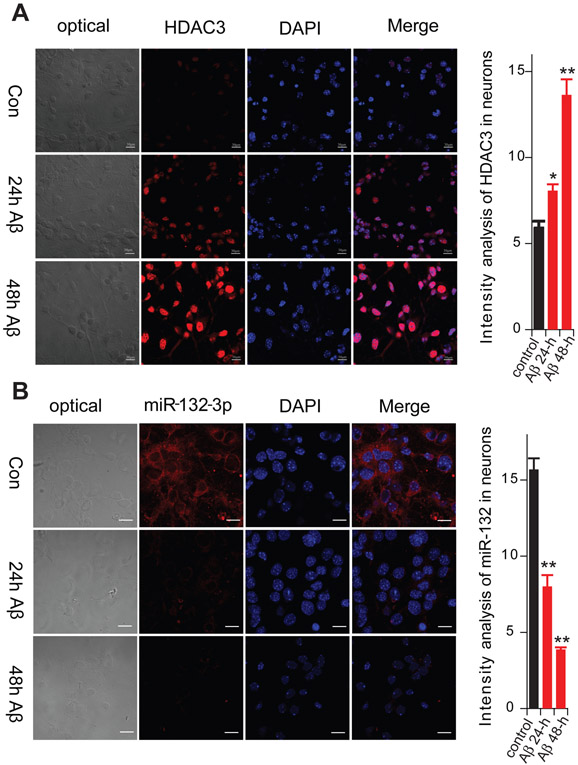
To investigate whether the Aβ oligomers, miR-132 and HDAC each affect LTP via independent mechanisms or through a shared pathway, we used mouse hippocampal primary neuron cultures to ascertain whether Aβ oligomers can increase HDAC levels and decrease miRNA-132 levels. The level of HDAC3 immunoreactivity in neurons was examined by immunofluorescence microscopy after applying pure aggregated Aβ1-42. We found that the HDAC3 levels rose significantly 24 hr and 48 hr after Aβ1-42 (1 μM) application (Fig. 4A). On the other hand, miR-132 expression, as detected by FISH in the cultured neurons, was significantly reduced by the Aβ1-42 exposure (Fig. 4B), consistent with our previous finding using qRT-PCR (El Fatimy et al., 2018). The results suggest that miR-132 downregulation and HDAC3 upregulation are intermediate steps in Aβ-induced LTP impairment.
Figure 4. The effects of synthetic Aβ1-42 oligomers on Hdac3 levels and miR-132 expression in cultured mouse primary neurons.
(A) Hdac3 immunoreactivity in response to aggregated Aβ1-42 was examined by immunofluorescence (Cy3). (B) miR-132-3p expression in response to Aβ1-42 was quantified by in situ hybridization. Data are from 3 independent experiments. Summary data are graphed on the right as means ± SEM. Statistical analysis by t test: * p<0.05. **p <0.01. Scale bar, 30 μm
HDAC3 is a target gene of miR-132-3p
These data led us to question whether miR-132 can regulate expression of HDAC3 directly. Although HDAC3 was not among validated targets of miR-132 in miRTarBase (Chou et al., 2018), several independent CLIP-Seq datasets (Gottwein et al., 2011; Kishore et al., 2011; Skalsky et al., 2012; Memczak et al., 2013) summarized in starBase v2.0 ( Li et al., 2014) suggest that miR-132 might bind to HDAC3 mRNA in vitro (Fig. 5A). Notably, the putative miR-132 seed binding site on HDAC3 mRNA is conserved between human and mouse (highlighted in bold in Fig. 5A). To validate that HDAC3 is a functional target of miR-132, we constructed a dual-luciferase reporter vector which contained either the HDAC3 3’UTR or its mutant 3’UTR (4 mutations in the seed binding region shown in red in Fig. 5A). The miR-132-3p mimics or negative control (NC) mimics were co-transfected with a luciferase reporter vector (empty psiCHECK-2 vector, vector with Hdac3 3’-UTR, or vector with mutant 3’-UTR) into HEK293T cells. Luminescence was quantified 24 hr later. Firefly luciferase (Fluc) was used as the internal reference, and the ratio of Renilla luciferase (Rluc) to Fluc quantified the miRNA effects. miR-132 did not affect luciferase activity of the empty vector, but significantly reduced the expression of Rluc gene bearing the HDAC3 3’-UTR (Fig. 5B). Importantly, such down-regulation was rescued by the specific mutations in the miR-132 binding site within the HDAC3 3’-UTR, indicating that miR-132 bound and regulated HDAC3 expression directly (Fig. 5B). To verify the effect of miR-132-3p on Hdac3 in primary neurons, the cells were transfected with miR-132-3p oligonucleotide inhibitor, a corresponding NC, miR-132-3p mimics or NC mimics, 24 hr prior to the addition of 1 μM synthetic Aβ1-42. After another 24 hr, total protein was extracted, and Hdac3 protein level was quantified by Western blot (Fig. 5C). Overexpressing miR-132 using mimics downregulated Hdac3, whereas knocking down miR-132 with the oligonucleotide inhibitor upregulated Hdac3 (Fig. 5C).
