Abstract
Aims
A robust inflammatory response to tissue injury is a necessary part of the repair process but the deposition of scar tissue is a direct downstream consequence of this response in many tissues including the heart. Adult zebrafish not only possess the capacity to regenerate lost cardiomyocytes but also to remodel and resolve an extracellular scar within tissues such as the heart, but this scar resolution process remains poorly understood. This study aims to characterize the scarring and inflammatory responses to cardiac damage in adult zebrafish in full and investigate the role of different inflammatory subsets specifically in scarring and scar removal.
Methods and results
Using stable transgenic lines, whole organ imaging and genetic and pharmacological interventions, we demonstrate that multiple inflammatory cell lineages respond to cardiac injury in adult zebrafish. In particular, macrophage subsets (tnfα+ and tnfα−) play prominent roles with manipulation of different phenotypes suggesting that pro-inflammatory (tnfα+) macrophages promote scar deposition following cardiac injury whereas tnfα− macrophages facilitate scar removal during regeneration. Detailed analysis of these specific macrophage subsets reveals crucial roles for Csf1ra in promoting pro-inflammatory macrophage-mediated scar deposition. Additionally, the multifunctional cytokine Osteopontin (Opn) (spp1) is important for initial scar deposition but also for resolution of the inflammatory response and in late-stage ventricular collagen remodelling.
Conclusions
This study demonstrates the importance of a correctly balanced inflammatory response to facilitate scar deposition during repair but also to allow subsequent scar resolution, and full cardiac regeneration, to occur. We have identified Opn as having both pro-fibrotic but also potentially pro-regenerative roles in the adult zebrafish heart, driving Collagen deposition but also controlling inflammatory cell resolution.
Keywords: Zebrafish, Regeneration, Scarring, Heart failure, Inflammation
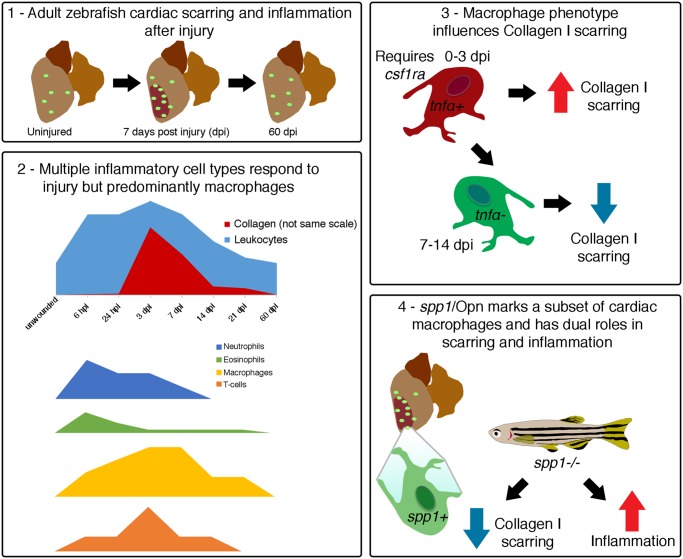
Graphical Abstract
Graphical Abstract.
1. Introduction
Complete tissue regeneration of multiple organs, including the heart, has been demonstrated in several vertebrate model systems such as the zebrafish.1–5 Mammals retain some regenerative capacity during early neonatal periods but later lose this ability.6,7 Instead, tissue injury in adult mammals, including ischaemic events such as a myocardial infarction, results in adverse tissue remodelling and irreversible scarring, producing a non-functional region of cardiac tissue. The inflammatory response to injury is necessary for correct and timely repair but also leads to activation of fibroblasts/stromal cells and deposition of scar collagen,6,8,9 limiting cardiac function and contributing to the progression of heart failure in patients.10
Multiple different immune cells are recruited to the ventricular myocardium following ischaemic injury in adult mammals.11,12 Macrophages play critical roles in tissue repair and scarring, but recent reports have suggested a, somewhat contradictory, role for macrophage subsets in complete regeneration of the neonatal mouse heart.13,14 Indeed, macrophage depletion at different time-points post-injury in a model of liver fibrosis can have dramatically different effects on fibrosis/scarring vs. regeneration.15 Additionally, recent studies in regenerative adult zebrafish and salamanders also suggest vital roles for macrophages in tissue regeneration with global reduction of numbers via clodronate liposome treatment resulting in impaired cardiac and limb regeneration.16–19 Therefore, there remains some controversy over the precise role of the inflammatory response in tissue regeneration and the factors that promote regeneration over fibrotic repair are not fully elucidated. Macrophages exist as a spectrum of different activation states that are controlled by a range of factors including transcriptional and epigenetic regulation, developmental pathways, and the local tissue microenvironment, which are incompletely understood.20–22 During the injury response, the appropriate balance of these activation states is thought to be vital for optimal wound healing and could be harnessed to promote regeneration.23,24
Here, we describe the timing, extent, and spatial distribution of the full inflammatory and scarring response to cardiac cryoinjury in a regenerative adult zebrafish model with a strong early recruitment of neutrophils followed by an influx of macrophages. Further investigation reveals two waves of macrophage populations, tnfα+ and tnfα− that predominate at different time-points post-injury and manipulations of each of these populations result in dramatic impairment of scar deposition and scar resolution, respectively. Additionally, we investigate the role of Osteopontin (Opn), a multifunctional glycoprotein expressed by fibroblasts and inflammatory cells and a potent fibrotic factor in mammals,25–27 in the response to cardiac injury. Importantly, we have identified bifunctional roles for Opn in both initial scar collagen deposition, similar to mammals, and subsequently as a regulator of macrophage phenotype driving scar and inflammatory cell resolution in our regenerative model.
2. Materials and methods
2.1 Zebrafish lines and cardiac injury
Details of transgenic lines and cardiac cryoinjury can be found in the Supplementary Material online. Animals were anaesthetized via immersion in 0.13% MS-222 (Sigma; A5040) in aquarium facility water for all procedures, once, for a maximum of 5 min. Animals were euthanized via immersion in an overdose of anaesthetic.
2.2 Immunofluorescence analysis, histology, in situ hybridization and imaging
Standard protocols were used for immunostaining, histology, and imaging of stable transgenic fluorescence. Details and antibodies used can be found in the Supplementary Material online. In situ hybridization was performed using a commercial spp1 probe (Dr-spp1; catalog no. 409501) and RNAscope® technology (ACD, USA) on 4% PFA fixed TgBAC(spp1:mCherry) larvae and adult hearts following manufacturers protocols. Following in situ, samples were labelled with an anti-RFP (1:250; MBL Life science; PM005) antibody using methods described in the Supplementary Material online.
2.3 Clodronate liposomes and drug treatments
Anaesthetized fish were intraperitoneally (IP) injected the day before cryoinjury (regime A) or at 3 days post-injury (dpi) (regime B) with 10 μL liposomes containing either clodronate or phosphate buffered saline (PBS) (10 mg/mL; FormuMax Sientific, Inc.; F70101C-A). For alteration of macrophage phenotype, anaesthetized zebrafish were IP injected with lipopolysaccharides (LPS) (5 μg in 10 μL PBS; Sigma; L2630) or recombinant zebrafish IL10 (10 μL of 6 M in PBS; Kingfisher Biotech/Cambridge Bioscience; RP1023Z) immediately after cardiac cryoinjury.
