Abstract
Background
Photoacoustic (PA) imaging is an emerging non-invasive biomedical imaging modality that could potentially be used to determine the borders of basal cell carcinomas (BCC) preoperatively in order to reduce the need for repeated surgery.
Methods
Two- and three-dimensional PA images were obtained by scanning BCCs using 59 wavelengths in the range 680–970 nm. Spectral unmixing was performed to visualize the tumor tissue distribution. Spectral signatures from 38 BCCs and healthy tissue were compared ex vivo.
Results and discussion
The PA spectra could be used to differentiate between BCC and healthy tissue ex vivo (p < 0.05). Spectral unmixing provided visualization of the overall architecture of the lesion and its border.
Conclusion
PA imaging can be used to differentiate between BCC and healthy tissue and can potentially be used to delineate tumors prior to surgical excision.
Keywords: Photoacoustic imaging, Basal cell carcinoma, Basalioma, Patients, Human, Tissue differentiation, Spectral unmixing
1. Introduction
Basal cell carcinoma (BCC) is the most common form of non-melanoma skin cancer. It is characterized by a slowly growing tumor originating from basal cells in the deepest layer of the epidermis, and is most common on the head, neck, and face [1]. The incidence of BCC is increasing worldwide [2], leading to a considerable economic burden on healthcare providers. While the risk of metastasis associated with BCC is low [3,4], local invasion of BCC lesions can lead to devastating destruction of important tissues.
The recommended technique for the diagnosis of BCC is excisional biopsy. When using a 4 mm clinically predetermined margin, excisional biopsies are non-radical in approximately 5% of cases, but the percentage varies depending on the subtype [5]. An alternative technique for the removal of BCC is Mohs surgery, which allows examination of the tumor margins by staged resection and simultaneous histopathological examination [6]. Mohs surgery is considered the most efficient method for the complete removal of high-risk and complicated BCCs. However, the procedure is time consuming and expensive, and dependent on the experience of the physician, which limits its practice to specific cases [[7], [8], [9]].
Preoperative, non-invasive delineation of a tumor could improve patient management and eliminate the need for further excision. Many non-invasive imaging modalities have been used for the in vivo assessment of BCCs, but several have significant limitations. Dermatoscopy is routinely used to examine subsurface tumor characteristics and has facilitated the diagnosis of BCC [10]. However, its penetration depth is limited by optical diffusion, and images cannot be obtained beyond the papillary dermis. Other optical imaging methods, such as confocal microscopy [[10], [11], [12], [13]], and two-photon microscopy [14,15], provide good contrast and resolution, but their penetration depth is also limited. Optical coherence tomography images skin structures down to a depth of about 2 mm and can be used to visualize typical features of skin tumors [[15], [16], [17]]. Although this would be enough to enable visualization of the average BCC, a portion of these tumors, especially the more aggressive subtypes, are thicker than 2 mm [[18], [19], [20]]. High-frequency ultrasound has good resolution at penetration depths greater than those possible with optical imaging, but the contrast is poor as the difference in acoustic impedance between tumors and the surrounding tissue is low [21,22]. Magnetic resonance imaging and positron emission tomography have been used in the assessment of skin tumors [23,24], but they have poor resolution in the skin, are expensive, and are not routinely available for clinical dermatological imaging.
Photoacoustic (PA) imaging is a non-invasive biomedical imaging modality that combines the advantages of optical and ultrasound imaging [25]. It can provide high-resolution 3D images of the molecular composition of the tissue. PA imaging employs a laser that emits nanosecond pulses at different wavelengths, typically in the infrared spectral range. The energy of the pulses is absorbed by the tissue, leading to a slight increase in the temperature, which in turn results in ultrasonic signals due to thermoelastic expansion. These ultrasonic waves are collected by an ultrasonic transducer and converted into a multispectral image of the tissue. Although the resolution of PA is not as high as for example optical coherence tomography, PA imaging can provide high-resolution images at a greater depth, making it superior to most optical imaging techniques [26].
So far, PA imaging has mainly been developed experimentally, and only a few studies have been carried out on human skin tumors, mostly melanoma [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36]]. In the present study, 3D PA imaging was performed ex vivo on BCC lesions from 35 patients immediately after excision. The spectra obtained from the tumors were compared to those from healthy tissue. Spectral unmixing was performed to visualize the distribution of the suspected BCC tissue.
2. Materials and methods
2.1. Ethics
The experimental protocol was approved by the Ethics Committee at Lund University, Sweden, prior to the start of the study. The participants were given both verbal and written information about the study and its voluntary nature. Written consent was obtained from all subjects.
2.2. Patients
Forty-one patients, with 44 suspected BCC lesions, were recruited from the Department of Dermatology at Skåne University Hospital in Lund, between September and November 2018, for ex vivo investigation of their lesions. The inclusion criteria were: at least 18 years of age and suspected BCC smaller than 20 × 20 mm. The size of the tumor was limited to allow measurements to be performed using the probe on the photoacoustic equipment available. Of the 41 patients recruited, 2 were excluded due to bleeding lesions, and 4 were excluded due to temporary failure of the PA laser. The characteristics and histological diagnosis of the lesions for the remaining 35 patients, presenting with a total of 38 lesions, are given in Table 1.
Table 1.
