Highlights
-
•
Primary vaginal cancer is rare, and endometrioid adenocarcinoma is an uncommon subtype.
-
•
Vaginal bleeding is the most common presenting symptom of vaginal endometrioid adenocarcinoma.
-
•
Endometriosis is associated with malignant transformation through oxidative stress, inflammation, and hyperestrogenism.
-
•
Inflammatory insults that could contribute to malignant transformation are inclusion cysts, sclerotherapy, and fistulas.
Keywords: Endometrioid adenocarcinoma, Primary vaginal carcinoma, Malignant transformation, Endometriosis
Abstract
Primary vaginal endometrioid adenocarcinoma is a rare cancer that is often associated with chronic endometriosis. We present the case of a 72-year-old female who underwent right salpingo-oophorectomy followed by hysterectomy with benign pathology 25 years prior to her cancer diagnosis. She had an extensive surgical history in the intervening years and several complicating factors including a history of endometriosis as well as a recurrent peritoneal inclusion cyst treated with ethanol sclerotherapy, followed by formation of a peritoneal-vaginal fistula. Endometriosis is associated with malignant transformation to endometrioid adenocarcinoma through genomic alteration, oxidative stress, inflammation, and hyperestrogenism. Frequency of surveillance examinations and imaging prior to diagnosis were based on patient symptoms, and ultimately a vaginal cuff mass was detected with invasion of the rectosigmoid colon, bladder and levators at time of diagnosis, necessitating infralevator total pelvic exenteration for removal.
1. Introduction
Vaginal cancer is a rare primary malignancy, accounting for 2% of all gynecologic cancers. Squamous cell carcinoma represents 80% of the histologic subtypes, and adenocarcinoma represents 15%, with endometrioid adenocarcinoma being the most common subtype (Creasman et al., 1998). A review in 1989 (Haskel et al., 1989) documented 7 cases of primary vaginal endometrioid adenocarcinoma, a case series in 2007 (Staats et al., 2007) reported 18 cases, and a case report in 2018 (Kondo et al., 2018) described a nonsurgical treatment approach. Table 1 summarizes the previously reported cases of primary vaginal endometrioid adenocarcinoma (Haskel et al., 1989, Staats et al., 2007, Kondo et al., 2018, Tewari et al., 2002, Eckert and Eckert, 2000, Adjetey et al., 2003, Somoye and Gull, 2005, Wirtheimer, 1964, Decelle, 1969, Bamford, 1967, Hyman, 1977, Kapp et al., 1982, Granai et al., 1984, Orr et al., 1989). While the diagnosis is well established, we present a unique insight into a patient’s development of primary vaginal endometrioid adenocarcinoma as she was followed for 35 years for benign disease complicated by a recurrent peritoneal inclusion cyst fistulized to her vagina before being diagnosed with vaginal endometrioid adenocarcinoma.
Table 1.
Summary of previously reported cases of primary vaginal endometrioid adenocarcinoma.
| Author | Number of cases | Gynecologic surgical history (n*) | Treatment (n) | Associated exposures/conditions (n) |
|---|---|---|---|---|
| Wirtheimer (1964) | 1 | None | TAH, BSO, mass removal | Endometriosis Endometrial cancer† |
| Decelle (1969) | 1 | None | TAH, BSO, LN sampling, cystectomy | Endometriosis |
| Bamford (1967) | 1 | None | TAH, vaginectomy | Endometriosis |
| Hyman (1977) | 1 | TAH, BSO 7 years prior to diagnosis | None (widespread metastases developed, and patient died) | Endometriosis Borderline left ovarian malignancy‡ |
| Kapp et al. (1982) | 1 | TAH, BSO 1 year prior to diagnosis | RT | Endometriosis Estrogen exposure |
| Granai et al. (1984) | 1 | LSO 27 years prior to diagnosis TAH, RSO 13 years prior to diagnosis |
Hormonal therapy (no effect), laparotomy with biopsies, and RT | Endometriosis Estrogen exposure |
| Haskel et al. (1989) | 7 | None | TAH, BSO, LN sampling, omental biopsy, partial colpectomy and RT | Endometriosis |
| Orr et al. (1989) | 1 | TAH, BSO 22 years prior to diagnosis | Laparotomy with mass excision and RT CT for recurrence |
Endometriosis Estrogen exposure |
| Tewari et al. (2002) | 1 | None | BSO, removal remnant uterine horns, LN sampling, excision of mass, adjunctive radiation Pelvic exenteration for recurrence (4 months) |
MRKH Syndrome |
| Eckert and Eckert (2000) | 1 | TAH, BSO 27 years prior to diagnosis | Mass resection, LN dissection | Endometriosis Estrogen exposure |
| Adjetey et al. (2003) | 1 | TAH, BSO 3 years prior to diagnosis | Excision of mass, adjuvant radiation Bone metastasis |
