Abstract
Background
Ketamine is a potent sedative drug that helps to maintain upper‐airway patency, due to its higher upper‐airway dilator muscular activity and higher level of duty cycle, as seen in rats. However, no clinical trials have tested passive upper‐airway collapsibility and changes in the inspiratory duty cycle against partial upper‐airway obstruction in humans. The present study evaluated both the passive mechanical upper‐airway collapsibility and compensatory response against acute partial upper‐airway obstruction using three different sedative drugs in a crossover trial.
Methods
Eight male volunteers entered this nonblinded, randomized crossover study. Upper‐airway collapsibility (passive critical closing pressure) and inspiratory duty cycle were measured under moderate sedation with ketamine, propofol, and dexmedetomidine. Propofol, dexmedetomidine, and ketamine anesthesia were induced to obtain adequate, same‐level sedation, with a BIS value of 50–70 and the OAA/S score of 2–3 and RASS score of −3.
Results
The median passive critical closing pressure of 0.08 [−5.51 to 1.20] cm H2O was not significantly different compared to that of propofol sedation (−0.32 [−1.41 to −0.19] cm H2O) and of dexmedetomidine sedation (−0.28 [−0.95 to −0.03] cm H2O) (p = .045). The median passive R US for ketamine 54.35 [32.00 to 117.50] cm H2O/L/s was significantly higher than that for propofol 5.50 [2.475 to 19.60] cm H2O/L/s; (mean difference, 27.50; 95% CI 9.17 to 45.83) (p = .009) and for dexmedetomidine 19.25 [4.125 to 22.05] cm H2O/L/s; (mean difference, 22.88; 95% CI 4.67 to 41.09) (p = .021). The inspiratory duty cycle increased significantly as the inspiratory airflow decreased in passive conditions for each sedative drug, but behavior differed among the three sedative drugs.
Conclusion
Our findings demonstrate that ketamine sedation may have an advantage of both maintained passive upper‐airway collapsibility and a compensatory respiratory response, due to both increase in neuromuscular activity and the increased duty cycle, to acute partial upper‐airway obstruction.
ketamine sedation may have an advantage of both maintained passive upper‐airway collapsibility and a compensatory respiratory response, due to the increased duty cycle, to acute partial upper‐airway obstruction.

1. INTRODUCTION
Upper airway obstruction during sedation can result from changes in either the passive structural properties of the pharynx or from disturbances in neuromuscular control (Ayuse, 2010, 2016; Ayuse et al., 2009; Hoshino et al., 2009; Kobayashi et al., 2011), similar to the mechanism in sleep (Patil et al., 2007; Schneider et al., 2009). Methods for quantifying the contribution of anatomic alterations have been established in both sleeping and anesthetized subjects.(Eastwood, Platt, Shepherd, Maddison, & Hillman, 2005; Hoshino et al., 2009; Isono, Remmers, et al., 1997; Patil et al., 2007) When the upper‐airway first becomes obstructed, the upper‐airway musculature remains in a relatively hypotonic or passive state Patil et al., 2007; Schwartz et al., 1998) Initially, mechanoreceptor activity of the airway pressure receptors and pulmonary stretch receptors can produce immediate alterations in respiratory timing in experimental animals and sleeping humans. It has been suggested that a prolongation of the inspiratory duty cycle (IDC) can help stabilize ventilation during periods of upper‐airway obstruction (Hoshino et al., (2009); Schneider et al., 2009; Tagaito, Schneider, O'Donnell, Smith, & Schwartz, 2002), as a compensatory response against partial upper‐airway obstruction. Thereafter, upper‐airway obstruction can elicit compensatory neural responses that can mitigate the initial obstruction during spontaneous breathing in sleeping and in anesthetized subjects. With persistent upper‐airway obstruction, disturbances in gas exchange ensue, leading to increases in upper‐airway neuromuscular activity, improvements in airway patency, and greater ventilatory stability (active state) (McGinley et al., 2008; Patil et al., 2007; Schwartz et al., 1993; Seelagy et al., 1994). When compensatory mechanisms are inadequate to stabilize ventilation, upper‐airway obstruction often terminates in an arousal from sleep, with prompt restoration of upper‐airway patency (Younes, 2004). Arousals can therefore interfere with the assessment of compensatory upper‐airway and respiratory timing responses. We have previously reported that passive measurements of upper‐airway collapsibility in sedated subjects were similar to those in non‐REM sleep (Ayuse et al., 2004; Hoshino et al., 2009; Inazawa et al., 2005; Kobayashi et al., 2011). Active compensatory neuromuscular responses to partial upper‐airway obstruction have also been characterized in anesthetized subjects (Hoshino et al., 2009).
The protocols for sedation using propofol, dexmedetomidine, and ketamine are well established according to age, the magnitude of surgical invasion, and the site of the procedure, for maintaining safety in different clinical contexts. However, each of these drugs have different characteristics in terms of maintaining upper‐airway patency during sedation. Previously, Eikermann et al. (2012) strongly suggested that ketamine anesthesia can stabilize breathing by increasing the genioglossus activity, duty cycle, and respiratory rate, as compared with sleep and propofol‐induced unconsciousness, in a rat experiment. Furthermore, a recent study by Lodenius et al. (2019) indicated that dexmedetomidine and propofol exhibit similar degrees of pharyngeal collapsibility and reductions in ventilatory drive. They concluded that dexmedetomidine sedation does not inherently protect against upper‐airway collapse. However, no previous study had tested both the passive structural properties of the pharynx and neuromuscular compensatory control during sedation with different sedative drugs.
We hypothesized that at comparable levels of moderate sedation as assessed clinically, upper airway collapsibility is less with ketamine than that with propofol or dexmedetomidine. To address this hypothesis, we examined passive upper‐airway properties and timing responses to acute and sustained periods of airflow obstruction under ketamine, propofol, and dexmedetomidine sedation.
2. METHODS
2.1. Subjects
All male participants with an American Society of Anesthesiologists physical status score of I or II were enrolled in this nonblinded randomized, crossover study (Figure 1). Because we have observed that there is a major influence of hormonal status in female subjects on upper airway patency, we have only recruited male subjects to eliminate these effects during sedation (Hoshino et al., 2011). Subjects were excluded if they were overweight or obese (body mass index more than 25 kg/m2), had a history of frequent or excessive snoring according to their bed‐partner, had abnormal sleep patterns assessed by Epworth Sleepiness score, or reported excessive daytime sleepiness (Epworth Sleepiness score > 10), or assessment of predisposition to sleep apnea by STOP‐BANG, had significant medical disease (cardiopulmonary pathology) or other clinical history (allergy to anesthetic), have a history of dysphagia, or reported tobacco use, or chronic alcohol or drug use. In addition, subjects were excluded if they had an anatomical deformation in the upper airway (e.g., deviated septum, retrognathia).
Figure 1.