Clinical and histological characteristics of the patients and tumors examined ex vivo.
| Patient characteristics | n | Percentage (%) | |
|---|---|---|---|
| Sex | Male/female | 18/17 | 51/49 |
| Median age (y) | 70 | Range 52−93 | |
| Skin type – Fitzpatrick scale (I–VI) | I/II | 5/30 | 14/86 |
| Diabetes mellitus | Type 1/type 2/no | 1/5/29 | 3/14/83 |
| Hypertension | Yes/no | 15/20 | 43/57 |
| Stroke | Yes/no | 5/30 | 14/86 |
| Smoking | Yes/no/previous | 4/24/7 | 11/69/20 |
| Histological characteristics | |||
| Subtype | Superficial/nodular/ morpheiform | 6/22/10 | 16/58/26 |
| Radical excision | Yes/no | 35/3 | 92/8 |
| Minimum margin stated | Yes/no | 18/20 | 47/53 |
| > 1 mm | 16 | 42 | |
| > 0.1 mm | 2 | 5 | |
| < 0.1 mm | 0 | 0 | |
2.3. Photoacoustic imaging equipment
A Vevo LAZR-X multimodal imaging system was used (FUJIFILM VisualSonics Inc., Toronto, ON, Canada), which allows examination using ultrahigh-frequency ultrasound and PA imaging. Diagnostic ultrasound is used as a guide during PA, and ultrasound images are interleaved with the laser pulses. The system has an ultrasound transducer and a fiberoptic bundle coupled to a 20-Hz tunable laser with a nanosecond pulse width. The laser was operated in the wavelength range 680–970 nm. Two planar light beams, located on either side of the ultrasound linear array, illuminate the skin surface. A 10-mm-thick Aquaflex Ultrasound Gel Pad (Parker Laboratories Inc., Fairfield, NJ, USA) was used to ensure an adequate distance between the laser fibers and the skin line. The photoacoustic waves were detected using an ultrasound linear array transducer MX400 (VisualSonics Inc.), with a central frequency of 30 MHz and bandwidth of 22−55 MHz, which provides axial and lateral resolutions of 50 and 110 μm, respectively. Three-dimensional hybrid images of ultrasound and photoacoustic waves were obtained by scanning the transducer with a linear stepping motor while capturing 2D images, using step sizes between 40 and 500 μm.
2.4. Ex vivo setup and measurements
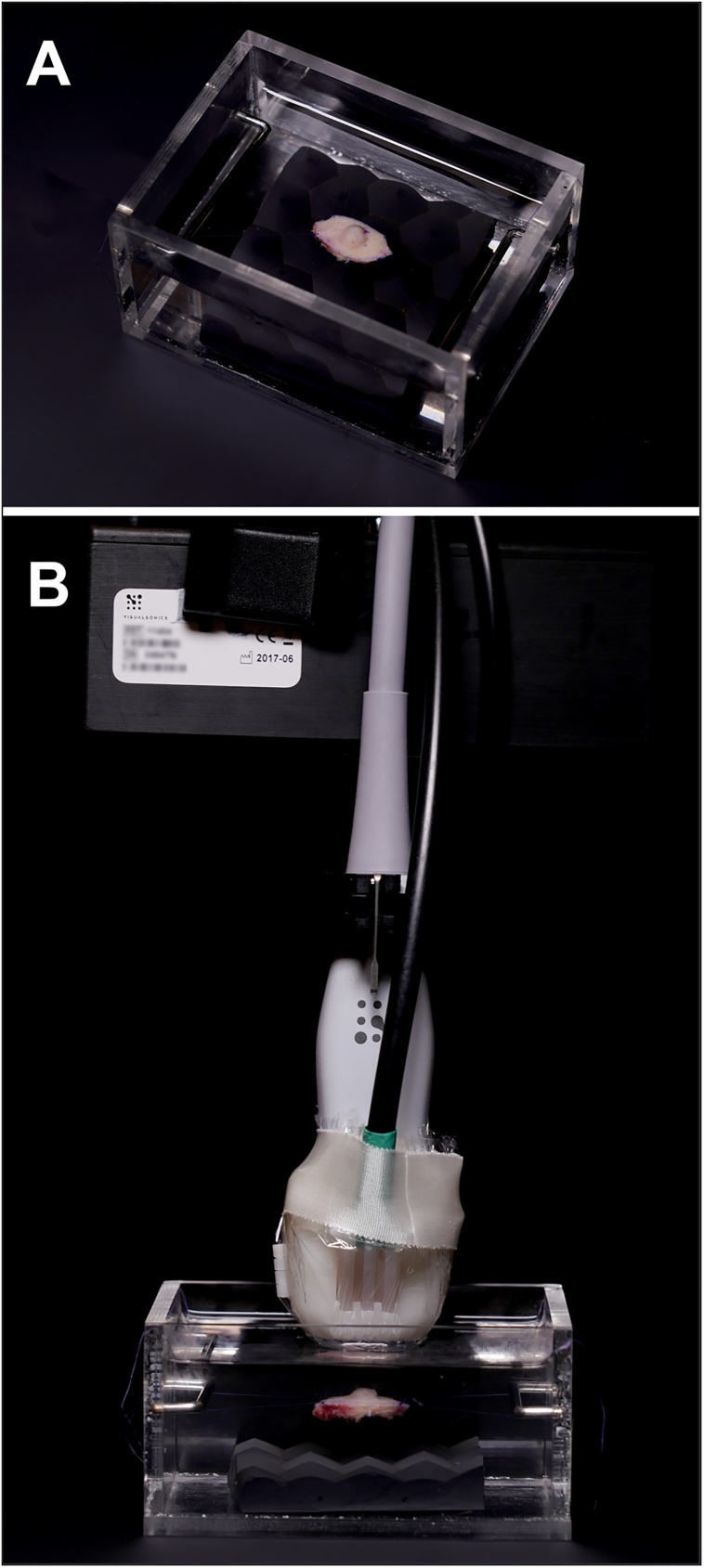
The skin lesions were surgically removed under local anesthesia, according to standard clinical procedure. Two Prolene 6-0 sutures were sewn to each end of the lesion, and it was then mounted in a 100 × 70 × 50 mm Perspex container, filled with buffered saline solution (Fig. 1A). The bottom of the container was covered with an ultrasound-attenuating material. A combined photoacoustic and ultrasound setup was used, as shown in Fig. 1B. The transducer was mounted on an adjustable arm (Mounting Accessory, GCX Corporation, Petaluma, CA, USA) to avoid motion artifacts caused by the examiner. The transducer was driven by a stepping motor (VisualSonics Inc., Toronto, Canada) allowing 3D images to be obtained.