Endometriosis Estrogen exposure |
| Somoye and Gull (2005) | 1 | TAH, BSO 20 years prior to diagnosis | Radiation therapy | Endometriosis Estrogen exposure |
| Staats et al. (2007) | 18 | (16) TAH and/or BSO 8–40 years prior to diagnosis | (8) Radical resection (8) Excisional biopsy or local resection (5) Resection alone (7) Adjuvant CT +/- RT (1) Neoadjuvant CT (1) Hormone therapy |
(14) Endometriosis (5) Estrogen exposure (1) OCP exposure (1) IVF exposure |
| Kondo et al. (2018) | 1 | RSO 16 years prior to diagnosis TAH, LSO 8 years prior to diagnosis |
CT (paclitaxel, carboplatin) Upper vaginectomy with mass excision and adjuvant CT for recurrence |
Endometriosis |
TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; LSO, left salpingo-oophorectomy; RSO, right salpingo-oophorectomy; LN, lymph node; CT, chemotherapy; RT, radiotherapy; OCP, oral contraceptive pill; IVF, in vitro fertilization.
Number of cases are included for the Staats et al. case series. In this case series, 4 cases did not have information on additional treatment available and the numbers given represent of those available.
Endometrial cancer found synchronously, but article is in French and unable to reviewed.
Borderline malignancy was found on review of TAH, BSO slides.
2. Case report
The patient is a 72-year-old G0P0 with a history of severe endometriosis and an extensive surgical history. Her endometriosis was first noted during an exploratory laparotomy, right salpingo-oophorectomy, left ovarian wedge resection, and lysis of adhesions at the age of 38 for bilateral endometriomas. At age 47, she underwent total abdominal hysterectomy and pelvic mass removal with resection of the remaining left ovary. The pathology was consistent with a benign uterine specimen and endometrioma, and the surgeon noted difficulty with the dissection due to endometriosis.
At age 64, the patient re-presented with rectal bleeding and left lower quadrant pain. She first underwent endoscopy, which revealed a biopsy-proven rectal ulcer, and a CT scan of the abdomen and pelvis revealed a 9 cm left pelvic cystic mass. She underwent robotic-assisted resection of the pelvic mass and repair of a colpotomy that occurred during the dissection. Pathology was consistent with a hemorrhagic cyst with cicatricial fibrosis and benign vaginal mucosa. Over the next 2 years, she intermittently complained of urinary retention and was found to have a recurrent pelvic cyst requiring CT-guided drainage twice; each time, cytology was benign and consisted of macrophages, acute inflammatory cells, and debris. At age 66, an exploratory laparotomy was performed for a symptomatic recurrent pelvic cyst. The cyst was removed from the pre-sacral space and involved the bilateral ureters, which were dissected off the cyst prior to its removal. The cyst was noted to rupture prior to removal. Pelvic washings prior to rupture were benign, and the final cyst pathology was consistent with benign fibroadipose tissue. The cyst reformed within 6 months, and repeat CT-guided drainage was consistent with abundant inflammatory cells and reactive mesothelial cells with rare mild atypical squamous cells.
Over the next 2 years, she had an additional CT-guided drainage procedure (benign cytology) and a colonoscopy with biopsy of sigmoid colon ulcer (benign colonic mucosa with focal changes suggestive of ischemia). She was managed for a few months with oral progestin and experienced periods of time without symptoms, but began having abdominal pain 2 years after her most recent surgery, at which time she decided to pursue ethanol sclerotherapy treatments. She underwent 3 cyst drainage and ethanol instillation procedures, after which she developed a peritoneal-vaginal fistula. She was managed with topical estrogen initially and the drainage decreased, but she continued to have intermittent vaginal drainage and a 0.5 cm vaginal cuff defect was visible and monitored on physical exam. Nearly a year after her sclerotherapy treatments, an MRI revealed a 7.9 cm complex pelvic cyst with areas of enhancing nodularity (Fig. 1), characteristics that were unchanged from previous imaging over the past 4 years. The impression was that of a recurrent peritoneal inclusion cyst. She was closely monitored with serial exams, and 1 year later, vaginal cuff biopsy was consistent with chronic inflammation with hemosiderin deposition but negative for malignancy.
