Abstract
Recurrence and metastasis remain the major cause of cancer mortality. Even for early-stage lung cancer, adjuvant chemotherapy yields merely slight increase to patient survival. EF-hand domain-containing protein D2 (EFHD2) has recently been implicated in recurrence of patients with stage I lung adenocarcinoma. In this study, we investigated the correlation between EFHD2 and chemoresistance in non-small cell lung cancer (NSCLC). High expression of EFHD2 was significantly associated with poor overall survival of NSCLC patients with chemotherapy in in silica analysis. Ectopic EFHD2 overexpression increased cisplatin resistance, whereas EFHD2 knockdown improved chemoresponse. Mechanistically, EFHD2 induced the production of NADPH oxidase 4 (NOX4) and in turn the increase of intracellular reactive oxygen species (ROS), consequently activating membrane expression of the ATP-binding cassette subfamily C member 1 (ABCC1) for drug efflux. Non-steroidal anti-inflammatory drug (NSAID) ibuprofen suppressed EFHD2 expression by leading to the proteasomal and lysosomal degradation of EFHD2 through a cyclooxygenase (COX)-independent mechanism. Combining ibuprofen with cisplatin enhanced antitumor responsiveness in a murine xenograft model in comparison with the individual treatment. In conclusion, we demonstrate that EFHD2 promotes chemoresistance through the NOX4-ROS-ABCC1 axis and therefore developing EFHD2-targeting strategies may offer a new avenue to improve adjuvant chemotherapy of lung cancer.
Keywords: EFHD2, Recurrence, Adjuvant chemotherapy, NOX4, ABCC1, Ibuprofen
Highlights
-
•
EFHD2 increases resistance of lung cancer to cisplatin.
-
•
EFHD2 enhances the NOX4-ROS-ABCC1signalingfor cisplatin efflux.
-
•
Ibuprofen suppresses EFHD2 through both proteasomal and lysosomal degradationmechanisms
1. Introduction
Non-small cell lung cancer (NSCLC) is one of the most common causes of cancer-related death worldwide [1]. Due to advances in diagnostic technology, early diagnosis of NSCLC has gradually increased in recent years [2]. Surgical resection remains the optimal therapeutic treatment for patients with early-stage NSCLC [3]. However, approximately one-third of these patients develop local and/or distant recurrence, which is the main cause of mortality in the postsurgical treatment of NSCLC [4]. Currently, adjuvant cisplatin-based chemotherapy is the standard of care following resection treatment to reduce the risk of recurrence. Nevertheless, the regimen only leads to a 4% increase in 5-year survival compared to patients not receiving the adjuvant chemotherapy, implying that intrinsic resistance to cisplatin could be a major obstacle for treatment response [5].
A cisplatin-refractory phenotype can be attributed to several possible mechanisms, including increased cellular efflux of cisplatin, alteration of cisplatin metabolism, and increased DNA self-repairing activity [6]. Drug export from cancer cells is a primary cause of cellular resistance that can lead to an initial treatment failure. The elevated ATP-binding cassette (ABC) transporter family has been implicated in intrinsic cisplatin resistance. Among them, multidrug resistance protein 1 (ABCB1), multidrug resistance-associated protein 1 (ABCC1), and ATP-binding cassette subfamily G member 2 (ABCG2) have been studied extensively in association with multidrug resistance [7].
Reactive oxygen species (ROS) serve as a second messenger in cellular signaling or induce oxidative stress in pathological state that depends on their cellular levels [8]. In comparison with normal tissues, most cancer cells exhibit higher levels of ROS that can promote tumor progression and development [9]. Elevated ROS has been implicated in drug resistance at multiple levels such as increased drug efflux, and now been considered a distinctive characteristic of drug resistance in cancer [10]. The enzyme NADPH oxidases (NOXs) have been identified as the key sources of ROS in mammalian cells [11]. The NOX family consists of seven members, which includes NOX1-NOX5, dual oxidase 1 (DUOX1), and DUOX2. NOXs integrate into plasma and endosome membrane, serving a variety of functions, including antimicrobial defense, biosynthetic processes, oxygen sensing, and redox-based cellular signaling [12]. Among them, NOX4 is the most frequently overexpressed isoform in cancer cells. NOX4 sustains apoptosis resistance and promotes tumor cell proliferation and metastasis in several cancer cells, including lung cancer [13]. Furthermore, NOX4 induces the expression of drug-efflux transporters such as P-glycoprotein, resulting in multidrug resistance [14].
EF hand domain-containing protein 2 (EFHD2) is a conserved calcium-binding protein that is highly expressed in the immune system [15] and the central nervous system [16]. EFHD2 is involved in immune cell activation [17] and the regulation of immune response [18]. The expression levels of EFHD2 can influence the behavior and cognitive phenotypes of individuals such as alcohol addiction [19] and susceptibility to motion sickness [20]. EFHD2 dysfunction is associated with autoimmune and neuropathological diseases, including Parkinson's disease and Alzheimer's disease [21]. In cancer research, EFHD2 enhances cancer cell migration and potentially leads to cancer metastasis [22]. Recently, we demonstrated that EFHD2 promoted epithelial-to-mesenchymal transition (EMT) in lung adenocarcinoma and was significantly associated with postsurgical recurrence of stage I lung cancer patients [23]. Due to inefficacy of adjuvant chemotherapy to reduce the risk of recurrence, we speculated that EFHD2 could enhance resistance of lung cancer cells to cisplatin. In the current study, we explored the EFHD2-mediated mechanism in modulating cisplatin resistance and tested combining ibuprofen with chemotherapeutic drug to lay foundation for pharmacological targeting of EFHD2 in a proof-of-concept preclinical setting.
2. Materials and methods
2.1. In silica survival analysis and correlation analysis
The effect of target genes on overall survival of lung cancer patients was evaluated by the Kaplan-Meier plotter server (http://kmplot.com/analysis/), which contained independent datasets from the Cancer Biomedical Informatics Grid (caBIG), the Gene Expression Omnibus (GEO), and the Cancer Genome Atlas (TCGA) repositories. The high versus low expression levels of mRNA of target genes such as EFHD2 and ABCC1 were split by the median value. Patients subjected with pan-chemotherapeutic drugs were included. The threshold of follow-up of patients was set as 60 months. The hazard ratio (HR) was given with 95% confidence intervals, and log rank P value was calculated and displayed on the webpage.
Pair-wise gene expression correlation analysis was performed at the Gene Expression Profiling Interactive Analysis (GEPIA) web server (http://gepia.cancer-pku.cn/) using TCGA and the Genotype-Tissue Expression (GTEx) expression data by a standard processing pipeline. The linear correlation between EFHD2 and ABCC1 expression was calculated by Pearson correlation coefficient.
2.2. Cell culture
Human lung cancer cells A549 and H1299 were obtained from the American Type Culture Collection (ATCC). H2981, CL1-0, and CL1-5/F4 (F4), which was established by selection of increasingly invasive cell populations from CL1-0 [24], were provided by Dr. Yuh-Pyng Sher. A549 and CL1-0 were maintained in RPMI 1640 media (Invitrogen), H2981 was maintained in DMEM media (Invitrogen), H1299 and F4 were cultured in DMEM/F-12 media (Invitrogen). All culture media were supplemented with 10% fetal bovine serum and 1% antibiotics (GIBCO). Lung cancer cells were grown in a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
Cisplatin-resistant lung tumor cells were generated by initially treating CL1-0 with 5 μM cisplatin, maintaining survival cells in cisplatin-containing media, and escalating doses of cisplatin (to 20 μM) to develop drug resistance.
2.3. Chemicals
Cisplatin (Cat.No. ALX-400-040-M050) was purchased from the Enzo Life Sciences; N-acetyl-l-cysteine (NAC; Cat.No. A7250) and ibuprofen (Cat.No. I4883) were purchased from the Sigma-Aldrich; bafilomycin A1 (Baf-A1; Cat.No. sc-201550), aspirin (Cat.No. sc-202471), diclofenac (Cat.No. sc-357332), ketorolac (Cat.No. sc-205360), mefenamic acid (Cat.No. sc-205380), piroxicam (Cat.No. sc-200576), sulindac (Cat.No. sc-202823), and MG132 (Cat.No. sc-351846) were purchased from the Santa Cruz Biotechnology; flurbiprofen (Cat.No. 344079) was purchased from the Millipore; naproxen (Cat.No. ALX-270-102-G005) was purchased from the Enzo Life Sciences; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Cat.No. M6494) and CM-H2DCFDA (Cat.No. C6827) were purchased from the Invitrogen; ketoprofen (Cat.No. J62702) was purchased from the Alfa Chemistry; GKT137831 (Cat.No. 9444) was purchased from the BioVision.
2.4. Mass spectrometry for proteomics
EFHD2-mediated protein changes were identified by mass spectrometric analysis (MS). Total proteins of H1299 and F4 cells were extracted using RIPA lysis and extraction buffer (Thermo Fisher) and quantified using the Bio-Rad Protein Assay kit by the measurement of absorbance at 595 nm. Total protein (20 μg) of each sample was separated using 10% SDS-PAGE and divided into eight gel fractions. After finely cutting (<1 mm3), gel pieces were subjected to in-gel digestion to produce tryptic peptides, followed our previously described method [25]. The linear ion trap-Fourier transform ion cyclotron resonance mass spectrometer (LTQ-FTICR MS, Thermo Fisher) was used for survey scan analysis (range: m/z 320–2000) with a mass resolution of 100,000 at m/z 400. Top ten most abundant multiply charged ions were sequentially isolated for tandem mass analysis using LTQ. Protein identification and label-free quantification were performed using the MaxQuant and MaxLFQ software [26], and the identification threshold was set to P < 0.01.