To further assess the physiological relevance of miR-132 regulation of HDAC3 in aging human and AD brains, we re-analyzed RNA expression data from the Religious Orders Study and Memory and Aging Project (ROSMAP) study (Patrick et al., 2017). In this longitudinal cohort (N=527), both miRNA and mRNA expression were profiled in dorsolateral prefrontal cortex of individuals at different stages of AD (Braak 0-VI) using microarray and RNAseq, respectively. Non-parametric Spearman’s rank correlation showed that miR-132 and HDAC3 mRNA were significantly inversely correlated (Fig. 5D). Similarly, miR-132 and HDAC3 mRNA, both quantified as log-transformed RNAseq data, correlated inversely (Supplementary Fig. S6) in a TCGA (The Cancer Genome Atlas) low grade glioma cohort (N=525) (Li et al., 2014). Taken together, these multiple lines of evidence indicated that miR-132 directly regulates HDAC3 expression in neurons and the brain.
An HDAC3 selective inhibitor mimics the EE effect and prevents oAβ-induced LTP inhibition in vivo
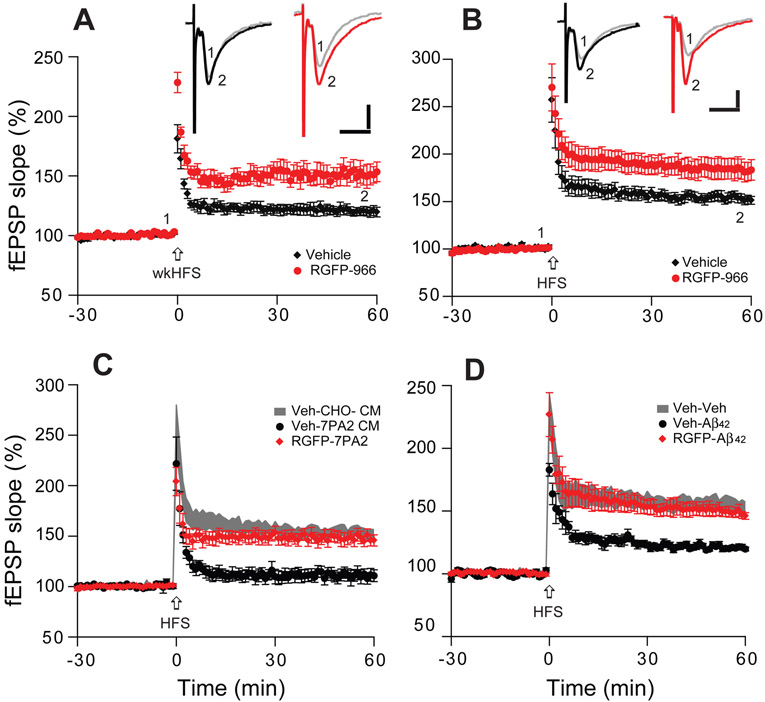
In view of the amelioration of oligomeric Aβ-mediated effects on synaptic plasticity by HDAC inhibitors in hippocampal slices, we extended this approach to in vivo supplementation. We asked whether HDAC3 played a role in the Aβ-neutralizing effects of EE. The HDAC3-selective inhibitor, RGFP-966 (35 μg), was intracerebroventricularly (icv) injected into SH mouse brain. Forty-eight hr after injection, hippocampal slices were prepared and electrophysiological recordings performed. In line with our non-selective HDAC inhibitors (Fig. 1) and reports of RGFP-966 used to treat slices in vitro (Sharma et al., 2015; Krishna et al., 2016), RGFP-966 enabled the weak-HFS to induce a robust LTP (Veh: 121 ± 4%, n=8/4, vs. RGFP-966:152 ± 9%, n=8/5, p<0.001) (Fig. 6A), and significantly increased LTP magnitude after HFS (184 ± 11%, n=8/6, vs. 154 ± 5%, n=8/5; p<0.001) (Fig. 6B).