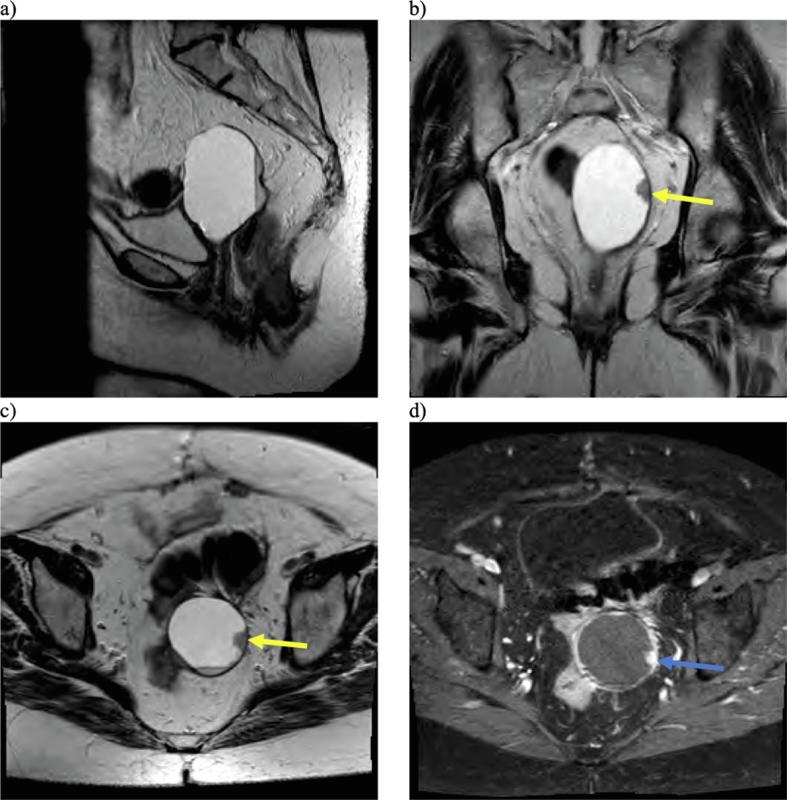
Fig. 1.
Multiplanar, multisequential MRI of the pelvis was obtained with T2-weighted sagittal (a), coronal (b), and axial (c) images demonstrating a cystic lesion arising from the vaginal cuff with peripheral nodularity (yellow arrows) and dependent debris. The area of nodularity also demonstrated enhancement on T1-weighted post-contrast imaging (blue arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
She was seen regularly for surveillance without notable changes, until 2 years later when she presented with an increase in vaginal discharge and bleeding. The vaginal defect was noted to have enlarged to 1 cm with fatty, friable granulation tissue surrounding the fistula, which demonstrated grade 1 endometrioid adenocarcinoma (Fig. 2). CT scan and MRI revealed a 5.2 cm vaginal cuff mass with central necrosis and invasion into the rectosigmoid colon, posterior bladder, left ureter, and levator (MRI images shown in Fig. 3). PET/CT was also performed and demonstrated significant uptake in the lesion consistent with malignancy (SUV 18.4) (Supplemental Fig. 1). She was discussed at an interdisciplinary tumor board, where the recommendation was made for pelvic exenteration. She underwent an infralevator total pelvic exenteration (no perineal phase required) with colonic conduit for urinary diversion, an end colostomy, omentectomy, and pelvic lymph node sampling. The extenteration specimen showed that the tumor replaced the (ulcerated) vaginal mucosa, invaded the vaginal wall, extended into perivesicular soft tissue, and invaded completely through the rectum. Margins were all negative, and pelvic, obturator, external iliac, and periaortic lymph nodes were benign. The vaginal endometrioid adenocarcinoma was grade 3, stage pT4N0Mx (FIGO stage IVA). She was recommended to undergo surveillance every 3 months for the first 2 years.
Fig. 2.
Primary vaginal endometrioid adenocarcinoma demonstrating back to back glands with cytoplasmic vacuoles (H&E 200X) (Fig. 2a). Estrogen receptor (ER) positive immunostaining supporting endometrioid tumor (200×) (Fig. 2b). Positive nuclear Pax 8 immunostaining supporting a gynecologic primary (40×) (Fig. 2c). P16 immunostaining is patchy, interpreted as negative, and supports the diagnosis of endometrioid tumor (200×) (Fig. 2d).