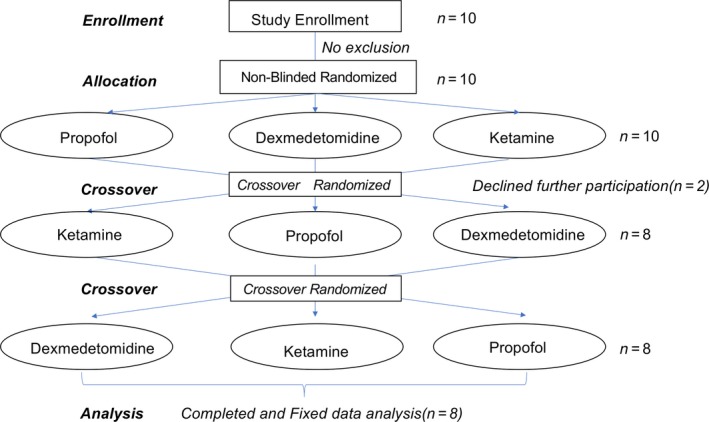
Standard flow diagram of the nonblinded, randomized crossover trial, showing the experimental enrollment criteria and number of individuals in the final analysis
Upper‐airway pressure‐flow relationships were assessed under the awake condition and under moderate sedation with ketamine, dexmedetomidine, and propofol in eight male subjects (median age [range], 29.0 [24.5–34.75] yr; body weight, 64.5 [60.25–70.00] kg; height, 168.0 [165.3–172.3] cm; body mass index, 22.05 [21.17–24.15] kg/m2.
The protocol was approved by the Human Investigation Committee of the Nagasaki University School of Dentistry (No.0506–9) and registered with UMIN clinical trials registry (UMIN000038127). The enrollment of subjects was performed from September 2019 to January 2020 at the research facilities at Nagasaki University and written informed consent was obtained from all subjects.
2.2. Experimental procedures and monitoring
2.2.1. Sedation level
All subjects underwent routine hemodynamic monitoring (systolic and diastolic blood pressure, as well as pulse rate). EEG signals were processed by the BIS monitor (Aspect Medical Systems, Inc., Natick, MA, USA) in order to determine the depth of anesthesia. The genioglossus electromyogram (EMGGG) was measured by surface electrode placed on the chin. Oxygen saturation was measured by pulse oximetry (SpO2). We required the subject's BIS value to be between 50 and 70 (Observer's Assessment of Alertness/Sedation (OAA/S) score 2–3: somnolence, response to tactile stimulation, or slow response to a loud voice) and that the subject scores a −3 on the Richmond Agitation‐Sedation Scale (RASS) in order to obtain an adequate and comparable moderate sedation level with each of propofol and dexmedetomidine. Because BIS is not a valid parameter for monitoring ketamine sedation, we used only the OAA/S score and the RASS scale to obtain a comparable moderate sedation level. At the conclusion of the measurements, all subjects remained in the supine position until they spontaneously emerged from anesthesia. Additionally, we will try to estimate the drug plasma concentration at stable sedative level 30 min after induction based on drug dosage and body weight, using the algorithm of pharmacokinetics of dexmedetomidine and ketamine using AnestAssist TM (Palma Healthcare Systems, USA).
2.2.2. Respiratory measurements
Airflow (V ¯) was measured using a pneumotachometer (model 3,830, Hans Rudolph, Inc., Kansas City, MO, USA) and nasal pressure (P N) was measured using a differential pressure transducer (model 1100, Hans Rudolph, Inc.). The outflow from the valve attached to the nasal mask was then connected in series to the pneumotachometer and nasal mask, as described in a previous study (Kobayashi et al., 2011). Air leaks from the mouth were prevented by applying surgical tape across the lips. The changes in P N could be made by utilizing a pressure generator (MAP/ResMed, Martinsried, Germany) operating over a−15 to +15 cm H2O range connected to the nasal mask. All measurements were displayed and recorded simultaneously on a computer using a data acquisition device (either Embla S7000 or A‐10, Medcare, Beverly Hills, CA, USA).
2.3. Experimental protocols
2.3.1. Propofol sedation
No premedication was given. Propofol anesthesia was induced with intravenous propofol (Diprivan; Astra Zeneca, Nether Alderley, UK), administered via a Diprifusor (Astra Zeneca) target‐controlled infusion system (Terumo TCI pump TE371, Tokyo, Japan). The system calculated the effect site concentration on the basis of a three‐compartment pharmacokinetic algorithm (Coetzee, Glen, Wium, & Boshoff, 1995; Eastwood et al., 2005; Marsh, White, Morton, & Kenny, 1991). The propofol target blood concentration was increased and kept constant between 1.5 and 2.0 μg/ml to obtain an adequate level of anesthesia.
2.3.2. Dexmedetomidine sedation
Dexmedetomidine was introduced at an initial load of 6 μg kg‐1 h‐1 for the first 10 min, and after 10 min, a dose of 0.2–0.7 μg kg‐1 h‐1 was used to maintain a constant sedation level.
2.3.3. Ketamine sedation
Ketamine was introduced at an initial dose of 0.5–1.0 mg/kg, maintained at a rate of 0.05 mg kg−1 min−1, and supplemented with 0.5 mg/kg as needed to maintain a constant sedation level.
2.4. Respiratory parameters
Respiratory parameters were obtained under the baseline awake condition and under the sedated condition with each sedative drug. The respiratory parameters evaluated were as follows: respiratory rate (fR), minute ventilation (V ¯ E), inspiratory duty cycle (IDC; IDC = T I/ T TOT), where T I is the duration of inspiration and T TOTAL is the duration of the inspiration and expiration, maximum inspiratory airflow (V I max) during airflow‐limited breathing, and mean inspiratory airflow (V I = V T/ T I), where V T is the tidal volume. Dead space volume (V DS) was calculated using the formula, 24 × height2 (inches)/703 ml. Alveolar ventilation (V ¯ ALV) was calculated by subtracting dead space ventilation, which is the product of V DS and fR, from minute ventilation, as shown in the following equation:
| (1) |
2.5. Primary outcome: upper airway collapsibility and passive critical closing pressure (PCRIT) during moderate sedation with ketamine, propofol, and dexmedetomidine
Following the establishment of an adequate level of stable sedation, the subjects were initially allowed to breathe under atmospheric pressure. P N was then gradually increased to a holding pressure where inspiratory airflow limitation was abolished (“passive state”), as previously described.(Boudewyns et al., 2000; Schwartz et al., 1998) To establish the passive P CRIT, P N was rapidly lowered from the holding pressure to specific levels, for five successive breaths, before returning to the holding pressure (Figure 2a,b). Nasal pressure levels traversed a range of pressures, also including zero flow (airway occlusion). Passive pressure‐flow relationships were generated from at least two series of pressure drops over this range. The upper‐airway pressure‐flow relationship was analyzed to determine upper‐airway collapsibility. At each level of P N, breaths were evaluated for the presence of inspiratory airflow limitation, as previously described (Ayuse et al., 2004; Boudewyns et al., 2000; Hoshino et al., 2009, 2011; Kobayashi et al., 2011; Schwartz, Smith, Wise, Gold, & Permutt, 1988). Breaths that were associated with arousal evaluated with sudden change of increase in BIS value associated with change if raw EEG trace was excluded from analysis. Non‐flow‐limited breaths from the baseline condition at holding pressure were analyzed to determine the peak inspiratory airflow (V I peak) at baseline. Maximal inspiratory flow (V I max) and the corresponding P N were obtained for each flow‐limited breath in the obstructed conditions. Least‐squares linear regression was used to generate the pressure‐flow relationship (Gold & Schwartz, 1996), which was fit by the following equation:
| (2) |
Figure 2.