Fig. 1.
Photographs of the ex vivo examination setup. (A) An excised lesion suspended in a saline solution. (B) The PA probe attached to an adjustable arm with the stepping motor, used for the examinations.
2.5. Spectral signature of BCCs
To obtain the mean photoacoustic signature of all tumors, four spectral scans were performed on each lesion: three in different parts of the tumor, and one outside the lesion in healthy tissue, as a reference. The first of the three scans of the tumor was chosen for further analysis if the signal-to-noise ratio was deemed adequate at visual inspection. If the signal quality was suboptimal, the two other scans were also analyzed and the one with best quality was selected for further use. This resulted in 59 PA images collected at different wavelengths (680−970 nm in steps of 5 nm) giving each ‘pixel’ of the ultrasound image a spectrum dependent on the optical properties of the tissue. It was hypothesized that these spectra could provide a fingerprint of typical BCC tissue.
2.6. Multiwavelength 3D scan
To obtain the spectral signals for the whole ex vivo tissue and to be able to perform spectral unmixing, a multiwavelength 3D scan was performed using the same 59 wavelengths (680−970 nm, in steps of 5 nm). The step length between each 2D image was 500 μm. The scanning time for a multiwavelength 3D scan was approximately 4 min.
2.7. Histopathology
After ex vivo imaging, the lesion was placed in formalin and sent for histological analysis using standard staining with hematoxylin and eosin (H&E).
2.8. Data analysis
All measurements were analyzed and assessed using VisualSonics Vevo LAB 3.1.0 software for data processing and storage. With the help of this software, regions of interest (ROIs) were defined in the ultrasound image. Freehand ROIs were drawn only on the most certain central part of the lesion to get a signal derived from the tumor tissue. The same was done from the most certain part of the healthy tissue outside of the lesion and was used as a reference. Each ROI could then be translated into the mean PA spectral signal of the corresponding area in the software, making it possible to compare the tumor signal with the signal from the surrounding healthy tissue.
The spectral signals from the tumors and healthy tissue were exported to MATLAB R2017b (MathWorks Inc., Natick, MA, USA) for further calculations and graph drawing. To calculate the mean signal for all the lesions, the signals were first min-max normalized into the 0–1 range. Graphs were created representing the mean of all the lesion spectra and the healthy tissue spectra.
2.9. Spectral unmixing
In addition to multiwavelength 3D scans, an imaging technique known as spectral unmixing was employed. The resulting mean PA tumor spectrum from the 38 lesions were used as the endmember spectrum. A linear spectral unmixing approach minimizing the mean squared error was then performed in every pixel for all collected 2D images, producing false-colored images that were overlaid the ultrasound images, mapping out the probable spatial distribution of BCC tissue [37]. Due to the complexity of the chromophoric composition of BCC, this analysis was thus based on the overall spectral dissimilarities between BCC and healthy tissue instead of identifying individual chromophores.
2.10. Statistical analysis
Comparison of the normalized spectral signals were analyzed using two-way analysis of variance (ANOVA) for repeated measures followed by the Sidak multiple comparison test. Significance was defined as: p < 0.05 (∗) and p > 0.05 (not significant, n.s.).
3. Results
3.1. Spectral signatures
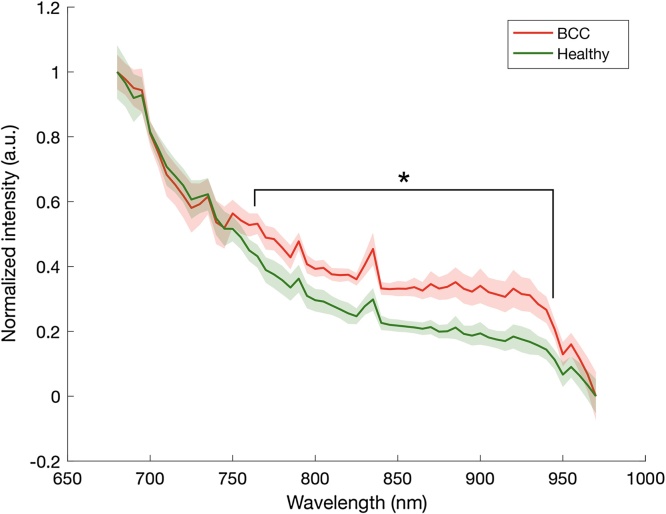
A clear difference was seen between the mean spectra from BCCs and healthy tissue ex vivo. This difference was statistically significant (p < 0.05) in the wavelength range 760–945 nm (Fig. 2). Individual spectra could vary, as indicated in the range, and no clear difference between the various BCC subtypes could be found. For instance, at 850 nm, mean tumor signal was 0.31 (range 0.16−0.51) and healthy tissue 0.20 (range 0.05−0.43). The difference between the two groups remained statistically significant also when using alternative methods for normalization, such as normalization at 920 nm.
Fig. 2.
The mean PA spectra obtained from BCCs and the surrounding healthy tissue from ex vivo measurements. The dispersion (± one standard deviation) is indicated by the shaded areas. A clear difference can be seen in the spectral signatures of the BCCs and healthy tissue (p < 0.05 for 760 to 945 nm, n=38).