2.5. CyTOF mass cytometry
Cisplatin, cis-diamminedichloroplatinum (II), is a platinum (Pt)-based chemotherapy drug with cytotoxic effect through inducing DNA damages and impairing DNA replication and transcription. To evaluate the intracellular cisplatin accumulation, Pt content in individual cell was directly determined using CyTOF® 2 mass cytometry operated with software v6.0.626 (Fluidigm Sciences) [27]. Basically, cells are atomized and ionized in a high temperature inductively coupled plasma. After excluding light atoms such as C, H, O, S, CyTOF MS measures heavy elements introduced into a cell, such as Pt. In this study, EFHD2-depleted H1299 and F4 and their control cells were treated with 5 μM cisplatin for 24 h and washed by cisplatin-free culture media twice (defined as 0 h post cisplatin treatment), and then incubated in cisplatin-free culture media for another 24 h (defined as 24 h post cisplatin treatment). To acquire MS information from intact cells, cell surfaces were stained with Maxpar® Intercalator-Ir solution (500 μM; Fluidigm Sciences), which can be covalently tethered to DNA molecules of living cells as a tracer for cell recognition. Prior to MS, cells were reconstituted in MaxPar® water (Fluidigm Sciences) containing EQ four element calibration beads (including 140/142Ce, 151/153Eu, 165Ho, and 175/176Lu; Fluidigm Sciences). For CyTOF® 2 analysis, 5 × 105 cells (in 1000 μL) were loaded into the instrument with injection speed of 45 μL/min and the acquired data were analyzed by the FlowJo software.
2.6. Intracellular H2O2 detection
For pHyPer-cyto vector detection, tumor cells (2 × 105 cells) were transfected with 2.5 μg pHyPer-cyto plasmid (Cat.No. FP941, Evrogen) with Lipofectamine 2000 reagent (Thermo Fisher) for 6 h, and then transferred to normal culture medium for 24 h. After PBS wash and Hoechst 33342 staining, the fluorescence generated by the binding of pHyPer-cyto vector and H2O2 was detected using Leica TCS SP8 X confocal spectral microscope imaging system.
For flow cytometer detection, trypsinized tumor cells (3 × 105 cells) were treated with 10 μM CM-H2DCFDA for 30 min, and then analyzed using BD FACSCalibur flow cytometer system and CellQuest software.
2.7. Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted with TRIzol reagent (Invitrogen) and then used to perform RT-PCR by MMLV first-strand synthesis kit (GeneDireX). The diluted RT-PCR products were applied for qPCR analysis using KAPA SYBR FAST qPCR Master Mix Kit (Kapa Biosystems) by the LightCycler 480 apparatus (Roche). GAPDH, 18S rRNA, and β-actin individually served as endogenous controls. The sequences of qPCR primers used in this study were listed in Supplementary Table 1. The expression of mRNA was estimated by the comparative Ct method using 2−ΔΔCt.
2.8. Western blot analysis
Protein expression levels were determined by SDS-PAGE separation and the following Western blot assay. Proteins were electro-blotted onto PVDF membrane at 400 V at 0 °C for 3 h in 25 mM Tris-HCl, 197 mM glycine, and 13.3% (v/v) methanol. Membranes were blocked with 5% (w/v) skim milk in TBST for 1 h, and incubated with primary antibodies at 4 °C for 16 h. The primary antibodies used in this study were listed in Supplementary Table 2. After gently agitating in three TBST washes for 15 min each, horseradish peroxidase-conjugated secondary antibodies were added to incubate at room temperature for 1 h. Immunoreactive signals were revealed using an enhanced ECL substrate Western Lighting Plus-ECL (PerkinElmer) and recorded by developing photographic film under optimum exposure.
2.9. Wound healing migration assay
In vitro migration assay was performed using the IncuCyte ZOOM system (ESSEN BioScience). Cancer cells were seeded into a 96-well microplate at a density of 4 × 104 cells/well and cultured overnight. The wound gap was created by manual scratch of ESSEN WoundMaker. The photographs of cell migration were recorded at 2 h intervals for 22–24 h. The relative wound density of cells migrating into a scratch was analyzed using the in-house Cell Migration/Invasion Software Module (ESSEN BioScience).
2.10. Matrigel invasion assay
For in vitro invasion assay, cancer cells (1.5 × 105 cells in 200 μL) were suspended in the upper half of a PET membrane transwell insert chamber (BD Biosciences), which was coated with Matrigel (1 mg/mL; BD Biosciences), on a 24-well plate. Medium without FBS supplement was added into the upper chamber, whereas medium with 10% FBS supplement was added into the lower chamber. After incubation at 37 °C for 24 h, cancer cells that passed through the insert were fixed with 3.7% formalin (Sigma-Aldrich) and stained with 0.1% crystal violet (Sigma-Aldrich).
2.11. Cell viability assay
The effect of cisplatin on cell viability was assayed using a methylthiazol tetrazolium (MTT) method. Cancer cells were seeded into a 24-well microplate at a density of 2 × 104 cells/well. For combination treatment, cancer cell were pretreated with reagents such as ibuprofen for 24 h, and then the designed doses of cisplatin were directly added to culture for another 24 h culture. After treatment, MTT solution (200 μL, 1 mg/mL in PBS) was added and incubated for further 4 h at 37 °C. Removing solution and 500 μL DMSO was used to dissolve an insoluble purple formazan. Cell viability was calculated by the optical density (OD) at the wavelength of 570 nm, and the viability rate was defined as: cell viability (%) = (experiment OD570/control OD570) x 100%.
2.12. Immunofluorescence assay
Cells grew on slides were fixed using 4% paraformaldehyde in PBS for 10 min at room temperature. Permeabilization of cell membrane was created by incubating cells with 0.25% Triton X-100 in PBS for 5 min. The blocking reaction was performed using 1% BSA-containing PBST for 30 min, and then cells were incubated with primary and secondary antibodies. Mounting medium contains DAPI for nuclear DNA staining. The fluorescence signals were analyzed using Leica TCS SP8 X confocal spectral microscope imaging system.
2.13. Mouse xenograft tumor model and antitumor assay
The animal procedure (CMUIACUC-2018-177) was approved by the Institutional Animal Care and Use Committee (IACUC) at China Medical University Hospital (Taichung, Taiwan). H1299 cells (1 × 106 cells) were mixed with matrigel and subcutaneously inoculated into right flank of 5-week-old male BALB/c nude mice (BALB/cAnN.Cg-Foxn1nu/CrlNarl). Until tumor was visible (100–200 mm3), animals were randomly assigned into four groups (weak 0; W0) (N = 6 for each group), including (1) control group, (2) ibuprofen treatment group, (3) cisplatin treatment group, and (4) ibuprofen and cisplatin treatment group. For one treatment cycle in a week (starting from W1), cisplatin (5 mg/kg, intraperitoneal injection, 1 time) and ibuprofen (25 mg/kg, oral administration, 3 times) were given. Total three treatment cycles were conducted in this experiment. The body weight of mice and tumor growth were recorded weekly.
2.14. Immunohistochemical (IHC) assay
H1299 lung adenocarcinoma paraffin sections from mouse xenograft tumors were deparaffinized, hydrated, and heated to 95–100 °C to induce antigen retrieval. After inactivating endogenous peroxidase activity, rabbit anti-human EFHD2 and ABCC1 polyclonal antibodies were used for IHC staining, which was performed by an automatic BenchMark XT staining machine using iVIEW 3,3-diaminobenzidine (DAB) detection kit (Ventana Medical Systems). Tumor sections were finally incubated with iVIEW copper to enhance signal intensity. Samples were then counterstained with hematoxylin, dehydrated, mounted, and examined using a Leica DM2000 LED microscope.
2.15. Statistical analysis
The data were displayed as the means ± SD. The significance of differences was examined by Student's t-test. P < 0.05 was considered statistically significant.