2.4 FACS
Ventricles of wildtype Tg(mpeg1:mCherry) were collected into PBS containing 10 mM HEPES, 30 mM taurine, and 5.5 mM glucose and cells dissociated by the addition of 0.25% trypsin, 12.5 M CaCl2, and 5 mg/mL Collagenase II (Worthington Biochemical Corp; LS004176). Dissociated cells were suspended in Leibovitz medium L-15 containing 0.3 mM glutamine (GIBCO), 0.8 mM CaCl2, Pen 50 U/mL/Strep 0.05 mg/mL, and 2% FCS. Mpeg1+ macrophages were sorted for mCherry expression on a BD Biosciences InFlux high-speed fluorescence activated cell sorter (San Jose, CA, USA).
2.5 Generation of TgBAC(spp1:mCherry) SN:374 and spp1−/− fish
To generate the TgBAC(spp1:mCherry)SN:374 reporter line, BAC recombination was performed as described.28 A BAC covering the full spp1 coding sequence was obtained [CH73-213K3; BACPAC Resources Centre (BPRC)] and the following primers were used to insert mCherry downstream of the spp1 start site (5′–3′): forward, tttagtttttctctctctgtttctcttctgttttagaatattttgcacacACCATGGTGAGCAAGGGCGAGGAG; reverse, ggtacacagaagactgtggcgacgaggagtgttaaaacaataatagatttTCAGAAGAACTCGTCAAGAAGGCG (lowercase indicates the BAC homology arms and uppercase indicates the mCherry cassette). To generate a spp1 mutant line a TALEN site was designed to target exon 7 of zebrafish spp1. TALEN plasmids were synthesized by ToolGen Genomics Toolmaker (Seoul, South Korea). The target site sequence was TACCACCATCATCCCAGTCACAGTCGATCCCACGCTGGGTCCCATTATCAACA, where TACCACCATCATCCCAGTCA represents the left TALEN and GCTGGGTCCCATTATCAACA represents the right. The red highlighted residue is deleted in the C331del line and the blue highlighted residues are deleted in the CGAT327-330del line. The TALEN plasmids were linearized, transcribed individually into TALEN mRNA using mMESSAGE mMACHINE T7 ULTRA kit (Ambion). Equal amounts (2 nL) of left and right TALEN mRNA were microinjected together into single-cell zebrafish embryos.
2.6 Statistics
In all cases n numbers refer to biological replicates. All experiments were repeated at least twice. Raw data recording and analysis was conducted using GraphPad Prism6/7. Power calculations (90% power at 5% significance) indicate that six animals are adequate for each parameter for statistical significance. This is also the number of fish used per time-point by others working on repair of the heart.2 Due to the variability of biological replicates we found that increased numbers were sometimes required to clarify findings. In some cases, fewer biological replicates were used whenever possible. For quantification of the inflammatory and scarring responses all heart regions beyond the ventricle (atrium, bulbous arteriosus, etc., including the heart valves) were manually removed from the image (×10 objective) in ImageJ. For quantification of the scarring response the percentage of the ventricle positive for Collagen I was calculated by thresholding the fluorescent signal of the whole ventricle (DAPI) and the Collagen I alone using ImageJ. The results are presented as the fold change compared to unwounded hearts. For quantification of the inflammatory response a threshold was applied to images of the whole ventricle (either sections or whole mount images) and the number of positive cells determined in ImageJ. For quantification of the scarring and inflammatory responses in sections, an average of three sections through the injury site were taken for each heart, to ensure a representative value was determined. The numbers of eosinophils and T-cells in whole mount images were counted manually due to the dim fluorescent signal. To determine the percentage of pro-inflammatory macrophages, images were taken just of the injury site (×25 objective) and the numbers of mpeg1+ cells co-expressing tnfα determined manually. All GFP+ cells, regardless of intensity, were counted and all cells with clear outlines were counted in each image (typically 250–600 cells). Counts were taken from maximum projections of z-stacks to ensure maximum numbers of cells were analysed. Only cells where clear co-localization (e.g. by cell shape/outline) of both fluorophores was apparent were counted to prevent errors regarding overlap of cells. For all data sets a ROUT outlier test was performed and any significant outliers [Q (maximum false discovery) = 1%] were removed. Statistical significance was determined via non-parametric Mann–Whitney or Kruskal–Wallis/Dunn’s multiple comparison tests (details provided in figure legends). In all cases error bars represent SD. Indicated significance = *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001.
2.7 Study approval
All experiments were conducted with approval from the local ethical review committee at the University of Bristol and in accordance with UK Home Office regulations.
3. Results
3.1 Characterization of the scarring and total inflammatory responses to cardiac injury
Cryoinjury to the adult zebrafish ventricle leads to repair with transient scarring that resolves over time (Figure 1A3). Mammalian studies have suggested a critical role for the inflammatory response in the formation of an extracellular scar8,9 but the precise involvement of immune cell lineages in scar deposition/resolution in a regenerative model has not been fully elucidated. We first determined the timing of the total inflammatory and scarring response to sham and cryoinjuries in comparison to unwounded tissue (Figure 1B–K). In unwounded hearts a tissue resident population of L-plastin+ immune cells resided predominantly around the edge of the ventricle (Figure 1B and C). L-plastin is a pan-leucocyte marker in zebrafish and mammals.29,30
Figure 1.
Analysis of the inflammatory and scarring response to cardiac cryoinjury. (A) Schematic showing the process of cardiac scarring and scar resolution in adult zebrafish following cryoinjury to the ventricle. (B–I) Representative images of immunofluorescence analysis of the pan-leucocyte marker, L-plastin, and the major scar matrix component, Collagen I, in cardiac tissues of unwounded and cryoinjured fish. The boxes in B, D, F, and H demark the approximate position of C, E, G, and I, respectively. The dashed lines in D and F demark the extent of the injured region. (B and C) In unwounded adult zebrafish hearts a sparse population of L-plastin+ leucocytes (arrowed in C) is observed. Some Collagen I expression is observed at low levels around the edge of the ventricle and at much higher levels in the cardiac valves (asterisks in B). (D–G) At 3 (D and E) and 7 days post-injury (dpi) (F and G), significant inflammatory and scarring responses are observed in the ventricle. (H and I) At 60 dpi both responses have resolved and the ventricle resembles that of unwounded hearts. (J and K) Quantification of the number of L-plastin+ cells (J) and the fold change of the percentage of the ventricle positive for Collagen I staining (K) within sections through the heart for the injury type and time-points indicated. Three sections through the injury area were quantified and an average taken for each fish. For each time-point and injury type n numbers are indicated. For statistical analyses in J and K, analysis of differences between sham and cryoinjuries were performed with a Kruskal–Wallis test at each time-point. Significant differences between sham and cryoinjury are shown beneath the cryoinjury line for relevant time-points. Kruskal–Wallis/Dunn’s multiple comparisons tests were used to analyse all data against unwounded (significance bars shown above graphs). All other values were not significant. Ep, epicardium; myo, myocardium. Scale bars: B, D, F, H = 250 μm, C, E, G, I = 20 μm.