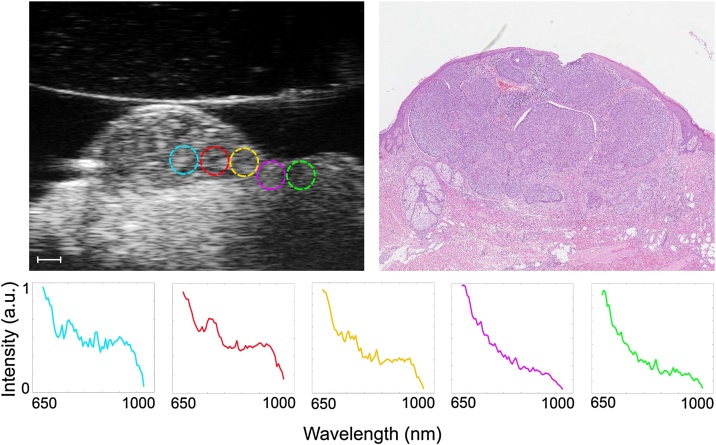
The gradual change in the PA spectrum, from lesion to normal tissue, was investigated by defining several small ROIs in a row, starting at the center of the tumor and extending out into healthy tissue (Fig. 3). This provides further evidence that the PA signal could be used to differentiate cancerous tissue from healthy tissue.
Fig. 3.
Representative example of an ex vivo scan of a nodular BCC. The image on the upper left shows the small ROIs superimposed on the ultrasound image. The scale bar is 1 mm. The PA spectra obtained from the ROIs are given below, showing the change from cancerous to healthy tissue. The image on the upper right shows the same H&E-stained histological sample.
3.2. Multiwavelength 3D scanning and spectral unmixing
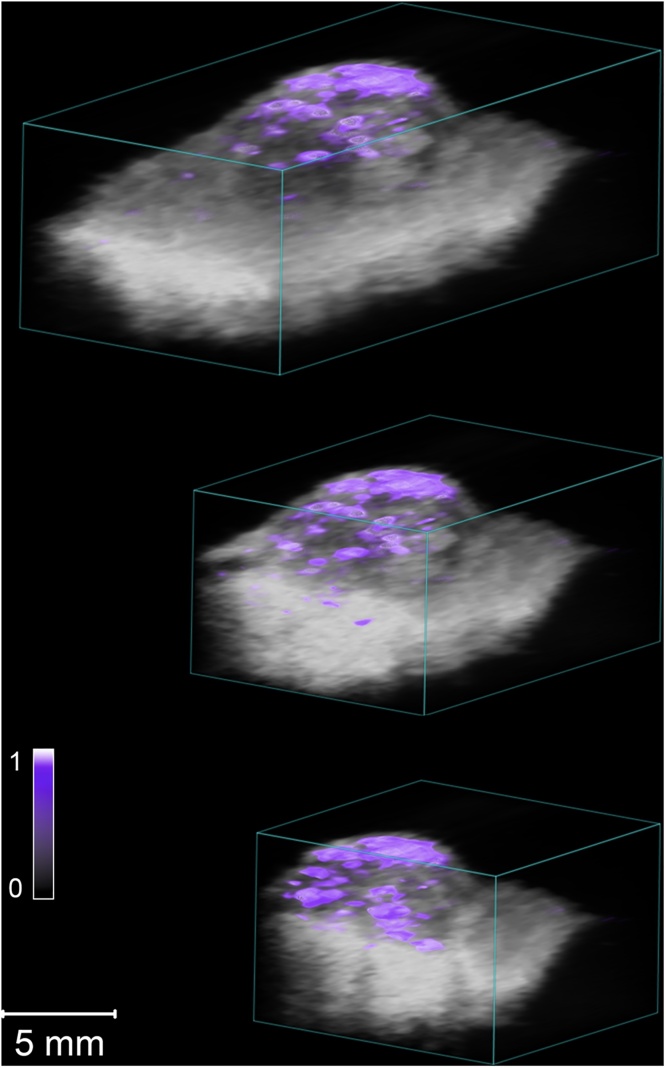
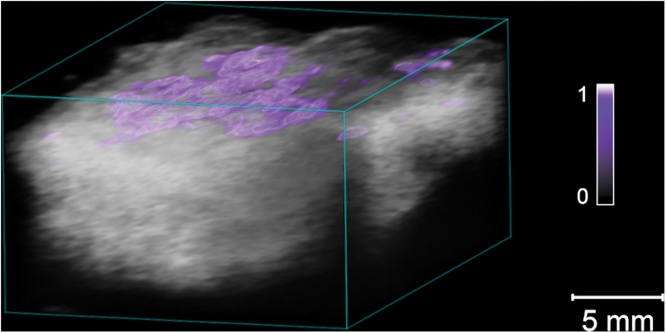
Multiwavelength 3D scanning was performed over the whole excised lesion, providing a map of the overall lesion architecture. To obtain more information on BCC tissue distribution in the lesion, spectral unmixing was performed on the multiwavelength recordings using the mean absorption spectrum for all 38 tumors. Fig. 4 shows a spectrally unmixed 3D sequence of a nodular BCC lesion and Fig. 5a superficial BCC, illustrating how this can be used to differentiate the tumor tissue from the surrounding healthy tissue.
Fig. 4.
Representative example of a spectrally unmixed 3D multiwavelength PA image of a nodular BCC lesion examined ex vivo. The suspected cancerous tissue is shown as a purple overlay. The colorbar represents the unmixed signal for BCC tissue (a.u.). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
Representative example of a spectrally unmixed 3D multiwavelength PA image of a superficial BCC lesion examined ex vivo. The suspected cancerous tissue is shown as a purple overlay. The colorbar represents the unmixed signal for BCC tissue (a.u.). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
Photoacoustic imaging constitutes a new optical technique that can be used to obtain real-time 2D and 3D images, with high resolution and depth, for the evaluation of skin tumors. The main strength of PA imaging is its ability to image molecular changes, and thus differentiate healthy from diseased tissue. The high resolution and imaging depth may make it possible to determine the tumor borders in three dimensions. However, mainly preclinical studies have been performed to date. In this study, we have demonstrated the feasibility of PA imaging in differentiating between BCC and healthy tissue by analyzing the spectral signatures. Furthermore, using this information for spectral unmixing, the distribution of tumor tissue could be visualized. This shows promise for future presurgical definition of the tumor, but further development of the spectral unmixing algorithms is needed before this can be studied.