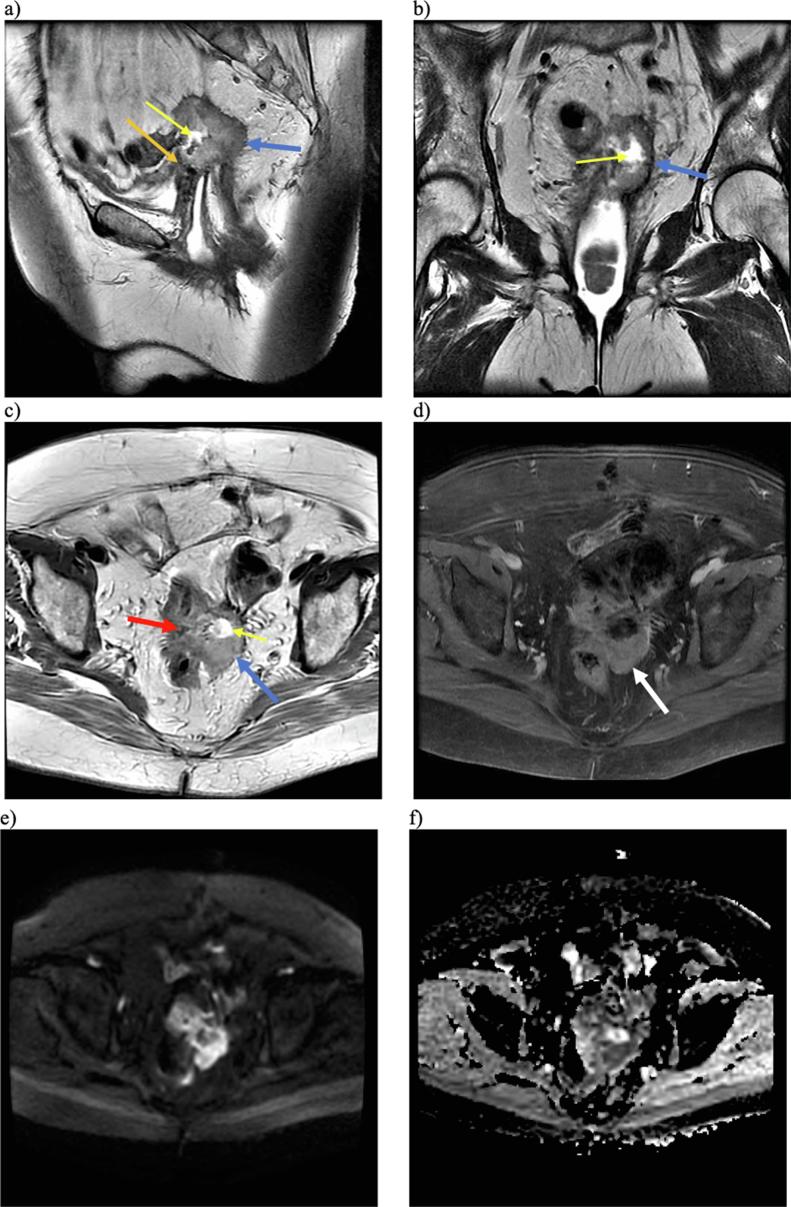
Fig. 3.
Multiplanar, multisequential MRI of the pelvis with T2-weighted sagittal (a), T2 coronal (b), and T2 axial (c) images demonstrating a slightly T2 hyperintense predominantly solid lesion (blue arrows) with central necrosis (yellow arrows) on the vaginal cuff with invasion of the rectosigmoid (red arrow), posterior urinary bladder (orange arrow), and left ureter (not pictured). T1-weighted post-contrast imaging (d) demonstrated enhancement of the solid component (white arrow). Bright signal on DWI (e) and dark signal on ADC (f) sequences is also suggestive of malignancy. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Discussion
This patient experienced many inflammatory processes over her 4 decades of treatment outlined above, including endometriosis, four surgical excision procedures, a recurrent peritoneal inclusion cyst, three ethanol sclerotherapy treatments, and a peritoneal-vaginal fistula. Thus, there were numerous insults that could have contributed to oncologic tumorigenesis.