The scarring response was assessed by immunofluorescence analysis and subsequent quantification of Collagen I, suggested to be the major scar component.31 Collagen I was observed at low levels beneath the epicardium and at high levels in the cardiac valves in unwounded hearts (Figure 1B and C). Following sham injury, no significant inflammatory or scarring responses were observed (Figure 1J and K). By contrast, cryoinjury elicited a significant inflammatory response at 3 dpi, and a significant scarring response at 3 and 7 dpi (Figure 1D–G, J, and K). Inflammatory cell influx and scarring were significantly elevated in cryoinjured hearts compared to sham injury at 3 and 7 dpi (Figure 1J and K). At 3 dpi, L-plastin+ cells are distributed throughout the myocardium, accumulating close to the injury site where considerable Collagen I deposition is observed (Figure 1D and E). By 7 dpi, L-plastin+ cells are restricted almost exclusively to the injury site where significant Collagen I is still detected (Figure 1F and G). At 14, 21, and 60 dpi both the inflammatory and scarring responses are reduced (Figure 1H–K). Indeed, at 60 dpi the heart resembles that of an unwounded fish with the deposited Collagen I completely resolved (Figure 1H and I).
Comparison of Collagen I and acid fuchsin orange G (AFOG) histological staining, which is commonly used to label scar collagen in injured hearts, on adjacent slides revealed similar distribution of collagens with both methods, particularly at early time-points (Supplementary material online, Figure S1). Collagen I staining generally gave a clearer result that was easier to quantify and less variable between different experiments. Interestingly, at 60 dpi (when scar tissue was only present in a few fish) Collagen I appears more restricted than collagen labelled with AFOG (Supplementary material online, Figure S1E and F) suggesting a different composition of these rare late scars, supporting previous results suggesting that interstitial fibroblasts express collagens other than Collagen I at later time-points.32 These preliminary examinations provide an accurate description of the time-line of repair and regeneration in relation to scarring and the total inflammatory response in the adult zebrafish heart.
3.2 Multiple inflammatory cell lineages respond to cardiac injury
Previous reports have highlighted the importance of different innate and adaptive inflammatory cell populations following cardiac injury and have suggested that some may be required for cardiac regeneration in different species.11–14,17,19,24 We next sought to determine the predominant inflammatory cell types that are present at different time-points post-injury in adult zebrafish cardiac tissue. Various transgenic zebrafish lines expressing fluorescently labelled immune cell lineages were cryoinjured and hearts imaged at 6 h post-injury (hpi), 1, 3, 7, 14, and 21 dpi (Figure 2). Neutrophils are generally considered to be the first responders to injury9 and analysis of either Tg(mpx:GFP)i114 or Tg(lyz:DsRed2) transgenic zebrafish reveals rapid recruitment of neutrophils following cardiac cryoinjury, peaking at 6 hpi, but with almost complete resolution by 7 dpi (Figure 2A and C). Although the vast majority of L-plastin+ cells at 6 hpi are neutrophils (Figure 2A; Supplementary material online, Figure S2A), analysis of Tg(gata2a:eGFP) transgenic fish reveals a significant recruitment of eosinophils to early zebrafish cardiac injury also (Figure 2H and I). Analysis of double transgenic Tg(gata2a:eGFP); Tg(lyz:DsRed2) fish demonstrates two separate cell populations confirming the specificity of the Tg(gata2a:eGFP) fish for eosinophils (Figure 2H).
Figure 2.
Individual immune cell lineages are predominant at different times post-injury. Representative images of whole hearts dissected from Tg(mpx:GFP) (A), Tg(lyz:dsRED2) (B), Tg(c-fms:GFP); Tg(mpeg1:mCherry) (D and E), Tg(lck:GFP) (G), and Tg(gata2a:GFP); Tg(lyz:dsRED2) (H) transgenic zebrafish at the time-points post-injury as indicated. The types of inflammatory cells labelled by the different transgenics are shown above the images. Quantification of the different inflammatory cells across a timeline from unwounded to 21 dpi (C, F, and I). In C the total L-plastin+ cells present at each timepoint is shown in blue. L-plastin+ data also shown in F and I to allow comparison to total inflammatory cell numbers (same data, grey in F and I). (A–C) Neutrophils [marked by Tg(mpx:GFP) (A) and Tg(lyz:dsRED2) (B)] peak at 6 hpi and resolve by 7 dpi (C). L-plastin labelling for all leucocytes (in magenta) is also shown in A. (D–F) Macrophages, marked by Tg(c-fms:GFP) and Tg(mpeg1:mCherry), are present in large numbers at all time-points and show significant infiltration compared to unwounded hearts at 7 dpi (F). The majority of macrophages express both transgenic reporters (inset in D). (G) Analysis of Tg(lck:GFP) fish reveals significant numbers of T-cells at 7 dpi.33 Eosinophils, labelled with Tg(gata2a:GFP) (H), are rarely observed in unwounded hearts but are significantly recruited at 6 hpi (I) and at later time-points (7, 14, and 21 dpi) (I). Tg(gata2a:GFP); Tg(lyz:dsRED2) double transgenic fish demonstrate two distinct populations of cells present at 24 hpi (inset in H). Boxed regions demark the approximate position of the insets for each panel. Dashed lines in A, B, and E–H demark the injured region. In C, F, and I, colour coded n numbers are provided for each time-point and transgenic reporter. L-plastin n numbers are only shown in C for clarity. For statistical analyses Kruskal–Wallis/Dunn’s multiple comparisons tests were used to analyse all data against unwounded for each data set. All other values were not significant. Scale bars: A, B, D, E, G, H = 250 μm; insets = 50 μm.
Analysis of two macrophage marker lines, Tg(mpeg1:mCherry) and Tg(c-fms:GFP) (csf1ra), revealed high numbers of macrophages present at all time-points (Figure 2D–F). In unwounded hearts, analysis of L-plastin+, mpeg1+, and c-fms+ cell numbers suggests that almost all tissue resident, L-plastin+ cells are macrophages (Figure 2D and F; Supplementary material online, Figure S2B). Significantly elevated numbers of macrophages were present at 7 dpi corresponding to the commencement of resolution (Figures 1K and 2E and F). This pattern is very similar to what has been reported for neonatal mice.13 Analysis of images in this way provides spatial information on inflammatory cell populations within the heart as well as enabling representative numbers of cells to be quantified. However, to determine the total numbers of macrophages present within the heart, ventricles of Tg(mpeg1:mCherry) fish were analysed by FACS at different time-points post-injury (Supplementary material online, Figure S2C and D) revealing an average of 0.5 ± 0.1% [unwounded (unw)], 1.2 ± 0.3% (1 dpi), and 7 ± 5% (3 dpi) mpeg1+ cells per fish (Supplementary material online, Figure S2C and D).
Recent reports have suggested a potential role for T-cells in the response to tissue injury34,35 so we investigated the number of T-cells responding to cryoinjury in adult zebrafish using a Tg(lck:GFP) transgenic reporter. Significant numbers of lck+ cells were recruited to the damaged ventricle, peaking at 7 dpi (Figure 2G and I) and were almost completely resolved by 14 dpi (Figure 2I). Additionally, significant numbers of eosinophils were also observed at 7, 14, and 21 dpi (Figure 2I). Interestingly, cumulative numbers of mpx+ (neutrophils), gata2a+ (eosinophils), mpeg1+ (macrophages), and lck+ (T-cells) present at each time-point does not notably differ from the total numbers of L-plastin+ cells suggesting that the cell types labelled by these transgenic markers represent the majority of all inflammatory cells responding to cardiac injury in adult zebrafish (Supplementary material online, Figure S2E). Collectively, this analysis demonstrates that multiple different inflammatory cell types respond to cardiac injury in adult zebrafish and are present at similar time points and in similar ratios as observed in mammals. The main difference to adult mammals is the rapid attenuation of the inflammatory response between 7 and 14 dpi.