So far, only two other research groups have used PA imaging to study BCCs [27,30,31,38]. A research group at the National Skin Center in Singapore has shown that PA can be used to visualize and measure the depth and length of BCCs in vivo in a small group of patients (Supplementary Table S1 in [31]). They performed 3D reconstructions using 10 wavelengths between 700 and 900 nm with an inVision 512-echo opto-acoustic imaging system (iThera Medical, GmbH, Munich, Germany) [27,31]. The majority of the patients in their study manifested with pigmented BCCs, and the tumor depth and length were obtained by calculating the distance between the non-baseline values of the melanin signals after spectral unmixing. While pigmentation of BCCs is seen in 50% of Blacks, Hispanics, and Japanese, only 6% of BCCs are pigmented in Caucasians [39]. The patients in the present study were defined as Fitzpatrick skin type I or II (i.e. fair-skinned), and none had a pigmented BCC. Most clinical in vivo and ex vivo studies using PA have been performed on melanoma lesions [28,29,34,36,40]. Melanoma lesions contain higher amounts of melanin, which is an endogenous chromophore with a known absorption spectrum [41].
Photoacoustic imaging relies on the absorption of light in the tissue by chromophores, and the degree of absorption varies with the concentration of chromophores in the tissue. The difference in spectral signatures between BCCs and healthy tissue thus expectedly provides an indication that the molecular compositions are different. The lack of pigmentation in the BCCs investigated in the present study suggests that melanin alone is not responsible for the difference in the spectral signatures of tumors and healthy tissue. The cellular origin of BCC has not been completely elucidated, but it has been reported that BCC can arise from multiple epidermal and follicular compartments [42,43]. Cytokeratin is expressed in all types of epithelial cells, and is often used as an immunohistological marker for non-melanoma skin tumors [42,44]. In addition, BCCs often contain arborizing blood vessels. These features, together with others, may explain the changes in the spectrum seen in the wavelength interval 745−965 nm. A larger number of wavelengths (59) and a broader spectral range (680−970 nm) were used in the present study than in previous studies. This provides a broader view of the molecular composition of the tissue, increasing the probability of finding differences between cancerous and healthy tissue.
The PA examinations in the present study were performed on surgically excised BCCs. This provided high-resolution spectra, free from motion artifacts and other sources of interference. One of the most important tasks in the translation of the PA technique from the ex vivo to the in vivo setting is the reduction of motion artefacts. We have recently published a paper describing a setup allowing for in vivo 3D PA scanning in patients [45]. In an effort to reduce motion artifacts, the patients were examined lying down, and the area to be examined was stabilized with a vacuum pillow. The transducer was mounted on an adjustable arm and moved by a stepping motor, instead of using a hand-held transducer. A few examinations have been carried out on BCCs in vivo with this setup and the results are encouraging (data not shown).
The spectral signal was found to change distinctly at the border when moving from the center of the tumor to the surrounding healthy tissue. This not only confirms that there is a difference between the signals from a BCC and healthy tissue, but also indicates the possibility of using this modality to define tumor borders in the future. With the aid of machine-learning techniques, it may be possible to define the tumor margins, avoiding operator subjectivity, learning curves and variations in the examiner’s assessment. To identify and mark the tumor border, it would be necessary to develop a probe that is capable of this, as this does not exist today. A first step toward the implementation of PA in the clinical setting, could be to excise the tumor in the routine way, and then immediately scan the lesion ex vivo to determine whether it has been radically removed. This would be faster than conventional Mohs surgery. Further developments and studies on a larger number of patients with different kinds of skin tumors will be required before the method can be implemented in the clinic.
One limitation of the present study was that it was not possible to separate the different sub-types of BCCs due to the limited sample size. There was some interindividual variability in the spectra obtained from the lesions, which could have been partially due to different subtypes of BCCs. Further studies are required on larger groups of histologically different BCCs to investigate the spectra for specific subtypes of BCCs. Surgery involves external trauma to the body, and autolysis is initiated a certain time after excision, resulting in the release of metabolites into the tissue and edema. This could affect the results, however the time between excision and measurement was short, which makes this improbable. Furthermore, the spectrum from the tumor tissue was compared to that of surrounding healthy tissue that has experienced the same trauma, which should eliminate this source of error.
In summary, the spectral signatures from BCCs and healthy tissue were found to be statistically significantly different. Spectral unmixing could be used to visualize the probable distribution of BCC tissue in three dimensions. Our findings provide further evidence of the potential of this new imaging method in guiding surgical intervention to achieve more precise excision with better clearance, reducing the need for repeated surgery. It may also be possible to shorten the duration of traditional Mohs surgery by using PA imaging instead of histopathological examination of the excised lesion. Further studies are necessary to develop the technique so that it can be used to identify the tumor margins prior to surgery.
Funding source
All sources of funding should also be acknowledged and you should declare any involvement of study sponsors in the study design; collection, analysis and interpretation of data; the writing of the manuscript; the decision to submit the manuscript for publication. If the study sponsors had no such involvement, this should be stated.
Declaration of Competing Interest
A conflicting interest exists when professional judgement concerning a primary interest (such as patient’s welfare or the validity of research) may be influenced by a secondary interest (such as financial gain or personal rivalry). It may arise for the authors when they have financial interest that may influence their interpretation of their results or those of others. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
Acknowledgements
This study was supported by the Swedish Government Grant for Clinical Research (ALF), Skåne University Hospital (SUS) Research Grants,Skåne County Council Research Grants,Lund University Grant for Research Infrastructure, the Swedish Cancer Foundation Crown Princess Margaret's Foundation (KMA), the Foundation for the Visually Impaired in the County of Malmöhus, The Nordmark Foundation for Eye Diseases at Skåne University Hospital, Lund Laser Center Research Grant,IngaBritt and Arne Lundberg Research Foundation, Carmen and Bertil Regnér Foundation and the Swedish Eye Foundation. The authors would like to thank John Albinsson, Josefin Andersson, Bo Baldetorp, Cassandra Hennström, Katarina Lundqvist, Helen Sheppard and the surgical staff at the Department of Dermatology in Lund for valuable help with this project.