Figure 6. In vivo injection of a selective HDAC3 inhibitor significantly enhances LTP and prevents LTP impairment by soluble Aβ oligomers.
(A) Hippocampal LTP induced by a weak HFS stimulation (arrow) in the CA1 region of SH hippocampal slices 48 hr after RGFP-966 icv injection (red circles, n=7 slices/from 5 mice) or vehicle injection (black diamonds, n=7/4). (B) As in (A), but LTP induced by a regular HFS. (C) Effects of soluble human Aβ oligomers (7PA2 CM) on hippocampal LTP induced by HFS in SH brain slices after injection of RGFP-966 (red) or vehicle; (D) As in (C) but using aggregated synthetic Aβ1-42. Inset traces are typical fEPSPs recorded before (gray) and after (black, red) HFS for each condition. Horizontal calibration bars: 10 ms; vertical bars: 0.5 mV.
To ascertain whether the in vivo administration of the HDAC3-selective inhibitor can prevent oAβ-induced LTP inhibition, we added soluble Aβ oligomer-rich 7PA2 CM to hippocampal slices of SH mice that had undergone RGFP-966 (or vehicle) i.c.v. injections 48 hr earlier. LTP was significantly impaired by 7PA2 CM in vehicle-injected mice but persevered in the RGFP-966 injected mice (Veh: 111 ± 6%, n=7/5; RGFP: 149 ± 6%, n=8/6; p<0.001) (Fig. 6C). Similar results were seen upon treatment of slices from the two groups with pure, synthetic Aβ1-42 (200 nM) (V: 121 ± 3%, n=8/4; RGFP: 150 ± 4%, n=8/6; p<0.001) (Fig. 6D). These findings suggest that inhibition of HDAC3 activity may beneficially modulate hippocampal synaptic plasticity and reduce synaptotoxic effects of Aβ oligomers, in ways comparable to those provided by a novel environment.
Discussion
Here, we investigated the molecular mechanisms of hippocampal LTP enhanced by environmental enrichment or impaired by soluble Aβ oligomers. Most importantly, we found that exposure to EE for 4 wk upregulated levels of miR-132 in hippocampal slices, whereas treating slices from SH mice with soluble Aβ oligomers reduced miR-132. Further experiments demonstrated that HDAC3 was a direct target of miR-132, and Aβ oligomers also increased neuronal HDAC3 levels. Overexpressing miR-132 or inhibiting HDAC3 in SH mice in vivo each mimicked the effects of EE in enhancing hippocampal LTP and preventing LTP impairment by Aβ oligomers. Our findings add new evidence to the epigenetic mechanisms involved in the AD pathogenesis and provide a direct link between miRNA and epigenetic regulation in AD.
Most AD cases represent the late-onset, ‘sporadic’ form of the disease without a deterministic genetic alteration. Epigenetic mechanisms are essential for normal brain functions (including learning and memory), and epigenetic alterations have been linked to the molecular pathogenesis of neurological disorders such as Huntington’s and Alzheimer’s disease (Fischer 2014; Lardenoije et al. 2015; Kim and Kaang, 2017). Growing evidence indicates that histone acetylation plays an important role in the persistence of long-term memory, as it allows chromatin to relax and thus increases access of transcription factors driving gene expression. For example, pan-HDAC inhibitors such as TSA and SB have been shown to enhance LTP in hippocampal slices and enhance memory consolidation in vivo during contextual fear conditioning (Levenson et al., 2004; Vecsey et al., 2007; Guan et al., 2009; Gräff et al., 2014) and to facilitate spatial memory (Dagnas et al., 2015; Pandey et al., 2015 ). Present study focus on early-phase LTP (E-LTP) that are widely used and sensitive to EE and HDAC inhibitors treatment in vitro (Li et al., 2013; Levenson et al., 2004; Vecsey et al., 2007) , while late-phase LTP (L-LTP) that requires new mRNA and protein synthesis is less studied in acute brain slices (Reymann & Frey 2007). Further study will be needed to find whether miR-132 also require for L-LTP in EE mice. The present study confirms and extends previous findings that TSA and SB can mimic the effect of EE in enhancing hippocampal LTP and preventing the LTP impairment induced by soluble Aβ oligomers.