While malignant transformation of endometriosis is rare, having chronic endometriosis increases the relative risk for developing malignancy through a combination of genomic alterations, oxidative stress, inflammation, and hyperestrogenism (Robinson et al., 2019, Nezhat et al., 2008). In a review of 18 cases of vaginal endometrioid adenocarcinoma, only 4 cases did not have a documented history of endometriosis (Staats et al., 2007). This does not prove that malignant transformation took place, though. It is possible that the same processes that contribute to the development of endometriosis also contribute to the development of endometrioid adenocarcinoma. One theory of endometriosis is metaplasia, or mesothelial-epithelial transition (Kajihara et al., 2012). Just as the ovarian surface has cortical inclusion cysts, the peritoneum can develop inclusion cysts, which are benign, fluid-filled cysts that often form in the setting of inflammation and can be precipitated by surgery, endometriosis, infection, or inflammatory bowel disease (Vallerie et al., 2009). Our patient developed an open connection between the vagina and a peritoneal inclusion cyst. Although she did not have any recent surgical pathology with endometriosis, her presentation is suggestive of the metaplasia theory of endometriosis in a cellular environment suitable for malignant transformation. Furthermore, she had pathology-documented endometriosis in the past. Alternatively, our patient had many general inflammatory insults present, and there is ample evidence for inflammation such as chronic hepatitis, H. pylori, obesity, and tobacco smoke in the development of cancer (Greten and Grivennikov, 2019).
Vaginal endometrioid adenocarcinoma most often presents with vaginal bleeding (Staats et al., 2007). Our patient initially sought care from many specialists at three area hospital systems until the last three years of her care, when she was more aggressively managed with frequent physical exams. Case reports clearly favor surgical treatment once extragonadal endometrioid adenocarcinoma is diagnosed. In a review of vaginal cases, 8/18 underwent radical resection and 8/18 underwent excisional biopsy or local resection. Information on adjuvant treatment was available for 14 cases, 7 of which underwent adjuvant chemotherapy or radiation therapy (Staats et al., 2007). In a review of rectosigmoid endometriosis-associated malignancies, 9/10 underwent surgical resection, with 8/10 receiving radiation and/or chemotherapy (Yang et al., 2019). While there has been one case report of a patient successfully treated with chemotherapy alone (Kondo et al., 2018), this appears to be the exception. Table 1 outlines the previously reported cases of primary vaginal endometrioid adenocarcinoma with their treatments and associated exposures that may have contributed to the development of malignancy.
Vaginal endometrioid adenocarcinoma is a rare primary malignancy with a reported association with endometriosis, but presence of an inclusion cyst in the setting of processes that increase the inflammatory cellular environment, such as multiple surgeries and sclerotherapy, could also lead to metaplastic changes and malignant transformation.
4. Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Jennifer Wolf: Investigation, Writing - original draft, Writing - review & editing. Amanda Jackson: Writing - review & editing. Thomas Herzog: Writing - review & editing. Ady Kendler: Resources, Writing - review & editing. Shaun A. Wahab: Resources, Writing - review & editing. Caroline Billingsley: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2020.100585.
Contributor Information
Jennifer Wolf, Email: wolf2je@ucmail.uc.edu.
Amanda Jackson, Email: jacks2a6@ucmail.uc.edu.
Thomas Herzog, Email: herzogtj@ucmail.uc.edu.
Ady Kendler, Email: kendlea@ucmail.uc.edu.
Shaun A. Wahab, Email: wahabsa@ucmail.uc.edu.