Biographies

Ulf Dahlstrand received his medical degree in 2009 and his PhD in 2020 at Lund University, Lund, Sweden. His main area of interest is the need for better noninvasive techniques for tumor margin delineation, both in the periorbital area as well as on the rest of the skin. He is an ophthalmologist at the Department of Ophthalmology, Skåne University Hospital, Lund, Sweden, specializing in oculoplastic and strabismus surgery.

Rafi Sheikh was born in Visby, Sweden in 1980. He received his MD degree in 2009 and his PhD in 2018 at Lund University, Lund, Sweden. His main areas of research interest are currently oculoplastic surgery, microvascular blood flow, neuro-ophthalmology and photoacoustic imaging. He works clinically as an ophthalmologist, specialized in cataract and vitreoretinal surgery, at Skåne University Hospital.

Aboma Merdasa was born in Örebro, Sweden in 1982. He received his M.Sc. in engineering physics (Lund University, 2010) focusing on optical detection of malaria infected blood cells without the need for chemical staining. He received his PhD degree in chemical physics (Lund University, 2017) with his thesis topic on super-resolution optical microscopy of functional materials. After a two year post-doc at the Helmholtz Center Berlin working on spectroscopic characterization of energy materials, he is currently pursuing his research interest in biomedical physics employing a diverse range of spectroscopy and imaging characterization methods at Lund University and Skåne University Hospital.

Rehan Chakari is currently a medical student at the University of Lund. His research focus is the use of photoacoustic imaging for the detection and delineation of various skin tumors.

Bertil Persson was born in Helsingborg, Sweden in 1958. He received his M.D. degree from Lund University in 1983 and became specialist in dermato-venereology in 1992. He was head of the Department of Dermato-venereology, Skåne University hospital, Lund Sweden, between 2003 and 2013. He is currently senior consultant at the department and head of the units of skin tumor diagnostics, Mohs- and dermatosurgery. His research interests include tumor imaging and treatment, and photoacoustic imaging.

Magnus Cinthio received his M.Sc. degree in biomedical engineering and his PhD degree in electrical measurements from Lund University, Lund, Sweden, in 1999 and 2004, respectively. In 2010, he joined the Faculty of Engineering, Lund University, as an Associate Professor. He was a visiting researcher at Tohoku University, Sendai, Japan, in 2007, and at Florence University, Florence, Italy, in 2012. In 2013, he joined the Department of Biomedical Engineering, Lund University, as a University Lecturer. His research interests include the longitudinal movement and the resulting intramural shearing of the arterial wall, ultrasonic tissue motion measurements, photoacoustic imaging, as well as arterial, cerebral, and intestinal characterization.

Tobias Erlöv received his M.Sc. degree in engineering physics and his PhD degree in biomedical engineering from Lund University, Lund, Sweden, in 2009 and 2015, respectively. His PhD thesis was on the characterization of the arterial wall and carotid plaques. He is currently a post-doctoral researcher in photoacoustic imaging at the Department of Biomedical Engineering, Lund University. His research interests include ultrasound signal processing, tissue characterization, motion tracking, and photoacoustic imaging.

Bodil Gesslein is a vascular scientist with a special interest in ophthalmology and neuroscience. She received the M.Sc. degree in Biomedicine from Lund University, Lund, Sweden, in 2006. In 2010 she received the PhD degree in Experimental Vascular Research with a focus on retinal ischemia from Department of Ophthalmology at Lund University. Between 2011 and 2017 she pursued a post doc at Department of Neuroscience and Pharmacology at University of Copenhagen, Copenhagen, Denmark where she was involved in studying capillary blood flow regulation in the brain. Since 2018 she has returned to Department of Ophthalmology at Lund University working as a senior researcher. Her current research interest includes photoacoustic imaging and non-invasive imaging techniques for monitoring blood perfusion and vascular anatomy.

Malin Malmsjö is an internationally recognized expert in oculoplastic surgery, an expertise that she has honored in her role as Professor and Senior Consultant in ophthalmology, focusing on cancer surgery at Skåne University Hospital, Lund, Sweden. She is currently the Head of the Department of Ophthalmology. She has written over 140 scientific publications and book chapters and is also the inventor and patent holder of award-winning medical devices for heart and vascular surgery. Her research in ophthalmology focuses on the development of novel noninvasive imaging techniques for tumor margin delineation and optimizing periorbital cancer surgery by monitoring blood perfusion.