HDAC inhibitors have been used clinically for a wide variety of disorders from cancer to cardiovascular disease (Yoon et al., 2016); however, the use of such agents is limited due to their low specificity. Targeting an individual HDAC would diminish the nonspecific effects of pan-HDAC inhibitors. A growing number of reports show a critical role of HDAC3 in synaptic plasticity and memory function in AD model mice (Sharma, et al., 2015; Krishna et al., 2016; Zhu et al., 2017; Janczura et al., 2018; Shu et al., 2018). These studies demonstrated that inhibition or deletion of HDAC3 results in significant neuroprotective effects, including reduced amyloidogenic APP processing and tau phosphorylation, and attenuates the activation of microglia in the hippocampus. Other evidence indicates that downregulating HDAC3 can convert a subthreshold learning event into a more persistent long-term memory (McQuowen et al., 2011; Malvaez et al., 2013; Kwapis et al., 2017). Our study supports a critical role for HDAC3 in synaptic plasticity and the protective effect of its inhibitors against soluble Aβ oligomers.
While the detailed molecular mechanisms of HDAC3 dysregulation in mice exposed to EE or in neurons treated with Aβ oligomers remain to be pinpointed, our new work strongly suggests a link to miRNA-mediated control. MicroRNAs are post-transcriptional regulators that control mRNA stability and translation (Shukla et al.,2011), and they are known to be dysregulated in AD (e.g., Ullah et al., 2014; Femminella et al., 2015; Quinlan et al., 2017; Patrick et al., 2017). Here, we show that the levels of miR-132, a miRNA enriched in neurons, are increased significantly by EE and decreased by exposure to soluble Aβ oligomers. In this context, it was previously reported that miR-132 was significantly downregulated in the brain tissue of MCI and AD patients, and its reduced levels were quantitatively associated with both Aβ and tau pathology (Wong at al. 2013; Lau et al. 2013; Patrick et al., 2017; Salta et al.,2017). miR-132 upregulation in rodent hippocampus after EE exposure has been supported by a recent report (Benito et al., 2018). Therefore, understanding the miR-132 signaling cascades could provide new insights into regulatory gene circuits in AD and lead to the development of novel miRNA-based therapeutic approaches.
Notably, miR-132 is one of the best-characterized miRNAs in the CNS, and its roles in neuronal development and synapse function have been extensively studied (Siegel et al., 2011; Wanet et al., 2012). miR-132 promotes dendritic arborization and neurite outgrowth and increases the width of dendritic spines (Magill et al., 2010; Edbauer et al., 2010). Direct miR-132 targets that mediate dendritic spine maturation and axonal extension include the spine inhibitor GTPase, p250GAP, and the sensor of axon guidance cues, RAS p21 protein activator 1 (RASA1) (Hancock et al., 2014). On the other hand, miR-132 transcription, processing, and decay are dynamically regulated by beneficial neuronal activity (Remenyi et al., 2013; Aten et al., 2016). For example, HFS-induced LTP results in a 50-fold increase of pre-miR-132 and the accumulation of mature miR-132 in anesthetized rats (Wibrand et al., 2010), whereas delta-burst HFS-LTP reduces the levels of mature miR-132 in freely moving rats by post-transcriptional mechanisms (Joilin et al., 2014). The knockout of miR-132 in mice impairs synaptic transmission, plasticity (Remenyi et al.,2013), and learning and memory (Hansen et al., 2016). Our current data show that miR-132 levels increase after 4 weeks, but not 2 weeks, of EE exposure, in agreement with the kinetics of the LTP induction. Collectively, these results indicate that miR-132 is regulated by neuronal activity and controls the expression of certain synaptic signaling genes, thereby contributing to feedback regulation of synaptic functions.