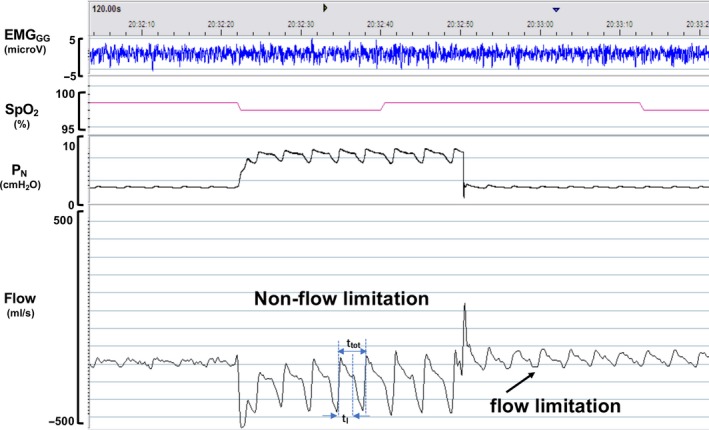
A schematic of the experimental protocol for producing upper airflow obstruction in the passive state. The polysomnographic recording in one subject is shown: surface genioglossus electromyogram (EMGG), oxyhemoglobin saturation (SpO2), nasal mask pressure (P N), and pneumotach airflow (Flow). P N is abruptly reduced from an elevated holding pressure to a level that induced airflow limitation (plateau in airflow) within two or three steps, without causing an increase in EMGGG activity and any reduction of SpO2 under sustained airflow limitation. This maneuver was repeated at least three times to generate passive pressure‐flow relationships. Note that, the drop in P N from the holding pressure to lower pressure levels decreases the flow. The arrows indicate the occurrence of flow limitation as PN decreases. Ttot (the duration of inspiration) and TI (the duration of the inspiration and expiration) indicate each segment to calculate IDC in each breath
where P CRIT is the estimated extrapolated zero critical closing pressure (P N at zero flow) and R US cm H2O/L/s is the resistSance of the portion of the tube upstream to the site of collapse (Figure 3).
Figure 3.
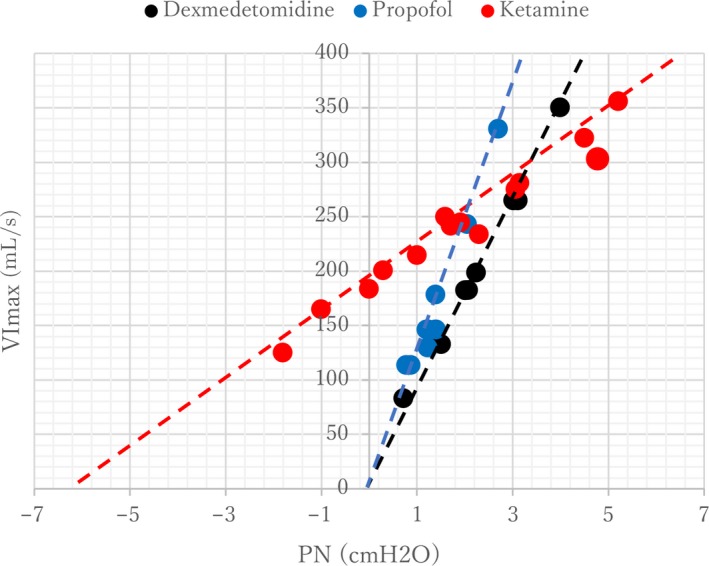
Representative trace of pressure‐flow relationships in one subject for each sedated condition: dexmedetomidine, propofol, and ketamine. The extrapolated estimated zero closing pressure was obtained by a linear regression curve between PN and V I max. The equation of the linear regression is Y = 117x + 1.2, R 2 = 0.95 for propofol, Y = 82x + 15, R 2 = 0.99 for dexmedetomidine, and Y = 29x + 186, R 2 = 0.97 for ketamine
2.6. Inspiratory duty cycle due to severity of upper‐airway obstruction
The IDC was obtained from non‐flow‐limited breaths at baseline and two levels of V I ¯ obtained from breaths during passive conditions, as described in our previous study (Hoshino et al., 2009). The severity of upper‐airway obstruction was established based on the specific ranges of V̄I above and below a clinically and physiologically relevant cutoff of approximately 150 ml/s. When V̄I is below this cutoff, upper‐airway obstruction is known to lead to periodic obstructive hypopneas, whereas breathing patterns stabilize when V İ exceeds this cutoff (Hoshino et al., 2009). Mild flow limitation was thereforeS defined by a V I max of greater than 150 ml/s and severe flow limitation was approximated as a V I max between 25 and 150 ml/s.
2.7. Sample size analysis
The sample size was calculated to determine what difference in P CRIT was of clinical significance. We established the standard effect size by calculating individual differences from the raw data obtained from a previous study, which showed that head elevation with fixed‐jaw condition reduced P CRIT to −7.2 ± 3.2 (mean ± SD) cm H2O, from a baseline of −2.8 ± 2.6 (mean ± SD) cm H2O in healthy volunteers (Kobayashi et al., 2011). Therefore, eight subjects were needed in each group to detect a difference in mean passive P CRIT of 3.5–4.0 cm H2O with a type I error of 0.05 and a power of 0.9, based on a two‐tailed paired t‐test. Anticipating a drop‐out rate of 10%–15%, we enrolled 10 subjects in each group.
Subjects were nonblinded and were randomly scheduled to undergo sedation with propofol, dexmedetomidine, or ketamine by an independent researcher using an envelope method. A crossover design was used for all subjects, with at least a 1‐week interval between each sedative drug administration. The allocation sequence was generated by the statistician, and the research staff opened the sequentially numbered envelope containing the randomization assignment. The research assistant evaluated eligibility, obtained informed consent, and enrolled the participants in this study.
2.8. Statistical analysis
Continuous normal distribution data are presented as median [interquartile range] or mean ± SD. Categorical nonparametric data are presented as median [interquartile range] or numbers. Normal distribution of data was assessed using histograms and Q‐Q plots. To evaluate how obstruction level, drug type, and obstruction level × drug interaction affected IDC results, data were analyzed using a linear mixed model. We also set the drug type and obstruction level as fixed effects and individuals as a random effect. In post hoc testing, Tukey's multiple comparison test was applied to examine differences compared with drug type by obstruction level, and obstruction level by drug level. The passive P CRIT, R US for each drug and the value of V T, fR, V̄E, V̄ALV, and SpO2 between the awake and sedative condition for each drug were analyzed by Student's paired t‐test. Statistical analysis was performed with R (version 3.2.4, R Foundation for Statistical Computing, Vienna, Austria). A p‐value of less than .05 was considered to be statistically significant.
3. RESULTS
Complete data sets were obtained from eight subjects and there was no missing data.
3.1. Effect of sedation on breathing parameters under THREE sedated conditions
For propofol sedation, the mean target blood concentration of propofol was 1.54 ± 0.23 μg/ml propofol, which produced adequate anesthesia and a BIS score of 66.3 ± 2.4. There was no significant difference in the BIS score between propofol and dexmedetomidine (64.3 ± 9.3, p = .33). An OAA/S score of 2–3 was confirmed for all three sedative drugs: average 2.3 ± 0.5 for propofol, 2.5 ± 0.5 for dexmedetomidine, and 2.5 ± 0.5 for ketamine. An RASS scale of −3 was confirmed for all three sedative drugs, indicating that comparable levels of moderate sedation were achieved with the value of average −3.0 ± 0.5 for propofol, −2.9 ± 0.4 for dexmedetomidine, and −2.8 ± 0.5 for ketamine. We obtained estimated plasma concentration of approximately 575.38 ± 48.89 ng/ml in ketamine sedation and approximately 0.61 ± 0.11 ng/ml in dexmedetomidine. We used TCI for propofol of 1.5 ~ 2.0 μg/ml.
Respiratory parameters, such as V ˙ I max, f R, VT, V̄E, and V̄ALV, are represented for the baseline awake condition and each sedated condition in Table 1. During stable sedated conditions with each of the sedative drugs, f R was significantly higher than during the awake condition. V T was significantly decreased under all sedated conditions than under the awake condition. There was no significant difference in V̄E and V̄ ALV under sedation compared to under quiet wakefulness.