3.3 Macrophage ablation at different time-points significantly alters scar deposition or resolution
As macrophages are the most abundant inflammatory cell type present in the heart at the crucial scar deposition and Collagen I regression time-points we sought to determine the role(s) of this population. To assess the early function of macrophages (up to 3 dpi), Tg(c-fms:GFP) zebrafish received IP injections of clodronate or PBS containing liposomes36 on the day prior to cryoinjury with cardiac tissue collected at 3 dpi [Figure 3A (regime A)]. To assess the later function of macrophages (3–7 dpi), fish were first cryoinjured and then allowed to recover until 3 dpi when the peak inflammatory and scarring responses are occurring, and only then injected with clodronate or PBS liposomes, and here the repairing cardiac tissue collected at 7 dpi [Figure 3B (regime B)]. In both cases the number of c-fms+ cells was significantly reduced when compared to controls at 3 days post-treatment (Figure 3A′ and B′ and Supplementary material online, Figure S3).
Figure 3.
Macrophage ablation results in altered levels of Collagen I. (A and B) Schematics describing the liposome treatment procedure for early (A) and late (B) ablation of macrophages. (A′ and B′) Quantification of the number of c-fms+ macrophages following either early (regime A; A and A′) or late (regime B; B and B′) ablation with clodronate liposomes as compared to PBS liposomes. (C–J) Immunofluorescence analysis of Collagen I and subsequent quantification of the fold change compared to unwounded hearts (regime A, D, H, and K). The quantity of Collagen I is increased in relation to PBS liposome injected following late ablation from 3 to 7 dpi (regime B, F, J and K). There is no significant difference between control Collagen I levels at 3 dpi and clodronate treated at 7 dpi suggesting a lack of resolution (K). The boxed regions in C–F demark the approximate position of G–J. Dashed lines in C–F demark the injured region. For statistical analyses in A′ and B′, Mann–Whitney tests were used. For statistical analyses in K, Mann–Whitney tests were used to analyse PBS vs. Clodronate liposome data in each regime and a Kruskal–Wallis/Dunn’s multiple comparisons test was used to analyse regime A against regime B. Scale bars: C–J = 250 μm.
Reducing the number of phagocytic cells during the early injury responses (Figure 3A) reduced Collagen I deposition within the myocardium suggesting a role for macrophages in scar formation in line with previous reports (Figure 3D, H, and K8,15). Conversely, depletion during the later stages of the injury response (Figure 3B), resulted in significantly more Collagen I at 7 dpi compared to control fish but no significant difference to control fish at 3 dpi, suggesting a failure in Collagen I resolution (Figure 3F, J, and K). These observations support previous reports from mammals that macrophages play a role in scar deposition.8,15 In addition, removal of phagocytic macrophages after Collagen I deposition results in a failure of scar resolution suggesting that macrophages are also pivotal in this more novel aspect of the healing process.
3.4 A more pro-inflammatory environment affects scarring
Macrophages exist as a spectrum of different activated phenotypes ranging from pro-inflammatory through to anti-inflammatory/pro-resolution and these subpopulations can, at least partially, be defined by the expression of cytokines and other markers.20–24,37 Next, we used a transgenic reporter line, TgBAC(tnfα:GFP), to assess the activation state of the macrophages responding to cardiac injury (Figure 4). In unwounded fish, very few tnfα+ cells were observed whereas there was rapid accumulation of tnfα+ cells following cryoinjury with highly significant numbers observed at 1 and 3 dpi (Figure 4A and B). The number of tnfα+ cells peaked with the early wave of macrophage influx at 3 dpi but reduced by 7 dpi when peak total macrophage numbers are observed and when scar resolution is commencing (Figures 1K, 2F and 4B).
Figure 4.
tnfα+ macrophages respond to cardiac injury and LPS treatment affects Collagen I scarring and results in changes in macrophage phenotype. (A and B) Representative images and quantification of whole hearts from TgBAC(tnfα:GFP) fish at different time-points post-injury. The dashed line in A demarks the extent of the injured region. L-plastin, mpx, and mpeg1 data from Figure 2C and F (greys) are provided as a reference (B). (C) Representative image of a heart from a Tg(mpeg1:mCherry); TgBAC(tnfα:GFP) double transgenic fish at 7 dpi (higher magnification shown in F). The boxed region demarks the approximate area imaged in D–F and all subsequent ventricular apex images. (D–F) Representative images of the ventricular apex of unwounded Tg(mpeg1:mCherry); TgBAC(tnfα: GFP) fish (D) and the injury area at 3 (E) and 7 dpi (F). Very few tnfα+ mpeg1+ cells are observed in unwounded hearts (D). (D′–F′) For each image, single channel panels and the merge of the boxed region is provided. (G) Quantification of the percentage of mpeg1+ macrophages at the injury site expressing tnfα at 3, 7, and 14 dpi with and without LPS treatment. (H and I) Representative images of the injury site of Tg(mpeg1:mCherry); TgBAC(tnfα:GFP) fish (H) and following LPS treatment (I) at 14 dpi. (J) Quantification of the Collagen I fold change as compared to unwounded fish in control and LPS treated fish. (K–L) Representative images of sections through the injury site of control (K) and LPS treated fish at 14 dpi (L). Quantification in B and G; statistical analysis by Kruskal–Wallis/Dunn’s multiple comparisons tests. n numbers for mpx+, mpeg1+, and L-plastin+ are shown in Figure 2. Quantification in J; statistical analysis by Mann–Whitney tests at each time-point. un-w, unwounded. Scale bars: A, C, K, L = 250 μm; D–F, H, I = 50 μm; inset in A, D′–F′ = 20 μm.
To further evaluate the potential opposing roles of these different macrophage populations, we analysed Tg(mpeg1:mCherry); TgBAC(tnfα:GFP) double transgenic fish in more detail (Figure 4C–H). In unwounded fish, very few tnfα+ macrophages were observed (Figure 4D and D′). Following cardiac cryoinjury, 91 ± 2% of mpeg1+ cells were also tnfα+ at 3 dpi (Figure 4E, E′, and G). At 7 and 14 dpi, however, this number was significantly reduced (65 ± 5% and 34 ± 9%, respectively; Figure 4F–H). We next sought to determine if a more pro-inflammatory environment might influence the scarring response. Treating Tg(mpeg1:mCherry); TgBAC(tnfα:GFP) fish with a single dose of LPS at the time of injury resulted in significantly more mpeg1+ macrophages expressing tnfα at 7 and 14 dpi when compared to untreated fish (Figure 4G–I), although the total number of macrophages was not significantly altered (Supplementary material online, Figure S4). Use of LPS to elicit a pro-inflammatory response has recently been validated in zebrafish.38 Analysis of the scarring response following these manipulations reveals significantly more Collagen I in the repairing ventricles of fish treated with LPS compared to controls at 7 and 14 dpi, suggesting an association between tnfα+ macrophages and scar deposition and that limiting the tnfα+ response is required for scar resolution to occur correctly. With these experiments we cannot completely rule out effects of other cell types (Figure 4J–L).