The present findings identify the miR-132/HDAC3 pathway as capable of ameliorating the impairment of synaptic plasticity by soluble Aβ oligomers; moreover, they suggest miR-132 as an innate HDAC3 inhibitor. It is tempting to hypothesize that HDAC3 functions as a critical target of miR-132 and that this helps mediate the benefits of EE. At the same time, miR-132 is known as a multifactorial regulator of neuronal health that, in addition to synaptic function, also controls tau metabolism and even overall neuronal viability (Wong et al.,2013; El Fatimy et al.,2018). Recently validated direct miR-132 targets such as acetyltransferase p300, tau modifiers GSK3β, calpain 2 and Sirt1 and Mapk1/ERK2 may also contribute to the signaling cascades of EE and Aβ oligomers (El Fatimy et al.,2018; Hernandez-Rapp et al., 2016). Overall, considering their neuroprotective properties counteracting toxic Aβ oligomers, selective HDAC3 inhibitors alone, or in combination with miR-132 mimic, might pride a novel approach to treat the cognitive deficits of AD and perhaps other neurodegenerative diseases.
Supplementary Material
Acknowledgements
Supported by NIH grants AG 027443 and AG 036694 (to D.J.S.), AG060019 and Rainwater Foundation/Tau Consortium grants (to A.M.K), a grant (to S.L.) from the Massachusetts Alzheimer’s Disease Research Center (5P50 AG 005134), the MGH Neurology Clinical Trials Units and the Harvard NeuroDiscovery Center and grants (to S.Q.) from the National Natural Science Foundation of China (U1603281 and 81870991).
Abbreviations:
- AD
Alzheimer’s disease
- EE
enriched environment
- fEPSP
field excitatory postsynaptic potentials
- HDAC
histone deacetylase
- LTP
long-term potentiation
- oAβ
soluble Aβ oligomers
- SH
standard housing
- TSA
Trichostatin-A
Footnotes
Declaration of Interests
The authors declare no competing interests.
Any Conflict of Interest: No
References
- Aten S, Hansen KF, Hoyt KR, Obrietan K. (2016) The miR-132/212 locus: a complex regulator of neuronal plasticity, gene expression and cognition. RNA Dis. 3(2). pii: e1375. Epub 2016 Aug 2. [PMC free article] [PubMed] [Google Scholar]
- Barak B, Shvarts-Serebro I, Modai S, Gilam A, Okun E, Michaelson DM, et al. , (2013) Opposing actions of environmental enrichment and Alzheimer's disease on the expression of hippocampal microRNAs in mouse models. Transl Psychiatry. 2013 September 10;3:e304. doi: 10.1038/tp.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito E, Kerimoglu C, Ramachandran B, Pena-Centeno T, Jain G, Stilling RM, et al. , (2018) RNA-Dependent Intergenerational Inheritance of Enhanced Synaptic Plasticity after Environmental Enrichment. Cell Rep. 23(2):546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, et al. , (2018) miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018 January 4;46(D1):D296–D302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnas M, Micheau J, Decorte L, Beracochea D, Mons N. (2015) Post-training, intrahippocampal HDAC inhibition differentially impacts neural circuits underlying spatial memory in adult and aged mice. Hippocampus. 25(7):827–837. [DOI] [PubMed] [Google Scholar]
- de la Tremblaye PB, Cheng JP, Bondi CO, Kline AE.(2018) Environmental enrichment, alone or in combination with various pharmacotherapies, confers marked benefits after traumatic brain injury. Neuropharmacology. pii: S0028-3908(18)30098-4. [DOI] [PubMed] [Google Scholar]
- Dehghani R, Rahmani F, Rezaei N.(2018) MicroRNA in Alzheimer's disease revisited: implications for major neuropathological mechanisms. Rev Neurosci. 29(2):161–182. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, et al. , (2010) Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 5(3):373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fatimy R, Li S, Chen Z, Mushannen T, Gongala S, Wei Z, et al. , (2018) MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol. 136(4):537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femminella GD, Ferrara N, Rengo G.(2015) The emerging role of microRNAs in Alzheimer's disease. Front Physiol. 6:40. doi: 10.3389/fphys.2015.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. (2007) Recovery of learning and memory is associated with chromatin remodelling. Nature. 447(7141):178–182. [DOI] [PubMed] [Google Scholar]
- Fischer A(2014) Epigenetic memory: the Lamarckian brain. EMBO J. 33(9):945–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A(2016) Environmental enrichment as a method to improve cognitive function. What can we learn from animal models? Neuroimage. 131:42–47. [DOI] [PubMed] [Google Scholar]