3. Results
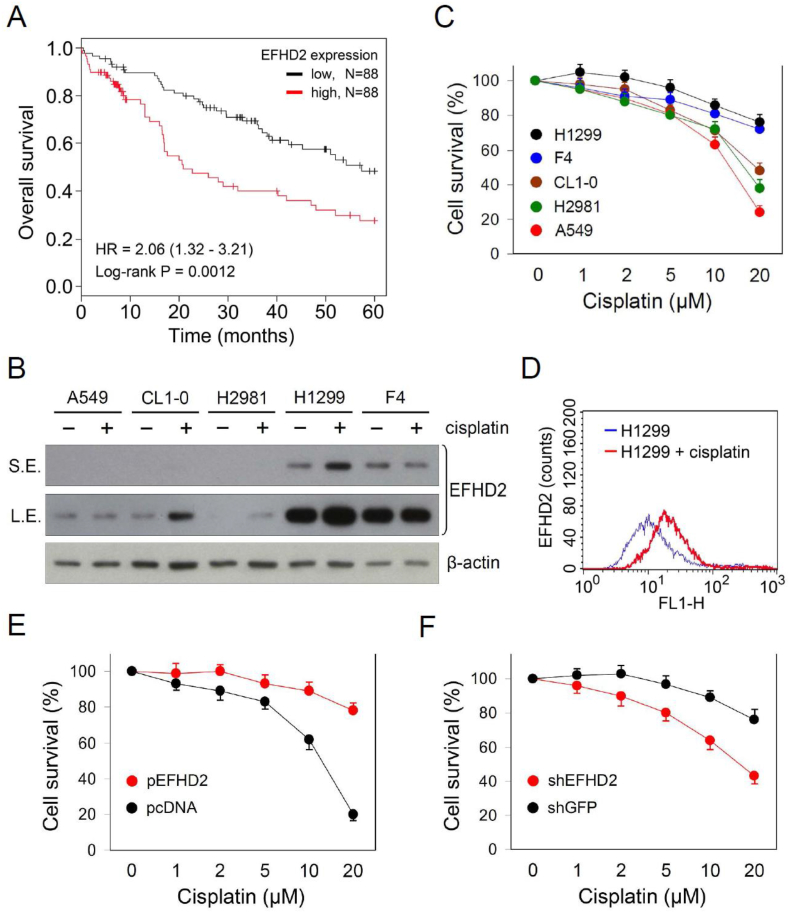
3.1. EFHD2 increases cisplatin resistance of lung cancer
To evaluate the association between EFHD2 and chemotherapeutic resistance, we examined the effect of EFHD2 on overall survival of lung cancer patients subjected to chemotherapy. The search result of the Kaplan-Meier-plotter cancer database [28] revealed that EFHD2 mRNA levels were significantly correlated with poor overall survival of lung cancer patients with chemotherapy (Fig. 1A). Cisplatin is the mainstay of chemotherapeutic drug for adjuvant therapy to prevent cancer recurrence of early-stage NSCLC. EFHD2 expression levels were negatively correlated with the sensitivity of lung tumor cells to cisplatin (Fig. 1B and C). Cisplatin induced the expression of EFHD2 within 24 h exposure depending on lung cancer cell lines (Fig. 1B) as well as increasing the signal intensity of EFHD2 antibody immunofluorescence staining of lung cancer cells in flow cytometry assay (Fig. 1D). Furthermore, ectopic EFHD2 overexpression (EFHD2-OE) promoted resistance to cisplatin in A549 cells that express relatively low levels of endogenous EFHD2 (Fig. 1E). In contrast, EFHD2 knockdown (EFHD2-KD) by shRNA (shRNA information: Supplementary Table 3) enhanced sensitivity to cisplatin in EFHD2-expressing H1299 cells (Fig. 1F). Except cisplatin, EFHD2 was ineffective to treatment efficacy of other therapeutic drugs such as etoposide, alimta, taxol, and vinorelbine in our in vitro testing (Supplementary Fig. 1). Collectively, our findings suggest that EFHD2 can not only enhance resistance of lung cancer to cisplatin but may also contribute to the development of acquired resistance.
Fig. 1.
EFHD2 increases resistance of lung cancer cells to cisplatin. A) The correlation between EFHD2 mRNA levels and overall survival of lung cancer patients with chemotherapy was analyzed using the Kaplan-Meier-plotter cancer database. The high versus low expression levels of EFHD2 mRNA were split by the median value. B) Lung tumor cells were treated with 5 μM cisplatin for 24 h, and EFHD2 expression was determined by Western blot assay. S.E., short exposure; L.S., long exposure. β-actin, loading control. C) Lung cancer cells were treated with indicated doses of cisplatin for 24 h, cell survival was determined by MTT assay. D) EFHD2 expression in the control and cisplatin-treated (5 μM for 24 h) H1299 cells was examined by flow cytometry assay. E) EFHD2-OE and the control A549 cells and F) EFHD2-KD and the control H1299 cells were treated with indicated doses of cisplatin for 24 h. Cell viability was determined by MTT assay.
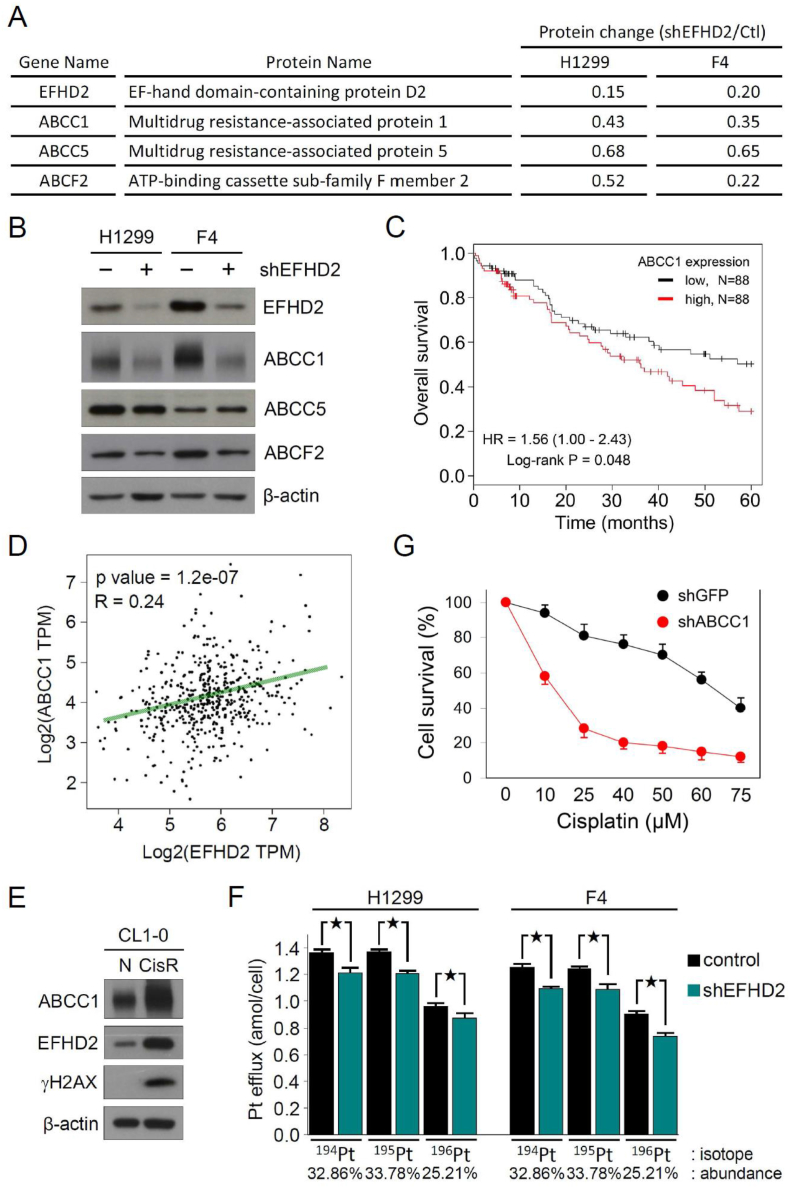
3.2. ABCC1 is involved in EFHD2-induced cisplatin resistance
To gain insight of how EFHD2 modulates cisplatin sensitivity, comparative proteomic analyses of parental as well as EFHD2-KD H1299 and F4 cells were performed. Several ABC transporters were downregulated in both EFHD2-KD cell lines (Fig. 2A; Supplementary Tables 4 and 5). Among them, ABCC1 was the most affected as confirmed by Western blot assay (Fig. 2B). The in silica search of Kaplan-Meier-plotter cancer database indicated that high ABCC1 levels were significantly correlated with poor overall survival of NSCLC patients with chemotherapy (Fig. 2C). In addition, the in silica gene expression analysis revealed a significantly positive correlation between EFHD2 and ABCC1 in NSCLC patients using TCGA RNA-Seq database [29] (Fig. 2D). The positive correlation of both proteins was also found in cisplatin-resistant CL1-0 (CL1-0/CisR), which expressed higher levels of EFHD2 and ABCC1 in comparison with the parental cells (Fig. 2E). Due to a long-lasting treatment, CL1-0/CisR had an increased expression of γH2AX, which was caused by cisplatin-induced DNA damage [30].
Fig. 2.
ABCC1 contributes to EFHD2-mediated resistance to cisplatin. A) Change of ABC transporter levels between EFHD2-KD and the control H1299 and F4 cells in comparative proteomic analyses. B) Expression levels of ABC transporters in EFHD2-KD and the control H1299 and F4 cells were validated by Western blot assay. C) The correlation between ABCC1 mRNA levels and overall survival of lung cancer patients with chemotherapy was analyzed using the Kaplan-Meier-plotter cancer database. The high versus low expression levels of EFHD2 mRNA were split by the median value. D) The correlation of EFHD2 and ABCC1 gene expression was analyzed at GEPIA web server by TCGA RNA-Seq database. The linear correlation between EFHD2 and ABCC1 expression was calculated by Pearson correlation coefficient. E) Change of EFHD2 and ABCC1 expression between cisplatin-resistant and the control CL1-0 cells was determined by Western blot assay. β-actin, loading control. F) After 5 μM cisplatin treatment for 24 h, EFHD2-KD and the control H1299 and F4 cells were transferred to cisplatin-free media for another 24 h culture. Intracellular Pt content was analyzed by CyTOF MS, and the amounts of Pt efflux from cells during post cisplatin treatment period were calculated. ★, <0.05. G) ABCC1-KD and the control H1299 cells were treated with indicated doses of cisplatin for 24 h. Cell viability was determined by MTT assay.