References
- 1.Lomas A., Leonardi-Bee J., Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 2.Cameron M.C., Lee E., Hibler B., Barker C.A., Mori S., Cordova M., Nehal K.S., Rossi A.M. Basal cell carcinoma: part 1. J. Am. Acad. Dermatol. 2018 doi: 10.1016/j.jaad.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 3.Lewis K.G., Weinstock M.A. Nonmelanoma skin cancer mortality (1988–2000): the Rhode island follow-back study. Arch. Dermatol. 2004;140(7):837–842. doi: 10.1001/archderm.140.7.837. [DOI] [PubMed] [Google Scholar]
- 4.Mohan S.V., Chang A.L. Advanced basal cell carcinoma: epidemiology and therapeutic innovations. Curr. Dermatol. Rep. 2014;3:40–45. doi: 10.1007/s13671-014-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas D.J., King A.R., Peat B.G. Excision margins for nonmelanotic skin cancer. Plast. Reconstr. Surg. 2003;112(1):57–63. doi: 10.1097/01.PRS.0000067479.77859.31. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu D., Fathi R., Srivastava D., Nijhawan R.I. Current perspectives on Mohs micrographic surgery for melanoma. Clin. Cosmet. Investig. Dermatol. 2018;11:309–320. doi: 10.2147/CCID.S137513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deinlein T., Richtig G., Schwab C., Scarfi F., Arzberger E., Wolf I., Hofmann-Wellenhof R., Zalaudek I. The use of dermatoscopy in diagnosis and therapy of nonmelanocytic skin cancer. J. Dermatol. Ges. 2016;14(2):144–151. doi: 10.1111/ddg.12903. [DOI] [PubMed] [Google Scholar]
- 8.Newlands C., Currie R., Memon A., Whitaker S., Woolford T. Non-melanoma skin cancer: united kingdom national multidisciplinary guidelines. J. Laryngol. Otol. 2016;130(S2):S125–S132. doi: 10.1017/S0022215116000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker P., Hill D. Surgical treatment of basal cell carcinomas using standard postoperative histological assessment. Australas. J. Dermatol. 2006;47(1):1–12. doi: 10.1111/j.1440-0960.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 10.Benvenuto-Andrade C., Dusza S.W., Agero A.L., Scope A., Rajadhyaksha M., Halpern A.C., Marghoob A.A. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch. Dermatol. 2007;143(3):329–338. doi: 10.1001/archderm.143.3.329. [DOI] [PubMed] [Google Scholar]
- 11.Rajadhyaksha M., Grossman M., Esterowitz D., Webb R.H., Anderson R.R. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J. Invest. Dermatol. 1995;104(6):946–952. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 12.Segura S., Puig S., Carrera C., Palou J., Malvehy J. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J. Am. Acad. Dermatol. 2009;61(2):216–229. doi: 10.1016/j.jaad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Xiong Y.D., Ma S., Li X., Zhong X., Duan C., Chen Q. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J. Eur. Acad. Dermatol. Venereol. 2016;30(8):1295–1302. doi: 10.1111/jdv.13712. [DOI] [PubMed] [Google Scholar]
- 14.Dimitrow E., Ziemer M., Koehler M.J., Norgauer J., Konig K., Elsner P., Kaatz M. Sensitivity and specificity of multiphoton laser tomography for in vivo and ex vivo diagnosis of malignant melanoma. J. Invest. Dermatol. 2009;129(7):1752–1758. doi: 10.1038/jid.2008.439. [DOI] [PubMed] [Google Scholar]
- 15.Gambichler T., Regeniter P., Bechara F.G., Orlikov A., Vasa R., Moussa G., Stucker M., Altmeyer P., Hoffmann K. Characterization of benign and malignant melanocytic skin lesions using optical coherence tomography in vivo. J. Am. Acad. Dermatol. 2007;57(4):629–637. doi: 10.1016/j.jaad.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 16.von Braunmuhl T., Hartmann D., Tietze J.K., Cekovic D., Kunte C., Ruzicka T., Berking C., Sattler E.C. Morphologic features of basal cell carcinoma using the en-face mode in frequency domain optical coherence tomography. J. Eur. Acad. Dermatol. Venereol. 2016;30(11):1919–1925. doi: 10.1111/jdv.13704. [DOI] [PubMed] [Google Scholar]
- 17.Pelosini L., Smith H.B., Schofield J.B., Meeckings A., Dithal A., Khandwala M. A novel imaging approach to periocular basal cell carcinoma: in vivo optical coherence tomography and histological correlates. Eye. 2015;29(8):1092–1098. doi: 10.1038/eye.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welsch M.J., Troiani B.M., Hale L., DelTondo J., Helm K.F., Clarke L.E. Basal cell carcinoma characteristics as predictors of depth of invasion. J. Am. Acad. Dermatol. 2012;67(1):47–53. doi: 10.1016/j.jaad.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 19.Pyne J.H., Myint E., Barr E.M., Clark S.P., Hou R. Basal cell carcinoma: variation in invasion depth by subtype, sex, and anatomic site in 4,565 cases. Dermatol. Pract. Concept. 2018;8(4):314–319. doi: 10.5826/dpc.0804a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wetzel M., Strickley J., Haeberle M.T., Brown T.S. Depth of invasion of aggressive and nonaggressive basal cell carcinoma. J. Clin. Aesthet. Dermatol. 2019;12(3):12–14. [PMC free article] [PubMed] [Google Scholar]
- 21.Wassef C., Rao B.K. Uses of non-invasive imaging in the diagnosis of skin cancer: an overview of the currently available modalities. Int. J. Dermatol. 2013;52(12):1481–1489. doi: 10.1111/ijd.12159. [DOI] [PubMed] [Google Scholar]
- 22.Dummer W., Blaheta H.J., Bastian B.C., Schenk T., Brocker E.V., Remy W. Preoperative characterization of pigmented skin lesions by epiluminescence microscopy and high-frequency ultrasound. Arch. Dermatol. 1995;131(3):279–285. [PubMed] [Google Scholar]
- 23.Bittoun J., Querleux B., Darrasse L. Advances in MR imaging of the skin. NMR Biomed. 2006;19(7):723–730. doi: 10.1002/nbm.1101. [DOI] [PubMed] [Google Scholar]
- 24.Belhocine T.Z., Scott A.M., Even-Sapir E., Urbain J.L., Essner R. Role of nuclear medicine in the management of cutaneous malignant melanoma. J. Nucl. Med. 2006;47(6):957–967. [PubMed] [Google Scholar]