- Fischer FR, Peduzzi JD.(2007) Functional recovery in rats with chronic spinal cord injuries after exposure to an enriched environment. J Spinal Cord Med. 30(2):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis YI, Fà M, Ashraf H, Zhang H, Staniszewski A, Latchman DS, et al. , (2009) Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer's disease. J Alzheimers Dis. 18(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Manero S, Pichardo-Casas I, Arias C, Vaca L, Zepeda A. (2014) Selective distribution and dynamic modulation of miRNAs in the synapse and its possible role in Alzheimer's Disease. Brain Res. 1584:80–93. [DOI] [PubMed] [Google Scholar]
- Gottwein E, Corcoran DL, Mukherjee N, Skalsky RL, Hafner M, Nusbaum JD, et al. ,.(2011) Viral microRNA targetome of KSHV-infected primary effusion lymphoma cell lines. Cell Host Microbe. 10(5):515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A. (2011) Sodium butyrate improves memory function in an Alzheimer's disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis. 26(1):187–197. [DOI] [PubMed] [Google Scholar]
- Gräff J, Joseph NF, Horn ME, Samiei A, Meng J, Seo J, et al. , (2014) Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell. 201156(1-2):261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griñan-Ferré C, Puigoriol-Illamola D, Palomera-Ávalos V, Pérez-Cáceres D, Companys-Alemany J, Camins A, et al. , (2016) Environmental Enrichment Modified Epigenetic Mechanisms in SAMP8 Mouse Hippocampus by Reducing Oxidative Stress and Inflammaging and Achieving Neuroprotection. Front Aging Neurosci. 8:241. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. , (2009) HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 459(7243):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock ML, Preitner N, Quan J, Flanagan JG. (2014) MicroRNA-132 is enriched in developing axons, locally regulates Rasa1 mRNA, and promotes axon extension. J Neurosci. 34(1):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan AJ. (2014) Environmental enrichment and brain repair: harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol Appl Neurobiol. 40(1):13–25. [DOI] [PubMed] [Google Scholar]
- Hansen KF, Sakamoto K, Aten S, Snider KH, Loeser J, Hesse AM, et al. , (2016) Targeted deletion of miR-132/-212 impairs memory and alters the hippocampal transcriptome. Learn Mem. 23(2):61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, La H, Plise E, Chen YH, Ding X, Hanania T, et al. , (2013) SAHA enhances synaptic function and plasticity in vitro but has limited brain availability in vivo and does not impact cognition. PLoS One. 8(7):e69964. doi: 10.1371/journal.pone.0069964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rapp J, Rainone S, Goupil C, Dorval V, Smith PY, Saint-Pierre M, et al. (2016) microRNA-132/212 deficiency enhances Aβ production and senile plaque deposition in Alzheimer's disease triple transgenic mice. Sci Rep. 6:30953. doi: 10.1038/srep30953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rapp J, Rainone S, Hébert SS. (2017) MicroRNAs underlying memory deficits in neurodegenerative disorders. Prog Neuropsychopharmacol Biol Psychiatry. 73:79–86. [DOI] [PubMed] [Google Scholar]
- Janczura KJ, Volmar CH, Sartor GC, Rao SJ, Ricciardi NR, Lambert G, et al. , (2018) Inhibition of HDAC3 reverses Alzheimer's disease-related pathologies in vitro and in the 3xTg-ADmouse model. Proc Natl Acad Sci U S A. 115(47):E11148–E11157. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Joilin G, Guévremont D, Ryan B, Claudianos C, Cristino AS, Abraham WC, et al. , (2014) Rapid regulation of microRNA following induction of long-term potentiation in vivo. Front Mol Neurosci. 7:98. doi: 10.3389/fnmol.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kaang BK.(2017) Epigenetic regulation and chromatin remodeling in learning and memory. Exp Mol Med. 49(1):e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore S, Jaskiewicz L, Burger L, Hausser J, Khorshid M, Zavolan M. (2011) A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat Methods. 8(7):559–564. [DOI] [PubMed] [Google Scholar]