3.5 Csf1ra controls macrophage phenotype and drives scarring
We next assessed the inflammatory and scarring response to cardiac injury in csf1raj4e1/j4e1 mutant zebrafish. Csf1ra has been suggested to be important for macrophage differentiation and polarization and null Csf1r mouse mutants have reduced numbers of macrophages.39 As expected, analysis of csf1raj4e1/j4e1; Tg(mpeg1:mCherry) zebrafish revealed significantly reduced numbers of mpeg1+ macrophages at 3 and 7 dpi across the whole ventricle (Figure 5A–C). Numbers of mpeg1+ macrophages present only at the injury site in csf1raj4e1/j4e1; Tg(mpeg1:mCherry) fish, however, were similar to control fish suggesting that the remaining macrophages are functional and responsive to injury (Figure 5B and D). Interestingly, analysis of csf1raj4e1/j4e1; Tg(mpeg1:mCherry); TgBAC(tnfα:GFP) fish revealed a significant reduction in the number of tnfα+ macrophages at the injury site at 3 dpi compared to wildtype (58 ± 3%; Figure 5E, F and H) and these fish exhibited significantly reduced collagen deposition (Figure 5I–K). LPS treatment of csf1raj4e1/j4e1; Tg(mpeg1:mCherry); TgBAC(tnfα:GFP) fish partially rescued the number of tnfα+ macrophages responding to cardiac injury (81 ± 3%; Figure 4G and H) and this rescue was associated with significantly increased Collagen I deposition at 3 dpi suggesting a rescue of the scarring response also (Figure 5I and L).
Figure 5.
csf1ra is required for correct recruitment and a pro-inflammatory phenotype in cardiac macrophages. (A–D) Representative images of wildtype Tg(mpeg1:mCherry) (A) and Tg(mpeg1:mCherry); csf1raj4e1/j4e1 fish (B) at 7 dpi and quantification of the total number of macrophages in the ventricle (C) and macrophages present at the injury site (D). (E–G) Representative images of the injury site of TgBAC(tnfα:GFP); Tg(mpeg1:mCherry) control fish (E), TgBAC(tnfα:GFP); Tg(mpeg1:mCherry); csf1raj4e1/j4e1 treated with PBS (F), and TgBAC(tnfα:GFP); Tg(mpeg1:mCherry); csf1raj4e1/j4e1 treated with LPS (G) at 3 dpi. (H) Quantification of the percentage of mpeg1+ macrophages at the injury site expressing tnfα in control, TgBAC(tnfα:GFP); Tg(mpeg1:mCherry); csf1raj4e1/j4e1, TgBAC(tnfα:GFP); Tg(mpeg1:mCherry); csf1raj4e1/j4e1 fish treated with PBS, TgBAC(tnfα:GFP); Tg(mpeg1:mCherry); csf1raj4e1/j4e1 fish treated with LPS and wildtype TgBAC(tnfα:GFP); Tg(mpeg1:mCherry) fish treated with recombinant zebrafish IL10. (I) Quantification of the Collagen I fold-change in wildtype, csf1raj4e1/j4e1 fish, csf1raj4e1/j4e1 fish treated with PBS, csf1raj4e1/j4e1 fish treated with LPS, and wildtype fish treated with recombinant zebrafish IL10 at 3 dpi. (J–L) Representative images of Collagen I staining in wildtype (J), csf1raj4e1/j4e1 (K), and csf1raj4e1/j4e1 fish treated with LPS (L). Dashed lines in A, B, and J–L demark the injured region. Boxed regions in J–L demark the approximate position of the inset. Quantification in C and D; statistical analysis by Mann–Whitney tests of control and csf1raj4e1/j4e1 data at each time-point. Quantification in H and I; statistical analysis by Kruskal–Wallis/Dunn’s multiple comparisons tests. Scale bars: A, B, J–L = 250 μm; E–G = 100 μm; inset in J–L = 100 μm.
To further address whether an initial macrophage pro-inflammatory response is required for scar deposition we treated wildtype Tg(mpeg1:mCherry); TgBAC(tnfα:GFP) fish with recombinant zfIL10, a cytokine that promotes anti-inflammatory polarization in macrophages, at the time of injury (Figure 5H and I and Supplementary material online, Figure S5A–D). This treatment reduced the number of tnfα+ macrophages at the injury at 3 dpi (58 ± 7%; Figure 5H and Supplementary material online, Figure S5A and B) and the amount of Collagen I, recapitulating the csf1raj4e1/j4e1 fish (Figure 5I and Supplementary material online, Figure S5C and D). We also determined the number of proliferating cells present in the ventricle of wildtype and csf1raj4e1/j4e1 fish at 7 dpi. No significant differences were observed (Supplementary material online, Figure S5E–G). Collectively, our data suggest that an early pro-inflammatory response in macrophages, which requires Csf1ra, is necessary to initiate the scarring response in adult zebrafish but loss of csf1ra does not affect proliferation. Also, between 3 and 7 dpi, macrophages reduce tnfα expression and inhibiting this switch in phenotype with LPS treatment blocks scar resolution.
3.6 Opn is expressed in a subset of macrophages during scar resolution
One gene shown to be downstream of the injury inflammatory response and causal of fibrosis in mouse is spp1, encoding Opn.26,27,40 Opn is a multifunctional protein that plays intra- and extracellular roles both as a transcription factor and as a secreted factor that can bind integrins.25,41–43 Opn is expressed by both macrophages and fibroblasts following tissue injury in mammals26 and Spp1 has been shown to be up-regulated in pro-resolving macrophages in the mouse heart;24 however, Opn function has not been studied during tissue regeneration. To determine the cell types expressing spp1 in response to cardiac cryoinjury, we generated a reporter line—TgBAC(spp1:mCherry) (Figure 6). Analysis of this reporter line reveals that mCherry expression recapitulates the previously described expression pattern of spp1 during development (Figure 6A; Supplementary material online, Figure S6A–C).44 Analysis of hearts from adult TgBAC(spp1:mCherry); Tg(c-fms:GFP) fish reveals spp1 expression specifically at the site of cardiac injury at 3 dpi, partly co-localizing with Tg(c-fms:GFP) (Figure 6B–D). In situ hybridization confirms the specificity of the TgBAC(spp1:mCherry) line in the craniofacial skeleton and the heart (Supplementary material online, Figure S6A–F).
Figure 6.
TgBAC(spp1:mCherry) fish reveal spp1 expression in a subset of macrophages following cardiac injury. (A) Lateral image of the head of a TgBAC(spp1:mCherry) fish at 14 dpi demonstrating spp1 expression in the craniofacial skeleton, as reported previously.44 Anterior is to the left. Op, Operculum, Cl, Cleithrum. (B–D) Images of an adult heart from a TgBAC(spp1:mCherry); Tg(c-fms:GFP) fish demonstrating spp1 expression specifically at the injury site at 3 dpi (B), partially co-localizing with c-fms expression (C and D). (E–G) Confocal images of the apex of the ventricle of unwounded (E), 3 dpi (F), and 7 dpi (G) hearts from TgBAC(spp1:mCherry); Tg(c-fms:GFP) double transgenic fish. Very little spp1 expression is seen in macrophages of unwounded fish (E). (H) Quantification of the percentage of c-fms+ macrophages at the injury site that are expressing spp1. (I) Single z-plane of the boxed region in D. Other cells in the injury site also express spp1 (arrowheads). (J–L) spp1 is expressed in a subset of macrophages in the injured heart at 3 dpi (asterisks in L). Open arrowheads indicate c-fms+ macrophages not expressing spp1. (M–O) Quantification and representative images of the injury site of TgBAC(spp1:mCherry); Tg(c-fms:GFP) double transgenic fish following treatment with PBS (Control; N) or LPS (O). Statistical analysis in H; Kruskal–Wallis/Dunn’s multiple comparisons test. In M, statistical analysis was performed by a Mann–Whitney test. Scale bars: A = 200 μm, B–D = 250 μm, E–G, I, N, O = 100 μm, J–L = 10 μm.