Caroline Billingsley, Email: billincn@ucmail.uc.edu.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Adjetey V., Ganesan R., Downey G.P. Primary vaginal endometrioid carcinoma following unopposed oestrogen administration. J. Obstet. Gynaecol. (Lahore). 2003;23(3):316–317. doi: 10.1080/01443610310000105975. [DOI] [PubMed] [Google Scholar]
- Bamford D.S. Primary adenocarcinoma of the Vagina. Proc. Roy. Soc. Med. 1967;60:999–1000. doi: 10.1177/003591576706001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasman W.T., Phillips J.L., Menck H.R. The national cancer data base report on cancer of the Vagina. Cancer. 1998;83:1033–1040. [PubMed] [Google Scholar]
- Decelle J. Cancerization simultanee de I’endometre “in situ” et ectopique. Bull. Sot. R Belg. Gynecol. Obs. 1969;36:241–254. [PubMed] [Google Scholar]
- Eckert R., Eckert R. Adenocarcinoma arising in endometriosis. Am. Fam. Physician. 2000;62(4):734–736. [PubMed] [Google Scholar]
- Granai C.O., Walters M.D., Safaii H., Jelen I., Madoc-Jones H., Moukhtar M. Malignant transformation of vaginal endometriosis. Obstet. Gynecol. 1984;64(4):592–595. [PubMed] [Google Scholar]
- Greten F.R., Grivennikov S.I. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskel S., Chen S.S., Spiegel G. Vaginal endometrioid adenocarcinoma arising in vaginal endometriosis: a case report and literature review. Gynecol. Oncol. 1989;34:232–236. doi: 10.1016/0090-8258(89)90149-2. [DOI] [PubMed] [Google Scholar]
- Hyman M.P. Extraovarian endometrioid carcinoma: review of the literature and report of two cases with unusual features. Am. J. Clin. Pathol. 1977;68:522–527. doi: 10.1093/ajcp/68.4.522. [DOI] [PubMed] [Google Scholar]
- Kajihara H., Yamada Y., Shigetomi H., Higashiura Y., Kobayashi H. The dichotomy in the histogenesis of endometriosis-associated ovarian cancer: clear cell-type versus endometrioid-type adenocarcinoma. Int. J. Gynecol. Pathol. 2012;31:304–312. doi: 10.1097/PGP.0b013e318243a97b. [DOI] [PubMed] [Google Scholar]
- Kapp D.S., Merino M., Livolsi V. Adenocarcinoma of the Vagina arising in endometriosis: long-term survival following radiation therapy. Gynecol. Oncol. 1982;14:271–278. doi: 10.1016/0090-8258(82)90099-3. [DOI] [PubMed] [Google Scholar]
- Kondo E., Maki S., Nii M., Yoshida K., Tabata T., Ikeda T. Long-term survival of a patient with malignant transformation of extragonadal endometriosis treated solely with chemotherapy: a case report. J. Obstet. Gynaecol. Res. 2018;44(12):2186–2189. doi: 10.1111/jog.13773. [DOI] [PubMed] [Google Scholar]
- Nezhat F., Datta M.S., Hanson V., Pejovic T., Nezhat C., Nezhat C. The relationship of endometriosis and ovarian malignancy: a review. Fertil. Steril. 2008;90(5):1559–1570. doi: 10.1016/j.fertnstert.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Orr J.W., Holimon J.L., Sisson P.F. Vaginal adenocarcinoma developing in residual pelvic endometriosis: a clinical dilemma. Gyn. 1989;33:96–98. doi: 10.1016/0090-8258(89)90611-2. [DOI] [PubMed] [Google Scholar]
- Robinson K.A., Menias C.O., Chen L. Understanding malignant transformation of endometriosis: imaging features with pathologic correlation. Abdom. Radiol. 2019 doi: 10.1007/s00261-019-01914-7. [DOI] [PubMed] [Google Scholar]
- Somoye G.O., Gull S. Adenocarcinoma of the vaginal vault following prolonged unopposed oestrogen hormone replacement therapy. J. Obstet. Gynaecol. (Lahore). 2005;25(2):220–221. doi: 10.1080/01443610500050991. [DOI] [PubMed] [Google Scholar]
- Staats P.N., Clement P.B., Young R.H. Primary endometrioid adenocarcinoma of the Vagina: a clinicopathologic study of 18 cases. Am. J. Surg. Pathol. 2007;31(10):1490–1501. doi: 10.1097/PAS.0b013e31804a7e9a. [DOI] [PubMed] [Google Scholar]
- Tewari D.S., McHale M.T., Kuo J.V., Monk B.J., Burger R.A. Primary invasive vaginal cancer in the setting of the Mayer-Rokitansky-Kuster-Hauser syndrome. Gynecol. Oncol. 2002;85(2):384–387. doi: 10.1006/gyno.2002.6637. [DOI] [PubMed] [Google Scholar]
- Vallerie A.M., Lerner J.P., Wright J.D., Baxi L.V. Peritoneal inclusions cysts: a review. Obstet. Gynecol. Surv. 2009;64(5):321–334. doi: 10.1097/OGX.0b013e31819f93d4. [DOI] [PubMed] [Google Scholar]
- Wirtheimer C. Deux cas d’adenocarcinoma endometrial d’origine differente. Bull. Sot. R Gynecol. Obs. 1964;34:117–129. [PubMed] [Google Scholar]
- Yang H., Gu J., Qi Y., Zhao W., Wang X. Endometrioid adenocarcinoma of the rectovaginal septum with invasion of the rectum: a case report and review of literature. World J. Surg. Oncol. 2019;17(206) doi: 10.1186/s12957-019-1743-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.