To evaluate the functional consequence of EFHD2-induced ABCC1, cisplatin efflux was assessed by measuring the intracellular cisplatin content using CyTOF MS [27], an inductively coupled plasma mass spectrometry (ICP-MS) coupling with cell sorting function. After 24 h incubation in 5 μM cisplatin, the average amounts of the most abundance of platinum isotope (195Pt) in individual cell were 1 × 106 vs. 9.6 × 105 and 8.9 × 105 vs. 8.5 × 105 in the control and EFHD2-KD H1299 and F4 cells, respectively (Supplementary Fig. 2). The penetrating rate of cisplatin into cell was approximately 5 × 10−4, which did not have significant difference between these cancer cells. The major copper influx transporter, copper transporter 1 (CTR1), is now considered the principal gateway for the entrance of cisplatin into cancer cells [31]. EFHD2-KD had no obvious effect on CTR1 expression, consistent with the current finding that similar intracellular cisplatin content was detected in these cancer cells (Supplementary Figs. 3A and 3B). After 24 h post cisplatin treatment, EFHD2-KD significantly decreased Pt efflux, resulting in higher levels of intracellular Pt content and could consequently enhance sensitivity to cisplatin (Fig. 2F). ABCC1-KD did not change EFHD2 expression (Supplementary Fig. 3C), but it significantly enhanced cell killing by cisplatin in MTT assay (Fig. 2G), indicating the important role of ABCC1 in EFHD2-mediating cisplatin resistance. Together, these results suggest that EFHD2 promotes cisplatin resistance through activating ABCC1 expression.
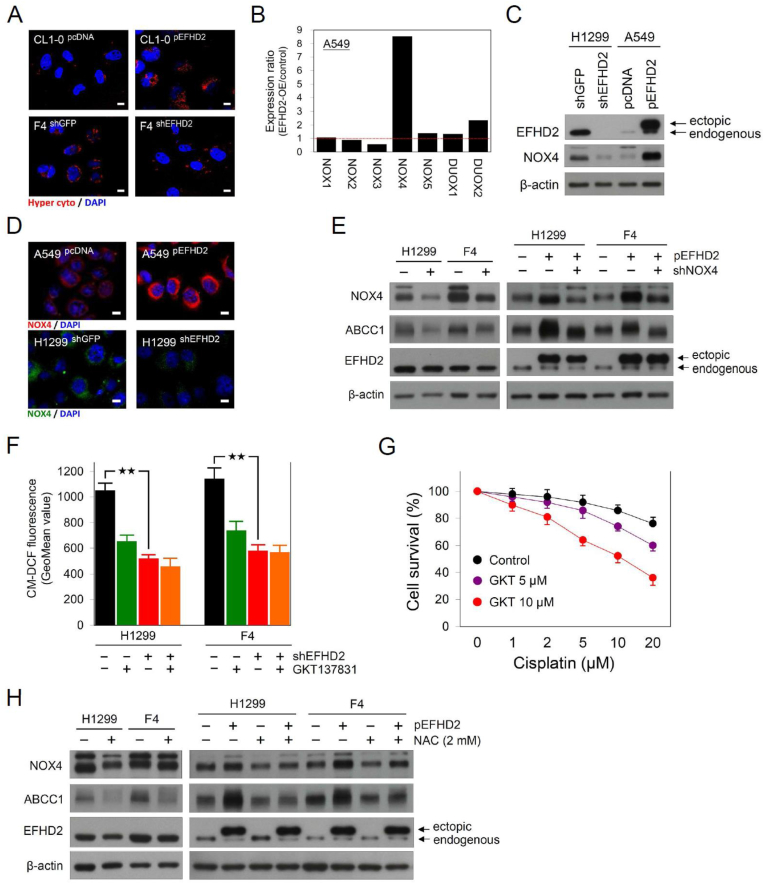
3.3. EFHD2 enhances ABCC1 through activating NOX4-ROS pathway
Given that elevated ROS levels are a distinct characteristic of drug resistance in cancer [10], we determined the role of EFHD2 in regulating the intracellular ROS levels. The cytosolic H2O2 levels were evaluated by the genetically encoded fluorescent sensor p-HyPer-Cyto vector [32], which consists a yellow fluorescent protein inserted into the regulatory domain of H2O2 sensing protein OxyR. After H2O2 binding, the conformational change of OxyR alters the excitation fluorescence that can be detected in confocal microscopic assay. The result revealed that cytosolic H2O2 levels were positively correlated with EFHD2 expression (Fig. 3A). The NOX family is one of the key sources of ROS in mammalian cells as well as the enzyme activity of NOXs is tightly associated with various hallmarks of cancer including angiogenesis and metastasis [11]. Thus we examined whether the levels of NOXs were affected by EFHD2 expression. The qPCR analysis revealed that EFHD2-OE dramatically increased NOX4 expression (Fig. 3B; Supplementary Fig. 4A). Western blot assay further verified the regulation between of EFHD2 and NOX4 that EFHD2-OE increased NOX4 levels, whereas EFHD2-KD decreased NOX4 levels (Fig. 3C; Supplementary Fig. 4B). The microscopic fluorescence analysis showed the similar observation (Fig. 3D).
Fig. 3.
EFHD2 enhances ABCC1 through NOX4-ROS activation. A) Cytosolic H2O2 in EFHD2-OE or EFHD2-KD and their control cells was monitored by the genetically encoded fluorescent sensor p-HyPer-Cyto, and fluorescence was detected using Leica TCS SP8 confocal microscope. Scale bar, 10 μm. B) The mRNA levels of NOX family including NOX1-NOX5, DUOX1, and DUOX2 in EFHD2-OE and the control A549 cells were analyzed by qPCR. GAPDH served as internal control. C) Western blot assay and D) confocal microscopic assay were used to analyze NOX4 protein expression in EFHD2-OE or EFHD2-KD and their control cells. Scale bar, 10 μm. E) The effect of NOX4-KD on ABCC1 expression was examined in parental and EFHD2-OE H1299 and F4 cells. F) Intracellular H2O2 levels of H1299 and F4 cells with EFHD2-KD or 10 μM GKT137831 treatment alone or combination both treatments were determined by CM-H2DCFDA and flow cytometric assay. ★★, <0.01. G) After GKT137831 pretreatment (0, 5, or 10 μM) for 24 h, indicated doses of cisplatin were added into culture media for another 24 h. Cell viability was determined by MTT assay. H) Parental and EFHD2-OE H1299 and F4 cells were treated with 2 mM NAC for 24 h, its effect on EFHD2, NOX4, and ABCC1 was determined by Western blot assay. β-actin, loading control.
Next, we verified whether EFHD2 induced ABCC1 through activating NOX4 expression. NOX4-KD suppressed ABCC1 expression in both H1299 and F4 cells (Fig. 3E). EFHD2-OE enhanced NOX4 and ABCC1 expression, while NOX4-KD rescued ABCC1 expression in EFHD2-OE cells (Fig. 3E). Besides the genetic method, we use a pharmacological approach to determine the causal role of EFHD2-NOX4 signaling in promoting ABCC1. The potent NOX4 inhibitor GKT137831 [33] decreased intracellular H2O2 levels in both H1299 and F4 cells (Fig. 3F). The inhibitory effect of GKT137831 on intracellular H2O2 was similar between EFHD2-KD and the control H1299 and F4 cells (Fig. 3F), implicating EFHD2-induced intracellular ROS is dependent on NOX4 activation. In addition, GKT137831 sensitized H1299 cells to cisplatin in a dose-dependent manner (Fig. 3G). Elevated ROS has been recognized to induce ABCC1 [34], thus we tested ROS scavenger N-acetylcysteine (NAC) in the EFHD2-induced ABCC1 signaling. Although NAC showed no obvious effect on EFHD2 and NOX4 expression, NAC dramatically inhibited ABCC1 as well as reverting ABCC1 expression in EFHD2-OE cancer cells (Fig. 3H). Together, these results suggest that EFHD2-NOX4 signaling enhances ABCC1 in a ROS-dependent manner.