TABLE 1.
Respiratory parameters for baseline awake condition and the sedative condition, with each sedative agent.
| Propofol | Dexmedetomidine | Ketamine | |||||
|---|---|---|---|---|---|---|---|
| Awake | Sedation | Awake | Sedation | Awake | Sedation | ||
| VT | 359 ± 51 | 268 ± 108* | 357 ± 83 | 271 ± 50* | 360 ± 38.5 | 283 ± 76* | |
| f R | 12.5 ± 4.7 | 18.4 ± 3.4* | 11.1 ± 4.4 | 18.0 ± 2.3* | 12.8 ± 4.3 | 14.0 ± 4.3 | |
|
|
4518 ± 1644 | 4747 ± 1145 | 3833 ± 1190 | 4804 ± 658 | 4602 ± 1730 | 3606 ± 1314 | |
|
|
2608 ± 910 | 1983 ± 1263 | 2137 ± 792 | 2081 ± 913 | 2662 ± 1111 | 1928 ± 1027 | |
| SpO2 | 98.8 ± 1.3 | 97.0 ± 1.1 | 98.6 ± 1.1 | 96.4 ± 2.0 | 98.3 ± 1.3 | 98.3 ± 1.9 | |
| BIS | 66.3 ± 2.4 | 64.3 ± 9.3 | |||||
| OAA/S | 2.3 ± 0.5 | 2.5 ± 0.5 | 2.5 ± 0.5 | ||||
The asterisk (*) indicates significant differences from the awake condition; p < .05. There is a significant difference in the respiratory rate (f R) under sedation with propofol and dexmedetomidine, but not with ketamine. There is a significant difference in the tidal volume (V T) among the three sedative conditions. There is no significant difference in alveolar ventilation ( )among the sedative drugs.
3.2. Passive upper‐airway properties
The median passive P CRIT values for each sedation condition are shown in Figure 4. The median passive P CRIT for ketamine [interquartile range], 0.08 [−5.51 to 1.20] cm H2O, was not significantly different compared to that of propofol −0.32 [−1.41 to −0.19] cm H2O (mean difference, −2.59; 95% CI −4.52 to −0.66) (p = .016) and dexmedetomidine −0.28 [−0.95 to −0.03] cm H2O (mean difference, −0.13; 95% CI −1.37 to 1.11) (p = .045). There was no significant difference in P CRIT between propofol and dexmedetomidine (p = .812). The median passive R US for ketamine 54.35 [32.00 to 117.50] cm H2O/L/s was significantly higher than that for propofol 5.50 [2.475 to 19.60] cm H2O/L/s; (mean difference, 27.50; 95% CI 9.17 to 45.83) (p = .009) and for dexmedetomidine 19.25 [4.125 to 22.05] cm H2O/L/s; (mean difference, 22.88; 95% CI 4.67 to 41.09) (p = .021) (Figure 5). There was no significant difference in R US between propofol and dexmedetomidine (p = .133).
Figure 4.
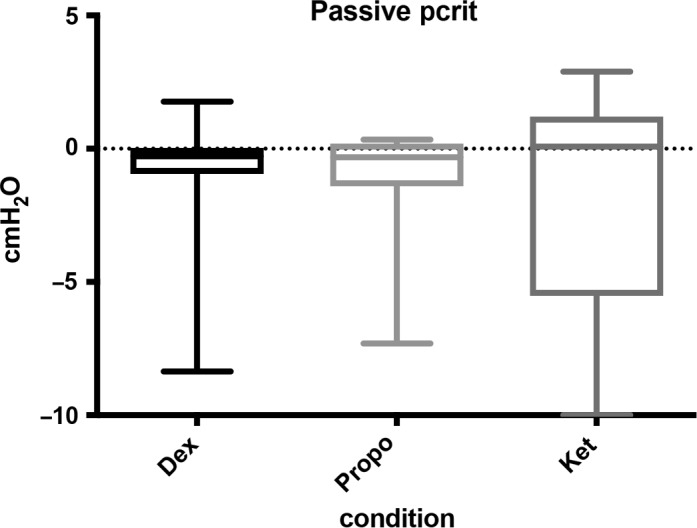
The median passive P CRIT values for each sedation condition are shown in Figure 4. The median passive P CRIT for ketamine [interquartile range], 0.08 [−5.51 to 1.20] cm H2O, was not significantly different compared to that of propofol −0.32 [−1.41 to −0.19] cm H2O (mean difference, −2.59; 95% CI −4.52 to −0.66) (p = .016) and dexmedetomidine −0.28 [−0.95 to −0.03] cm H2O (mean difference, −0.13; 95% CI −1.37 to 1.11) (p = .045). There was no significant difference in P CRIT between propofol and dexmedetomidine (p = .812)
Figure 5.
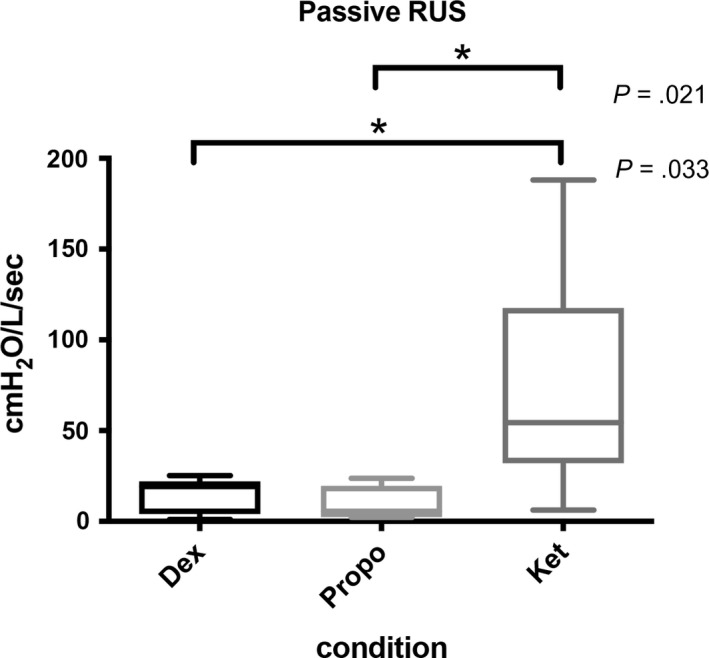
The median passive R US for ketamine 54.35 [32.00 to 117.50] cm H2O/L/s was significantly higher than that for propofol 5.50 [2.475 to 19.60] cm H2O/L/s; (mean difference, 27.50; 95% CI 9.17 to 45.83) (p = .009) and for dexmedetomidine 19.25 [4.125 to 22.05] cm H2O/L/s; (mean difference, 22.88; 95% CI 4.67 to 41.09) (p = .021) (Figure 5). There was no significant difference in R US between propofol and dexmedetomidine (p = .133)
3.3. Compensatory response to acute and sustained periods of airflow obstruction
The compensatory response to acute and sustained periods of airflow obstruction (baseline non‐flow‐limited, mild obstruction, and severe obstruction) was evaluated in terms of changes in IDC for each sedated condition. Figure 6 shows the change in IDC for different levels of V̄I under each sedated condition. The IDC increased significantly as V I max decreased in passive conditions, for each sedative drug. In the comparison of drug type by obstruction level, there was a significant difference in IDC response between propofol and ketamine (p = .025), and propofol and dexmedetomidine (p = .013) at a mild obstruction level. There was a significant difference in the IDC response between propofol and ketamine (p < .001), and between propofol and dexmedetomidine (p = .025) at a severe obstruction level. When we compared the obstruction level by the drug level, there was a significant difference (p < .001) among obstruction levels for all three sedation conditions, except between baseline and mild obstruction during propofol sedation (p = .196).