Detailed analysis of TgBAC(spp1:mCherry); Tg(c-fms:GFP) fish demonstrates a subset of spp1+ c-fms+ macrophages following cardiac injury (Figure 6E–L) with a significant increase at 7 dpi (Figure 6E–H). Very few spp1+ c-fms+ cells are observed in unwounded hearts or at 1 dpi (Figure 6E and H). At 3 dpi, another subset of spp1+ cells are c-fms− and are most likely interstitial fibroblasts (Figure 6I26). Indeed, analysis of TgBAC(spp1:mCherry); ET37 fish suggests localization of spp1 in a subset of cardiac fibroblasts (Supplementary material online, Figure S6G). The enhancer trap line, ET37, has been shown to label larval mesenchymal cells and we have further verified this line as labelling cardiac fibroblasts (Supplementary material online, Figure S745). Analysis of TgBAC(spp1:mCherry); TgBAC(tnfα:GFP) fish at 3 and 7 dpi reveals double spp1+; tnfα+ and single positive cells suggesting dynamic expression changes of these two macrophage phenotype markers (Supplementary material online, Figure S6H and I). To further identify the macrophages expressing spp1 following cardiac injury we treated TgBAC(spp1:mCherry); Tg(c-fms:GFP) fish with LPS at the time of injury (Figure 6M–O) which significantly reduced the number of spp1+ macrophages suggesting these are more anti-inflammatory cells. Together, our data suggest that TgBAC(spp1:mCherry) fish recapitulate the developmental expression pattern of spp1, demonstrate expression in a subset of cell types in the heart, specifically after injury and that this expression is reduced with LPS treatment.
3.7 Opn is required for macrophage phenotypic switching, inflammatory response resolution, and scar remodelling
To determine whether zebrafish Opn plays a role in regulating macrophage phenotype during cardiac repair/regeneration, we generated spp1 mutant lines [spp1C331del/C331del/spp1CGAT327-330del/C327-330del (referred to as spp1−/−); Figure 7 and Supplementary material online, Figure S8]. These mutations result in frameshifts and a premature STOP codon that removes the majority of Opn functional domains predicted from mammals (Supplementary material online, Figure S8A and B) although we cannot currently confirm a complete lack of protein in these fish. The resulting adult fish appear grossly normal (Supplementary material online, Figure S8C and D). Analysis of the scarring phenotype in spp1−/− fish reveals a significant reduction in Collagen I deposition following cryoinjury at 3 and 7 dpi, similar to that described for Opn inhibition following skin wounding in mouse (Figure 7A–E26). Interestingly, the amount of scarring in the ventricle was significantly increased at later time points of 28 and 60 dpi by either Collagen I immunofluorescence or AFOG, which labels all collagens (Figure 7A and F–I), suggesting that zebrafish Opn plays a role in scar resolution also. To assess effects of spp1 mutation on cardiomyocyte regeneration we assessed proliferation in wildtype and spp1−/− fish at 7 dpi (Supplementary material online, Figure S8E–G). No significant differences were observed.
Figure 7.
Loss of spp1 results in an extended inflammatory response and altered scarring. (A) Quantification of Collagen I in the ventricle of wildtype vs. spp1−/− mutants at the time-points indicated. (B–I) Representative images of immunofluorescence analysis for Collagen I (B, C, F, and G) and AFOG (collagen in blue; D, E, H, and I) on sections through the ventricle of wildtype (B, D, F, and H) and spp1−/− mutants (C, E, G, and I) at 3, 7, 28, and 60 dpi. (J) Quantification of the number of L-plastin+ cells present in the ventricle of wildtype and spp1−/− mutants at the time-points indicated. (K and L) Representative images of immunofluorescence analysis of L-plastin and Collagen I on sections through the ventricle of wildtype (K) and spp1−/− mutants (L) at 14 dpi. (M) Quantification of the percentage of mpeg1+ macrophages at the injury site expressing tnfα in wildtype TgBAC(tnfα:GFP); Tg(mpeg1:mCherry) and spp1−/−; TgBAC(tnfα:GFP); Tg(mpeg1:mCherry) fish at 3 and 7 dpi. (N and O) Representative images of the injury site of wildtype TgBAC(tnfα:GFP); Tg(mpeg1:mCherry) (N) and spp1−/−; TgBAC(tnfα:GFP); Tg(mpeg1:mCherry) fish at 7 dpi (O). (P) Quantification of the number of mpeg1+ macrophages at the injury site in wildtype and spp1−/− mutants at 3 and 7 dpi. Dashed lines in B–E, I, K, and L demark the injured region. Boxed regions in B, C, F, and G demark the approximate position of the inset. Statistical analysis in A, J, M, and P by Mann–Whitney tests between wildtype and mutant at each time-point. Scale bars: B–I, K, L = 250 μm; inset in B, C, F, G, K, L = 50 μm; N, O = 100 μm.
Quantification of L-plastin+ cells responding to cardiac injury in spp1−/− fish reveals significantly higher numbers compared to wildtype at 7 and 14 dpi (Figure 7J–L) suggesting a failure in the resolution of the inflammatory response in the absence of Opn. Further analysis of spp1−/−; Tg(mpeg1:mCherry); TgBAC(tnfα:GFP) fish reveals increased numbers of tnfα+ macrophages at 3 and 7 dpi compared to wildtype fish (Figure 7M–O) although normal numbers of total macrophages are observed at the injury site (Figure 7P). This suggests that Opn is critical for controlling the switch in macrophage phenotype between 3 and 7 dpi from tnfα+ to tnfα− and that this is required for later resolution of inflammation and may impact on scar remodelling/resolution. Together, these data suggest bifunctional roles for Opn in both fibrosis/scar deposition, as in mammals, but also in regulation of inflammation and promotion of scar resolution, highlighting a previously unidentified role of Opn as a contributor to tissue regeneration.
4. Discussion
Cardiac scarring following tissue damage is a major contributing factor to the progression of heart failure, a leading cause of death in the western world. One major therapeutic goal is to improve the capacity of mammalian hearts to undergo regenerative rather than fibrotic repair. Interventions under investigation include generation of induced pluripotent stem cells (iPSCs) or differentiated cardiomyocytes and subsequent implantation into the infarcted region of the heart.46,47 Currently, however, the efficacy of these interventions remains limited.47 One factor limiting the success of these therapeutic strategies could be the adverse environment in which these cells are implanted. Scar tissue represents an abnormal, stiffened extracellular architecture that limits the connectivity and function of the cells embedded within it.48 Therefore, combined therapeutics that both provide replacement cells/trophic signals and limit the changes to, or improve, the extracellular environment at the site of an infarct could have major benefits.