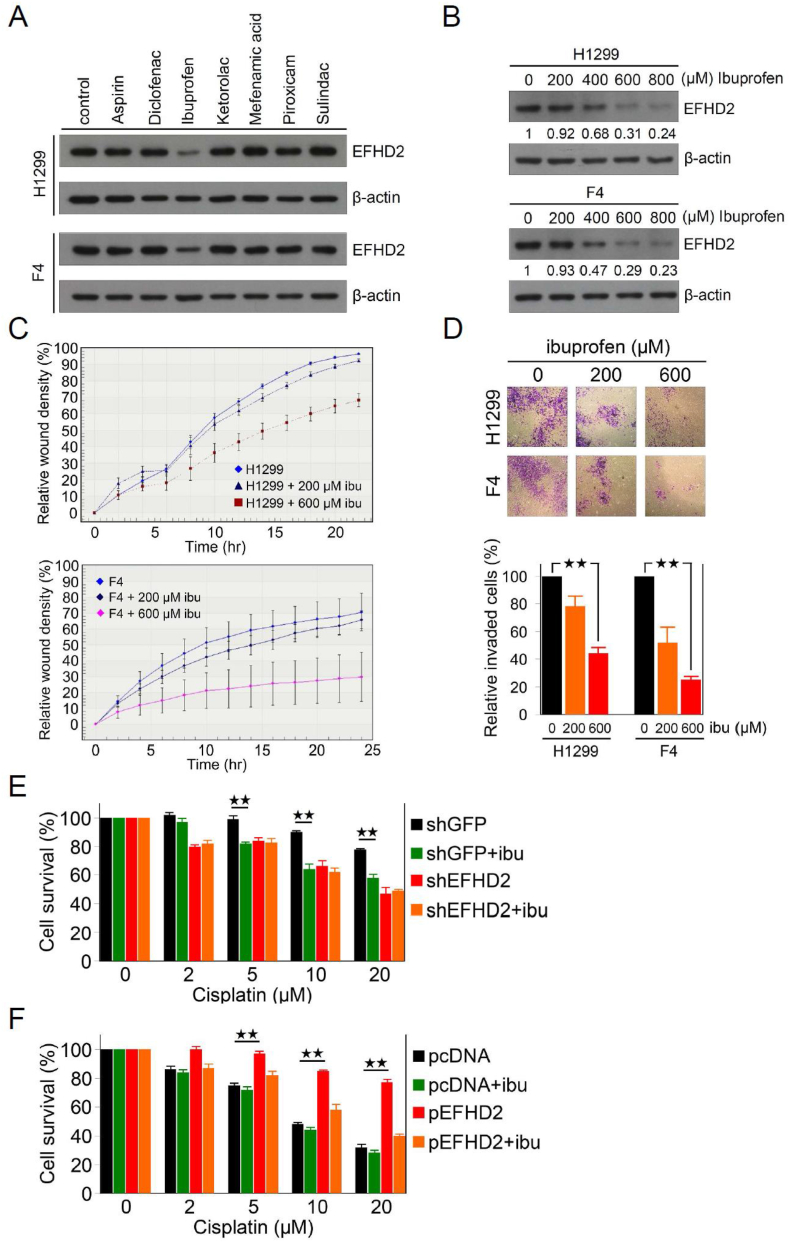
3.4. Ibuprofen sensitizes lung cancer to cisplatin through suppression of EFHD2
Due to the functions of EFHD2 in chemoresistance, developing EFHD2-targeting approaches can enhance responsiveness to adjuvant chemotherapy of lung cancer. While small molecule inhibitors specific for EFHD2 are unavailable yet, a recent research reported that ibuprofen, a non-steroidal anti-inflammatory drug (NSAID), downregulated EFHD2 in the hippocampus of mice [35]. Intriguingly, when a series of NSAIDs were tested in sub-pharmacological doses (approximately 90% viability; Supplementary Fig. 5), only ibuprofen showed the ability of EFHD2 inhibition in a does-dependent manner (Fig. 4A and B). In addition, ibuprofen attenuated the migration and invasion abilities of lung cancer cells (Fig. 4C and D), could resulting from inhibition of EFHD2 [23]. Importantly, ibuprofen pretreatment significantly sensitized H1299 cells to cisplatin in comparison to mock-treated cells, similar to the effect of EFHD2-KD, and the sensitization effect of ibuprofen was abolished when EFHD2-KD (Fig. 4E). In consistent with this result, although ibuprofen had no obvious sensitization effect in A549 cells whose endogenous EFHD2 levels were low, ibuprofen pretreatment reverted the killing effect of cisplatin in EFHD2-OE A549 cells (Fig. 4F). These results suggest that the sensitization effect of ibuprofen on lung cancer to cisplatin is EFHD2 dependent. To further clarify whether the inhibition of cyclooxygenase (COX) activity is important for cisplatin sensitization, we tested aspirin, a non-selective and irreversible inhibitor of COX1 and COX2, to sensitize lung cancer cells to cisplatin. Aspirin showed no effect on cisplatin sensitization (Supplementary Fig. 6), suggesting that EFHD2 inhibition and cisplatin sensitization of ibuprofen was independent on COX inhibition.
Fig. 4.
Ibuprofen suppresses EFHD2 and sensitizes lung cancer cells to cisplatin. A) NSAIDs, aspirin 500 μM, diclofenac 40 μM, ibuprofen 600 μM, ketorolac 10 μM, mefenamic acid 2 μM, piroxicam 10 μM, and sulindac 20 μM, were used to treat both H1299 and F4 cells for 24 h. EFHD2 levels were determined by Western blot assay. B) H1299 and F4 cells were treated with indicated dose of ibuprofen. The relative EFHD2 levels in each sample were normalized to the control cell. β-actin, loading control. C) After 24 h pretreatment with indicated doses of ibuprofen, migration ability of H1299 and F4 cells was analyzed by real time quantification using the IncuCyte system. D) After 24 h pretreatment with indicated doses of ibuprofen, invasion ability of H1299 and F4 cells was analyzed by matrigel transwell system. Invaded cell number was normalized with cell viability and the relative invasion was normalized with the control cells. E) EFHD2-KD and the control H1299 cells and F) EFHD2-OE and the control A549 cells were pretreated with or without 600 μM ibuprofen for 24 h, and then indicated doses of cisplatin were added into culture media for another 24 h. Cell viability were determined by MTT assay. ★★, <0.01.
3.5. Ibuprofen activates both proteasomal and lysosomal EFHD2 degradation
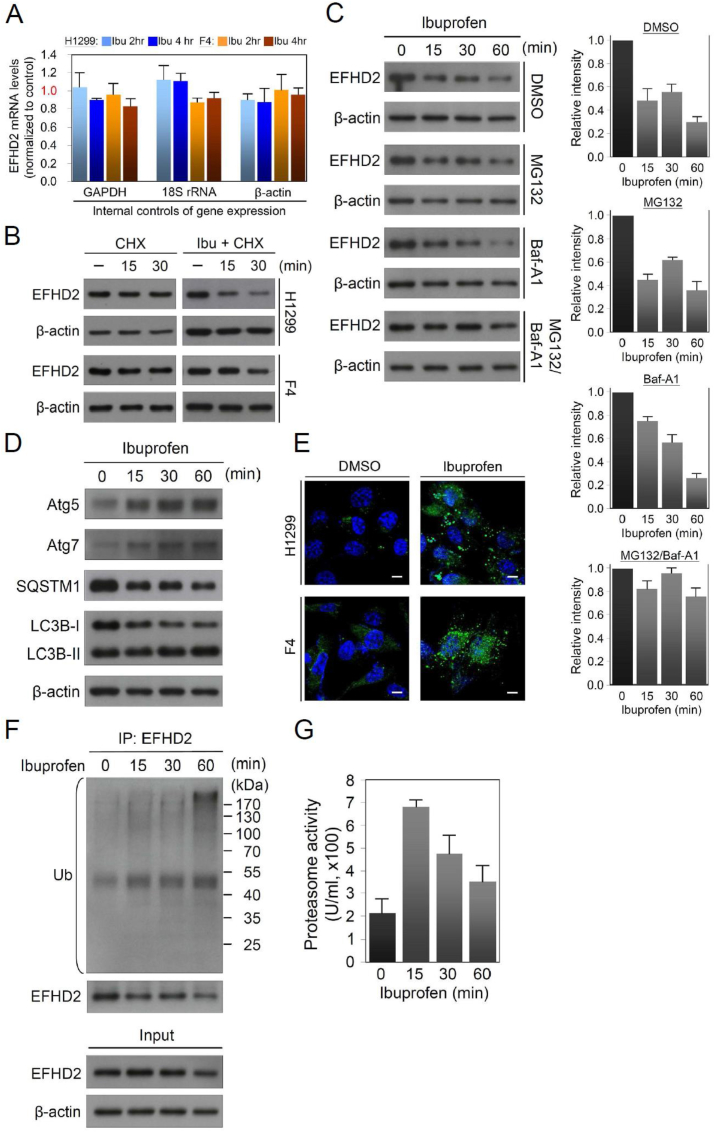
To understand how ibuprofen suppresses EFHD2 expression, EFHD2 mRNA levels of lung cancer cells with or without ibuprofen treatment were measured by qPCR. We found no significant effect on EFHD2 mRNA levels by ibuprofen treatment (Fig. 5A). On the other hand, pulse-chase experiment revealed that the protein stability of EFHD2 was dramatically reduced by ibuprofen treatment (Fig. 5B). Ubiquitin-proteasome pathway and autophagy-lysosome pathway are two major systems responsible for cellular protein degradation. To test which system was involved in ibuprofen-mediated EFHD2 degradation, MG132 and bafilomycin A1 (Baf-A1) were used to inhibit proteasomal and autophagic protein degradation, respectively. MG132 or Baf-A1 alone was incapable of reducing ibuprofen-induced EFHD2 degradation, but combination of MG132 and Baf-A1 stabilized EFHD2 (Fig. 5C). The results strongly suggest that ibuprofen-induced EFHD2 degradation through both proteasomal- and lysosomal-dependent mechanism. To verify the functions of ibuprofen in activation of lysosomal degradation system, we determined the expression of critical components in the degradation pathway. Ibuprofen enhanced autophagy-related protein 5 (Atg5) and Atg7, which are involved in the elongation and closure of the autophagosomal membrane [36], in a time-dependent manner (Fig. 5D). An increased of microtubule-associated proteins 1 light chain 3B (LC3B) II/I ratio, an indicator of autophagic activity [37], was detected in line with the puncta accumulation of LC3 after ibuprofen treatment (Fig. 5D and E). Moreover, a decreased SQSTM1 (p62) level was observed (Fig. 5D), which correlates with autophagic activation and an entire autophagic flux [37]. To confirm the involvement of proteasome pathway, we performed immunoprecipitation of EFHD2 after ibuprofen treatment. EFHD2 ubiquitylation had an increase in the time course and peaked at 60 min (Fig. 5F). The proteasomal activity was enhanced within 15 min treatment (Fig. 5G), and gradually reduced activities could reflect increased ubiquitination signals during the experimental period. Collectively, we demonstrate that ibuprofen induces EFHD2 degradation through activation of both ubiquitin-proteasome and autophagy-lysosome mechanisms.