- Krishna K, Behnisch T, Sajikumar S.(2016) Inhibition of Histone Deacetylase 3 Restores Amyloid-β Oligomer-Induced Plasticity Deficit in Hippocampal CA1 Pyramidal Neurons. J Alzheimers Dis. 51(3):783–791. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Alaghband Y, López AJ, White AO, Campbell RR, Dang RT, et al. , (2017) Context and Auditory Fear are Differentially Regulated by HDAC3 Activity in the Lateral and Basal Subnuclei of the Amygdala. Neuropsychopharmacology. 42(6):1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardenoije R, Iatrou A, Kenis G, Kompotis K, Steinbusch HW, Mastroeni D, et al. , (2015) The epigenetics of aging and neurodegeneration. Prog Neurobiol. 131:21–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau P, Bossers K, Janky R, Salta E, Frigerio CS, Barbash S, et al. , (2013) Alteration of the microRNA network during the progression of Alzheimer's disease. EMBO Mol Med. 5(10):1613–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD.(2004) Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 279(39):40545–59. [DOI] [PubMed] [Google Scholar]
- Li JH, Liu S, Zhou H, Qu LH, Yang JH. (2014) starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014 January;42(Database issue):D92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Dolios G, Wang R, Liao FF. (2014) Soluble beta-amyloid peptides, but not insoluble fibrils, have specific effect on neuronal microRNA expression. PLoS One. 2014 March 4;9(3):e90770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Zhang D, Yang T, Koeglsperger T, Fu H, et al. , (2013) Environmental novelty activates β2-adrenergic signaling to prevent the impairment of hippocampal LTP by Aβ oligomers. Neuron. 77(5):929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ (2011). Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 31, 6627–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Jin M, Liu L, Dang Y, Ostaszewski BL, Selkoe DJ.(2018) Decoding the synaptic dysfunction of bioactive human AD brain soluble Aβ to inspire novel therapeutic avenues for Alzheimer's disease. Acta Neuropathol Commun. 2018 November 8;6(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, et al. , (2010) microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 107(47):20382–20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, et al. , (2013) HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner. Proc Natl Acad Sci U S A. 110(7):2647–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, et al. , (2011) HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 31(2):764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. , (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 495(7441):333–338. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ.(2006) Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 7(9):697–709 [DOI] [PubMed] [Google Scholar]
- Pandey K, Sharma KP, Sharma SK.(2015) Histone deacetylase inhibition facilitates massed pattern-induced synaptic plasticity and memory. Learn Mem. 22(10):514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick E, Rajagopal S, Wong HA, McCabe C, Xu J, Tang A, et al. , (2017) Dissecting the role of non-coding RNAs in the accumulation of amyloid and tau neuropathologies in Alzheimer's disease. Mol Neurodegener. 12(1):51. doi: 10.1186/s13024-017-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, et al. , (1995) Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 270(16):9564–9570. [DOI] [PubMed] [Google Scholar]
- Prado Lima MG, Schimidt HL, Garcia A, Daré LR, Carpes FP, Izquierdo I, et al. , (2018) Environmental enrichment and exercise are better than social enrichment to reduce memory deficits in amyloid beta neurotoxicity. Proc Natl Acad Sci U S A. 115(10):E2403–E2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan S, Kenny A, Medina M, Engel T, Jimenez-Mateos EM (2017) MicroRNAs in Neurodegenerative Diseases. Int Rev Cell Mol Biol. 334:309–343. [DOI] [PubMed] [Google Scholar]
- Reymann KG, Frey JU. (2007) The late maintenance of hippocampal LTP: requirements, phases, 'synaptic tagging', 'late-associativity' and implications. Neuropharmacology. 52(1):24–40. [DOI] [PubMed] [Google Scholar]
- Remenyi J, van den Bosch MW, Palygin O, Mistry RB, McKenzie C, Macdonald A, et al. , (2013) miR-132/212 knockout mice reveal roles for these miRNAs in regulating cortical synaptic transmission and plasticity. PLoS One. 8(4):e62509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricobaraza A, Cuadrado-Tejedor M, Marco S, Pérez-Otaño I, García-Osta A. (2012)Phenylbutyrate rescues dendritic spine loss associated with memory deficits in a mouse model of Alzheimer disease. Hippocampus. 22(5):1040–1050. [DOI] [PubMed] [Google Scholar]
- Saab BJ, Mansuy IM. (2014) Neuroepigenetics of memory formation and impairment: the role of microRNAs. Neuropharmacology. 80:61–9. [DOI] [PubMed] [Google Scholar]