Figure 6.
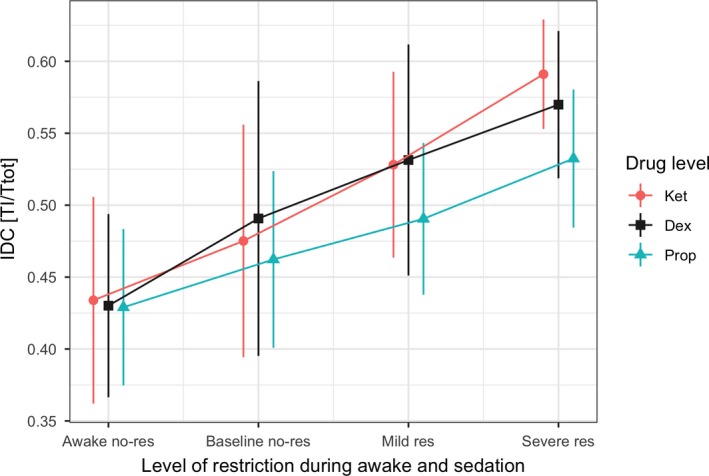
Compensatory response to acute and sustained periods of airflow restriction (awake no‐restriction, baseline no‐restriction, mild restriction, and severe restriction) was evaluated in terms of change in inspiratory duty cycle (IDC) under each sedative condition. The change in IDC at different levels of V̄I under each sedative condition is shown. The IDC increased significantly, as V I decreased in passive conditions for each sedative drug. There is a significant difference in the IDC response between propofol and ketamine (p = .025), and between propofol and dexmedetomidine (p = .013) at a mild restriction level. There is also a significant difference in the IDC response between propofol and ketamine (p < .001), and between propofol and dexmedetomidine (p = .025) at the severe restriction level. There is significant difference in IDC between awake and baseline no‐restriction, mild restriction, and severe restriction levels for all three sedation drugs (p < .001). There is no significant difference between baseline and mild restriction during propofol sedation (p = .196). awake no‐restriction (awake no‐res), baseline no‐restriction (baseline no‐res), mild restriction (mild res), and severe restriction (severe rest), propofol (Prop), dexmedetomidine (Dex), ketamine (Ket)
4. DISCUSSION
The present study compared both passive mechanical upper‐airway collapsibility and compensatory responses to acute partial upper‐airway obstruction under three different sedative drugs. Ketamine sedation maintained upper‐airway patency with similar collapsibility, as assessed by passive P CRIT, compared to propofol and dexmedetomidine sedation. However, the mean passive R US for ketamine was significantly higher than propofol and dexmedetomidine. The activated compensatory response to partial upper‐airway obstruction, as demonstrated by increased IDC, remained intact with ketamine. Upper‐airway obstruction induced immediate, progressive IDC increases with increased airflow obstruction (V̄I) severity, for all three sedative drugs. As confirmed by the existence of compensatory response to restore ventilation during non‐REM sleep (Patil et al., 2007), the compensatory ventilatory responses as well as timing parameters were partially preserved with ketamine, similarly or superior to propofol and dexmedetomidine sedation.
There are substantial increases in pharyngeal collapsibility (Patil et al., 2007) in spontaneously breathing patients during sedation and anesthesia (Drummond, 1996; Oshima, Masaki, & Toyooka, 1999). Although anesthesia and/or neuromuscular blockade can elevate upper‐airway collapsibility (Eastwood et al., 2005; Isono, Tanaka, Tagaito, Sho, & Nishino, 1997b), passive P CRIT (−3.8 cm H2O) under ketamine sedation was comparable to that during non‐REM stage 2 sleep (−4.5 cm H2O) in healthy subjects (Kirkness et al., 2008; Patil et al., 2007). In contrast, the P CRIT under propofol (−1.2 cm H2O) and dexmedetomidine sedation (−1.1 cm H2O) was similar to that under midazolam sedation (−1.9 cm H2O) (Ayuse et al., 2006), and was more negative than that under isoflurane anesthesia (1.2 cm H2O) (Eastwood, Szollosi, Platt, & Hillman, 2002), which produced notable decreases in pharyngeal neuromuscular tone and patency. The passive P CRIT values of this study were consistent with the reported values for moderate sedation with dexmedetomidine (0.3 [−9.2 to 1.4] cm H2O) and propofol (−0.6 [−7.7 to 1.3] cm H2O) (Lodenius et al., 2019). Therefore, it is likely that hypotonic pharynx collapsibility during ketamine sedation is similar to that observed during natural non‐REM sleep. Passive upper‐airway patency may be moderately influenced under propofol and dexmedetomidine sedation.
Interestingly, RUS was significantly different under ketamine compared to propofol and dexmedetomidine sedation, indicating resistance of the airway region upstream to the site of collapse. We had not found a RUS change in our previous study, although P CRIT differed significantly. This may indicate increased muSscle activity in the upper‐airway dilator muscle, as suggested by Eikermann et al. (2012) Upper‐airway dilator muscle (e.g., genioglossus) activation can mitigate airway obstruction (Fogel et al., 2001; Malhotra et al., 2000; Mezzanotte, Tangel, & White, 1992). Despite depression of dilator activity under propofol anesthesia, dilator responses to sustained upper‐airway obstruction were evident, and could account for increased upper‐airway patency (increased V̇I max) and decreased P CRIT. These responses could also have facilitated V I max maintenance under the mildly flow‐limited active condition, at levels comparable to baseline (non‐flow‐limited) V I peak levels. Airflow became limited during sustained reductions in nasal pressure, because negative tracheal pressure swings increased as ventilatory drive increased. Decreased airflow caused by nasal pressure reduction (Schwartz et al., 1988) might reflect progressive decreases in intraluminal pressures (Rowley, Williams, Smith, & Schwartz, 1997). These findings imply that neuromuscular responses stabilized upper‐airway patency during sustained airflow obstruction. The time‐course of these compensatory responses is more consistent with chemoreceptor than with mechanoreceptor stimulation (Seelagy et al., 1994).
During periods of inspiratory airflow limitation, the IDC increased immediately and progressively as airflow obstruction severity increased during sedation, consistent with the time‐course of mechano‐ rather than chemoreflex responses (Chow, Xi, Smith, Saupe, & Dempsey, 1994; Dejours, 1962; Iber, Simon, Skatrud, Mahowald, & Dempsey, 1995; Kimoff, Sforza, Champagne, Ofiara, & Gendron, 2001; Leevers, Simon, Xi, & Dempsey, 1993; Manchanda et al., 1996). The increased IDC (T I/ T TOT) restores ventilation at any given level of upper‐airway obstruction, as described by the relationship: V̄E = V T / T I × T I / T TOT, where V T/T I represents the mean inspiratory flow rate. The IDC initially increased with upper‐airway obstruction development and decreased as obstruction abated (as V̇I max rose), with the activation of pharyngeal neuromuscular responses (Schneider et al., 2003, 2009; Tagaito et al., 2002). Thus, distinct physiological mechanisms govern the IDC and upper‐airway responses to airway obstruction. The IDC response offers immediate relief from nocturnal hypoventilation during upper‐airway obstruction periods in sleep (Schneider et al., 2009), and is partially preserved during propofol, dexmedetomidine, and ketamine sedation. Interestingly, as the passive mechanical properties are similar under propofol and dexmedetomidine sedation, the IDC response to partial upper‐airway obstruction is also similar to the response under dexmedetomidine sedation, which has a higher baseline IDC value. Furthermore, the IDC response under ketamine sedation is more functional, as indicated by the steeper IDC response curve, than that with other sedative drugs. During sedation, ventilation can only be preserved by prolonging the IDC (Schneider et al., 2003; Younes, 2003), which maintains and stabilizes ventilation during periods of inspiratory flow limitation. We observed increases in f R (Table 1) for a given IDC under sedated conditions, which would decrease V T, increase the dead space fraction, and decrease V ALV accordingly. Although we did not observe any significant V ALV reduction during sedation, compared to under the awake condition, V ALV might change as the upper‐airway becomes obstructed under moderate to deep sedation. Thus, IDC and fR responses to a given level of upper‐airway obstruction may determine the degree of hypoventilation during sedation. This IDC response could be an advantage of dexmedetomidine and ketamine sedation, providing a well‐preserved compensatory response to acute partial upper‐airway obstruction.