Fig. 5.
Ibuprofen activates both proteasomal and lysosomal degradation of EFHD2. A) EFHD2 mRNA levels of H1299 cells with or without 600 μM ibuprofen treatment for indicated time were determined by qPCR. GAPDH, 18S rRNA, and β-actin mRNA individually served as internal control of gene expression, respectively. B) After 10 mg/mL cycloheximide (CHX) reaction for 1 h, H1299 and F4 cells were treated with or without 600 μM ibuprofen for indicated time. EFHD2 expression in ibuprofen-treated and the control cells was determined by Western blot assay. C) After reaction with 1 nM MG132 and/or 5 nM Baf-A1 for 0.5 h, H1299 cells were treated with 600 μM ibuprofen for indicated time. EFHD2 expression was determined by Western blot assay. The relative signal intensities were analyzed by ImageJ software. D) H1299 cells were treated with 600 μM ibuprofen for indicated time, and then autophagy-related proteins were examined by Western blot assay. E) Both H1299 and F4 cells were treated with 600 μM ibuprofen for 2 h, and then confocal images of antibody to LC3B were analyzed. Scale bar, 10 μm. F) After 600 μM ibuprofen treatment for indicated time, EFHD2 proteins were immunoprecipitated from 1 mg total protein of H1299 cells with EFHD2-specific antibody. The immunoprecipitated EFHD2 and ubiquitin (Ub)-conjugated proteins were examined by Western blot assay. β-actin, loading control. G) After 600 μM ibuprofen treatment for indicated time, proteasome activities of H1299 cells were determined by the Proteasome Activity Fluorometric Assay Kit (BioVision).
3.6. Ibuprofen enhances cisplatin efficacy in mouse model
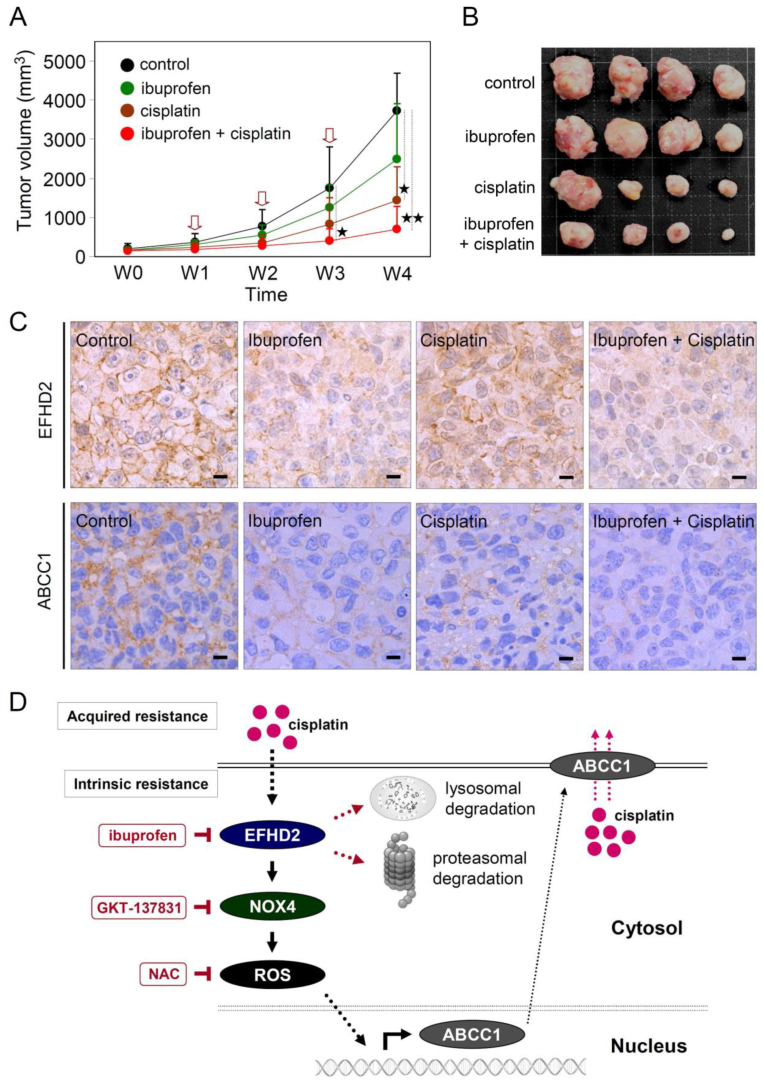
To evaluate the effect of ibuprofen on sensitizing lung cancer to cisplatin in vivo, H1299 cells (1 × 106) were inoculated into BALB/c nude mice by subcutaneous injection. After tumor size reached approximately 100 mm3, animals were randomly assigned into four groups, the control, cisplatin or ibuprofen alone, and combination of cisplatin and ibuprofen, for three treatment cycles. When it is given alone, as expected, cisplatin alone showed a superior efficacy of tumor suppression compared with ibuprofen alone treatment. Co-treatment with ibuprofen and cisplatin significantly improved the responsiveness of lung cancer to cisplatin (Fig. 6A and B). The function of ibuprofen in inhibiting EFHD2 and ABCC1 expression was observed using the formalin-fixed/paraffin-embedded tissues in IHC analysis (Fig. 6C), consistent with the previous in vitro analyses. This result corroborate with the mechanism that ibuprofen treatment sensitize cisplatin through suppression of EFHD2, forming the basis of targeting EFHD2 by small compound inhibitors in a proof-of-concept setting. On the basis of our current findings, the representative working model was proposed in Fig. 6D.
Fig. 6.
Ibuprofen enhances cisplatin efficacy in mouse model. A) Male BALB/c nude mice (5 weeks of age) were injected with 1 × 106 H1299 cells. When tumor volumes reached approximately 100–200 mm3 (defined as weak 0, W0), cisplatin (5 mg/kg) was intraperitoneally injected one time a week and ibuprofen (25 mg/kg) was orally administrated three times a week. Total three treatment cycles were conducted, which was indicated by red arrows. Tumor volumes were determined once per week. ★, <0.05; ★★, <0.01. B) Tumor masses of individual experimental group were shown after sacrifice at W4. C) The representative working model in this study. EFHD2 contributes to intrinsic and acquired resistance of NSCLC to cisplatin through activating the NOX4-ROS-ABCC1 pathway to increase cisplatin efflux. Ibuprofen treatment leads to the proteasomal and lysosomal degradation of EFHD2. NOX4 inhibitor GKT137831 and ROS scavenger NAC were potentially capable of inhibiting ABCC1 in lung cancer. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Recurrence is responsible for the main mortality in early-stage NSCLC patients with complete surgical resection [4]. Besides clinical and pathologic parameters, molecular biomarkers have been proposed to precisely identify patients with high risk of recurrence. Recent works have uncovered several predictive biomarkers, including the expression of specific protein, the gene signature variation of cell cycle genes and immune-related genes, and circulating tumor DNA [23,[38], [39], [40]]. Adjuvant therapy is currently used to reduce recurrence risk of patients harbor occult metastasis. While targeted therapies such as inhibitors of mutant EGFR have been available for patients with high risk of recurrence, only a small proportion of patients have the targetable mutations [41]. Immunotherapies such as humanized PD-1 and PD-L1 antibodies are now assessed for both first and second-line treatment in patients with metastatic lung cancer, while adjuvant and neoadjuvant immunotherapy trials are still ongoing [42]. Cisplatin-based adjuvant chemotherapy presently remains the standard of care for completely resected NSCLC. However, this treatment merely yields an unsatisfied improvement in patient outcome, a roughly 4% increase in 5-year survival [5]. Accordingly, several molecular-based management strategies have been explored to identify patients who likely benefit from adjuvant chemotherapy [43,44]. Recently, we found that EFHD2 promoted EMT and was significantly associated with postsurgical recurrence of patients with stage I lung adenocarcinoma [23]. In this study, we demonstrate that EFHD2 is involved in intrinsic chemoresistance of lung cancer and therefore leads to low responsiveness to cisplatin-mediating killing effect. Thus, we develop an EFHD2-targeting strategy to sensitize lung cancer to adjuvant chemotherapy in a proof-of-concept preclinical testing.
Clinical chemoresistance is a major obstacle for cancer therapy response [45]. Cancer cells can acquire chemoresistance by increasing cellular efflux or metabolism of drugs, producing antioxidants against drug-induced oxidative damage, or enhancing DNA self-repairing activity [6]. Elevated ROS has been recognized as a major cause of drug resistance in cancer [10]. In the current study, EFHD2 activated NOX4 expression and resulted in an increase of intracellular ROS. NOX4 has been identified to promote NSCLC cell proliferation and metastasis through positive regulation of PI3K/Akt signaling [46]. Whether the PI3K/Akt signaling is also involved in the regulation between EFHD2 and NOX4 remains to be further determined. NOX4 is frequently overexpressed in cancer cells; the more NOX4 expression is significantly increased along with cancer progression and associated with poor prognosis [47]. NOX4 participates in the regulation of angiogenesis, EMT, notch signaling, and anoikis resistance [48], which is essential for successful metastasis. Consequently, the regulation axis of EFHD2-NOX4 may potentially influence occult metastasis of cancer cells on developing recurrence. Accumulating evidence shows that ROS enhances multidrug resistance by inhibiting degradation of pyruvate kinase M2 isoform to regulate metabolism [14] or activating redox-sensing transcription factors such as nuclear factor-erythroid 2 related factor 2 (NRF2), forkhead box O (FOXO) proteins, and apurinic-apyrimidinic endonuclease 1 (APE1) to promote the expression of drug efflux transporters [49]. In consistent with the current knowledge, our findings indicate that EFHD2-mediated chemoresistance depends on NOX4-derived ROS that in turn promotes transporter ABCC1 expression to increase drug efflux in lung cancer.