The inflammatory response to tissue injury is a necessary consequence but one that drives subsequent fibrosis and scar formation.6,8,9 In this study, we demonstrate that adult zebrafish exhibit similar relative contributions of inflammatory cell types and scarring following cardiac injury to mammals; however, the inflammatory response is much more rapidly attenuated and subsequent scar resolution occurs. Our data indicates that different macrophage populations promote both the formation of a cardiac scar and subsequent scar resolution/regeneration. This supports recent reports demonstrating a requirement for macrophages as a whole for cardiac and limb regeneration.17–19 These recent reports described global leucocyte and neutrophil and macrophage infiltration to the heart and used depletion of macrophages with clodronate at the time of injury to demonstrate a requirement for these cells in promoting cardiomyocyte proliferation and in neutrophil clearance.17,19 Our findings complement and extend these previous studies by demonstrating the involvement of multiple inflammatory cell types and highlighting the importance of macrophage phenotype in regenerative processes. By manipulating macrophage subsets, either by global reduction or by influencing activation state, we demonstrate that tnfα+ macrophages promote scar deposition, but a more anti-inflammatory phenotype is necessary to allow scar resolution. Csf1ra is required to maintain normal numbers of macrophages in the whole heart but the numbers responding to injury are relatively normal in csf1raj4e1/j4e1 mutants. However, these fish exhibit significantly reduced tnfα+ macrophages and scarring, supporting a role for these pro-inflammatory cells in promoting scar deposition, although the overall reduced numbers of cardiac macrophages may contribute to the reduced scarring observed in these fish. In vitro data suggests that human pro-inflammatory (‘M1’) macrophages are less fibrotic than alternatively activated (‘M2’) macrophage populations;49 however, our data suggest that this view may be too simplistic and that more dynamically activated macrophage subpopulations may influence the balance between a pro- and anti-fibrotic milieu and that zebrafish are uniquely placed to decipher these dynamic activation states.
We have also identified a role for zebrafish Opn both in promoting scarring but also as a pro-regenerative factor in the heart. OPN is a multifunctional protein shown to be a pro-fibrotic factor downstream of the inflammatory response in mammals.26,27,40 OPN regulates mammalian haematopoietic stem cell homing to the bone marrow niche,50 a model of cellular regeneration, but its role in tissue regeneration is not known. We demonstrate that Opn is expressed in a subset of macrophages at the injury, with an increase in spp1+ cardiac macrophages at 7 dpi, a reciprocal expression pattern from that observed for tnfα. Studies in mammals have described contradictory roles for OPN in controlling macrophage phenotype; it may be a marker of alternatively activated ‘M2’ macrophages in the mouse heart24 but loss of OPN promotes a more anti-inflammatory phenotype in a model of muscular dystrophy.51 We have used tnfα and spp1 expression to define macrophage activation state, but further investigation will be required to delineate the precise phenotypes of macrophage populations in the adult zebrafish heart and to determine differences from mammalian macrophages, perhaps further illustrating why scar removal is so much more effective in zebrafish than in mammals.
spp1 −/− fish exhibit reduced collagen deposition at 3 and 7 dpi but they exhibit significantly more scarring at 28 and 60 dpi when compared to wildtype fish, indicating a failure in resolution. Our data supports a role for Opn in specifically promoting Collagen I deposition, as observed in mammals, as this is the predominant collagen present at early stages post-injury. Interestingly, a recent report suggests collagens other than Collagen I are expressed during zebrafish cardiac fibrosis and scarring, particularly at later stages,32 and our data supports this. Further work will be required to determine if Opn effects Collagen I and other collagens differently. We have shown that Opn promotes scarring, but also functions as a regenerative factor by facilitating the rapid attenuation of the inflammatory response, which characterizes the zebrafish response to cardiac injury. spp1−/− fish exhibit an uncontrolled and expanded inflammatory response at 7 and 14 dpi and a concurrent increase in the number of tnfα+ macrophages, further supporting the role of Opn in control of the inflammatory response to cardiac injury. Analysis of proliferation rates in either the whole ventricle or specifically in Tropomyosin+ cardiomyocytes did not reveal significant differences between csf1raj4e1/j4e1, spp1−/−, and wildtype fish suggesting cardiomyocyte regenerative capacity is unaffected by the loss of csf1ra or spp1 and that this regenerative process is uncoupled from scarring and scar resolution. However, spp1−/− fish fail to completely remove scar tissue demonstrating that modulation of abnormal deposited scarring is a pre-requisite for complete tissue regeneration, regardless of cardiomyocyte proliferative ability.
Collectively, our data suggest that adult zebrafish possess an intricately balanced inflammatory response to cardiac injury, with similarities to that described in mammals. However, zebrafish utilize an initial pro-inflammatory response to drive Collagen I scar deposition rapidly following ventricular damage and we have demonstrated that csf1ra is crucial for this early pro-inflammatory response and scarring. Subsequently, zebrafish macrophages exhibit a rapid phenotypic switch to reduce tnfα and increase spp1 expression and that this switch is necessary to allow inflammatory and scar resolution to occur. Our data provide evidence to suggest that the subtle regulation and balance of the inflammatory response and in particular of macrophage phenotypic state is crucial for scar resolution and subsequent full cardiac regeneration.
Translational perspective
Currently, curative treatments for severe cardiac scarring/heart failure include complex surgical interventions and, ultimately, a complete heart transplant. Therapeutic interventions that aim to improve the limited intrinsic regenerative potential of mammalian hearts or to reconstitute lost cardiomyocytes via exogenous stem cells or iPSCs are under intense investigation but have shown limited success, potentially due to the adverse scarred microenvironment these cells are placed into.46,47 Combining these therapies with future treatments that promote beneficial modulation of the extracellular microenvironment of the heart could have major benefits for the field of cardiovascular regenerative medicine.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the assistance of the Wolfson Bioimaging Facility for imaging expertise, Andrew Herman and Lorena Sueiro Ballesteros for cell sorting in the University of Bristol Faculty of Biomedical Sciences Flow Cytometry Facility, and Ashish Maurya for initial guidance in generating the spp1−/− line.
Conflict of interest: none declared.
Funding
This work was supported by a BHF Intermediate Fellowship to R.J.R. (FS/15/2/31225), BHF project grant to P.R.R. and P.M., a Wellcome Trust Investigator award to P.M. and MRC funding of a pre-clinical in-vivo functional imaging platform for translational regenerative medicine. Support for R.J.R. and P.M. was also provided by the BHF Oxbridge Centre of Regenerative Medicine (RM/13/03/30159). The work in BV’s lab was supported by the Biomedical Research Council of A*STAR, Singapore.