There were several limitations in this study. For example, the results may be imprecise due to errors in outcome measurements, misdiagnosis, or misclassification of events. The potential influence of sources of bias may also substantially impact the interpretation of the trial.
First, we could not produce maximum activation of the respiratory response based on IDC measurement, because subjects’ exposure to hypoxemia was limited for ethical reasons. This may have attenuated the degree of activation during the stepwise decreases in nasal pressure, leading to underestimation of the full strength of the active response based on IDC changes.
Second, sedation levels, based on evaluation of the OAA/S score, RASS score, and BIS monitoring, with propofol, dexmedetomidine, and ketamine might differ slightly pharmacologically. However, the BIS value obtained in this study was consistent with that previously reported for propofol and dexmedetomidine (Lodenius et al., 2019). That study reported BIS at the time of critical pharyngeal pressure measurements as 57 ± 16 and 39 ± 12 during moderate infusion rates of dexmedetomidine and propofol, respectively. Therefore, we considered the sedation level obtained in this study (BIS: 50–70, OAA/S score 2–3, and RASS score −3) as a clinically relevant moderate sedation level. Third, this study did not reveal any severe adverse events associated with ketamine‐only administration that may counter the potential benefit of maintained airway patency including dysphoria and increased salivation/secretions; however, the patients were monitored for these adverse events for only a short period of time.
Fourth, the differences in upper airway mechanics could really be due to subtle differences between the drugs’ mode of anesthetic action, despite ostensible similarities in clinical sedation level. In this study, we found the maintained a compensatory response as an increase in the IDC and increase in neuromuscular activity as an increase in R US in ketamine sedation. Although the combination of two fundamentally different neural compensatory mechanisms, that is instantaneous prolongation of the IDC and increase neuromuscular activity, might be the most important factor for both maintaining passive upper airway collapsibility and ventilation sedation, further study to test different level of compensatory mechanism depends on anesthetic depth.
Our findings have two major clinical implications.
First, in addition to the advantage of ketamine over propofol and dexmedetomidine in maintaining passive upper airway patency in a given clinical setting, ketamine may also be more suitable than propofol for sedation during invasive procedures due to its synergistic effect with local anesthesia. However, it has been suggested that ketamine should be used in combination with benzodiazepines in order to avoid the side effects of ketamine, such as nightmares; the effects of such combinations on upper‐airway patency should be further investigated. Drummond (1996) suggested that ketamine had beneficial effects on airway patency. Furthermore, ketamine is recommended in difficult airway situations where spontaneous respiration needs to be preserved in adults (Craven, 2007). In critically ill children, a lower incidence of airway obstruction was observed under ketamine than under propofol sedation (Vardi, Salem, Padeh, Paret, & Barzilay, 2002). Ketamine combines potent analgesic with hypnotic actions (Bourgoin et al., 2003). Further research is needed to test upper‐airway patency under ketamine sedation combined with other agents such as potent analgesics with hypnotic actions.
Second, the preservation of compensatory neuromuscular responses involved in respiratory control suggests that upper‐airway patency can be restored during sedation with all three sedative drugs, and particularly with ketamine sedation. Our previous study suggested that immediate IDC responses can prevent a precipitous decrease in ventilation during propofol anesthesia, whereas more sustained periods of upper‐airway obstruction in the absence of arousal are required for the restoration of upper‐airway patency (Hoshino et al., 2009).
Ketamine sedation may preserve upper‐airway patency, although the effect of ketamine on upper‐airway patency in humans is poorly understood at present. Eikermann et al. (2012) hypothesized that spontaneously breathing animals, when administered ketamine, maintain breathing by two mechanisms: augmentation of airway dilator muscle activity and increased IDC. The IDC determines ventilatory stability; an increase in the IDC can compensate for partial airway obstruction Schneider et al. (2009). Ketamine induces a dose‐dependent increase in the IDC. This mechanism can compensate for partial airway obstruction (Schneider et al., 2009). Of note, in a previous study, a dose‐dependent IDC increase was observed in animals breathing normally. Accordingly, we concluded that the increase in IDC was not a compensatory mechanism for partial upper‐airway obstruction, but rather represented a direct and beneficial drug effect on inspiratory time.
Although the threshold for arousal responses and surface genioglossus electromyogram (EMGGG) activation is significantly depressed with propofol, some compensatory mechanisms may still persist. Previously, Eastwood et al.(2005) reported that increasing depths of propofol anesthesia are associated with increased upper‐airway collapsibility. Furthermore, Lodenius et al.(2019) indicated that passive pharyngeal critical pressure may have similar effects during low or moderate sedation with dexmedetomidine and propofol. Although they concluded that dexmedetomidine sedation does not protect against upper airway collapse, there might be some advantage of maintaining a compensatory response to partial upper airway obstruction, based on our finding regarding IDC response. They concluded that dexmedetomidine sedation does not protect against upper‐airway collapse. We strongly support their suggestion that continuous respiratory monitoring, such as capnography and pulse oximetry, is advisable during any level of sedation, to detect early stages of upper‐airway obstruction. Furthermore, risk factors of positional change and background disease, such as obstructive sleep apnea syndrome, should be considered in future studies.
In conclusion, we found that passive upper airway patency with less collapsibility is maintained and compensatory neuromuscular responses to upper‐airway obstruction remained intact during ketamine sedation, as compared to a similar level of propofol or dexmedetomidine sedation. Moreover, distinct mechanisms underlie ventilation maintenance during upper‐airway obstruction, with specific latencies to responses in the IDC (immediate) and inspiratory airflow (delayed). Partitioning the upper‐airway properties into structural and neuromuscular components could facilitate establishing ways to evaluate and maintain upper‐airway patency and ventilation under sedation with different anesthetic agents, and ultimately prevent perioperative respiratory complications of anesthesia.
CONFLICT OF INTEREST
GM., TS., SS., MK, SK., and TA. have no declaration of interest.
AUTHOR CONTRIBUTIONS
GM, TS, SK, MK, and TA are responsible for conceiving and designing the trial, planning data analysis, drafting the manuscript, and approving the final manuscript. GM and TS are responsible for participating in data collection and are in charge of recruitment and treatment of patients. SS is responsible for planning data analysis and analyzing the data resulting from the trial. All authors will have access to the interim results as well as the capacity to discuss, revise, and approve the final manuscript.