- Salta E, De Strooper B.(2017) microRNA-132: a key noncoding RNA operating in the cellular phase of Alzheimer's disease. FASEB J. 31(2):424–433. [DOI] [PubMed] [Google Scholar]
- Sharma M, Shetty MS, Arumugam TV, Sajikumar S.(2015) Histone deacetylase 3 inhibition re-establishes synaptic tagging and capture in aging through the activation of nuclear factor kappa B. Sci Rep. 5:16616. doi: 10.1038/srep16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu G, Kramár EA, López AJ, Huynh G, Wood MA, Kwapis JL.(2018) Deleting HDAC3 rescues long-term memory impairments induced by disruption of the neuron-specific chromatin remodeling subunit BAF53b. Learn Mem. 25(3):109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla G C, Singh J, Barik S. (2011) MicroRNAs: Processing, Maturation, Target Recognition and Regulatory Functions. Mol Cell Pharmacol. 3(3):83–92. [PMC free article] [PubMed] [Google Scholar]
- Siegel G, Saba R, Schratt G.(2011) microRNAs in neurons: manifold regulatory roles at the synapse. Curr Opin Genet Dev. 21(4):491–497. [DOI] [PubMed] [Google Scholar]
- Sim SE, Bakes J, Kaang BK. (2014) Neuronal activity-dependent regulation of MicroRNAs. Mol Cells. 37(7):511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Corcoran DL, Gottwein E, Frank CL, Kang D, Hafner M, et al. , (2012) The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog. 8(1):e1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart KE, King AE, Fernandez-Martos CM, Dittmann J, Summers MJ, Vickers JC. (2017) Mid-life environmental enrichment increases synaptic density in CA1 in a mouse model of Aβ-associated pathology and positively influences synaptic and cognitive health in healthy ageing. J Comp Neurol. 525(8):1797–1810. [DOI] [PubMed] [Google Scholar]
- Tong H, Zhang X, Meng X, Xu P, Zou X, Qu S. (2017) Amyloid-beta peptide decreases expression and function of glutamate transporters in nervous system cells. Int J Biochem Cell Biol. 85:75–84. [DOI] [PubMed] [Google Scholar]
- Ullah S, John P, Bhatti A. (2014) MicroRNAs with a role in gene regulation and in human diseases. Mol Biol Rep. 41(1):225–232. [DOI] [PubMed] [Google Scholar]
- Vadnal J, Houston S, Bhatta S, Freeman E, McDonough J. (2012) Transcriptional signatures mediated by acetylation overlap with early-stage Alzheimer's disease. Exp Brain Res. 221(3):287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, et al. , (2007) Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 27(23):6128–6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanet A, Tacheny A, Arnould T, Renard P. (2012) miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res. 40(11):4742–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei CW, Luo T, Zou SS, Wu AS.(2017a) Research progress on the roles of microRNAs in governing synaptic plasticity, learning and memory. Life Sci. 188:118–122. [DOI] [PubMed] [Google Scholar]
- Wei Z, Batagov AO, Schinelli S, Wang J, Wang Y, El Fatimy R, et al. , (2017b) Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat Commun. 8(1):1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wibrand K, Panja D, Tiron A, Ofte ML, Skaftnesmo KO, Lee CS, et al. , (2010) Differential regulation of mature and precursor microRNA expression by NMDA and metabotropic glutamate receptor activation during LTP in the adult dentate gyrus in vivo. Eur J Neurosci. 31(4):636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Veremeyko T, Patel N, Lemere CA, Walsh DM, Esau C, et al. (2013) De-repression of FOXO3a death axis by microRNA-132 and −212 causes neuronal apoptosis in Alzheimer's disease. Hum Mol Genet. 22(15):3077–3092. [DOI] [PubMed] [Google Scholar]
- Yang WM, Tsai SC, Wen YD, Fejer G, Seto E.(2002) Functional domains of histone deacetylase-3. J Biol Chem. 277(11):9447–9454. [DOI] [PubMed] [Google Scholar]
- Yoon S, Eom GH (2016) HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases. Chonnam Med J. 52(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, He X, Wu X, Lei M, Wei Z, Zhang X, et al. , (2017) Rapamycin upregulates glutamate transporter and IL-6 expression in astrocytes in a mouse model of Parkinson's disease. Cell Death Dis. 8(2):e2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang S, Yu L, Jin J, Ye X, Liu Y, et al. , (2017) HDAC3 negatively regulates spatial memory in a mouse model of Alzheimer's disease. Aging Cell. 16(5):1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.