References
- 1. Poss KD, Wilson LG, Keating MT.. Heart regeneration in zebrafish. Science (New York, N.Y.) 2002;298:2188–2190. [DOI] [PubMed] [Google Scholar]
- 2. Chablais F, Veit J, Rainer G, Jaźwińska A.. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol 2011;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N.. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 2011;138:1663–1674. [DOI] [PubMed] [Google Scholar]
- 4. Schnabel K, Wu CC, Kurth T, Weidinger G.. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PloS One 2011;6:e18503.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang J, Panáková D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin Y-F, Sabeh MK, Werdich AA, Yelon D, MacRae CA, Poss KD.. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 2011;138:3421–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redd MJ, Cooper L, Wood W, Stramer B, Martin P.. Wound healing and inflammation: embryos reveal the way to perfect repair. Phil Trans R Soc Lond B 2004;359:777–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA.. Transient regenerative potential of the neonatal mouse heart. Science (New York, N.Y.) 2011;331:1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stramer BM, Mori R, Martin P.. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J Invest Dermatol 2007;127:1009–1017. [DOI] [PubMed] [Google Scholar]
- 9. Eming SA, Wynn TA, Martin P.. Inflammation and metabolism in tissue repair and regeneration. Science (New York, N.Y.) 2017;356:1026–1030. [DOI] [PubMed] [Google Scholar]
- 10. Talman V, Ruskoaho H.. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res 2016;365:563–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van der Laan AM, Nahrendorf M, Piek JJ.. Healing and adverse remodelling after acute myocardial infarction: role of the cellular immune response. Heart (British Cardiac Society) 2012;98:1384–1390. [DOI] [PubMed] [Google Scholar]
- 12. Chen B, Frangogiannis NG.. Immune cells in repair of the infarcted myocardium. Microcirculation (New York, N.Y.: 1994) 2017;24:e12305. [DOI] [PubMed] [Google Scholar]
- 13. Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN.. Macrophages are required for neonatal heart regeneration. J Clin Invest 2014;124:1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL.. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA 2014;111:16029–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP.. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 2005;115:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Godwin JW, Pinto AR, Rosenthal NA.. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA 2013;110:9415–9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Preux Charles A-S, Bise T, Baier F, Marro J, Jaźwińska A.. Distinct effects of inflammation on preconditioning and regeneration of the adult zebrafish heart. Open Biol 2016;6:160102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Godwin JW, Debuque R, Salimova E, Rosenthal NA.. Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen Med 2017;2: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lai S-L, Marín-Juez R, Moura PL, Kuenne C, Lai JKH, Tsedeke AT, Guenther S, Looso M, Stainier DY.. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. eLife 2017;6: e25605. doi: 10.7554/eLife.25605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ginhoux F, Jung S.. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014;14:392–404. [DOI] [PubMed] [Google Scholar]
- 21. Schultze JL, Schmidt SV.. Molecular features of macrophage activation. Semin Immunol 2015;27:416–423. [DOI] [PubMed] [Google Scholar]
- 22. Amit I, Winter DR, Jung S.. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol 2016;17:18–25. [DOI] [PubMed] [Google Scholar]
- 23. Novak ML, Koh TJ.. Macrophage phenotypes during tissue repair. J Leukoc Biol 2013;93:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, Adachi H, Yashiro K, Suzuki K.. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest 2016;126:2151–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Denhardt DT, Guo X.. Osteopontin: a protein with diverse functions. FASEB J 1993;7:1475–1482. [PubMed] [Google Scholar]
- 26. Mori R, Shaw TJ, Martin P.. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med 2008;205:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Singh M, Foster CR, Dalal S, Singh K.. Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol 2010;48:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bussmann J, Schulte-Merker S.. Rapid BAC selection for tol2-mediated transgenesis in zebrafish. Development 2011;138:4327–4332. [DOI] [PubMed] [Google Scholar]
- 29. Chen H, Mocsai A, Zhang H, Ding R-X, Morisaki JH, White M, Rothfork JM, Heiser P, Colucci-Guyon E, Lowell CA, Gresham HD, Allen PM, Brown EJ.. Role for plastin in host defense distinguishes integrin signaling from cell adhesion and spreading. Immunity 2003;19:95–104. [DOI] [PubMed] [Google Scholar]
- 30. Cvejic A, Hall C, Bak-Maier M, Flores MV, Crosier P, Redd MJ, Martin P.. Analysis of WASp function during the wound inflammatory response–live-imaging studies in zebrafish larvae. J Cell Sci 2008;121:3196–3206. [DOI] [PubMed] [Google Scholar]
- 31. Lovvorn HN, Cheung DT, Nimni ME, Perelman N, Estes JM, Adzick NS 3rd. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg 1999;34:218–223. [DOI] [PubMed] [Google Scholar]
- 32. Sanchez-Iranzo H. Transient fibrosis resolves via fibroblast inactivation in the regenerating zebrafish heart. Proc Natl Acad Sci USA 2018;115:4188–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hui SP. Zebrafish regulatory T cells mediate organ-specific regenerative programs. Dev Cell 2017;43:659–672.e655. [DOI] [PubMed] [Google Scholar]
- 34. Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, Kerkau T, Frantz S.. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 2012;125:1652–1663. [DOI] [PubMed] [Google Scholar]
- 35. Weirather J, Hofmann UDW, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S.. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res 2014;115:55–67. [DOI] [PubMed] [Google Scholar]
- 36. van Rooijen N, van Nieuwmegen R.. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res 1984;238:355–358. [DOI] [PubMed] [Google Scholar]
- 37. Nguyen-Chi M, Laplace-Builhe B, Travnickova J, Luz-Crawford P, Tejedor G, Phan QT, Duroux-Richard I, Levraud J-P, Kissa K, Lutfalla G, Jorgensen C, Djouad F.. Identification of polarized macrophage subsets in zebrafish. eLife 2015;4:e07288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forn-Cuni G, Varela M, Pereiro P, Novoa B, Figueras A.. Conserved gene regulation during acute inflammation between zebrafish and mammals. Sci Rep 2017;7:41905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dai XM. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood 2002;99:111–120. [DOI] [PubMed] [Google Scholar]
- 40. Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OHL, Apstein CS, Colucci WS, Singh K.. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res 2001;88:1080–1087. [DOI] [PubMed] [Google Scholar]
- 41. Butler WT. The nature and significance of osteopontin. Connect Tissue Res 1989;23:123–136. [DOI] [PubMed] [Google Scholar]
- 42. Hu DD, Lin EC, Kovach NL, Hoyer JR, Smith JW.. A biochemical characterization of the binding of osteopontin to integrins alpha v beta 1 and alpha v beta 5. J Biol Chem 1995;270:26232–26238. [DOI] [PubMed] [Google Scholar]
- 43. Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CAG, Sodek J.. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. Journal of Cellular Physiology 2000;184:118–130. [DOI] [PubMed] [Google Scholar]
- 44. Laue K, Janicke M, Plaster N, Sonntag C, Hammerschmidt M.. Restriction of retinoic acid activity by Cyp26b1 is required for proper timing and patterning of osteogenesis during zebrafish development. Development 2008;135:3775–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feitosa NM, Zhang J, Carney TJ, Metzger M, Korzh V, Bloch W, Hammerschmidt M.. Hemicentin 2 and Fibulin 1 are required for epidermal-dermal junction formation and fin mesenchymal cell migration during zebrafish development. Dev Biol 2012;369:235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li M, Izpisua Belmonte JC.. Mending a faltering heart. Circ Res 2016;118:344–351. [DOI] [PubMed] [Google Scholar]
- 47. Cahill TJ, Choudhury RP, Riley PR.. Heart regeneration and repair after myocardial infarction: translational opportunities for novel therapeutics. Nat Rev Drug Discov 2017; 16:699–717. [DOI] [PubMed] [Google Scholar]
- 48. Moore L, Fan D, Basu R, Kandalam V, Kassiri Z.. Tissue inhibitor of metalloproteinases (TIMPs) in heart failure. Heart Fail Rev 2012;17:693–706. [DOI] [PubMed] [Google Scholar]
- 49. Song E, Ouyang N, Hörbelt M, Antus B, Wang M, Exton MS.. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol 2000;204:19–28. [DOI] [PubMed] [Google Scholar]
- 50. Nilsson SK. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 2005;106:1232–1239. [DOI] [PubMed] [Google Scholar]
- 51. Capote J, Kramerova I, Martinez L, Vetrone S, Barton ER, Sweeney HL, Miceli MC, Spencer MJ.. Osteopontin ablation ameliorates muscular dystrophy by shifting macrophages to a pro-regenerative phenotype. J Cell Biol 2016;213:275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.