ACKNOWLEDGMENTS
The authors thank Ichiro Okayasu, Division of Clinical Physiology, Department of Translational Medical Sciences, Nagasaki University Graduate School of Biomedical Sciences, Nagasaki, Japan, and Toshihiro Watanabe, Mari Kawai, Tachi Mizuki, Kensuke Kiriishi, Nanako Ito, Erika Tatsuta, Department of Dental Anesthesiology, Nagasaki University Hospital, Nagasaki, Japan, for assistance in conduction of the trial.
Mishima G, Sanuki T, Sato S, Kobayashi M, Kurata S, Ayuse T. Upper‐airway collapsibility and compensatory responses under moderate sedation with ketamine, dexmedetomidine, and propofol in healthy volunteers. Physiol Rep. 2020;8:e14439 10.14814/phy2.14439
Funding information
The work was aided by institutional support.
REFERENCES
- Ayuse, T. (2010). Understanding mechanism of upper airway obstruction. Masui, 59(Suppl), S81–101. [PubMed] [Google Scholar]
- Ayuse, T. (2016). Pathogenesis of upper airway obstruction and mechanical intervention during sedation and slee. Journal of Dental Sleep Medicine, 3, 11–19. [Google Scholar]
- Ayuse, T. , Hoshino, Y. , Inazawa, T. , Oi, K. , Schneider, H. , & Schwartz, A. R. (2006). A pilot study of quantitative assessment of mandible advancement using pressure‐flow relationship during midazolam sedation. Journal of Oral Rehabilitation, 33, 813–819. 10.1111/j.1365-2842.2006.01627b.x [DOI] [PubMed] [Google Scholar]
- Ayuse, T. , Hoshino, Y. , Kurata, S. , Ayuse, T. , Schneider, H. , Kirkness, J. P. , … Oi, K. (2009). The effect of gender on compensatory neuromuscular response to upper airway obstruction in normal subjects under midazolam general anesthesia. Anesthesia and Analgesia, 109, 1209–1218. 10.1213/ane.0b013e3181b0fc70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuse, T. , Inazawa, T. , Kurata, S. , Okayasu, I. , Sakamoto, E. , Oi, K. , … Schwartz, A. R. (2004). Mouth‐opening increases upper‐airway collapsibility without changing resistance during midazolam sedation. Journal of Dental Research, 83, 718–722. 10.1177/154405910408300912 [DOI] [PubMed] [Google Scholar]
- Boudewyns, A. , Punjabi, N. , Van de Heyning, P. H. , De Backer, W. A. , O'Donnell, C. P. , Schneider, H. , … Schwartz, A. R. (2000). Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest, 118, 1031–1041. 10.1378/chest.118.4.1031 [DOI] [PubMed] [Google Scholar]
- Bourgoin, A. , Albanese, J. , Wereszczynski, N. , Charbit, M. , Vialet, R. , & Martin, C. (2003). Safety of sedation with ketamine in severe head injury patients: Comparison with sufentanil. Critical Care Medicine, 31, 711–717. [DOI] [PubMed] [Google Scholar]
- Chow, C. M. , Xi, L. , Smith, C. A. , Saupe, K. W. , & Dempsey, J. A. (1994). A volume‐dependent apneic threshold during NREM sleep in the dog. Journal of Applied Physiology, 76, 2315–2325. 10.1152/jappl.1994.76.6.2315 [DOI] [PubMed] [Google Scholar]
- Coetzee, J. F. , Glen, J. B. , Wium, C. A. , & Boshoff, L. (1995). Pharmacokinetic model selection for target controlled infusions of propofol. Assessment of Three Parameter Sets. Anesthesiology, 82, 1328–1345. 10.1097/00000542-199506000-00003 [DOI] [PubMed] [Google Scholar]
- Craven, R. (2007). Ketamine. Anaesthesia, 62(Suppl 1), 48–53. 10.1111/j.1365-2044.2007.05298.x [DOI] [PubMed] [Google Scholar]
- Dejours, P. (1962). Chemoreflexes in breathing. Physiological Reviews, 42, 335–358. 10.1152/physrev.1962.42.3.335 [DOI] [PubMed] [Google Scholar]
- Drummond, G. B. (1996). Comparison of sedation with midazolam and ketamine: Effects on airway muscle activity. British Journal of Anaesthesia, 76, 663–667. [DOI] [PubMed] [Google Scholar]
- Eastwood, P. R. , Platt, P. R. , Shepherd, K. , Maddison, K. , & Hillman, D. R. (2005). Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology, 103, 470–477. 10.1097/00000542-200509000-00007 [DOI] [PubMed] [Google Scholar]
- Eastwood, P. R. , Szollosi, I. , Platt, P. R. , & Hillman, D. R. (2002). Comparison of upper airway collapse during general anaesthesia and sleep. The Lancet, 359, 1207–1209. 10.1016/S0140-6736(02)08224-7 [DOI] [PubMed] [Google Scholar]
- Eikermann, M. , Grosse‐Sundrup, M. , Zaremba, S. , Henry, M. E. , Bittner, E. A. , Hoffmann, U. , & Chamberlin, N. L. (2012). Ketamine activates breathing and abolishes the coupling between loss of consciousness and upper airway dilator muscle dysfunction. Anesthesiology, 116, 35–46. 10.1097/ALN.0b013e31823d010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel, R. B. , Malhotra, A. , Pillar, G. , Edwards, J. K. , Beauregard, J. , Shea, S. A. , & White, D. P. (2001).Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. American Journal of Respiratory and Critical Care Medicine, 164, 2025–2030. 10.1164/ajrccm.164.11.2102048 [DOI] [PubMed] [Google Scholar]
- Gold, A. R. , & Schwartz, A. R. (1996). The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest, 110, 1077–1088. 10.1378/chest.110.4.1077 [DOI] [PubMed] [Google Scholar]
- Hoshino, Y. , Ayuse, T. , Kobayashi, M. , Kurata, S. , Kawai, M. , Schneider, H. , … Oi, K. (2011). The effects of hormonal status on upper airway patency in normal female subjects during propofol anesthesia. Journal of Clinical Anesthesia, 23, 527–533. 10.1016/j.jclinane.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Hoshino, Y. , Ayuse, T. , Kurata, S. , Ayuse, T. , Schneider, H. , Kirkness, J. P. , … Oi, K. (2009). The compensatory responses to upper airway obstruction in normal subjects under propofol anesthesia. Respiratory Physiology & Neurobiology, 166(1), 24–31. 10.1016/j.resp.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber, C. , Simon, P. , Skatrud, J. B. , Mahowald, M. W. , & Dempsey, J. A. (1995). The Breuer‐Hering reflex in humans. Effects of pulmonary denervation and hypocapnia. American Journal of Respiratory and Critical Care Medicine, 152, 217–224. 10.1164/ajrccm.152.1.7599827 [DOI] [PubMed] [Google Scholar]
- Inazawa, T. , Ayuse, T. , Kurata, S. , Okayasu, I. , Sakamoto, E. , Oi, K. , … Schwartz, A. R. (2005). Effect of mandibular position on upper airway collapsibility and resistance. Journal of Dental Research, 84, 554–558. 10.1177/154405910508400613 [DOI] [PubMed] [Google Scholar]
- Isono, S. , Remmers, J. E. , Tanaka, A. , Sho, Y. , Sato, J. , & Nishino, T. (1997). Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. Journal of Applied Physiology, 82, 1319–1326. 10.1152/jappl.1997.82.4.1319 [DOI] [PubMed] [Google Scholar]
- Isono, S. , Tanaka, A. , Tagaito, Y. , Sho, Y. , & Nishino, T. (1997). Pharyngeal patency in response to advancement of the mandible in obese anesthetized persons. Anesthesiology, 87, 1055–1062. 10.1097/00000542-199711000-00008 [DOI] [PubMed] [Google Scholar]