The role of ABC transporters in multidrug resistance has been well recognized, ABCC1 is one of the most widely studied efflux transporters in cancer cells. Loss of ABCC1 enhanced the response to chemotherapy and significantly delayed tumor growth in vivo [50]. Elevated ABCC1 levels were associated with poor patient outcome in acute myeloid leukemia, acute lymphoblastic leukemia, breast cancer, and lung cancer [51]. Moreover, high ABCC1 gene levels are significantly correlated with shorter tumor-free survival and overall survival in postsurgical NSCLC patients receiving cisplatin-based adjuvant chemotherapy [52]. Several small molecules such as tricyclic isoxazoles [53] and flavonoid derivatives [54] are developed to specifically inhibit ABCC1, but these compounds remain to be evaluated in preclinical setting. Although reverse drug resistance by targeting ABC transporters is an attractive strategy, there is still lack of successful case to date [55].
The functions of cisplatin depend on the drug uptake and transport into cell nucleus to generate Pt-DNA adducts, thus cellular drug accumulation is crucial for therapeutic efficacy. Intracellular cisplatin content after treatment is traditionally monitored by highly sensitive elemental techniques, such as ICP MS [56], which measure the average cellular Pt levels. In the current study, we measure intracellular Pt amounts by the CyTOF MS, which takes advantage of coupling with sorting system for intact single cell [27]. The intracellular Pt levels measured in our study were approximately 1 μg/g cell similar to the previous reports [[56], [57], [58]]. Although Pt content has no dramatic difference between EFHD2-KD and the control cells, EFHD2-KD significantly increases intracellular Pt levels of lung cancer cells at 24 h post cisplatin treatment, implicating the important role of EFHD2-incuded ABCC1 in cisplatin efflux.
Ibuprofen has been shown to enhance the anticancer activity of cisplatin in lung cancer cells by inhibiting the chaperon heat shock 70 kDa protein (HSP70) [59], which is involved in protein homeostasis for preventing the misfolding and aggregation of normal proteins. Due to a high degradation rate of EFHD2 (Fig. 5B), ibuprofen could interfere the correct folding of EFHD2 through HSP70 inhibition, however this speculation remains to be examined. Using molecular docking analysis, EFHD2 molecule revealed two potential ibuprofen binding sites with a moderate to good binding energy (data not shown). Therefore, ibuprofen may mechanically enhance cisplatin sensitization through two kinds of EFHD2-dependent inhibition, directly binding to change EFHD2 molecular structure or indirectly making EFHD2 unstable by suppressing HSP70 activity.
In conclusion, recurrence and metastasis remain the major cause of cancer mortality. Even for early-stage lung cancer, adjuvant chemotherapy merely slightly increases patient survival. The critical roles of EFHD2 in cancer progression and abolishing therapeutic efficacy have been largely unaddressed. The current study highlights the novel functions of EFHD2 in promoting chemoresistance as well as impairing chemotherapeutic response in lung cancer. Therefore, the development of EFHD2-targeting strategy combined with chemotherapeutic drugs will potentially make tangible impact to survival of lung cancer patients.
Author contributions
CCF, STT, CYL, JCY, LCC, GYC, and WCC performed experiments. LCC, YPS, SCW, and MH advised on most experiments. YPS, SCW, MH, and WCC designed experiments, analyzed data and wrote the manuscript. All authors discussed results and commented on the manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
We thank the National RNAi Core Facility (Academia Sinica, Taipei, Taiwan) for providing the shRNAs. We are grateful to the National Center for High-Performance Computing for computer time and facilities. We also thank the Center for Resources, Research, and Development of Kaohsiung Medical University for the ChemBioOffice technical support. This research was supported by Ministry of Science and Technology (MOST, Grant No. 106-2314-B-039-023, 107-2314-B-039-066, and 108-2314-B-039-018) and China Medical University Hospital (Grant No. DMR-106-031, DMR-108-028, DMR-108-BC-6, DMR108-N-11, and DMR-109-134), Taiwan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101571.
Contributor Information
Michael Hsiao, Email: mhsiao@gate.sinica.edu.tw.
Wei‐Chao Chang, Email: t21443@mail.cmuh.org.tw.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. Ca - Cancer J. Clin. 2017;57:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Goldstraw P., Ball D., Jett J.R., Le Chevalier T., Lim E., Nicholson A.G. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 3.Scott W.J., Howington J., Feigenberg S., Movsas B., Pisters K. American College of Chest Physicians, Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:234S–242S. doi: 10.1378/chest.07-1378. [DOI] [PubMed] [Google Scholar]
- 4.Paximadis P., Beebe-Dimmer J.L., George J., Schwartz A.G., Wozniak A., Gadgeel S. Comparing treatment strategies for stage I small-cell lung cancer. Clin. Lung Canc. 2018;19:e559–e565. doi: 10.1016/j.cllc.2018.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NSCLC Meta-analysis Collaborative Group Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–1571. doi: 10.1016/S0140-6736(13)62159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L., Vitale I., Michels J., Brenner C., Szabadkai G., Harel-Bellan A. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis. 2014;5:e1257. doi: 10.1038/cddis.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Li Q., Zhou L., Xie N., Nice E.C., Zhang H. Cancer drug resistance: redox resetting renders a way. Oncotarget. 2016;7:42740–42761. doi: 10.18632/oncotarget.8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan C., Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat. Rev. Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorrini C., Harris I.S., Mak T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 10.Bosc C., Selak M.A., Sarry J.E. Resistance is futile: targeting mitochondrial energetics and metabolism to overcome drug resistance in cancer treatment. Cell Metabol. 2017;26:705–707. doi: 10.1016/j.cmet.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Skonieczna M., Hejmo T., Poterala-Hejmo A., Cieslar-Pobuda A., Buldak R.J. NADPH oxidases: insights into selected functions and mechanisms of action in cancer and stem cells. Oxid. Med. Cell. Longev. 2017;2017:9420539. doi: 10.1155/2017/9420539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leto T.L., Morand S., Hurt D., Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxidants Redox Signal. 2009;11:2607–2619. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang B., Liu Z., Hu X. Inhibiting cancer metastasis via targeting NAPDH oxidase 4. Biochem. Pharmacol. 2013;86:253–266. doi: 10.1016/j.bcp.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Shanmugasundaram K., Nayak B.K., Friedrichs W.E., Kaushik D., Rodriguez R., Block K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance. Nat. Commun. 2017;8:997. doi: 10.1038/s41467-017-01106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vuadens F., Rufer N., Kress A., Corthésy P., Schneider P., Tissot J.D. Identification of swiprosin 1 in human lymphocytes. Proteomics. 2004;4:2216–2220. doi: 10.1002/pmic.200300779. [DOI] [PubMed] [Google Scholar]
- 16.Purohit P., Perez-Branguli F., Prots I., Borger E., Gunn-Moore F., Welzel O. The Ca2+ sensor protein swiprosin-1/EFhd2 is present in neurites and involved in kinesin-mediated transport in neurons. PloS One. 2014;9 doi: 10.1371/journal.pone.0103976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brachs S., Turqueti-Neves A., Stein M., Reimer D., Brachvogel B., Bösl M. Swiprosin-1/EFhd2 limits germinal center responses and humoral type 2 immunity. Eur. J. Immunol. 2014;44:3206–3219. doi: 10.1002/eji.201444479. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S., Tu Y., Sun Y.M., Li Y., Wang R.M., Cao Y. Swiprosin-1 deficiency impairs macrophage immune response of septic mice. JCI Insight. 2018;3 doi: 10.1172/jci.insight.95396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mielenz D., Reichel M., Jia T., Quinlan E.B., Stöckl T., Mettang M. EFhd2/Swiprosin-1 is a common genetic determinator for sensation-seeking/low anxiety and alcohol addiction. Mol. Psychiatr. 2018;23:1303–1319. doi: 10.1038/mp.2017.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z.B., Han P., Tong L.C., Luo Y., Su W.H., Wei X. Low level of swiprosin-1/EFhd2 in vestibular nuclei of spontaneously hypersensitive motion sickness mice. Sci. Rep. 2017;7:40986. doi: 10.1038/srep40986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vega I.E. EFhd2, a protein linked to Alzheimer's disease and other neurological disorders. Front. Neurosci. 2016;10:150. doi: 10.3389/fnins.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huh Y.H., Oh S., Yeo Y.R., Chae I.H., Kim S.H., Lee J.S. Swiprosin-1 stimulates cancer invasion and metastasis by increasing the Rho family of GTPase signaling. Oncotarget. 2015;6:13060–13071. doi: 10.18632/oncotarget.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan C.C., Cheng W.C., Huang Y.C., Sher Y.P., Liou N.J., Chien Y.C. EFHD2 promotes epithelial-to-mesenchymal transition and correlates with postsurgical recurrence of stage I lung adenocarcinoma. Sci. Rep. 2017;7:14617. doi: 10.1038/s41598-017-15186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y.W., Yang P.C., Yang S.C., Shyu Y.C., Hendrix M.J., Wu R. Selection of invasive and metastatic subpopulations from a human lung adenocarcinoma cell line. Am. J. Respir. Cell Mol. Biol. 1997;17:353–360. doi: 10.1165/ajrcmb.17.3.2837. [DOI] [PubMed] [Google Scholar]