- 25.Wang L.V. Prospects of photoacoustic tomography. Med. Phys. 2008;35(12):5758–5767. doi: 10.1118/1.3013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu D., Yang S., Wang Y., Gu Y., Xing D. Noninvasive and high-resolving photoacoustic dermoscopy of human skin. Biomed. Opt. Express. 2016;7(6):2095–2102. doi: 10.1364/BOE.7.002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attia A.B.E., Chuah S.Y., Razansky D., Ho C.J.H., Malempati P., Dinish U.S., Bi R., Fu C.Y., Ford S.J., Lee J.S., Tan M.W.P., Olivo M., Thng S.T.G. Noninvasive real-time characterization of non-melanoma skin cancers with handheld optoacoustic probes. Photoacoustics. 2017;7:20–26. doi: 10.1016/j.pacs.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breathnach A., Concannon E., Dorairaj J.J., Shaharan S., McGrath J., Jose J., Kelly J.L., Leahy M.J. Preoperative measurement of cutaneous melanoma and nevi thickness with photoacoustic imaging. J. Med. Imaging. 2018;5(1) doi: 10.1117/1.JMI.5.1.015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breathnach A., Concannon L., Aalto L., Dorairaj J., Subhash H.M., Kelly J., Leahy M. Assessment of cutaneous melanoma and pigmented skin lesions with photoacoustic imaging. Proc. SPIE. 2015;9303 [Google Scholar]
- 30.Chuah S.Y., Attia A.B., Long V., Ho C.J., Malempati P., Fu C.Y., Ford S.J., Lee J.S., Tan W.P., Razansky D., Olivo M., Thng S. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography. Skin Res. Technol. 2017;23(2):221–226. doi: 10.1111/srt.12326. [DOI] [PubMed] [Google Scholar]
- 31.Chuah S.Y., Attia A.B.E., Ho C.J.H., Li X., Lee J.S., Tan M.W.P., Yong A.A., Tan A.W.M., Razansky D., Olivo M., Thng S.T.G. Volumetric multispectral optoacoustic tomography for 3-dimensional reconstruction of skin tumors: a further evaluation with histopathologic correlation. J. Invest. Dermatol. 2018 doi: 10.1016/j.jid.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 32.Favazza C.P., Jassim O., Cornelius L.A., Wang L.V. In vivo photoacoustic microscopy of human cutaneous microvasculature and a nevus. J. Biomed. Opt. 2011;16(1) doi: 10.1117/1.3528661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grootendorst D.J., Jose J., Wouters M.W., van Boven H., Van der Hage J., Van Leeuwen T.G., Steenbergen W., Manohar S., Ruers T.J. First experiences of photoacoustic imaging for detection of melanoma metastases in resected human lymph nodes. Lasers Surg. Med. 2012;44(7):541–549. doi: 10.1002/lsm.22058. [DOI] [PubMed] [Google Scholar]
- 34.Kim J., Kim Y.H., Park B., Seo H.M., Bang C.H., Park G.S., Park Y.M., Rhie J.W., Lee J.H., Kim C. Multispectral ex vivo photoacoustic imaging of cutaneous melanoma for better selection of the excision margin. Br. J. Dermatol. 2018;179(3):780–782. doi: 10.1111/bjd.16677. [DOI] [PubMed] [Google Scholar]
- 35.Valluru K.S., Wilson K.E., Willmann J.K. Photoacoustic imaging in oncology: translational preclinical and early clinical experience. Radiology. 2016;280(2):332–349. doi: 10.1148/radiol.16151414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y., Tripathi S.V., Rosman I., Ma J., Hai P., Linette G.P., Council M.L., Fields R.C., Wang L.V., Cornelius L.A. Noninvasive determination of melanoma depth using a handheld photoacoustic probe. J. Invest. Dermatol. 2017;137(6):1370–1372. doi: 10.1016/j.jid.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luke G.P., Nam S.Y., Emelianov S.Y. Optical wavelength selection for improved spectroscopic photoacoustic imaging. Photoacoustics. 2013;1(2):36–42. doi: 10.1016/j.pacs.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeitouni N.C., Rohrbach D.J., Aksahin M., Sunar U. Preoperative ultrasound and photoacoustic imaging of nonmelanoma skin cancers. Dermatol. Surg. 2015;41(4):525–528. doi: 10.1097/DSS.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 39.Gloster H.M., Jr., Neal K. Skin cancer in skin of color. J. Am. Acad. Dermatol. 2006;55(5):741–760. doi: 10.1016/j.jaad.2005.08.063. quiz 761-4. [DOI] [PubMed] [Google Scholar]
- 40.Stoffels I., Morscher S., Helfrich I., Hillen U., Leyh J., Burton N.C., Sardella T.C., Claussen J., Poeppel T.D., Bachmann H.S., Roesch A., Griewank K., Schadendorf D., Gunzer M., Klode J. Metastatic status of sentinel lymph nodes in melanoma determined noninvasively with multispectral optoacoustic imaging. Sci. Transl. Med. 2015;7(317) doi: 10.1126/scitranslmed.aad1278. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz M., Buehler A., Aguirre J., Ntziachristos V. Three-dimensional multispectral optoacoustic mesoscopy reveals melanin and blood oxygenation in human skin in vivo. J. Biophotonics. 2016;9(1–2):55–60. doi: 10.1002/jbio.201500247. [DOI] [PubMed] [Google Scholar]
- 42.Sellheyer K. Basal cell carcinoma: cell of origin, cancer stem cell hypothesis and stem cell markers. Br. J. Dermatol. 2011;164(4):696–711. doi: 10.1111/j.1365-2133.2010.10158.x. [DOI] [PubMed] [Google Scholar]
- 43.Thieu K., Ruiz M.E., Owens D.M. Cells of origin and tumor-initiating cells for nonmelanoma skin cancers. Cancer Lett. 2013;338(1):82–88. doi: 10.1016/j.canlet.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alessi E., Venegoni L., Fanoni D., Berti E. Cytokeratin profile in basal cell carcinoma. Am. J. Dermatopathol. 2008;30(3):249–255. doi: 10.1097/DAD.0b013e31816c828a. [DOI] [PubMed] [Google Scholar]
- 45.Sheikh R., Cinthio M., Dahlstrand U., Erlöv T., Naumovska M., Hammar B., Zackrisson S., Jansson T., Reistad N., Malmsjö M. Clinical translation of a novel photoacoustic imaging system for examining the temporal artery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2019;66(Ma(3)):472–480. doi: 10.1109/TUFFC.2018.2868674. [DOI] [PubMed] [Google Scholar]