- Kimoff, R. J. , Sforza, E. , Champagne, V. , Ofiara, L. , & Gendron, D. (2001). Upper airway sensation in snoring and obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 164, 250–255. 10.1164/ajrccm.164.2.2010012 [DOI] [PubMed] [Google Scholar]
- Kirkness, J. P. , Schwartz, A. R. , Schneider, H. , Punjabi, N. M. , Maly, J. J. , Laffan, A. M. , … Patil, S. P. (2008). Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. Journal of Applied Physiology, 104, 1618–1624. 10.1152/japplphysiol.00045.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, M. , Ayuse, T. , Hoshino, Y. , Kurata, S. , Moromugi, S. , Schneider, H. , … Oi, K. (2011). Effect of head elevation on passive upper airway collapsibility in normal subjects during propofol anesthesia. Anesthesiology, 115, 273–281. 10.1097/ALN.0b013e318223ba6d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers, A. M. , Simon, P. M. , Xi, L. , & Dempsey, J. A. (1993). Apnoea following normocapnic mechanical ventilation in awake mammals: A demonstration of control system inertia. Journal of Physiology, 472, 749–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodenius, A. , Maddison, K. J. , Lawther, B. K. , Scheinin, M. , Eriksson, L. I. , Eastwood, P. R. , … Walsh, J. H. (2019). Upper airway collapsibility during dexmedetomidine and propofol sedation in healthy volunteers: A nonblinded randomized crossover study. Anesthesiology, 131(5), 962–973. 10.1097/ALN.0000000000002883 [DOI] [PubMed] [Google Scholar]
- Malhotra, A. , Pillar, G. , Fogel, R. B. , Beauregard, J. , Edwards, J. K. , Slamowitz, D. I. , … White, D. P. (2000). Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. American Journal of Respiratory & Critical Care Medicine, 162, 1058–1062. 10.1164/ajrccm.162.3.9912067 [DOI] [PubMed] [Google Scholar]
- Manchanda, S. , Leevers, A. M. , Wilson, C. R. , Simon, P. M. , Skatrud, J. B. , & Dempsey, J. A. (1996). Frequency and volume thresholds for inhibition of inspiratory motor output during mechanical ventilation. Respiration Physiology, 105, 1–16. 10.1016/0034-5687(96)00037-0 [DOI] [PubMed] [Google Scholar]
- Marsh, B. , White, M. , Morton, N. , & Kenny, G. N. (1991). Pharmacokinetic model driven infusion of propofol in children. British Journal of Anaesthesia, 67, 41–48. 10.1093/bja/67.1.41 [DOI] [PubMed] [Google Scholar]
- McGinley, B. M. , Schwartz, A. R. , Schneider, H. , Kirkness, J. P. , Smith, P. L. , & Patil, S. P. (2008). Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. Journal of Applied Physiology, 105, 197–205. 10.1152/japplphysiol.01214.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte, W. S. , Tangel, D. J. , & White, D. P. (1992). Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). Journal of Clinical Investigation, 89, 1571–1579. 10.1172/JCI115751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima, T. , Masaki, Y. , & Toyooka, H. (1999). Flumazenil antagonizes midazolam‐induced airway narrowing during nasal breathing in humans. British Journal of Anaesthesia, 82, 698–702. 10.1093/bja/82.5.698 [DOI] [PubMed] [Google Scholar]
- Patil, S. P. , Schneider, H. , Marx, J. J. , Gladmon, E. , Schwartz, A. R. , & Smith, P. L. (2007). Neuromechanical control of upper airway patency during sleep. Journal of Applied Physiology, 102, 547–556. 10.1152/japplphysiol.00282.2006 [DOI] [PubMed] [Google Scholar]
- Rowley, J. A. , Williams, B. C. , Smith, P. L. , & Schwartz, A. R. (1997). Neuromuscular activity and upper airway collapsibility. Mechanisms of action in the decerebrate cat. American Journal of Respiratory & Critical Care Medicine, 156, 515–521. 10.1164/ajrccm.156.2.9607115 [DOI] [PubMed] [Google Scholar]
- Schneider, H. , Krishnan, V. , Pichard, L. E. , Patil, S. P. , Smith, P. L. , & Schwartz, A. R. (2009). Inspiratory duty cycle responses to flow limitation predict nocturnal hypoventilation. European Respiratory Journal, 33, 1068–1076. 10.1183/09031936.00063008 [DOI] [PubMed] [Google Scholar]
- Schneider, H. , Patil, S. P. , Canisius, S. , Gladmon, E. A. , Schwartz, A. R. , O'Donnell, C. P. , … Tankersley, C. G. (2003). Hypercapnic duty cycle is an intermediate physiological phenotype linked to mouse chromosome 5. Journal of Applied Physiology (1985), 95(1), 11–19. 10.1152/japplphysiol.01144.2002 [DOI] [PubMed] [Google Scholar]
- Schwartz, A. R. , O'Donnell, C. P. , Baron, J. , Schubert, N. , Alam, D. , Samadi, S. D. , & Smith, P. L. (1998). The hypotonic upper airway in obstructive sleep apnea: Role of structures and neuromuscular activity. American Journal of Respiratory & Critical Care Medicine, 157, 1051–1057. [DOI] [PubMed] [Google Scholar]
- Schwartz, A. R. , Smith, P. L. , Wise, R. A. , Gold, A. R. , & Permutt, S. (1988). Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. Journal of Applied Physiology, 64, 535–542. 10.1152/jappl.1988.64.2.535 [DOI] [PubMed] [Google Scholar]
- Schwartz, A. R. , Thut, D. C. , Brower, R. G. , Gauda, E. B. , Roach, D. , Permutt, S. , & Smith, P. L. (1993). Modulation of maximal inspiratory airflow by neuromuscular activity: Effect of CO2. Journal of Applied Physiology, 74, 1597–1605. [DOI] [PubMed] [Google Scholar]
- Seelagy, M. M. , Schwartz, A. R. , Russ, D. B. , King, E. D. , Wise, R. A. , & Smith, P. L. (1994). Reflex modulation of airflow dynamics through the upper airway. Journal of Applied Physiology, 76, 2692–2700. 10.1152/jappl.1994.76.6.2692 [DOI] [PubMed] [Google Scholar]
- Tagaito, Y. , Schneider, H. , O'Donnell, C. P. , Smith, P. L. , & Schwartz, A. R. (2002). Ventilating with tracheal gas insufflation and periodic tracheal occlusion during sleep and wakefulness. Chest, 122, 1742–1750. 10.1378/chest.122.5.1742 [DOI] [PubMed] [Google Scholar]
- Vardi, A. , Salem, Y. , Padeh, S. , Paret, G. , & Barzilay, Z. (2002). Is propofol safe for procedural sedation in children? A prospective evaluation of propofol versus ketamine in pediatric critical care. Critical Care Medicine, 30, 1231–1236. 10.1097/00003246-200206000-00010 [DOI] [PubMed] [Google Scholar]
- Younes, M. (2003). Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. American Journal of Respiratory and Critical Care Medicine, 168, 645–658. 10.1164/rccm.200302-201OC [DOI] [PubMed] [Google Scholar]
- Younes, M. (2004). Role of arousals in the pathogenesis of obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine, 169, 623–633. 10.1164/rccm.200307-1023OC [DOI] [PubMed] [Google Scholar]