- 25.Tsai S.T., Wang P.J., Liou N.J., Lin P.S., Chen C.H., Chang W.C. ICAM1 is a potential cancer stem cell marker of esophageal squamous cell carcinoma. PloS One. 2015;10 doi: 10.1371/journal.pone.0142834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox J., Hein M.Y., Luber C.A., Paron I., Nagaraj N., Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espina M., Corte-Rodríguez M., Aguado L., Montes-Bayón M., Sierra M.I., Martínez-Camblor P. Cisplatin resistance in cell models: evaluation of metallomic and biological predictive biomarkers to address early therapy failure. Metall. 2017;9:564–574. doi: 10.1039/c7mt00014f. [DOI] [PubMed] [Google Scholar]
- 28.Győrffy B., Surowiak P., Budczies J., Lánczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PloS One. 2013;8 doi: 10.1371/journal.pone.0082241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Z., Li C., Kang B., Gao G., Li C., Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukas J., Lukas C., Bartek J. More than just a focus: the chromatin response to DNA damage and its role in genome integrity maintenance. Nat. Cell Biol. 2011;13:1161–1169. doi: 10.1038/ncb2344. [DOI] [PubMed] [Google Scholar]
- 31.Holzer A.K., Manorek G.H., Howell S.B. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol. Pharmacol. 2006;70:1390–1394. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 32.Belousov V.V., Fradkov A.F., Lukyanov K.A., Staroverov D.B., Shakhbazov K.S., Terskikh A.V. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods. 2006;3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 33.Reis J., Massari M., Marchese S., Ceccon M., Aalbers F.S., Corana F. A closer look into NADPH oxidase inhibitors: validation and insight into their mechanism of action. Redox Biol. 2020;32:101466. doi: 10.1016/j.redox.2020.101466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laberge R.M., Karwatsky J., Lincoln M.C., Leimanis M.L., Georges E. Modulation of GSH levels in ABCC1 expressing tumor cells triggers apoptosis through oxidative stress. Biochem. Pharmacol. 2007;73:1727–1737. doi: 10.1016/j.bcp.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Matsuura K., Otani M., Takano M., Kadoyama K., Matsuyama S. The influence of chronic ibuprofen treatment on proteins expressed in the mouse hippocampus. Eur. J. Pharmacol. 2015;752:61–68. doi: 10.1016/j.ejphar.2015.01.047. [DOI] [PubMed] [Google Scholar]
- 36.Kroemer G., Mariño G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizushima N., Yoshimori T., Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aramini B., Casali C., Stefani A., Bettelli S., Wagner S., Sangale Z. Prediction of distant recurrence in resected stage I and II lung adenocarcinoma. Lung Canc. 2016;101:82–87. doi: 10.1016/j.lungcan.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Li B., Cui Y., Diehn M., Li R. Development and validation of an individualized immune prognostic signature in early-stage nonsquamous non-small cell lung cancer. JAMA Oncol. 2017;3:1529–1537. doi: 10.1001/jamaoncol.2017.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbosh C., Birkbak N.J., Wilson G.A., Jamal-Hanjani M., Constantin T., Salari R. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545:446–451. doi: 10.1038/nature22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Y.L., Herbst R.S., Mann H., Rukazenkov Y., Marotti M., Tsuboi M. ADAURA: phase III, double-blind, randomized study of osimertinib versus placebo in EGFR mutation-positive early-stage NSCLC after complete surgical resection. Clin. Lung Canc. 2018;19:e533–e536. doi: 10.1016/j.cllc.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Owen D., Chaft J.E. Immunotherapy in surgically resectable non-small cell lung cancer. J. Thorac. Dis. 2018;10:S404–S411. doi: 10.21037/jtd.2017.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodard G.A., Wang S.X., Kratz J.R., Zoon-Besselink C.T., Chiang C.Y., Gubens M.A. Adjuvant chemotherapy guided by molecular profiling and improved outcomes in early stage, non-small-cell lung cancer. Clin. Lung Canc. 2018;19:58–64. doi: 10.1016/j.cllc.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 44.Sharpnack M.F., Ranbaduge N., Srivastava A., Cerciello F., Codreanu S.G., Liebler D.C. Proteogenomic analysis of surgically resected lung adenocarcinoma. J. Thorac. Oncol. 2018;13:1519–1529. doi: 10.1016/j.jtho.2018.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka F., Yoneda K. Adjuvant therapy following surgery in non-small cell lung cancer (NSCLC) Surg. Today. 2016;46:25–37. doi: 10.1007/s00595-015-1174-7. [DOI] [PubMed] [Google Scholar]
- 46.Zhang C., Lan T., Hou J., Li J., Fang R., Yang Z. NOX4 promotes non-small cell lung cancer cell proliferation and metastasis through positive feedback regulation of PI3K/Akt signaling. Oncotarget. 2014;5:4392–4405. doi: 10.18632/oncotarget.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho S.Y., Kim J.S., Eun H.S., Kang S.H., Lee E.S., Kim S.H. Expression of NOX family genes and their clinical significance in colorectal cancer. Dig. Dis. Sci. 2018;63:2332–2340. doi: 10.1007/s10620-018-5121-5. [DOI] [PubMed] [Google Scholar]
- 48.Kim H., Sung J.Y., Park E.K., Kho S., Koo K.H., Park S.Y. Regulation of anoikis resistance by NADPH oxidase 4 and epidermal growth factor receptor. Br. J. Canc. 2017;116:370–381. doi: 10.1038/bjc.2016.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cort A., Ozben T., Saso L., De Luca C., Korkina L. Redox control of multidrug resistance and its possible modulation by antioxidants. Oxid. Med. Cell. Longev. 2016;2016:4251912. doi: 10.1155/2016/4251912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burkhart C.A., Watt F., Murray J., Pajic M., Prokvolit A., Xue C. Small-molecule multidrug resistance-associated protein 1 inhibitor reversan increases the therapeutic index of chemotherapy in mouse models of neuroblastoma. Canc. Res. 2009;69:6573–6580. doi: 10.1158/0008-5472.CAN-09-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li J., Li Z.N., Yu L.C., Bao Q.L., Wu J.R., Shi S.B. Association of expression of MRP1, BCRP, LRP and ERCC1 with outcome of patients with locally advanced non-small cell lung cancer who received neoadjuvant chemotherapy. Lung Canc. 2010;69:116–122. doi: 10.1016/j.lungcan.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Li X.Q., Li J., Shi S.B., Chen P., Yu L.C., Bao Q.L. Expression of MRP1, BCRP, LRP and ERCC1 as prognostic factors in non-small cell lung cancer patients receiving postoperative cisplatin-based chemotherapy. Int. J. Biol. Markers. 2009;24:230–237. doi: 10.1177/172460080902400403. [DOI] [PubMed] [Google Scholar]
- 53.Norman B.H., Lander P.A., Gruber J.M., Kroin J.S., Cohen J.D., Jungheim L.N. Cyclohexyl-linked tricyclic isoxazoles are potent and selective modulators of the multidrug resistance protein (MRP1) Bioorg. Med. Chem. Lett. 2005;15:5526–5530. doi: 10.1016/j.bmcl.2005.08.075. [DOI] [PubMed] [Google Scholar]
- 54.Obreque-Balboa J.E., Sun Q., Bernhardt G., König B., Buschauer A. Flavonoid derivatives as selective ABCC1 modulators: synthesis and functional characterization. Eur. J. Med. Chem. 2016;109:124–133. doi: 10.1016/j.ejmech.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Fletcher J.I., Williams R.T., Henderson M.J., Norris M.D., Haber M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updates. 2016;26:1–9. doi: 10.1016/j.drup.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Corte-Rodríguez M., Espina M., Sierra L.M., Blanco E., Ames T., Montes-Bayón M. Quantitative evaluation of cellular uptake, DNA incorporation and adduct formation in cisplatin sensitive and resistant cell lines: comparison of different Pt-containing drugs. Biochem. Pharmacol. 2015;98:69–77. doi: 10.1016/j.bcp.2015.08.112. [DOI] [PubMed] [Google Scholar]
- 57.Riisom M., Gammelgaard B., Lambert I.H., Stürup S. Development and validation of an ICP-MS method for quantification of total carbon and platinum in cell samples and comparison of open-vessel and microwave-assisted acid digestion methods. J. Pharmaceut. Biomed. Anal. 2018;158:144–150. doi: 10.1016/j.jpba.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 58.Petruzzella E., Sirota R., Solazzo I., Gandin V., Gibson D. Triple action Pt(iv) derivatives of cisplatin: a new class of potent anticancer agents that overcome resistance. Chem. Sci. 2018;9:4299–4307. doi: 10.1039/c8sc00428e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye M.X., Zhao Y.L., Li Y., Miao Q., Li Z.K., Ren X.L. Curcumin reverses cis-platin resistance and promotes human lung adenocarcinoma A549/DDP cell apoptosis through HIF-1α and caspase-3 mechanisms. Phytomedicine. 2012;19:779–787. doi: 10.1016/j.phymed.2012.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.